Abstract
Background
For endovascular rescue therapy (ERT) of cerebral vasospasm (CVS) due to spontaneous subarachnoid haemorrhage (sSAH), non-compliant (NCB) and compliant (CB) balloons are used with both balloon types bearing the risk of vessel injury due to specific mechanical properties. Although severe delayed arterial narrowing after transluminal balloon angioplasty (TBA) for CVS has sporadically been described, valid data concerning incidence and relevance are missing. Our aim was to analyse the radiological follow-up (RFU) of differently TBA-treated arteries (CB or NCB).
Methods
Twelve patients with utilisation of either NCB or CB for CVS were retrospectively analysed for clinical characteristics, ERT, functional outcome after 3 months and RFU. Compared with the initial angiogram, we classified delayed arterial narrowing as mild, moderate and severe (<30%, 30%–60%, respectively >60% calibre reduction).
Results
Twenty-three arteries were treated with CB, seven with NCB. The median first RFU was 11 months after TBA with CB and 10 after NCB. RFU was performed with catheter angiography in 18 arteries (78%) treated with CB and in five (71%) after NCB; magnetic resonance angiography was acquired in five vessels (22%) treated with CB and in two (29%) after NCB. Mild arterial narrowing was detected in three arteries (13%) after CB and in one (14%) after NCB. Moderate or severe findings were neither detected after use of CB nor NCB.
Conclusion
We found no relevant delayed arterial narrowing after TBA for CVS after sSAH. Despite previous assumptions that CB provides for more dilatation in segments adjacent to CVS, we observed no disadvantages concerning long-term adverse effects. Our data support TBA as a low-risk treatment option.
Keywords: aneurysm, complication, balloon, technique, intervention
Introduction
Cerebral vasospasm (CVS) after spontaneous subarachnoid haemorrhage (sSAH) can result in delayed cerebral ischaemia (DCI) and CVS-associated infarction, both worsening patients outcome. For endovascular rescue therapy (ERT), the intra-arterial (IA) administration of vasodilators and the use of transluminal balloon angioplasty (TBA) are proven techniques to treat severe and refractory CVS.1 2 In this context, TBA is implemented in addition to spasmolytic drugs in proximal intracranial arteries. The precise influence of TBA on the spastic vessel wall is still unclear, but it is believed to lead to prolonged dilation.3–5 Disadvantageously, TBA can lead to vessel injury and poses the risk of severe complications.6
There are two different balloon types used in connection with ERT for CVS: compliant balloons (CB) can be more easily implemented and are assumed to produce stronger dilative pressure in adjacent vessel parts because of variations in the final inflation diameter with expansion into the section of the artery with a larger calibre and potentially have a higher risk of edge dissections than non-compliant balloons (NCB).7 8 NCB, in turn, exert a stronger radial force;8 thus, their overdilation can lead to damage in the smaller spastic part of the vessel itself, whereas the final inflation diameter of NCB is well defined9 (figure 1).
Figure 1.
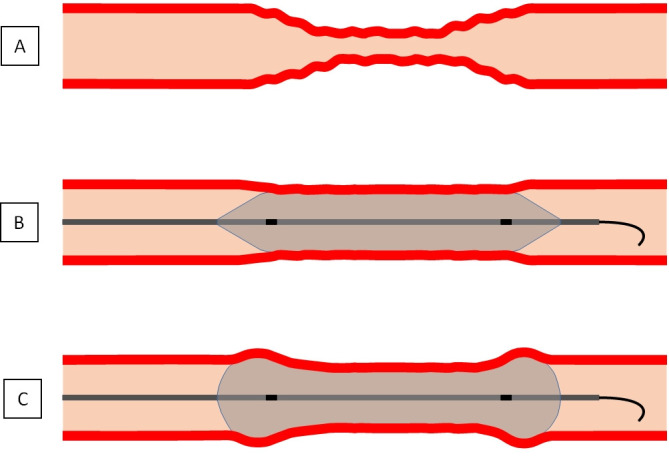
Illustration of (A) cerebral vasospasm and its endovascular treatment with angioplasty using a (B) non-compliant or a (C) compliant (with hypothetical ‘dog-bone’ phenomenon) balloon.
Similar effects for the local treatment of CVS with CB and NCB on angiographic and clinical outcomes have already been demonstrated.8 However, there are only a few clinical studies and case reports on severe delayed arterial narrowing after TBA for CVS.10–14 Systematic investigations that compare long-term effects of CB and NCB are still lacking. Thus, we reviewed for the first time radiological follow-up (RFU) in vessel segments formerly treated with TBA for CVS with CB or NCB in this case series.
Methods
All analyses were carried out on anonymised patient data and thus exempt from informed consent after institutional review board approval.
In our single-centre university clinic setting, we retrospectively reviewed all consecutive patients from 2011 to 2018 with sSAH who developed symptomatic CVS and underwent TBA as part of ERT. Patient data were collected with regard to clinical characteristics (gender, age, clinical grading (World Federation of Neurosurgical Societies (WFNS) and Fisher scale)), localisation of the aneurysm, the initially chosen treatment modality and the distribution of TBA in connection with severe CVS in large proximal arteries from the electronic patient chart.
ERT with TBA was analysed concerning localisation, the preceded execution of IA nimodipine infusion, the chosen balloon type (CB or NCB) and any complications (bleeding, dissection, vascular occlusion or thromboembolic events).
Furthermore, we assessed the occurrence of CVS-associated infarction within 48 hours after TBA in CT, in-hospital mortality and functional outcome after 3 months using the modified Rankin scale (mRS) (dichotomised into favourable (mRS 0–2) or unfavourable (mRS 3–6) outcome). In CT analyses, preexisting ischaemia related to former stroke, in association with the initial endovascular treatment, as a result of intracerebral haemorrhage or after ventricular catheter placement were not included.15
For each patient, we analysed the first acquired RFU after patient’s discharge concerning the chosen imaging modality and in that the presence of delayed arterial narrowing in a formerly TBA-treated vessel segment.
Indication for and technique of ERT with transluminal balloon angioplasty
After the event of sSAH, all patients were monitored on a neurointensive care unit. Nimodipine was applied routinely for prevention of CVS at a dosage of 240 mg/day orally. All patients received daily transcranial Doppler (TCD) measurements. If patients were conscious, hourly neurological assessments were performed, while intubated and sedated patients were monitored by multimodal invasive neuromonitoring including continuous intracranial pressure, cerebral perfusion pressure and regional brain tissue oxygenation (PBrO2) measurements. We routinely performed volume perfusion CT (VPCT) including a baseline examination on the day of admission and subsequently every 4 days, according to study results of Sanelli et al and Westermaier et al,16 17 except in awake and clinically unremarkable patients. In cases with hydrocephalus, external ventricular drainage was placed to drain cerebrospinal fluid.
DCI was defined by the presence of one of the following signs:
Sudden clinical deterioration with a decrease in consciousness and/or the presence of new neurological deficits.15
Refractory decrease in PBrO2 below 15 mm Hg and/or an increase in TCD mean flow velocities (FV) of ≥150 cm/s or an increase in FV by 50 cm/s over 24 hours.18
If DCI was suspected as a result of one of the abovementioned signs, we performed haemodynamic therapy (rise in mean arterial pressure to 90–110 mm Hg) in addition to orally applied nimodipine. If signs of DCI persisted, VPCT or catheter angiography was performed. We defined symptomatic CVS as the presence of arterial narrowing in the angiograms in patients with DCI.15
We carried out all ERT under general anaesthesia with a biplane X-ray system (Allura Xper FD20/20; Philips Healthcare) via femoral access. TBA was conducted only in severe findings (≥50% narrowing of the affected vessel compared with the baseline angiogram) in large cerebral arteries (including the proximal part of the second segment of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA)). We performed TBA either primarily in case of recurrent CVS after previous IA nimodipine instillation or secondarily as an addition to IA nimodipine, if there was poor angiographic response. For nimodipine instillation up to 3 mg per upstream vessel (internal carotid artery (ICA) or vertebral artery) were given. TBA was performed via 5 French (F) or 6F catheters utilising a 0.014 inch microwire. CB or NCB were chosen at the neuroradiologist’s discretion. We routinely used a roadmap mask and performed TBA under continuous fluoroscopy with careful and gentle stepwise grading and repeated inflations (≤3 per vessel segment, duration ≤10 s) until a ‘good’ response (defined as ≥50% dilation after TBA compared with the initial angiogram of the procedure) was achieved. Furthermore, we reviewed the final angiogram for complications (bleeding, dissection, vascular occlusion or thromboembolic events).
Radiographic follow-up and arterial calibre measurements
Under capture of the vessel segments formerly treated with TBA, the first acquired radiological control examination with visualisation of the cerebral arteries via catheter angiography or alternative three dimensional time-of-flight magnetic resonance angiography (TOF MRA) on 1.5T or 3T scanners (Achieva or Ingenia; Philips Healthcare), in some cases also with additional contrast-enhanced MRA, were considered. Furthermore, RFU was analysed by two experienced neuroradiologists with respect to vessel calibre changes or signs of damage in formerly TBA-treated arteries. In RFU, we defined delayed arterial narrowing by reduction in vessel calibre compared with the initial and final angiogram of the TBA procedures. Calibre reduction of <30% was classified as ‘mild’, from 30% to 60% as ‘moderate’ and >60% as ‘severe’.
Statistics
Statistical analysis was performed using the software IBM SPSS V.24.0. Functional outcome (mRS) was dichotomised into favourable outcome (mRS 0–2) and poor outcome (mRS 3–6). Categorical variables were analysed in contingency tables using χ2 test. Mann-Whitney U test was used to compare continuous variables. Statistical significance was accepted at p<0.05.
Results
Clinical characteristics
The median age of the 12 selected patients was 38.5 years (range 25–78). Nine were female (75%). In all cases of a detected bleeding source, treatment of the aneurysm consisted in endovascular obliteration. Four of 12 patients (33%) were initially classified as poor-grade SAH (WFNS grade 4 or 5). Fisher grades were predominantly high (grade 3 or 4 in 11 of 12 patients) with no clear difference between the subgroups of patients with favourable (mean Fisher grade 3) and unfavourable outcome (mean Fisher grade 3.5). WFNS grades were higher in the subgroup with unfavourable outcome with a mean of 3.5 versus 1.5 in the subgroup with favourable outcome.
The localisation of the endovascularly treated aneurysm, its method of obliteration and the distribution of TBA for CVS are listed in table 1.
Table 1.
Patient data, clinical characteristics, endovascular rescue therapy and outcome
| Patient number | Clinical characteristics | Endovascular rescue therapy | Outcome | ||||||||||
| Age | Gender | WFNS grade | Fisher scale | Aneurysm localisation | Aneurysm obliteration | Localisation of TBA | Intra-arterial nimodipine | Balloon type (NCB, CB) | Complications | CVS-associated infarction | In-hpospital mortality | mRS after 3 months | |
| 1 | 51 | f | 2 | 3 | BA | Coiling | MCA | + | NCB 2.0×9 mm Gateway (Boston Scientific) | – | – | – | 4 |
| 2 | 33 | f | 4 | 4 | ICA | Coiling | ICA+ACA+MCA | + | CB 4.0×10 mm HyperGlide (Medtronic) | – | – | – | 2 |
| 3 | 35 | m | 2 | 4 | SCA | Coiling | ICA | + | NCB 2.5×20 mm Ryujin Plus (Terumo) | – | – | – | 3 |
| 4 | 65 | f | 3 | 3 | MCA | Coiling | MCA | + | CB 4.0×7 mm TransForm (Stryker) | – | + | – | 4 |
| 5 | 46 | m | 5 | 3 | MCA | Coiling/stent-assisted | MCA | + | CB 4.0×7 mm TransForm (Stryker) | – | + | – | 4 |
| 6 | 39 | f | 1 | 3 | ACA | Coiling | ICA+ACA+MCA+VAS | + | CB 4.0×15 mm X-Pedion HyperGlide (Medtronic), NCB 2.5×9 mm Maverick (Boston Scientific) |
– | – | – | 2 |
| 7 | 33 | m | 1 | 3 | Angiogram-negative | – | ICA+MCA | + | CB 4.0×7 mm X-Pedion HyperForm (Medtronic) | – | – | – | 0 |
| 8 | 30 | f | 1 | 2 | BA | Coiling/stent-assisted | ACA | – | CB 4.0×10 mm X-Pedion HyperGlide (Medtronic) | – | – | – | 2 |
| 9 | 78 | f | 5 | 4 | PCOM | Coiling/stent-assisted | MCA | – | CB 4.0×7 mm TransForm (Stryker) | – | + | + | 6 |
| 10 | 38 | f | 1 | 3 | ICA | Coiling | ACA+MCA | + | CB 4.0×10 mm TransForm (Stryker) | – | – | – | 1 |
| 11 | 52 | f | 4 | 4 | ICA | Coiling/stent-assisted | ACA+MCA | + | CB 4.0×10 mm TransForm (Stryker) | – | – | – | 4 |
| 12 | 25 | f | 1 | 3 | MCA | WEB-Device | MCA | + | CB 4.0×10 mm TransForm (Stryker) | – | – | – | 1 |
ACA, anterior cerebral artery; BA, basilar artery; CB, compliant balloon; CVS, cerebral vasospasm; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin scale; NCB, non-compliant balloon; PCOM, posterior communicating artery; SCA, superior cerebellar artery; TBA, transluminal balloon angioplasty; WFNS, World Federation of Neurosurgical Societies.
Endovascular rescue therapy
In connection with severe CVS of large intracranial arteries, we treated 23 vessel segments with CB and seven with NCB. In the majority of treated patients with RFU, IA nimodipine was first instilled (10/12) and TBA was carried out only with a CB (9/12; 75%). One patient was treated with both CB and NCB. No complications occurred in connection with TBA.
Tables 1 and 2 show the detailed presentation of all data concerning ERT.
Table 2.
Data on endovascular rescue therapy and radiological follow-up
| Compliant balloon |
Non-compliant balloon |
P-value | |
| No. of treated vessel segments | 23 | 7 | |
| Localisation | 0.194 | ||
| ICA | 3 (0.13) | 3 (0.43) | |
| ACA | 7 (0.30) | 0 (0.00) | |
| MCA | 11 (0.48) | 3 (0.43) | |
| VAS | 2 (0.09) | 1 (0.14) | |
| Median radiological follow-up (range) (in months) |
11 (5–26) | 10 (7–28) | 0.701 |
| Imaging modalities | 0.532 | ||
| Catheter angiography | 18 (0.78) | 5 (0.71) | |
| TOF MRA | 5 (0.22) | 2 (0.29) | |
| Arterial narrowing in radiological follow-up | 0.677 | ||
| Mild | 3 (0.13) | 1 (0.14) | |
| Moderate or severe | 0 (0.00) | 0 (0.00) |
ACA, anterior cerebral artery; ICA, internal carotid artery; MCA, middle cerebral artery; TOF MRA, time-of-flight magnetic resonance angiography; VAS, vertebrobasilar arterial system.
Outcome
Three patients (25%) developed CVS-associated infarction, one patient died in-hospital. These events were not related to TBA. Favourable outcome (mRS 0–2) after 3 months was noted in six patients (50%).
The complete outcome data are also listed in table 1.
Radiological follow-up
Twenty-three vessel segments formerly treated with CB and seven vessel segments formerly treated with NCB were analysed in RFU. The median time of RFU was 11 months after TBA with CB and 10 months after TBA with NCB. RFU was performed with catheter angiography in 18 vessel segments (78%) formerly treated with CB and TOF MRA was utilised in five vessel segments (22%) formerly treated with CB. RFU was performed with catheter angiography in five vessel segments (71%) formerly treated with NCB and performed with TOF MRA in two vessel segments (29%) formerly treated with NCB. Mild arterial narrowing was detected in 3 (13%) vessel segments after ERT with CB and in one vessel segment (14%) after ERT with NCB. Moderate or severe arterial narrowing was neither detected after CB nor NCB.
We present all data concerning RFU in table 2. Furthermore, examples of RFU are presented in figures 2 and 3.
Figure 2.
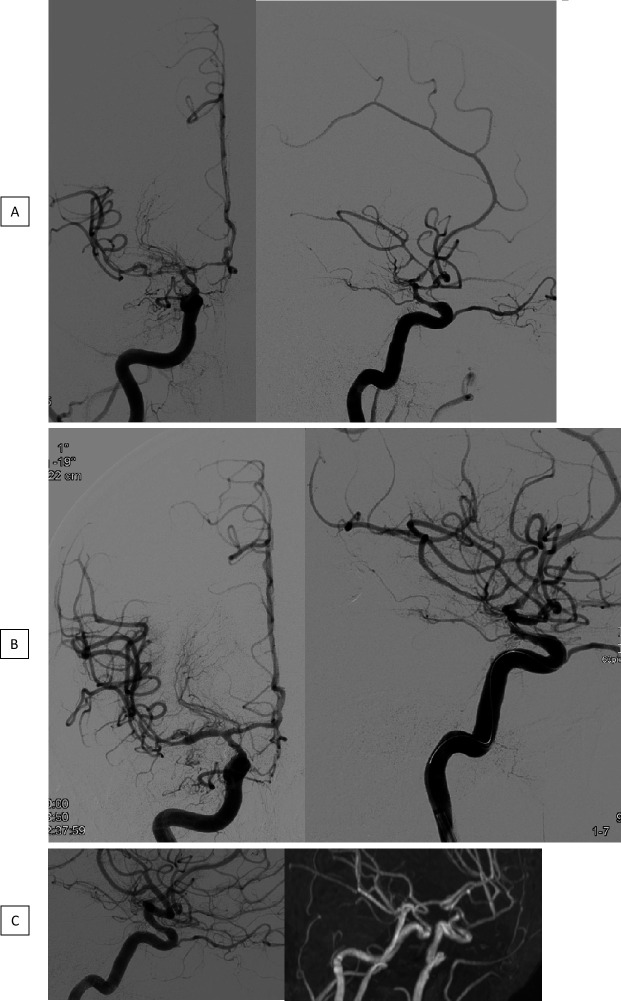
Angiogram before (A) and after (B) TBA with a CB in connection with severe CVS in the distal ICA as well as within the proximal segments of the MCA and ACA. Mild arterial narrowing in the catheter angiography and TOF MRA, 6 months later (C). ACA, anterior cerebral artery; CB, compliant balloons; CVS, cerebral vasospasm; ICA, internal carotid artery; MCA, middle cerebral artery; TBA, transluminal balloon angioplasty; TOF MRA, time-of-flight magnetic resonance angiography.
Figure 3.
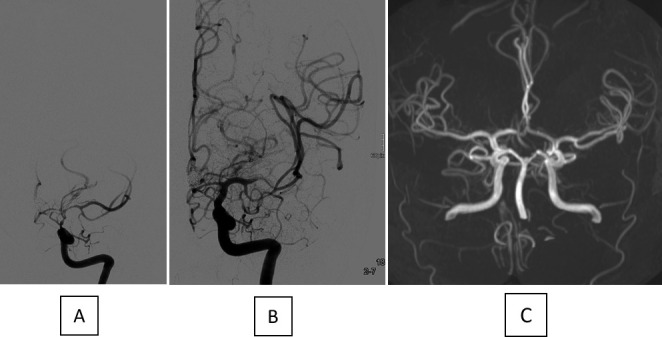
Angiogram before (A) and after (B) TBA with a CB in connection with severe CVS in the distal ICA as well as the proximal segments of the MCA and ACA. No arterial narrowing is visible in the TOF MRA 11 months later (C). ACA, anterior cerebral artery; CB, compliant balloon; CVS, cerebral vasospasm; ICA, internal carotid artery; MCA, middle cerebral artery; TBA; transluminal balloon angioplasty; TOF MRA, time-of-flight magnetic resonance angiography.
Discussion
So far, no prospective randomised clinical data on ERT for CVS after sSAH are available. The administration of nimodipine is well known to be evidence-based only when given orally (Class I, Level of Evidence A);19 in addition, there is no evidence that prophylactic use of TBA is beneficial.20 When medical therapy fails, many institutions implement IA vasodilators including the use of microcatheters with supraselective application;1 2 21 and/or TBA, whereby this method is mostly applied in proximal vessels, and also has Class IIa, Level of Evidence B according to the guidelines by Connolly and colleagues.19 More recent data on patients locally treated in connection with CVS support frequent and early ERT as favourable against the development of DCI and thus for good functional outcome, which provides Class III evidence.22
Another recent study on ERT in CVS suggests that severe findings within large intracranial arteries (especially of distal segments thereof) represent a major determinant of DCI and thus a potential therapeutic target.23 A further indication of the benefit of TBA is the data from Labeyrie et al, who showed that TBA in distal arteries (up to the second segments) decreased DCI and recurrent CVS in their series.24 On the other hand, a higher rate of complications has recently been reported in association with TBA for treatment of CVS.6 In contrast, recently published studies were able to show that patients with repetitive ERT due to recurrent CVS could achieve a favourable clinical course as well as that the outcome in patients undergoing multiple ERT procedures was similar to patients with only one endovascular treatment for CVS, even after taking all complications into account.18 25
In our experience, and in our opinion, the question is not whether one should perform additional ERT for symptomatic CVS after sSAH in general, but how to select the proper technique and the suited patient. Thus, an analysis of subgroups within the patient collective becomes necessary. To this end, both general ERT-related and recent TBA-specific risk factors were examined that are associated with an unfavourable course for CVS.26 27 There is a lack of discussion on the different types of balloons used, which represents a major cost factor for the materials while performing ERT. For example, the cost of CB used for ERT is up to 20-fold higher than that of NCB at our institution with a price range about between US$40 and US$927. Those different material costs should be weighted, especially as the indication for TBA potentially expands in the future. Up to now, there are no well-established indication criteria for TBA. Published criteria in literature vary, with the decision to perform TBA sometimes being described merely as operator dependent, but most often TBA is used, like we did in our study, in case of severe spasms of proximal large vessels with CVS refractory for respectively recurrent after IA nimodipine instillation.6 21 26 28 29 Compared with initial vasospasm, a good angiographic result could be achieved in all 12 cases of this study after the use of TBA.
The current study is the first to focus on RFU concerning delayed arterial narrowing in vessel segments treated with TBA according to the balloon type. The goal was to assist in clinical decision-making based on material-associated risk of complications and cost–benefit aspects.
However, TBA is a well-established method in the therapy of CVS that has been used for over 35 years.30 This technique has continuously undergone new developments.31 The differences between the mechanisms causing vasodilation for CB and NCB have already been explained above. One clear advantage in the more widely used NCB is the ability to very precisely control the balloon inflation; however, its rigidity can also present a risk factor.32 This point represents an advantage for CB, which provides for a gentler widening of the artery wall. CB have been associated with edge dissections, which is defined as stretching of the adjacent vessel segments and has been coined the ‘dog-bone’ phenomenon among literature on cardiological interventions using stents in arteriosclerotic stenosis.33 34 These findings cannot be directly applied to CVS in our opinion, as CVS is caused by temporary vasoconstriction and because cerebral arteries have different properties than coronary arteries.35 At our institution, we use CB for ERT in CVS much more often than NCB, as shown in this publication. This can be explained by the fact that our neurointerventionists often use the balloon remodelling method to treat aneurysms and are thus more familiar with the equipment and technique.36 The outcome of the patients in this study is rather poor with a favourable outcome of only 50%. The collective of the study consists understandably of patients, who show, compared with other SAH patients who do not need ERT, rather severe clinical courses. Although there is only a small number of patients which does not allow for profound statistical analysis, there can be noticed at least a trend concerning the clinical characteristics at time of admission with higher WFNS grades in the patients with unfavourable outcome (mean WFNS grade 3.5) and almost exclusively WFNS grade 1 in patients with favourable outcome (mean WFNS grade 1.5). Only in the subgroup of patients with unfavourable outcome, there occurred CVS-associated infarction (3 of 6). In the current study, there did not occur complications in any of the 12 cases associated with TBA in connection with ERT for CVS; one of our previous works on TBA reported on a single dissection related to an angioplasty with a NCB.27
Within this context, it should be mentioned again that previous studies have described the complication rate associated with TBA as a local treatment option for CVS in a very heterogeneous manner overall, so that it is impossible to derive a clear difference in risk between CB and NCB.6 28 29 Throughout the literature, a wide array of balloon catheters has been used. Adami et al reported on TBA-associated infarction in 5/88 patients (6%), both dissection-related and thromboembolism-related, without reporting on the materials used.6 One multicentre study on TBA for CVS using only NCB reported on no complications among the overall collective of 52 patients.28 Chaudry et al used only CB in connection with ERT of CVS and reported no TBA-associated complications in 35 treated segments of the ACA in 17 patients.37 Andaluz et al had similar results in their analyses of 50 patients with refractory CVS treated with CB.38 Further studies dedicated to CB were also able to demonstrate a complication-free course: Gross et al (18 patients, 64 vessels) and Heit et al (five patients, 11 vessels).7 39 We most often (6/12 patients with RFU) used the CB TransForm (Stryker, Fremont, CA, USA), which showed no complications in a prospective study on balloon-assisted coiling of aneurysms with at least one TBA for the treatment of CVS.40 A more recent study by Weiss et al on 33 consecutive patients with 54 ERT performed with NCB reported two cases of thrombus formation and three cases of postprocedural infarction within the realm of reported primary safety criteria.29 In contrast, in the formerly mentioned comparison study on CB versus NCB including 30 patients and 85 treated arteries with 34 CB and 51 NCB, no complications were reported for both balloon types.8
The last mentioned work by Miley et al did not include long-term follow-up in the vessels treated, but instead focused on the necessity of repetitive angioplasty, and was able to show that the different balloon types are equal in this respect.8 To our knowledge, comparative follow-up for CB/NCB in ERT for CVS has not been systematically evaluated to date. Srinivasan et al reported on long-term follow-up after TBA in CVS but only by measuring FVs with TCD.11 In that study, 28 patients were analysed with reevaluation at an average of 44 months after TBA. In addition to clinical data, TCD was used for follow-up examination, and all patients showed normal FV in the previously treated arteries. Umeoka et al analysed 32 patients who underwent successful TBA for CVS; however, only NCB was employed in this study.10 These patients underwent follow-up via CT angiography (CTA) and MRA with a follow-up range of 6 to 126 months and a mean of 12 months; restenosis were not detected in any patients.
In addition, several case reports can be found on this topic: Merchant et al described so-called delayed stenosis of the ICA bilaterally on CTA 6 months after local treatment of a patient with NCB for CVS.12 The case report by Sedat et al reported on a case of CVS treated with CB in which severe renarrowing in the origin of the ACA and MCA were detected 5 months later in catheter angiography as well as 12 months later in the CTA; these findings were clinically silent.13 Safain and Malek reported on delayed progressive bilateral stenosis in the supraclinoid ICA in a patient with a ruptured aneurysm of the basilar artery who underwent TBA with a CB for CVS within the ICA as demonstrated in the 3-month-follow-up after ERT with MRA and consecutive catheter angiography.14
The development of a severe stenosis of a cerebral artery after TBA presumably presupposes a significant mechanical damage to the vessel wall, which overall seems to be a rather rare event in spastic cerebral vessels undergoing TBA. The theoretically possible ‘dog-bone’ phenomenon with the risk of edge dissections when using CB occurs probably rather in rigid atherosclerotic vessels. Spastic cerebral vessel walls are presumably most often comparatively flexible, which unlikely results in a ‘dog-boning’ of the CB, respectively, in a vessel damage due to use of NCB, provided that it is used with caution. This might be an explanation for the fact that only single cases of severe delayed narrowing in vessels can be found on follow-up despite the large number of patients treated with TBA as part of ERT and supports our own results that did not show typical pathological findings of arterial narrowing in general as well as related to a specific balloon type. Our goal was never to exhaustively analyse every aspect of TBA, but rather to prompt further studies on this topic, especially with respect to materials used and complication rates and cost effectiveness.
Conclusion
In RFU, we found no evidence of relevant delayed arterial narrowing after previous TBA in connection with CVS after sSAH. Despite a formerly assumed stronger dilatation force of CB in vessel segments adjacent to CVS, we observed no significant disadvantages compared with NCB. Even after reviewing the literature, we deem the risks associated with TBA, especially in association with CB, to be very low. The choice of balloon involves advantages and disadvantages for each type and should be at the individual discretion of the neurointerventionist with the avoidance of complications.
Limitations
Retrospective design and a small case number are the main limitations of our study. One reason for the small number may be the often severe clinical course after SAH with loss of follow-up due to patients’ death. Furthermore, in our collective, there were only patients with endovascular obliteration of detected bleeding aneurysms. This could represent a selection bias as a result of a better control of the treated patients with our own initialisation of RFU, whereas after clipping RFU was only rarely acquired. There is heterogeneity in the method of RFU with cross-sectional angiography instead of catheter angiography being used in a subgroup of the study collective. Furthermore, with lack of a bigger collective, this study is not able to compare the different balloon types as well as to determine predictors for outcome or infarction. Finally, we were limited by short length of follow-up, as many of the patients were lost in the course due to the severe impact of SAH in general.
Footnotes
Contributors: AN had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AN, JK and HS. Acquisition, analysis or interpretation of data: AN, JK, CD, JL and HS. Drafting of the manuscript: KK. Critical revision of the manuscript and important intellectual contribution: PS. Statistical analysis: JK.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1. Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care 2016;20:277. 10.1186/s13054-016-1447-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li K, Barras CD, Chandra RV, et al. A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg 2019;126:513–27. 10.1016/j.wneu.2019.03.083 [DOI] [PubMed] [Google Scholar]
- 3. Chan PD, Findlay JM, Vollrath B, et al. Pharmacological and morphological effects of in vitro transluminal balloon angioplasty on normal and vasospastic canine basilar arteries. J Neurosurg 1995;83:522–30. 10.3171/jns.1995.83.3.0522 [DOI] [PubMed] [Google Scholar]
- 4. Megyesi JF, Findlay JM, Vollrath B, et al. In vivo angioplasty prevents the development of vasospasm in canine carotid arteries. pharmacological and morphological analyses. Stroke 1997;28:1216–24. 10.1161/01.str.28.6.1216 [DOI] [PubMed] [Google Scholar]
- 5. Megyesi JF, Vollrath B, Cook DA, et al. Long-term effects of in vivo angioplasty in normal and vasospastic canine carotid arteries: pharmacological and morphological analyses. J Neurosurg 1999;91:100–8. 10.3171/jns.1999.91.1.0100 [DOI] [PubMed] [Google Scholar]
- 6. Adami D, Berkefeld J, Platz J, et al. Complication rate of intraarterial treatment of severe cerebral vasospasm after subarachnoid hemorrhage with nimodipine and percutaneous transluminal balloon angioplasty: worth the risk? J Neuroradiol 2019;46:15–24. 10.1016/j.neurad.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 7. Gross BA, Tonetti DA, Weiner GM, et al. Septoplasty: scepter balloon angioplasty for vasospasm after aneurysmal subarachnoid hemorrhage. Interv Neurol 2017;6:229–35. 10.1159/000477467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miley JT, Tariq N, Souslian FG, et al. Comparison between angioplasty using compliant and noncompliant balloons for treatment of cerebral vasospasm associated with subarachnoid hemorrhage. Neurosurgery 2011;69:161–8. 10.1227/NEU.0b013e31822a8976 [DOI] [PubMed] [Google Scholar]
- 9. Grande A, Nichols C, Kha U. Treatment of post-hemorrhagic cerebral vasospasm: Role of endovascular therapy. : Early brain injury or cerebral vasospasm: volume 2: clinical management. Springer-Verlag Wien, 2011: 127–32. [DOI] [PubMed] [Google Scholar]
- 10. Umeoka K, Kominami S, Mizunari T, et al. Cerebral artery restenosis following transluminal balloon angioplasty for vasospasm after subarachnoid hemorrhage. Surg Neurol Int 2011;2:43. 10.4103/2152-7806.79758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srinivasan J, Moore A, Eskridge J, et al. Long-term follow up of angioplasty for cerebral vasospasm. Acta Neurochir Suppl 2001;77:195–7. 10.1007/978-3-7091-6232-3_41 [DOI] [PubMed] [Google Scholar]
- 12. Merchant A, Drazin D, Dalfino J, et al. Delayed stenosis as a consequence of angioplasty for subarachnoid hemorrhage-induced vasospasm. Case report. Neurosurg Focus 2009;26:E23. 10.3171/2009.2.FOCUS0912 [DOI] [PubMed] [Google Scholar]
- 13. Sedat J, Chau Y, Popolo M, et al. Restenosis after balloon angioplasty for cerebral vasospasm. Cardiovasc Intervent Radiol 2009;32:337–40. 10.1007/s00270-008-9419-0 [DOI] [PubMed] [Google Scholar]
- 14. Safain MG, Malek AM. Delayed progressive bilateral supraclinoid internal carotid artery stenosis in a patient with a ruptured basilar artery aneurysm. J Clin Neurosci 2015;22:368–72. 10.1016/j.jocn.2014.06.101 [DOI] [PubMed] [Google Scholar]
- 15. Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary Research Group. Stroke 2010;41:2391–5. 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 16. Sanelli PC, Jou A, Gold R, et al. Using CT perfusion during the early baseline period in aneurysmal subarachnoid hemorrhage to assess for development of vasospasm. Neuroradiology 2011;53:425–34. 10.1007/s00234-010-0752-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Westermaier T, Pham M, Stetter C, et al. Value of transcranial Doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care 2014;20:406–12. 10.1007/s12028-013-9896-0 [DOI] [PubMed] [Google Scholar]
- 18. Ditz C, Neumann A, Wojak J, et al. Repeated endovascular treatments in patients with recurrent cerebral Vasospasms after subarachnoid hemorrhage: a worthwhile strategy? World Neurosurg 2018;112:e791–8. 10.1016/j.wneu.2018.01.156 [DOI] [PubMed] [Google Scholar]
- 19. Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart Association/american stroke association. Stroke 2012;43:1711–37. 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 20. Zwienenberg-Lee M, Hartman J, Rudisill N, et al. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke 2008;39:1759–65. 10.1161/STROKEAHA.107.502666 [DOI] [PubMed] [Google Scholar]
- 21. Jun P, Ko NU, English JD, et al. Endovascular treatment of medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2010;31:1911–6. 10.3174/ajnr.A2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jabbarli R, Pierscianek D, Rölz R, et al. Endovascular treatment of cerebral vasospasm after subarachnoid hemorrhage: more is more. Neurology 2019;93:e458–66. 10.1212/WNL.0000000000007862 [DOI] [PubMed] [Google Scholar]
- 23. Brami J, Chousterman B, Boulouis G, et al. Delayed cerebral infarction is systematically associated with a cerebral vasospasm of large intracranial arteries. Neurosurgery 2020;86:E175–83. 10.1093/neuros/nyz340 [DOI] [PubMed] [Google Scholar]
- 24. Labeyrie M-A, Gaugain S, Boulouis G, et al. Distal balloon angioplasty of cerebral vasospasm decreases the risk of delayed cerebral infarction. AJNR Am J Neuroradiol 2019;40:1342–8. 10.3174/ajnr.A6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neumann A, Ditz C, Schacht H, et al. Symptomatic cerebral vasospasm after spontaneous subarachnoid hemorrhage: comparison of single and multiple intra-arterial treatment with respect to the functional outcome. J Neurol Surg A Cent Eur Neurosurg 2020;81:220–6. 10.1055/s-0039-1698434 [DOI] [PubMed] [Google Scholar]
- 26. Sokolowski JD, Chen C-J, Ding D, et al. Endovascular treatment for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: predictors of outcome and retreatment. J Neurointerv Surg 2018;10:367–74. 10.1136/neurintsurg-2017-013363 [DOI] [PubMed] [Google Scholar]
- 27. Schacht H, Küchler J, Boppel T, et al. Transluminal balloon angioplasty for cerebral vasospasm after spontaneous subarachnoid hemorrhage: a single-center experience. Clin Neurol Neurosurg 2020;188:105590. 10.1016/j.clineuro.2019.105590 [DOI] [PubMed] [Google Scholar]
- 28. Patel AS, Griessenauer CJ, Gupta R, et al. Safety and efficacy of noncompliant balloon angioplasty for the treatment of subarachnoid hemorrhage-induced vasospasm: a multicenter study. World Neurosurg 2017;98:189–97. 10.1016/j.wneu.2016.10.064 [DOI] [PubMed] [Google Scholar]
- 29. Weiss M, Conzen C, Mueller M, et al. Endovascular rescue treatment for delayed cerebral ischemia after subarachnoid hemorrhage is safe and effective. Front Neurol 2019;10:136. 10.3389/fneur.2019.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zubkov YN, Nikiforov BM, Shustin VA. Balloon catheter technique for dilatation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochir 1984;70:65–79. 10.1007/BF01406044 [DOI] [PubMed] [Google Scholar]
- 31. Alaraj A, Wallace A, Dashti R, et al. Balloons in endovascular neurosurgery: history and current applications. Neurosurgery 2014;74:S163–90. 10.1227/NEU.0000000000000220 [DOI] [PubMed] [Google Scholar]
- 32. Eskridge JM, McAuliffe W, Song JK, et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery 1998;42:510–6. 10.1097/00006123-199803000-00016 [DOI] [PubMed] [Google Scholar]
- 33. Romagnoli E, Sangiorgi GM, Cosgrave J, et al. Drug-eluting stenting: the case for post-dilation. JACC Cardiovasc Interv 2008;1:22–31. 10.1016/j.jcin.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 34. Hehrlein C, DeVries JJ, Arab A, et al. Role of the "dogbone" effect of balloon-expandable stents: quantitative coronary analysis of DUET and NIR stent implantation introducing a novel indexing system. J Invasive Cardiol 2002;14:59–65. [PubMed] [Google Scholar]
- 35. Barnard ZR, Alexander MJ. Update in the treatment of intracranial atherosclerotic disease. Stroke Vasc Neurol 2020;5:59–64. 10.1136/svn-2019-000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moret J, Cognard C, Weill A, et al. The "remodelling technique" in the treatment of wide neck intracranial aneurysms. angiographic results and clinical follow-up in 56 cases. Interv Neuroradiol 1997;3:21–35. 10.1177/159101999700300103 [DOI] [PubMed] [Google Scholar]
- 37. Chaudhry NS, Orning JL, Shakur SF, et al. Safety and efficacy of balloon angioplasty of the anterior cerebral artery for vasospasm treatment after subarachnoid hemorrhage. Interv Neuroradiol 2017;23:372–7. 10.1177/1591019917699980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andaluz N, Tomsick TA, Tew JM, et al. Indications for endovascular therapy for refractory vasospasm after aneurysmal subarachnoid hemorrhage: experience at the University of Cincinnati. Surg Neurol 2002;58:131–8. 10.1016/s0090-3019(02)00789-9 [DOI] [PubMed] [Google Scholar]
- 39. Heit JJ, Choudhri O, Marks MP, et al. Cerebral angioplasty using the Scepter XC dual lumen balloon for the treatment of vasospasm following intracranial aneurysm rupture. J Neurointerv Surg 2015;7:56–61. 10.1136/neurintsurg-2013-011043 [DOI] [PubMed] [Google Scholar]
- 40. Trivelato FP, Rezende MTS, Fonseca LV, et al. Preliminary experience with the transform occlusion balloon catheter: safety and potential advantages. Clin Neuroradiol 2018;28:25–31. 10.1007/s00062-016-0519-y [DOI] [PubMed] [Google Scholar]