Abstract
Background and purpose
Detection of atrial fibrillation (AF) after acute ischaemic stroke is pivotal for the timely initiation of anticoagulation to prevent recurrence. Besides heart rhythm monitoring, various blood biomarkers have been suggested as complimentary diagnostic tools for AF. We aimed to summarise data on the performance of cardiac natriuretic peptides for the diagnosis of covert AF after acute ischaemic stroke and to assess their potential clinical utility.
Methods
We searched PubMed and Embase for prospective studies reporting the performance of B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) for the diagnosis of covert AF after acute ischaemic stroke. Summary diagnostic performance measures were pooled using bivariate meta-analysis with a random-effect model.
Results
We included six studies focusing on BNP (n=1930) and three studies focusing on NT-proBNP (n=623). BNP had a sensitivity of 0.83 (95% CI 0.64 to 0.93), a specificity of 0.74 (0.67 to 0.81), a positive likelihood ratio of 3.2 (2.6 to 4.0) and a negative likelihood ratio of 0.23 (0.11 to 0.49). NT-proBNP had a sensitivity of 0.91 (0.65 to 0.98), a specificity of 0.77 (0.52 to 0.91), a positive likelihood ratio of 3.9 (1.8 to 8.7) and a negative likelihood ratio of 0.12 (0.03 to 0.48). Considering a pretest probability of 20%, BNP and NT-proBNP had post-test probabilities of 45% and 50%.
Conclusions
NT-proBNP has a better performance than BNP for the diagnosis of covert AF after acute ischaemic stroke. Both biomarkers have low post-test probabilities and may not be used as a stand-alone decision-making tool for the diagnosis of covert AF in patients with acute ischaemic stroke. However, they may be useful for a screening strategy aiming to select patients for long-term monitoring of the heart rhythm.
Keywords: stroke, statistics, embolic
Introduction
Atrial fibrillation (AF) is responsible for approximately 20% of all ischaemic strokes.1 Stroke secondary to AF is usually more severe and associated with greater recurrence and mortality rates.2 Oral anticoagulation is a readily available secondary prevention strategy. Therefore, timely and accurate diagnosis of AF after stroke is pivotal. The sensitivity of standard ECG for the detection of AF is low especially in patients with paroxysmal AF.3 Prolonged ECG monitoring has higher detection rates but is expensive, time consuming and hard to generalise to a routine clinical scenario.
Inflammation and haemodynamic stress are important pathophysiological substrates for AF and thrombus development in the left atrial appendage, and both factors trigger the production of natriuretic peptides by cardiomyocytes.4 The haemodynamic stress could result from underlying cardiac or systemic comorbidities but could also be a consequence of the catecholamine release after an acute stroke.5 6 Cardioembolic strokes usually cause larger brain lesions and are typically associated with higher levels of cardiac natriuretic peptides.7–9 Several studies have suggested that cardiac natriuretic peptides could serve as a useful biomarker for the diagnosis of AF in patients with ischaemic stroke.10 Previous meta-analyses have focused on the validity of cardiac natriuretic peptides for the diagnosis of cardioembolic stroke with documented paroxysmal or persistent AF or with evidence of other emboligenic heart diseases.11 12 However, a diagnostic biomarker is more relevant in patients with stroke in whom there is no pre-existing evidence of AF. We conducted this systematic review and meta-analysis to summarise data on the performance of cardiac natriuretic peptides for the diagnosis of covert AF after acute ischaemic stroke and to assess their potential clinical utility.
Methods
We searched PubMed and Embase, up to 15 December 2019, for prospective studies reporting the performance of cardiac natriuretic peptides for the diagnosis of covert AF after acute ischaemic stroke. Small cases series (<30 participants) and studies including patients with pre-existing or chronic AF were excluded. The search strategy is available in online supplemental table I. Agreement between reviewers for study selection was assessed using the Cohen’s kappa statistic which indicates the proportion of the possible beyond chance agreement that is attributable to the individual performance of the observers.13 Disagreements were solved through discussion and consensus. The Quality Assessment of Diagnostic Accuracy Studies-2 tool was used to assess the methodological quality of each study.14 Risk of bias and applicability concerns summary and graph were derived using the software RevMan (V.5.3, Cochrane Collaboration, London, UK).
svn-2020-000440supp001.pdf (408.4KB, pdf)
A bivariate meta-analysis with random-effects model was performed to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic OR (DOR) for B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP). A hierarchical summary receiver operating characteristic curve was derived and an area under the curve (AUC) calculated.15 16
The performances of BNP and NT-proBNP for the diagnosis of covert AF were analysed separately owing to substantial differences in primary structures, plasma levels, plasma half-lives, elimination pathways and antibodies used in detection assays.17–20 Although AF was not detected at the time of stroke onset or blood sampling in the included studies, the analyses of diagnostic accuracy were performed under the assumption that patients diagnosed with AF during follow-up already had an underlying paroxysmal AF that could be the cause of the stroke.
We investigated the interstudy heterogeneity by assessing the presence of a threshold effect. This was done by calculating the Spearman rank correlation coefficient between true positive and false positive rates, computing the I2 index, and performing the χ2 test on the Cochrane’s Q statistic.15 16 21 Publication bias was assessed by Deeks’ funnel plot asymmetry test.
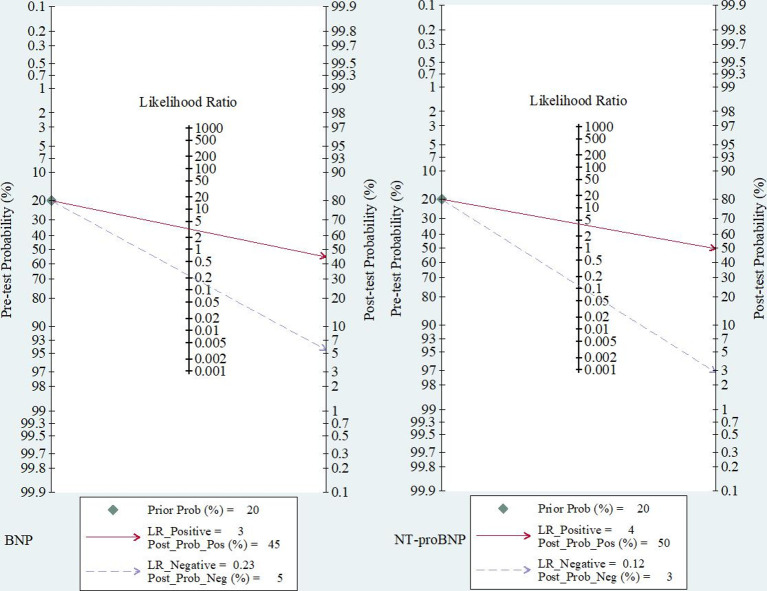
The clinical utility of the biomarkers was assessed by computing post-test probabilities on Fagan nomograms based on Bayes Theorem.22 23 We considered a pretest probability of 20% since it is the proportion of stroke attributed to AF and the proportion of patients without carotid disease who were diagnosed with AF after an average follow-up of 3.4 years in the New Approach Rivaroxaban Inhibition of factor Xa in the Global Trial vs Aspirin to Prevent Embolism in Embolic Stroke of Undetermined Source (NAVIGATE-ESUS) trial.1 24 We opted to use the most recent estimate from the NAVIGATE-ESUS trial rather than the 12.4% estimate from the Cryptogenic Stroke and Underlying AF (CRYSTAL-AF) trial25 because recent evidence suggests that patients with embolic stroke of undetermined source who have an ipsilateral mild carotid stenosis are less likely to have AF and may have a large-vessel stroke.26 In the CRYSTAL-AF trial, the lower incidence of AF despite the use of implantable cardiac monitors may be due to the inclusion of patients with mild carotid stenosis and the shorter duration of follow-up.27
All statistical tests were two-sided and statistical significance defined as p≤0.05. Data analysis was performed with the software STATA (V.13.0, StataCorp, College Station, Texas, USA) using the dedicated MIDAS module.23 This report is compliant with the Preferred Reporting Items for Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies guidelines.28 The database is available from the corresponding author on reasonable request.
Results
There were six studies of the diagnostic validity of BNP (n=1930)29–34 and three for NT-proBNP (n=623)35–37 (online supplemental figure I). There was 97.8% agreement between the investigators for study inclusion (κ=0.86).
Five studies recruited patients within 24 hours of stroke onset. In all studies, blood samples were collected on admission. All patients had a 12-lead ECG and a 24 hours Holter ECG at baseline, followed by single or repeated prolonged monitoring periods. Levels of cardiac natriuretic peptides were measured using electrochemiluminescence immunosorbent assay in five studies and chemiluminescence immunosorbent assay in four. The mean age of the participants was >60 years in eight studies and the mean time to AF diagnosis ranged from 3 to 36 days. The characteristics of the included studies are summarised in online supplemental table II. The overall quality of the included studies was high (online supplemental figures II and III).
The summary performance measures for the diagnosis of covert AF using BNP were as follows: sensitivity of 0.83 (95% CI 0.64 to 0.93), specificity of 0.74 (95% CI 0.67 to 0.81), PLR of 3.2 (95% CI 2.6 to 4.0), NLR of 0.23 (95% CI 0.11 to 0.49), DOR of 14 (95% CI 7 to 27) and AUC of 0.82 (95% CI 0.79 to 0.85) (figure 1 and online supplemental figure IV). Using a pretest probability of 20%, the post-test probability of AF was 45% for a positive test and 5% for a negative test (figure 2). There was a positive correlation between true and false positive rates (Spearman ρ=0.54), suggesting a threshold effect responsible for 64% of the observed heterogeneity (I2=97%, p<0.001). The Deeks’ Funnel plot asymmetry test was not significant, suggesting no publication bias (p=0.55, online supplemental figure V).
Figure 1.
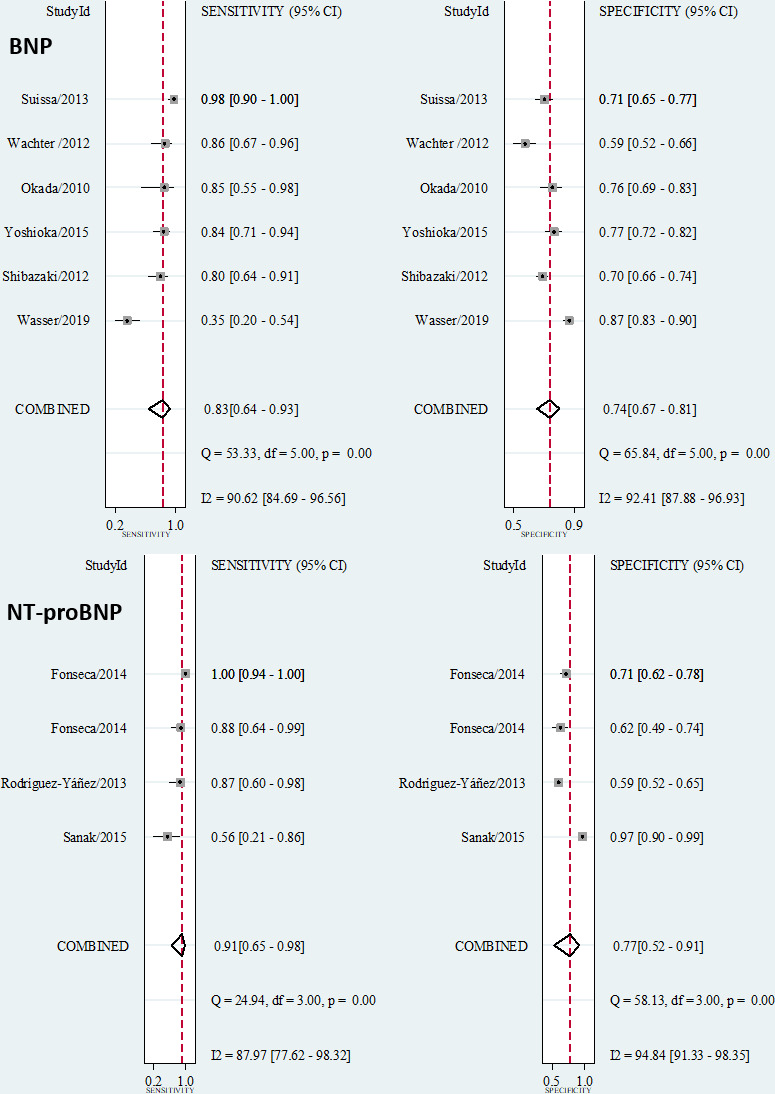
Forest plots showing study-specific and summary sensitivity and specificity with corresponding heterogeneity statistics. BNP, B-type natriuretic peptide.
Figure 2.
Fagan nomograms showing the post-test probability of atrial fibrillation after a positive or a negative test. Prior_prob, pre-test probability; LR, likelihood ratio; Post_prob_pos, post-test probability for a positive result; Post_prog_neg, post-test probability for a negative result.
The summary performance measures for diagnostic of covert AF using NT-proBNP were as follows: sensitivity of 0.91 (95% CI 0.65 to 0.98), specificity of 0.77 (95% CI 0.52 to 0.91), PLR of 3.9 (95% CI 1.8 to 8.7), NLR of 0.12 (95% CI 0.03 to 0.48), DOR of 34 (95% CI 8 to 138) and AUC of 0.91 (95% CI 0.88 to 0.93) (figure 1 and online supplemental figure IV). Using a pretest probability of 20%, the post-test probability of AF is 50% for a positive test and 3% for a negative test (figure 2). There was positive correlation between true and false positive rates (Spearman ρ=0.40), suggesting a threshold effect responsible for 47% of the observed heterogeneity (I2=90%, p<0.001). The Deeks’ Funnel plot asymmetry test was not significant, suggesting no publication bias (p=0.17, online supplemental figure V).
Discussion
NT-proBNP had a significantly higher AUC than BNP. NT-proBNP also had higher summary estimates of sensitivity, specificity, PLR and DOR although there was a wide overlap of CIs which may be explained by the small number of studies available for this meta-analysis. These results suggest that NT-proBNP has a better performance than BNP for the diagnosis of covert AF after acute ischaemic stroke. Our findings are consistent with the results of a recent individual patient data meta-analysis showing that the multivariable model including NT-proBNP had higher predictive value for cardioembolic stroke than the model including BNP in patients with stroke of undetermined cause at baseline.12 The better performance of NT-proBNP could be explained by the fact that it has a longer half-life than BNP (120 vs 20 min),19 thus increasing the chances of detecting a rise in plasma levels after a transient episode of AF.
Despite their high sensitivity, the clinical utility indicators of BNP and NT-proBNP remain suboptimal. It is considered that a PLR >10 and a NLR <0.1 provide strong evidence of the capacity of a test to rule in or rule out a diagnosis in most circumstances.38 Both biomarkers do not meet these requirements. Only about 50% of patients testing positive for BNP or NT-proBNP are likely to have AF if the pretest probability is 20%, no matter the cut-off used. Therefore, cardiac natriuretic peptides may not be used as a stand-alone decision-making tool for the diagnosis of covert AF in patients with acute ischaemic stroke. However, they may be useful for a screening strategy aiming to select patients for long-term monitoring of the heart rhythm.32 They could also be used to select high-risk patients prior to randomisation into anticoagulation trials as currently done in the atrial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke trial (NCT03192215).39 Further studies are needed to investigate if the performance of cardiac natriuretic peptides could be improved by the combination with transcriptomic biomarkers.40
Due to the limited number of included studies, subgroup and metaregression analyses could not be performed to explore how the diagnostic performance of BNP and NT-proBNP is influenced by patients’ clinical characteristics, notably age, presence of cardiovascular risk factors and cardiac comorbidities (heart failure, coronary atherosclerosis, valvular diseases), presence of kidney failure and use of diuretics, method of cardiac monitoring and time to diagnosis of AF, time from stroke onset to blood collection, size of brain infarction, treatment with beta-blockers and systemic inflammation.19 41 Analysis of data from ongoing trials and future observational studies may clarify if cardiac natriuretic peptides have a better clinical utility profile in specific subgroups of patients with acute ischaemic stroke.
Footnotes
Twitter: @JTatuene
KZ and JK-T contributed equally.
Contributors: KZ, JK-T, ML, and GCJ conceived the study. KZ, JK-T and ML searched the literature and selected studies. KZ, JK-T and ML extracted, analysed and interpreted the data. KZ and JK-T drafted the manuscript. ML and GCJ contributed to data interpretation and critically revised the manuscript. All authors approved the final version. JK-T is the guarantor of the review.
Funding: KZ is supported by the China Scholarship Council. JK-T is supported by graduate excellence scholarships from the Alberta Government and the Banque of Montreal Financial Group.
Competing interests: GCJ receives research support from Canadian Institutes of Health Research, the National Institutes of Health, The Heart and Stroke Foundation, the Canada Foundation for Innovation and the Alberta University Hospital Foundation.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hart RG, Diener H-C, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38. 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 2. Lin H-J, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke 1996;27:1760–4. 10.1161/01.STR.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 3. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol 2000;4:369–82. 10.1023/A:1009823001707 [DOI] [PubMed] [Google Scholar]
- 4. Kamel H, Okin PM, Elkind MSV, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 2016;47:895–900. 10.1161/STROKEAHA.115.012004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheitz JF, Nolte CH, Doehner W, et al. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 2018;17:1109–20. 10.1016/S1474-4422(18)30336-3 [DOI] [PubMed] [Google Scholar]
- 6. Méloux A, Béjot Y, Rochette L, et al. Brain-Heart interactions during ischemic processes: clinical and experimental evidences. Stroke 2020;51:679–86. 10.1161/STROKEAHA.119.027732 [DOI] [PubMed] [Google Scholar]
- 7. Lin C-H, Tsai Y-H, Lee J-D, et al. Magnetic resonance perfusion imaging provides a significant tool for the identification of cardioembolic stroke. Curr Neurovasc Res 2016;13:271–6. 10.2174/1567202613666160901143040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. García-Berrocoso T, Giralt D, Bustamante A, et al. B-Type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology 2013;81:1976–85. 10.1212/01.wnl.0000436937.32410.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katan M, Fluri F, Schuetz P, et al. Midregional pro-atrial natriuretic peptide and outcome in patients with acute ischemic stroke. J Am Coll Cardiol 2010;56:1045–53. 10.1016/j.jacc.2010.02.071 [DOI] [PubMed] [Google Scholar]
- 10. Kamtchum-Tatuene J, Jickling GC. Blood biomarkers for stroke diagnosis and management. Neuromolecular Med 2019;21:344–68. 10.1007/s12017-019-08530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bai J, Sun H, Xie L, et al. Detection of cardioembolic stroke with B-type natriuretic peptide or N-terminal Pro-BNP: a comparative diagnostic meta-analysis. Int J Neurosci 2018;128:1100–8. 10.1080/00207454.2017.1408612 [DOI] [PubMed] [Google Scholar]
- 12. Llombart V, Antolin-Fontes A, Bustamante A, et al. B-Type natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis. Stroke 2015;46:1187–95. 10.1161/STROKEAHA.114.008311 [DOI] [PubMed] [Google Scholar]
- 13. Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 2005;85:257–68. 10.1093/ptj/85.3.257 [DOI] [PubMed] [Google Scholar]
- 14. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15. Kim KW, Lee J, Choi SH, et al. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical Researchers-Part I. General guidance and tips. Korean J Radiol 2015;16:1175–87. 10.3348/kjr.2015.16.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee J, Kim KW, Choi SH, et al. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical Researchers-Part II. statistical methods of meta-analysis. Korean J Radiol 2015;16:1188–96. 10.3348/kjr.2015.16.6.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Espiner EA, Richards AM, Nicholls MG, et al. Physiology of natriuretic peptides. : Endocrinology of cardiovascular function. 1. Boston: Springer, 1998: 121–35. [Google Scholar]
- 18. Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide - Diagnostic role in stable coronary artery disease. Exp Clin Cardiol 2006;11:99–101. [PMC free article] [PubMed] [Google Scholar]
- 19. Goetze JP, Bruneau BG, Ramos HR, et al. Cardiac natriuretic peptides. Nat Rev Cardiol 2020. 10.1038/s41569-020-0381-0. [Epub ahead of print: 22 May 2020]. [DOI] [PubMed] [Google Scholar]
- 20. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–68. 10.1016/j.jacc.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 21. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. 10.1037/1082-989X.11.2.193 [DOI] [PubMed] [Google Scholar]
- 22. Safari S, Baratloo A, Elfil M, et al. Evidence based emergency medicine; part 4: pre-test and Post-test probabilities and Fagan's nomogram. Emerg 2016;4:48–51. [PMC free article] [PubMed] [Google Scholar]
- 23. Dwamena BA, Sylvester R, Carlos RC. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies [Tutorial], 2007. Available: https://www.researchgate.net/publication/4922515_MIDAS_Stata_module_for_meta-analytical_integration_of_diagnostic_test_accuracy_studies
- 24. Ntaios G, Perlepe K, Sirimarco G, et al. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology 2019;92:e2644–52. 10.1212/WNL.0000000000007611 [DOI] [PubMed] [Google Scholar]
- 25. Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 26. Kamtchum-Tatuene J, Wilman A, Saqqur M, et al. Carotid plaque with high-risk features in embolic stroke of undetermined source: systematic review and meta-analysis. Stroke 2020;51:311–4. 10.1161/STROKEAHA.119.027272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diederichsen SZ, Haugan KJ, Kronborg C, et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation 2020;141:1510–22. 10.1161/CIRCULATIONAHA.119.044407 [DOI] [PubMed] [Google Scholar]
- 28. McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 29. Okada Y, Shibazaki K, Kimura K, et al. Brain natriuretic peptide is a marker associated with thrombus in stroke patients with atrial fibrillation. J Neurol Sci 2011;301:86–9. 10.1016/j.jns.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 30. Shibazaki K, Kimura K, Fujii S, et al. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am J Cardiol 2012;109:1303–7. 10.1016/j.amjcard.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 31. Suissa L, Bresch S, Lachaud S, et al. Brain natriuretic peptide: a relevant marker to rule out delayed atrial fibrillation in stroke patient. J Stroke Cerebrovasc Dis 2013;22:e103–10. 10.1016/j.jstrokecerebrovasdis.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 32. Wasser K, Weber-Krüger M, Gröschel S, et al. Brain Natriuretic Peptide and Discovery of Atrial Fibrillation After Stroke: A Subanalysis of the Find-AFRANDOMISED Trial. Stroke 2020;51:395–401. 10.1161/STROKEAHA.119.026496 [DOI] [PubMed] [Google Scholar]
- 33. Yoshioka K, Watanabe K, Zeniya S, et al. A score for predicting paroxysmal atrial fibrillation in acute stroke patients: iPAB score. J Stroke Cerebrovasc Dis 2015;24:2263–9. 10.1016/j.jstrokecerebrovasdis.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 34. Wachter R, Lahno R, Haase B, et al. Natriuretic peptides for the detection of paroxysmal atrial fibrillation in patients with cerebral ischemia--the Find-AF study. PLoS One 2012;7:e34351. 10.1371/journal.pone.0034351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fonseca AC, Brito D, Pinho e Melo T, et al. N-Terminal pro-brain natriuretic peptide shows diagnostic accuracy for detecting atrial fibrillation in cryptogenic stroke patients. Int J Stroke 2014;9:419–25. 10.1111/ijs.12126 [DOI] [PubMed] [Google Scholar]
- 36. Rodríguez-Yáñez M, Arias-Rivas S, Santamaría-Cadavid M, et al. High Pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology 2013;81:444–7. 10.1212/WNL.0b013e31829d8773 [DOI] [PubMed] [Google Scholar]
- 37. Sanak D, Hutyra M, Kral M, et al. Paroxysmal atrial fibrillation in young cryptogenic ischemic stroke: a 3-week ECG Holter monitoring study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:283–7. 10.5507/bp.2015.019 [DOI] [PubMed] [Google Scholar]
- 38. Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004;329:168–9. 10.1136/bmj.329.7458.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamel H, Longstreth WT, Tirschwell DL, et al. The atrial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke 2019;14:1747493018799981:207–14. 10.1177/1747493018799981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jickling GC, Stamova B, Ander BP, et al. Prediction of cardioembolic, arterial, and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke 2012;43:2036–41. 10.1161/STROKEAHA.111.648725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fonseca AC, Matias JS, E Melo TP, et al. Time course of NT-proBNP levels after acute ischemic stroke. Acta Neurol Scand 2013;128:235–40. 10.1111/ane.12112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000440supp001.pdf (408.4KB, pdf)