Abstract
Background and purpose
The relationship of high-sensitive C-reactive protein (hs-CRP) levels and infarction numbers with the prognosis of stroke is uncertain. This study evaluated the association of different hs-CRP levels and infarction numbers with the prognosis of acute minor ischaemic stroke or transient ischaemic attack (TIA).
Methods
A subset of 807 patients with both hs-CRP measurement and baseline MRI was included from the Clopidogrel in High-risk Patients with Acute Non-disabling Cerebrovascular Events trial. The primary efficacy outcome was the occurrence of an ischaemic stroke at the 1-year follow-up. Infarction numbers were classified as multiple acute infarctions (MAIs), single acute infarction and no acute infarction (NAI). The association between different hs-CRP levels with different infarction numbers and the risk of any outcome was analysed using multivariable Cox regression models.
Results
Among the 807 patients, 84 (10.4%) patients had a recurrent ischaemic stroke within 1 year. After adjustment for conventional confounding factors, patients with both elevated hs-CRP levels and MAIs were associated with approximately 4.7-fold of risk of ischaemic stroke within 1 year (16.7% vs 3.5%, HR 4.68, 95% CI 1.54 to 14.23, p=0.007), compared with those with non-elevated hs-CRP levels and NAI. Similar results were observed for the composite events.
Conclusions
Combined elevated hs-CRP levels and MAIs may increase 1-year stroke risk stratification efficiency in patients with minor ischaemic stroke or TIA compared with using those markers alone, which indicated that the combination of inflammatory and imaging markers might improve the effectiveness of risk stratification concerning minor ischaemic stroke or TIA.
ClinicalTrials.gov Registry (NCT00979589).
Keywords: inflammation, MRI, stroke
Introduction
Patients with minor ischaemic stroke or transient ischaemic attack (TIA) have a high risk of stroke recurrence, especially in the early stage.1 2 Despite aggressive dual antiplatelet therapy, a fraction of patients with minor ischaemic stroke or TIA still developed recurrent stroke.3 4 Risk stratification is important for acute treatment and second stroke prevention in patients with minor ischaemic stroke or TIA. However, traditional risk stratification models, such as ABCD2 score,5 were usually based on clinical risk factors and limited to discriminating those at low or high risk of early recurrent stroke in patients with TIA,6–9 and there were limited risk stratification models for patients with minor ischaemic stroke.
Nowadays, neuroimaging parameters have become an important predictor for early recurrent cerebrovascular events in minor ischaemic stroke or TIA.10–12 TIA registry.org project demonstrated that patients with multiple acute infarctions (MAIs) had much higher risk of stroke recurrence than those with single acute infarction (SAI) or no acute infarction (NAI), which indicated that MAIs were important imaging markers to predict stroke recurrence,11 and this was confirmed in a Chinese study.13 MAIs often indicated the mechanism of artery-to-artery embolism in the non-cardioembolic ischaemic stroke,14 which indicated that MAIs represented unstable plaque.
Inflammatory markers were also important risk factors of stroke recurrence in ischaemic stroke and TIA.15–19 Elevated levels of high-sensitive C-reactive protein (hs-CRP) reflected the instability of atherosclerotic plaque.20 21 Previous studies showed that elevated hs-CRP levels predicted poor prognosis in patients with ischaemic stroke or TIA.19 22
However, whether the combination of the most representative markers of elevated hs-CRP and MAIs from two different dimensions could improve the effect of risk stratification in minor ischaemic stroke or TIA is unclear. Using data from the Clopidogrel in High-risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial, we investigated the outcomes of acute minor ischaemic stroke or TIA with different hs-CRP levels and infarction numbers.
Methods
Overview of the CHANCE trial
The detailed design and methods of the CHANCE trial have been previously described.4 23 CHANCE was a randomised, double-blind, placebo-controlled clinical trial conducted in 114 centres in China between October 2009 and July 2012. Patients in the trial met the following criteria: ≥40 years old and had been diagnosed as having an acute non-cardioembolic minor ischaemic stroke (NIH Stroke Scale (NIHSS) ≤3) or high-risk TIA (ABCD2≥4) within 24 hours after symptom onset. In total, 5170 patients were randomly assigned to either the clopidogrel plus aspirin group (clopidogrel at an initial dose of 300 mg, followed by 75 mg per day for 90 days, plus aspirin at 75 mg per day for the first 21 days) or placebo plus aspirin (75 mg per day for 90 days) group.
Patient consent
Written informed consent was obtained from all participants or their legal proxies.
Measurement of hs-CRP
The measurement of hs-CRP has been previously described.19 After randomisation, venous blood samples were drawn from fasting patients within 24±12 hours. Serum samples were extracted and stored at −80°C. Before testing, there was no occurrence of the freezing and thawing circle. Hs-CRP was centrally measured in the clinical laboratory of BeijingTiantan Hospital, using a Roche Modular P800 analyzer (Basel, Switzerland) and the turbidimetric immunoassay method (Ji’en Technique Co, Ltd, Shanghai, China). The laboratory personnel were blinded to the clinical and imaging data. The intra-assay and interassay coefficients of variation were 2.5% and 2.0%, respectively.19 Hs-CRP levels were stratified according to the relative risk category (low to medium risk, 0–3 mg/L; high risk, >3 mg/L) recommended by the Centers for Disease Control and Prevention and the American Heart Association, which was recommended for the risk assessment of cardiovascular disease.24 25
Patient screening and image analysis
All patients in the imaging substudy were asked to complete magnetic resonance (MR) examinations (3.0 or 1.5 Tesla) during hospitalisation. Patients with the following MR sequences were included in the substudy: T1-weighted imaging, T2-weighted imaging, diffusion-weighted imaging (DWI) and three-dimensional time-of-flight magnetic resonance angiography (MRA). Those without a baseline MR examination or any of the above sequences were excluded. The details of the CHANCE imaging substudy have been previously described.26
All MR images were collected from individual centres in a digital format and read centrally by two readers who were blinded to the patients’ baseline and outcome information. Any disagreement was resolved by a third reader. Patients were classified as NAI, SAI and MAIs, according to the infarction numbers. Acute infarctions were diagnosed with hyperintense lesions on DWI. Uninterrupted lesions visible in contiguous territories were defined as SAI, and more than one lesions that was topographically distinct (separated in space or discrete on contiguous slices) inside or outside a vascular territory were defined as MAIs, according to previous DWI studies.13 27 According to the Warfarin–Aspirin Symptomatic Intracranial Disease trial criteria, intracranial arterial stenosis (ICAS) was defined as 50%–99% stenosis28 or occlusion of at least one of the following arterial segments on MRA: intracranial portion of internal carotid arteries, middle cerebral arteries (M1/M2), basilar artery and intracranial portion of vertebral arteries.
Groups in this substudy
Patients were categorised into six groups according to the hs-CRP levels and infarction numbers: non-elevated hs-CRP levels (hs-CRP <3 mg/L) with NAI, non-elevated hs-CRP levels with SAI, non-elevated hs-CRP levels with MAIs, elevated hs-CRP levels (hs-CRP ≥3 mg/L) with NAI, elevated hs-CRP levels with SAI and elevated hs-CRP levels with MAIs.
Follow-up and outcomes
The original follow-up of the CHANCE trial was 90 days. However, we extended this to a 1-year follow-up period.29 All follow-up visits were undertaken by trained site coordinators. The primary efficacy outcome was an ischaemic stroke within the 1-year follow-up. The secondary efficacy outcomes were a composite event (ischaemic stroke, haemorrhagic stroke, myocardial infarction or vascular death) and TIA within the 1-year follow-up. Safety outcome was any bleeding event within the 1-year follow-up. The definitions of ischaemic stroke, composite event and bleeding were consistent with previously reported outcomes of the CHANCE trial.4 All reported outcomes were verified by a central adjudication committee which was blinded to the treatment assignments.
Statistical analysis
We presented categorical variables as percentages and continuous variables as median with IQR. Univariable analyses between the baseline variables and different hs-CRP levels with varying infarction numbers were described using the χ2 test for categorical variables and one-way analysis of variance or Kruskal-Wallis test for continuous variables. The time to the primary efficacy outcome event of each group was presented by using the Kaplan-Meier curves. We assessed the associations between different hs-CRP levels with varying infarction numbers and the prognosis of minor ischaemic stroke or TIA using multivariable Cox regression models. Adjusted HRs and their 95% CIs were calculated based on two models. In the first model, we adjusted only age and sex. In the second model, we included all the potential covariates listed in table 1. The levels of significance were p<0.05 (two-sided). All analyses were performed with SAS V.9.3 (SAS Institute Inc, Cary, North Carolina, USA).
Table 1.
Baseline characteristics of different hs-CRP levels and infarction numbers in patients with minor ischaemic stroke or TIA
| Characteristics | Non-elevated hs-CRP levels with NAI | Non-elevated hs-CRP levels with SAI | Non-elevated hs-CRP levels with MAIs | Elevated hs-CRP levels with NAI | Elevated hs-CRP levels with SAI | Elevated hs-CRP levels with MAIs | P value |
| (n=142) | (n=273) | (n=113) | (n=54) | (n=141) | (n=84) | ||
| Age, years, median (IQR) | 63.2 (54.7–71.0) | 61.0 (53.7–69.2) | 63.1 (54.5–74.0) | 65.6 (57.3–74.6) | 64.4 (56.9–72.8) | 67.0 (57.2–74.7) | 0.007 |
| Male, n (%) | 83 (58.5) | 184 (67.4) | 73 (64.6) | 35 (64.8) | 90 (63.8) | 60 (71.4) | 0.42 |
| Medical history, n (%) | |||||||
| Ischaemic stroke | 23 (16.2) | 41 (15.0) | 21 (18.6) | 12 (22.2) | 29 (20.6) | 14 (16.7) | 0.67 |
| TIA | 8 (5.6) | 4 (1.5) | 2 (1.8) | 5 (9.3) | 3 (2.1) | 4 (4.8) | 0.02 |
| Myocardial infarction | 0 (0) | 5 (1.8) | 3 (2.7) | 0 (0) | 1 (0.7) | 5 (6.0) | 0.02 |
| Known atrial fibrillation or flutter | 4 (2.8) | 2 (0.7) | 6 (5.3) | 0 (0) | 4 (2.8) | 2 (2.4) | 0.09 |
| Angina | 4 (2.8) | 5 (1.8) | 3 (2.7) | 3 (5.6) | 2 (1.4) | 6 (7.1) | 0.11 |
| Valvular heart disease | 1 (0.7) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 1 (1.2) | 0.55 |
| Hypertension | 92 (64.8) | 158 (57.9) | 68 (60.2) | 39 (72.2) | 108 (76.6) | 62 (73.8) | 0.001 |
| Diabetes mellitus | 22 (15.5) | 55 (20.1) | 24 (21.2) | 8 (14.8) | 24 (17.0) | 22 (26.2) | 0.36 |
| Hypercholesterolaemia | 21 (14.8) | 34 (12.5) | 12 (10.6) | 10 (18.5) | 18 (12.8) | 10 (11.9) | 0.76 |
| Current or previous smoking, n (%) | 49 (34.5) | 121 (44.3) | 50 (44.2) | 21 (38.9) | 60 (42.6) | 42 (50.0) | 0.27 |
| Time to randomisation, n (%) | 0.007 | ||||||
| <12 hours | 80 (56.3) | 129 (47.3) | 66 (58.4) | 38 (70.4) | 63 (44.7) | 45 (53.6) | |
| ≥12 hours | 62 (43.7) | 144 (52.7) | 47 (41.6) | 16 (29.6) | 78 (55.3) | 39 (46.4) | |
| NIHSS on admission, median (IQR) | 0 (0–1) | 2 (1–3) | 2 (1–2) | 0 (0–2) | 2 (1–3) | 2 (1–3) | <0.001 |
| ICAS, n (%) | 48 (33.8) | 93 (34.1) | 73 (64.6) | 22 (40.7) | 64 (45.4) | 58 (69.0) | <0.001 |
| Qualifying event, n (%) | <0.001 | ||||||
| TIA | 99 (69.7) | 27 (9.9) | 18 (15.9) | 36 (66.7) | 10 (7.1) | 12 (14.3) | |
| Minor stroke | 43 (30.3) | 246 (90.1) | 95 (84.1) | 18 (33.3) | 131 (92.9) | 72 (85.7) | |
| Group, n (%) | 0.32 | ||||||
| Aspirin only | 80 (56.3) | 130 (47.6) | 50 (44.2) | 25 (46.3) | 77 (54.6) | 42 (50.0) | |
| Clopidogrel+aspirin | 62 (43.7) | 143 (52.4) | 63 (55.8) | 29 (53.7) | 64 (45.4) | 42 (50.0) | |
| Medications, n (%) | |||||||
| Antihypertensive | 61 (43.3) | 121 (44.5) | 47 (41.6) | 25 (46.3) | 70 (50.0) | 35 (41.7) | 0.77 |
| Antidiabetic | 9 (6.4) | 44 (16.2) | 16 (14.2) | 7 (13.0) | 19 (13.6) | 15 (17.9) | 0.11 |
| Lipid lowering | 62 (44.0) | 139 (51.1) | 65 (57.5) | 28 (51.9) | 82 (58.6) | 47 (56.0) | 0.16 |
ICAS, intracranial arterial stenosis.hs-CRP, high-sensitive C-reactive protein; MAIs, multiple acute infarctions; NAI, no acute infarction; NIHSS, NIH Stroke Scale; SAI, single acute infarction; TIA, transient ischaemic attack.
Results
In the CHANCE trial, a total of 5170 patients with minor ischaemic stroke or TIA were recruited. Among them, 3044 patients provided eligible serum specimens, and 1089 patients underwent baseline MR examinations with all the image sequences as required. Totally, 807 patients from 31 centres with the required blood samples and MR examinations were retrieved for this subgroup analysis (online supplementary figure 1). Baseline characteristics of patients included and those not included in this subgroup analysis were basically similar, except that patients included in this subgroup were more likely to have a higher NIHSS on admission, minor stroke as a qualifying event, antihypertensive medications and lipid-lowering medications and less likely to have a prior ischaemic stroke (online supplementary table 1). Among the 807 included patients, 84 (10.4%) had elevated hs-CRP levels with MAIs, while 54 (6.7%), 141 (17.5%), 142 (17.6%), 273 (33.8%) and 113 (14.0%) had elevated hs-CRP levels with NAI, elevated hs-CRP levels with SAI, non-elevated hs-CRP levels with NAI, non-elevated hs-CRP levels with SAI and non-elevated hs-CRP levels with MAIs, respectively.
svn-2020-000369supp001.pdf (57.8KB, pdf)
svn-2020-000369supp002.pdf (77.7KB, pdf)
Table 1 shows the baseline characteristics of patients by status of hs-CRP levels and infarction numbers. Compared with those with non-elevated hs-CRP levels or NAI, patients with elevated hs-CRP levels and MAIs were more likely to be elder, a history of myocardial infarction and ICAS. Patients with elevated hs-CRP levels and SAI were more likely to have a history of hypertension. Patients with elevated hs-CRP levels and NAI were more likely to have a history of TIA and a shorter time to the randomisation of the trial treatment. Patients with infarction were more likely to have a higher NIHSS score on admission and a minor stroke as the qualifying event.
Risk of clinical events within 1 year after minor ischaemic stroke or TIA by the status of hs-CRP levels and infarction numbers are shown in table 2. In this subgroup analysis, 84 (10.4%) patients had recurrent ischaemic stroke within 1 year. Among all the patients with recurrent ischaemic stroke within 1 year, 14 (16.7%), 18 (12.8%), 3 (5.6%), 16 (14.2%), 28 (10.3%) and 5 (3.5%) had elevated hs-CRP levels with MAIs, elevated hs-CRP levels with SAI, elevated hs-CRP levels with NAI, non-elevated hs-CRP levels with MAIs, non-elevated hs-CRP levels with SAI and non-elevated hs-CRP levels with NAI, respectively.
Table 2.
Risk of clinical events by the status of hs-CRP levels and infarction numbers in patients with minor ischaemic stroke or TIA at 1-year follow-up
| Outcome | Group | No | Events at 1 year, n (%) | Model 1* | Model 1† | ||
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P Value |
||||
| Ischaemic stroke | Non-elevated hs-CRP levels with NAI | 142 | 5 (3.5) | Ref | Ref | ||
| Non-elevated hs-CRP levels with SAI | 273 | 28 (10.3) | 3.18 (1.23 to 8.24) | 0.02 | 3.23 (1.12 to 9.29) | 0.03 | |
| Non-elevated hs-CRP levels with MAIs | 113 | 16 (14.2) | 4.41 (1.61 to 12.04) | 0.004 | 3.93 (1.31 to 11.73) | 0.01 | |
| Elevated hs-CRP levels with NAI | 54 | 3 (5.6) | 1.62 (0.39 to 6.78) | 0.51 | 1.69 (0.40 to 7.22) | 0.48 | |
| Elevated hs-CRP levels with SAI | 141 | 18 (12.8) | 3.92 (1.45 to 10.57) | 0.007 | 3.33 (1.10 to 10.12) | 0.03 | |
| Elevated hs-CRP levels with MAIs | 84 | 14 (16.7) | 5.32 (1.91 to 14.83) | 0.001 | 4.68 (1.54 to 14.23) | 0.007 | |
| Composite events ‡ | Non-elevated hs-CRP levels with NAI | 142 | 5 (3.5) | Ref | Ref | ||
| Non-elevated hs-CRP levels with SAI | 273 | 28 (10.3) | 3.17 (1.22 to 8.22) | 0.02 | 3.29 (1.16 to 9.37) | 0.03 | |
| Non-elevated hs-CRP levels with MAIs | 113 | 18 (15.9) | 4.95 (1.84 to 13.34) | 0.002 | 4.62 (1.58 to 13.48) | 0.005 | |
| Elevated hs-CRP levels with NAI | 54 | 4 (7.4) | 2.16 (0.58 to 8.04) | 0.25 | 2.29 (0.60 to 8.70) | 0.22 | |
| Elevated hs-CRP levels with SAI | 141 | 18 (12.8) | 3.90 (1.45 to 10.52) | 0.007 | 3.44 (1.15 to 10.34) | 0.03 | |
| Elevated hs-CRP levels with MAIs | 84 | 16 (19.0) | 6.07 (2.21 to 16.63) | <0.001 | 5.48 (1.85 to 16.24) | 0.002 | |
| TIA | Non-elevated hs-CRP levels with NAI | 142 | 10 (7.0) | Ref | Ref | ||
| Non-elevated hs-CRP levels with SAI | 273 | 2 (0.7) | 0.10 (0.02 to 0.46) | 0.003 | 0.14 (0.03 to 0.70) | 0.02 | |
| Non-elevated hs-CRP levels with MAIs | 113 | 3 (2.7) | 0.38 (0.10 to 1.38) | 0.14 | 0.50 (0.12 to 2.09) | 0.34 | |
| Elevated hs-CRP levels with NAI | 54 | 4 (7.4) | 1.16 (0.36 to 3.73) | 0.80 | 1.04 (0.30 to 3.56) | 0.95 | |
| Elevated hs-CRP levels with SAI | 141 | 2 (1.4) | 0.21 (0.05 to 0.95) | 0.04 | 0.29 (0.05 to 1.52) | 0.14 | |
| Elevated hs-CRP levels with MAIs | 84 | 4 (4.8) | 0.73 (0.23 to 2.36) | 0.60 | 0.95 (0.23 to 3.89) | 0.95 | |
| Any bleeding | Non-elevated hs-CRP levels with NAI | 142 | 3 (2.1) | Ref | Ref | ||
| Non-elevated hs-CRP levels with SAI | 273 | 7 (2.6) | 1.33 (0.34 to 5.15) | 0.68 | 2.15 (0.43 to 10.71) | 0.35 | |
| Non-elevated hs-CRP levels with MAIs | 113 | 2 (1.8) | 0.81 (0.13 to 4.83) | 0.81 | 1.38 (0.18 to 10.52) | 0.76 | |
| Elevated hs-CRP levels with NAI | 54 | 0 (0) | 0 (0) | 0.99 | 0 (0) | 0.99 | |
| Elevated hs-CRP levels with SAI | 141 | 4 (2.8) | 1.23 (0.27 to 5.53) | 0.79 | 2.38 (0.40 to 14.37) | 0.34 | |
| Elevated hs-CRP levels with MAIs | 84 | 2 (2.4) | 1.01 (0.17 to 6.10) | 0.99 | 2.24 (0.30 to 16.73) | 0.43 | |
*Model 1: adjusted for age and sex.
†Model 2: adjusted for age, sex, history of ischaemic stroke, TIA, myocardial infarction, known atrial fibrillation or flutter, angina, valvular heart disease, hypertension, diabetes mellitus and hypercholesterolaemia, smoking status, time to randomisation, NIHSS on admission, ICAS, qualifying events, treatment classification, antihypertensive medications, antidiabetic medications and lipid-lowering medications.
‡Composite events: stroke, myocardial infarction or death from cardiovascular causes.
Hs-CRP, high-sensitive C-reactive protein; ICAS, intracranial arterial stenosis; MAIs, multiple acute infarctions; NAI, no acute infarction; NIHSS, NIH Stroke Scale; SAI, single acute infarction; TIA, transient ischaemic attack.
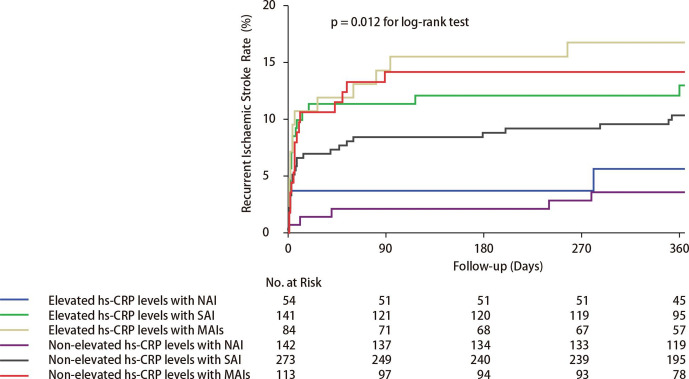
Compared with patients with non-elevated hs-CRP levels and NAI, patients with non-elevated hs-CRP levels and MAIs (HR 3.93, 95% CI 1.31 to 11.73, p=0.01), and patients with elevated hs-CRP levels and SAI (HR 3.33, 95% CI 1.10 to 10.12, p=0.03) were all associated with an increased risk of recurrent ischaemic stroke within 1 year, after adjustment for age, sex, history of ischaemic stroke, TIA, myocardial infarction, known atrial fibrillation or flutter, angina, valvular heart disease, hypertension, diabetes mellitus and hypercholesterolaemia, smoking status, time to randomisation, NIHSS on admission, ICAS, qualifying events, treatment classification, antihypertensive medications, antidiabetic medications and lipid-lowering medications. Compared with patients with non-elevated hs-CRP levels and NAI, patients with elevated hs-CRP levels and MAIs had approximately 4.7-fold increased the risk of recurrent ischaemic stroke within 1 year (HR 4.68, 95% CI 1.54 to 14.23, p=0.007), after adjusting for confounding factors. There was no interactive effect of the hs-CRP levels and infarction numbers on the primary outcome (p for interaction=0.89 in the multivariable adjusted model). The Kaplan-Meier curves show the cumulative hazard of recurrent ischaemic stroke (figure 1). Similar results were observed regarding the outcome of composite events. After adjusting for the confounding factors in model 1 and model 2, as compared with the reference group, there was no safety concern in any of the group.
Figure 1.
Cumulative probability of recurrent ischaemic stroke for patients by the status of hs-CRP levels and infarction numbers. Hs-CRP, high-sensitive C-reactive protein; MAIs, multiple acute infarctions; NAI, no acute infarction; SAI, single acute infarction.
Discussion
In this subgroup analysis of the CHANCE, combined hs-CRP levels and infarction numbers might increase 1-year stroke risk stratification efficiency in patients with minor ischaemic stroke or TIA than using those markers alone, and patients with both elevated hs-CRP and MAIs got the highest risk of recurrent ischaemic stroke and composite events within 1 year among groups.
More inflammation biomarkers from one pathway may provide an overlap of patients with cardiovascular disease.30 31 A prospective cohort study and systematic review found that additional inflammatory biomarkers did not add new prognostic value in patients with recent stroke.32 Previous studies showed that hs-CRP had crucial prognostic value in patients with ischaemic stroke, which indicated that inflammation played a pivotal role in the pathogenesis of poor prognosis after ischaemic stroke.15
MAIs were a significant independent predictor for vascular events in acute ischaemic stroke.27 TIA registry.org project showed that MAIs had a higher risk of stroke recurrence than NAI and SAI in minor ischaemic stroke or TIA.11 Previous studies showed that MAIs were usually related to large-artery atherosclerosis, cardio embolism and cryptogenic, according to the Trial of Org 10172 in Acute Stroke Treatment classification.33 34 Previous studies also showed that the pathogenesis of MAIs was usually likely to be caused by the embolism from major extracranial or intracranial vessels, or the heart.33–35 Microembolisation and haemodynamic failure were the most common pathogenesis of border-zone infarctions.36 Therefore, MAIs detected by DWI may indicate an unstable source of thromboembolism.
A previous study showed that inflammation could have a prothrombotic effect37 and was associated with microembolisation in patients with recently symptomatic carotid artery stenosis,38 39 which indicated that inflammation represented an unstable state. Infarction numbers could reflect the mechanism of ischaemic stroke.33 Multiple infarctions indicated embolismic mechanism, which also represented an unstable state.33–35 Multidimensional instability of ischaemic stroke may significantly increase the risk of stroke recurrence. Thus, the addition of inflammation and imaging markers to clinical features will yield the greatest accuracy for predicting poor outcomes in minor ischaemic stroke or TIA in the future stroke risk prediction models.
Our study had several limitations. First, since this subgroup analysis included only a small part of the patients in CHANCE, potential selection bias might exist. Second, additional imaging markers and inflammatory markers were not included in our study. Third, all patients in this substudy had non-cardioembolic minor ischaemic stroke which limited the generalisability of the findings to cardioembolic minor ischaemic stroke. Fourth, only one-point measurement of hs-CRP was available in this analysis. The accurate time of collecting blood sample was not recorded in the CHANCE. But a previous study showed that levels of hs-CRP appear to be stable for at least 28 days after first ischaemic stroke.40
Our results demonstrated that presence of both elevated hs-CRP levels and MAIs might increase 1-year stroke risk stratification efficiency in patients with minor ischaemic stroke or TIA than using those markers alone, which indicated that the combination of inflammatory and imaging markers might improve the effect of risk stratification in minor ischaemic stroke or TIA.
Footnotes
GW and JJ contributed equally.
Contributors: YoW and YiW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GW and JJ helped in study concept and design and
drafting of the manuscript. Critical revision of the manuscript for important intellectual content was done by YiW, XM, LL and HL. Statistical analysis was performed by YP, HY and HL. Study supervision and organisation of the project was done by YoW, YiW, XM, XZ and DZW.
Funding: This study was funded by National Science and Technology Major Project (Grant number: 2017ZX09304018); National Natural Science Foundation of China (Grant number: 81825007); Beijing Municipal Science and Technology Commission (Grant number: D171100003017001, D171100003017002); Beijing Outstanding Young Scientist Program (Grant number: BJJWZYJH01201910025030); Beijing Municipal Commission of Health and Family Planning (Grant number: No.2016-1-2041, SML20150502); the third batch of National Ten Thousand Talents Plan; Ministry of Science and Technology of the People's Republic of China (Grant number: 2016YFC0901002, 2017YFC1307900, 2017YFC1307905, 2018YFC1311706, 2018YFC1312903);Beijing Excellent Talents Training and Supporting- Top Youth Team by Beijing Municipal Science and Technology Commission (No. 2016000021223TD03);Youth Beijing Scholar Program.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Name of the ethics committee is IRB of Beijing Tiantan hospital, Capital Medical University. Ethics approval number of this study is ky2009-002. The trial was approved by the Ethics Committee of Beijing Tiantan Hospital and all the participating hospitals.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Coull AJ, Lovett JK, Rothwell PM, et al. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326. 10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901–6. 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Zhou M, Zhong X, et al. Dual versus mono antiplatelet therapy for acute non-cardioembolic ischaemic stroke or transient ischaemic attack: a systematic review and meta-analysis. Stroke Vasc Neurol 2018;3:107–16. 10.1136/svn-2018-000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 5. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–92. 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 6. Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015;85:373–80. 10.1212/WNL.0000000000001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coutts SB, Barrett KM. TIA risk stratification: what an event was and why it happened are more important than a score. Neurology 2015;85:304–5. 10.1212/WNL.0000000000001794 [DOI] [PubMed] [Google Scholar]
- 8. Edlow JA. Risk stratification in TIA patients: "It's the vascular lesion, stupid!". Neurology 2012;79:958–9. 10.1212/WNL.0b013e31825f9e68 [DOI] [PubMed] [Google Scholar]
- 9. Sanders LM, Srikanth VK, Blacker DJ, et al. Performance of the ABCD2 score for stroke risk post TIA: meta-analysis and probability modeling. Neurology 2012;79:971–80. 10.1212/WNL.0b013e31825f9d02 [DOI] [PubMed] [Google Scholar]
- 10. Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol 2016;73:572–8. 10.1001/jamaneurol.2015.4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amarenco P, Lavallée PC, Labreuche J, et al. One-Year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med 2016;374:1533–42. 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 12. Merwick A, Albers GW, Amarenco P, et al. Addition of brain and carotid imaging to the ABCD² score to identify patients at early risk of stroke after transient ischaemic attack: a multicentre observational study. Lancet Neurol 2010;9:1060–9. 10.1016/S1474-4422(10)70240-4 [DOI] [PubMed] [Google Scholar]
- 13. Jing J, Meng X, Zhao X, et al. Dual antiplatelet therapy in transient ischemic attack and minor stroke with different infarction patterns: subgroup analysis of the chance randomized clinical trial. JAMA Neurol 2018;75:711–9. 10.1001/jamaneurol.2018.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong KS, Gao S, Chan YL, et al. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol 2002;52:74–81. 10.1002/ana.10250 [DOI] [PubMed] [Google Scholar]
- 15. Zhong C, Zhu Z, Wang A, et al. Multiple biomarkers covering distinct pathways for predicting outcomes after ischemic stroke. Neurology 2019;92:e295–304. 10.1212/WNL.0000000000006717 [DOI] [PubMed] [Google Scholar]
- 16. Li J, Wang Y, Lin J, et al. Soluble CD40L is a useful marker to predict future strokes in patients with minor stroke and transient ischemic attack. Stroke 2015;46:1990–2. 10.1161/STROKEAHA.115.008685 [DOI] [PubMed] [Google Scholar]
- 17. Zhu B, Pan Y, Jing J, et al. Neutrophil counts, neutrophil ratio, and new stroke in minor ischemic stroke or TIA. Neurology 2018;90:e1870–8. 10.1212/WNL.0000000000005554 [DOI] [PubMed] [Google Scholar]
- 18. Kernagis DN, Laskowitz DT. Evolving role of biomarkers in acute cerebrovascular disease. Ann Neurol 2012;71:289–303. 10.1002/ana.22553 [DOI] [PubMed] [Google Scholar]
- 19. Li J, Zhao X, Meng X, et al. High-Sensitive C-reactive protein predicts recurrent stroke and poor functional outcome: subanalysis of the clopidogrel in high-risk patients with acute Nondisabling cerebrovascular events trial. Stroke 2016;47:2025–30. 10.1161/STROKEAHA.116.012901 [DOI] [PubMed] [Google Scholar]
- 20. Pleskovič A, Letonja Marija Šantl, Vujkovac AC, et al. C-reactive protein as a marker of progression of carotid atherosclerosis in subjects with type 2 diabetes mellitus. Vasa 2017;46:187–92. 10.1024/0301-1526/a000614 [DOI] [PubMed] [Google Scholar]
- 21. Zhao L, Zhai Z, Hou W. Analysis of carotid color ultrasonography and high sensitive C-reactive protein in patients with atherosclerotic cerebral infarction. Pak J Med Sci 2016;32:931–4. 10.12669/pjms.324.9731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elkind MSV, Tai W, Coates K, et al. High-Sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med 2006;166:2073–80. 10.1001/archinte.166.19.2073 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Johnston SC, CHANCE Investigators . Rationale and design of a randomized, double-blind trial comparing the effects of a 3-month clopidogrel-aspirin regimen versus aspirin alone for the treatment of high-risk patients with acute nondisabling cerebrovascular event. Am Heart J 2010;160:380–6. 10.1016/j.ahj.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 24. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American heart association. Circulation 2003;107:499–511. 10.1161/01.cir.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 25. Li J, Wang A, Zhao X, et al. High-sensitive C-reactive protein and dual antiplatelet in intracranial arterial stenosis. Neurology 2018;90:e447–54. 10.1212/WNL.0000000000004928 [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Wong KSL, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of chance. Neurology 2015;85:1154–62. 10.1212/WNL.0000000000001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wen HM, Lam WWM, Rainer T, et al. Multiple acute cerebral infarcts on diffusion-weighted imaging and risk of recurrent stroke. Neurology 2004;63:1317–9. 10.1212/01.WNL.0000140490.22251.B6 [DOI] [PubMed] [Google Scholar]
- 28. Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–6. [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Pan Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (chance) trial: one-year outcomes. Circulation 2015;132:40–6. 10.1161/CIRCULATIONAHA.114.014791 [DOI] [PubMed] [Google Scholar]
- 30. Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 2011;123:551–65. 10.1161/CIRCULATIONAHA.109.912568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bachmann KN, Wang TJ. Biomarkers of cardiovascular disease: contributions to risk prediction in individuals with diabetes. Diabetologia 2018;61:987–95. 10.1007/s00125-017-4442-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiteley W, Jackson C, Lewis S, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med 2009;6:e1000145. 10.1371/journal.pmed.1000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wessels T, Wessels C, Ellsiepen A, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol 2006;27:35–9. [PMC free article] [PubMed] [Google Scholar]
- 34. Kang D-W, Chalela JA, Ezzeddine MA, et al. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 2003;60:1730–4. 10.1001/archneur.60.12.1730 [DOI] [PubMed] [Google Scholar]
- 35. Takahashi K, Kobayashi S, Matui R, et al. The differences of clinical parameters between small multiple ischemic lesions and single lesion detected by diffusion-weighted MRI. Acta Neurol Scand 2002;106:24–9. 10.1034/j.1600-0404.2002.01319.x [DOI] [PubMed] [Google Scholar]
- 36. Joinlambert C, Saliou G, Flamand-Roze C, et al. Cortical border-zone infarcts: clinical features, causes and outcome. J Neurol Neurosurg Psychiatry 2012;83:771–5. 10.1136/jnnp-2012-302401 [DOI] [PubMed] [Google Scholar]
- 37. Esmon CT. Inflammation and thrombosis. J Thromb Haemost 2003;1:1343–8. 10.1046/j.1538-7836.2003.00261.x [DOI] [PubMed] [Google Scholar]
- 38. Nasr N, Ruidavets JB, Arnal JF, et al. Association of neutrophil count with microembolization in patients with symptomatic carotid artery stenosis. Atherosclerosis 2009;207:519–23. 10.1016/j.atherosclerosis.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 39. Yin R, Ma A, Pan X, et al. Biomarkers of cerebral microembolic signals. Clin Chim Acta 2017;475:164–8. 10.1016/j.cca.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 40. Elkind MSV, Coates K, Tai W, et al. Levels of acute phase proteins remain stable after ischemic stroke. BMC Neurol 2006;6:37. 10.1186/1471-2377-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000369supp001.pdf (57.8KB, pdf)
svn-2020-000369supp002.pdf (77.7KB, pdf)