Abstract
The reduction in skeletal muscle mass (SMM) is a common phenomenon in older adults. It is associated with several diseases, a reduction in physical fitness, longer periods of hospitalization and high rates of mortality. We aimed to identify the reliability of simple tools for screening for reduced SMM among older adult males in Lebanon. The Tanita MC-780MA bioimpedance analyzer (BIA) was used to assess body composition in a population of 102 community-dwelling elderly males with overweight or obesity, in order to be then categorized as with or without reduced SMM. Participants also performed the handgrip strength test and the 4 m gait speed test. Of the total sample of 102 participants (mean age 67.4 ± 6.96 years; BMI 30.8 6 ± 4.04 kg/m2), 32 (31.4%) met the criteria for reduced SMM. Partial correlation analysis showed that handgrip strength (ρ = 0.308, p = 0.002) and 4 m gait speed (ρ = 0.284, p = 0.004) were both associated with low SMM. Receiver operating characteristic (ROC) curve analysis identified discriminating cut-off points of 1.1 m/s for the 4 m gait speed test and 32.0 kg for the handgrip strength test. Our study showed that participants displayed a substantial prevalence of reduced SMM. Reduced 4 m gait speed and handgrip strength were associated with low SMM. Clear cut-off points for strength and functional tests for screening for this condition in Lebanese older men were identified.
Keywords: skeletal muscle mass, muscle strength, obesity, physical fitness, physical performance
1. Introduction
In the last three decades, much research has been published on body composition in people with overweight and obesity [1,2], particularly focusing on body fat mass and its distribution [3]. However, there is a lack of research on the reduction in skeletal muscle mass (SMM) in this population (i.e., overweight and obesity) [2,4]. In fact, SMM is an important component of a phenotype termed sarcopenic obesity (SO) [5], defined as the increase in body fat mass deposition and the reduction in SMM and muscle strength, which seems to be an ignored outcome and needs further investigation [5].
In the same way, an SMM deficit, especially in older populations, is associated with several serious medical consequences, including low levels of vitamin D [6], osteoporosis and fractures [7], a reduction in physical fitness [8], earlier disability onset, longer periods of hospitalization [9], and mortality [10,11].
Excess weight is a condition defined as an abnormal increased fat deposition and it is associated with an increased risk of chronic morbidity, disability and mortality [12]. The coexistence of overweight and obesity with reduced SMM may exacerbate their respective effects on cardio metabolic disorders [11,13]. A recent study from our group found that SMM reduction in young adult male patients with overweight and obesity was strongly associated with cardiovascular and metabolic diseases [14]. Based on these considerations, the early identification of SMM reduction is of major clinical importance [15]. This will allow the introduction of potential therapeutic strategies, such as dietary and physical activity interventions [16], that aim to limit SMM deterioration in individuals with overweight and obesity, especially among older adults [17].
An important methodological consideration is that the definitions of reduced SMM in overweight and obesity based only on free fat mass (lean mass) value, without considering body mass (body weight) [18], are imprecise. This is a problem, because it is widely known that individuals with overweight and obesity tend to have a relatively higher lean mass [18]. The criteria for low SMM that are based only on the absolute value of free fat mass may not be met in this population, meaning that the prevalence of reduced SMM may be strongly underestimated [18].
The aim of the current study is to assess the prevalence of SMM reduction in Lebanese community-dwelling older adult males with overweight and obesity, using the Oh et al. definition [19], which considers body weight in addition to appendicular SMM [19]. We also aimed to evaluate the reliability of simple measurements, i.e., muscle strength and physical performance tests, in screening for low SMM in this population. Within this scope, the hypothesis is that a high prevalence of low SMM among our population will be found, and this can be easily screened by means of strength and performance tests that do not require specialized instruments.
2. Materials and Methods
2.1. Participants
The study was conducted in the Department of Nutrition and Dietetics at Beirut Arab University (BAU) in Lebanon, during the period March 2018–February 2020. A total of 102 male participants were recruited from the general population through a simple, random, community e-mail-based survey, sent to members of BAU and other mailing lists. Recruitment focused on self-sufficient older adults of ≥60 years old, living independently in community dwellings. The inclusion criteria were (i) age ≥ 60 years of age, (ii) male and (iii) BMI ≥ 25.0 kg/m2. The exclusion criteria were (i) the inability to move without crutches, a walker or other assistive devices, (ii) the presence of artificial limbs or limb prosthesis, and (iii) the presence of active cancer, congestive heart failure, chronic obstructive pulmonary disorder, chronic renal failure, cirrhosis or liver failure.
The study was approved by the Institutional Review Board of BAU (No. 2019H-0063-HS-M-0318), and all participants gave informed, written consent for the use of their anonymized personal data. A questionnaire was administered to elicit information regarding medical history and lifestyle, as well as demographic and social conditions.
2.2. Measures
Body weight was measured by a trained dietician involved in the study, using an electronic weighing scale (SECA 2730-ASTRA, Hamburg, Germany). Height was measured using a stadiometer. BMI was then calculated according to the standard formula.
Body composition was measured in the morning in our clinics by a trained dietician, using a segmental body composition analyzer (MC-780MA, Tanita Corp., Tokyo, Japan) [20]. Participants wore their own clothes and weight adjustment for clothing was applied. This method allowed a bioelectrical impedance measurement of the whole body and of each body part (head, trunk, right leg, left leg, right arm and left arm) at a single frequency. Gender, age and height information was then entered into the device and participants were asked to stand in a stable position in bare feet. Their toes and heels were placed in contact with the anterior and posterior electrodes of the weighing platform, respectively. This device provides separate body mass readings for different segments of the body and uses an algorithm incorporating impedance, age and height to estimate the total and regional body fat and fat-free mass. Total fat, lean mass percentages and the appendicular lean mass (ALM) were calculated using standard formulas. Low SMM was defined based on the definition of Oh et al. ((ALM/weight) × 100%) with a cut-off score of 29.60 in males [19].
Participants’ muscle strength and physical performance were evaluated by the handgrip strength test [21] and the 4 m gait speed test [22], respectively, and by adhering to international guidelines. The handgrip strength test was performed as one trial in each hand, using a calibrated dynamometer (Camry Digital Hand Dynamometer Grip Strength, Model EH101–37, China) [23], and the maximum value (kg) was recorded. The 4 m gait speed test was performed on a precisely identified flat course, in a room situated in our clinic, with the 4 m distance marked out with tape [22]. Timing was taken with a chronometer that started when the participant began to move and stopped when he completely crossed the 4 m line with his first foot [22].
Cardiometabolic disease in this study is defined as the presence of any diseases, such as type 2 diabetes, cardiovascular diseases (coronary heart disease, stroke, transient ischaemic attack, and peripheral arterial disease) and dyslipidemia (lowered level of high-density lipoprotein cholesterol, and increased level of low-density lipoprotein cholesterol and triglycerides). These were self-reported to be experienced either simultaneously or separately.
2.3. Statistical Analysis
Descriptive statistics are presented as means and standard deviations or frequencies and proportions for continuous and categorical variables, respectively. Data were tested for normality using the Kolmogorov–Smirnov test and the Quantile-Quantile (Q-Q) normality plot. Means were compared via the Student t-test or Mann–Whitney test as appropriate. The chi-squared test for independence was used for proportions. To test the diagnostic performance of muscle strength and gait speed in detecting reduced SMM based on the Oh et al. definition, ALM by weight percentage was used as a gold standard. Classification analysis was done by calculating sensitivity and specificity, and the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The criterion values of muscle strength and gait speed with maximum sensitivity and specificity were selected for the cut-off points. An AUC > 0.8 indicates an excellent discrimination ability; an AUC of 70–80 is considered acceptable. The cut-off scores achieving 90% sensitivity and their corresponding specificities were also calculated. All values were considered significant at p < 0.05.
NCSS 12.0.2 (NCSS, NCSS, LLC. Kaysville, UT, USA) was used for the statistical analysis. Power analysis for the sample size was determined using PASS software (PASS 11. NCSS, LLC. Kaysville, Utah, USA). For the sample of 32 patients classified with reduced SMM vs. 70 with normal SMM, an alpha of 0.05 and an AUC of 0.697 or 0.679, the power was >0.90.
3. Results
The sociodemographic and body composition characteristics of the study participants are shown in Table 1. The sample comprised 102 males with a mean age of 67.4 ± 6.96 years, range 60–84 years. All participants were either living with overweight or obesity, with a mean BMI of 30.86 ± 4.04 kg/m2. Based on Oh et al.’s definition, 31.4% of the participants were confirmed with reduced SMM. Participants with reduced SMM were more likely to have low income compared to those with normal SMM (75% vs. 51.4%; p = 0.025). Those with reduced SMM were significantly heavier (89.10 kg vs. 82.70 kg; p = 0.014), with higher BMI (33.20 kg/m2 vs. 28.81 kg/m2; p < 0.0001), body fat (27.45 kg vs. 20.40 kg; p < 0.0001) and body fat percentage (30.82 ± 4.40% vs. 24.78 ± 4.13%; p < 0.0001), lower gait speed (0.91 ± 0.24 vs. 1.08 ± 0.24 m/s; p = 0.002) and lower muscle strength (30.56 ± 6.42 vs. 35.05 ± 6.75 kg; p = 0.002).
Table 1.
Socio-demographic, anthropometric and cardiometabolic characteristics of the study sample (n = 102).
| Demographics | Total N = 102 |
Normal SMM N = 70 |
Reduced SMM N = 32 |
Significance |
|---|---|---|---|---|
| Age (Years) | 67.64 (6.96) | 66.13 (5.93) | 70.94 (7.96) | p = 0.004 |
| Marital status | X2 = 3.075; p = 0.079 | |||
| Not married | 11 (10.8) | 5 (7.1) | 6 (18.8) | |
| Married | 91 (89.2) | 65 (92.9) | 26 (81.3) | |
| Level of education | X2 = 1.155; p = 0.283 | |||
| Lower education | 83 (81.4) | 55 (78.6) | 28 (87.5) | |
| Higher education | 19 (18.6) | 15 (21.4) | 4 (12.5) | |
| Employment | X2 = 0.073; p = 0.787 | |||
| Not employed | 65 (63.7) | 44 (62.9) | 21 (65.6) | |
| Employed | 37 (36.3) | 26 (37.1) | 11 (34.4) | |
| Salary | X2 = 5.038; p = 0.025 | |||
| <LL 1 million | 60 (58.8) | 36 (51.4) | 24 (75.0) | |
| >LL 1 million | 42 (41.2) | 34 (48.6) | 8 (25.0) | |
| Smoking | X2 = 0.415; p = 0.520 | |||
| Non smoker | 59 (57.8) | 39 (55.7) | 20 (62.5) | |
| Smoker | 43 (42.2) | 31 (44.3) | 12 (37.5) | |
| Place of residence | X2 = 0.164; p = 0.686 | |||
| Outside Beirut | 8 (7.8) | 6 (8.6) | 2 (6.3) | |
| Inside Beirut | 94 (92.2) | 64 (91.4) | 30 (93.8) | |
| Weight (kg) § | 83.75 (76.78–92.02) | 82.70 (75.73–88.25) | 89.10 (78.88–106.10) | p = 0.014 |
| Body Fat (BF) § | 22.25 (18.18–26.42) | 20.40 (17.18–27.51) | 27.45 (21.78–35.10) | p < 0.0001 |
| Body fat percentage (BF%) | 26.68 (5.05) | 24.78 (4.13) | 30.82 (4.40) | p < 0.0001 |
| Body mass index (BMI) § | 29.89 (28.00–33.07) | 28.81 (27.51–31.60) | 33.20 (29.58–38.71) | p < 0.0001 |
| Gait speed | 1.02 (0.25) | 1.08 (0.24) | 0.91 (0.24) | p = 0.002 |
| Handgrip strength | 33.64 (6.94) | 35.05 (6.75) | 30.56 (6.42) | p = 0.002 |
| Cardiometabolic disease | X2 = 0.012; p = 0.913 | |||
| No | 55 (53.9) | 38 (54.3) | 17 (53.1) | |
| Yes | 47 (46.1) | 32 (45.7) | 15 (46.9) |
Values are N (%) for categorical variables and Mean (SD) or § Median (IQR) for continuous variables; X2 = Chi Square.
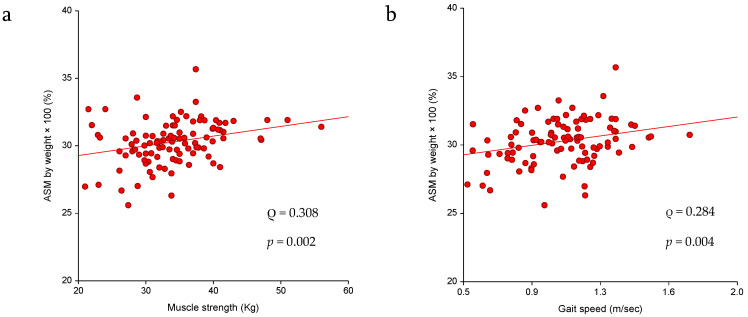
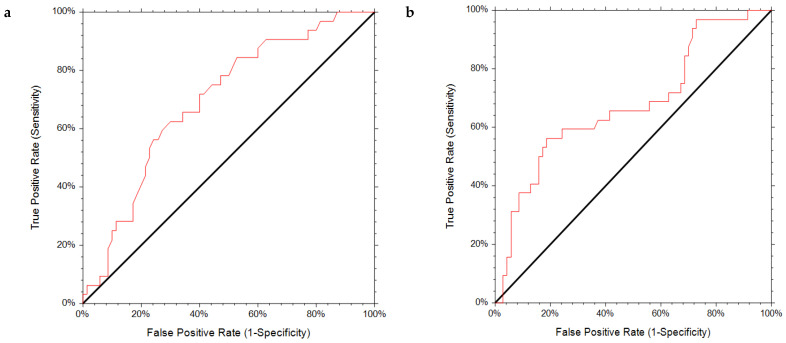
A significant association was observed for reduced SMM with lower muscle strength (ρ = 0.308, p = 0.002) and reduced gait speed (ρ = 0.284, p = 0.004) (Figure 1a,b). The results of the ROC analysis for the diagnostic performance are shown in Table 2 and Figure 2a,b. The AUC for muscle strength and gait speed indicates acceptable discriminating ability with an almost 70% chance of discriminating an individual with reduced SMM. The optimal handgrip strength for discriminating reduced ALM was 32.00 kg, with 62.5% sensitivity and 70% specificity, indicating a lower chance for false negatives and false positives. The optimal gait speed cut-off point was 1.01 with a sensitivity and specificity of 63%. The cut-off points at 90% sensitivity were 36.88 kg for handgrip strength and 1.19 m/s for gait speed, with the relatively low specificity of 37.7% and 28.9%, respectively, indicating a higher tendency toward false positives. According to the determined cut-off points for handgrip strength and gait speed, reduced lean body mass (LBM) affected 40.2% and 44% of the study participants, respectively.
Figure 1.
(a) Association between low SMM and handgrip strength (kg) and also (b) gait speed (m/s) in the study sample (n = 102).
Table 2.
Diagnostic performance of the handgrip strength (kg) and gait speed cut-off points to detect low SMM in the study population (n = 102).
| AUC | 95%CI | p Value | Sensitivity | Specificity | Cut Off | Specificity at 90% Sensitivity | Cut-Off at 90% Sensitivity | |
|---|---|---|---|---|---|---|---|---|
| Muscle strength (kg) | 0.696 | 0.573–0.788 | 0.0002 | 0.625 | 0.700 | 32.00 | 0.377 | 36.88 |
| Gait speed (m/s) | 0.679 | 0.543–0.779 | 0.0014 | 0.625 | 0.629 | 1.01 | 0.289 | 1.19 |
Figure 2.
Receiver operating characteristic (ROC) curves for (a) handgrip strength test and (b) 4 m gait speed test.
4. Discussion
In our population of community-dwelling Lebanese older males affected by overweight or obesity, across a wide age range from 60–84 years, the prevalence of low SMM was over 30%. Reduced SMM can be easily screened by means of simple, cheap and accessible measures, i.e., muscle strength and physical performance tests. Individuals with reduced SMM took significantly longer to perform the 4 m gait speed test and demonstrated lower performance in the handgrip strength test than those with normal SMM. We also established cut-off scores for these two measures: a gait speed of 1.1 m/s and a handgrip strength of 32 kg. Individuals with decreased SMM can be easily identified in different settings by means of these simple tests.
The clinical implications of these findings can have a profound impact on health care choices. First, awareness of the prevalence of reduced SMM in older Lebanese adult males with overweight or obesity in community dwellings should be raised through education campaigns using different platforms, e.g., television and social media. Second, the implementation of simple measures in primary care settings, particularly the handgrip strength and 4 m gait speed tests, is extremely important, considering the recent findings on the impact of muscle mass in middle age that can predict the subsequent risk of poor cardiovascular health, especially in males [24]. This is in addition to the association between reduced SMM and the increased risk of insulin resistance [25], as well as the higher levels of circulating inflammatory markers [26]. These functional tests should be considered as initial screening tools to identify those with a high risk of reduced SMM and who would benefit from further investigation via more precise tools, i.e., BIA and dual-energy X-ray absorptiometry (DXA) scans.
Our study has a number of strengths. To our knowledge, it is the first study to assess SMM reduction considering ALM and weight in older adults males with overweight and obesity in Lebanon. Second, our study established cut-off points on both performance tests, which are useful for identifying older individuals who are more likely to have low SMM and who are at higher risk of worsening their health problems. Third, we assessed a reduction in SMM using the Oh et al. definition [19], which has been shown to be of clinical relevance for the Lebanese population [14].
Our study also has some limitations. First, no blood testing was performed; this means that we are not in the position to understand the mechanisms of reduced SMM in this population. Second, our sample is composed only of males and our findings cannot be extended to females of the same population. Third, we assessed body composition using BIA instead of DXA, which is considered the gold standard technique for SMM assessment. However, multi-frequency BIA has been found to be a very accurate measurement and is widely used in different clinical settings. Moreover, it has been shown to be strictly correlated with DXA SMM measurements in healthy adults [27,28], and has been widely validated in individuals with overweight and obesity [29,30]. Finally, the cross-sectional nature of our study has to be considered a further limitation.
5. Conclusions
Our study provides evidence that community-dwelling older adult males with overweight or obesity in Lebanon have a substantial prevalence of low SMM. This can be simply screened for by the handgrip strength and 4 m gait speed tests. These tests are easy-to-use measures that can be implemented in several healthcare settings.
Acknowledgments
We thank the participants of this study.
Author Contributions
All authors claim authorship, and have approved and made substantial contributions to the conception, drafting, and final version of the paper. The study was designed by M.E.G., M.P. and A.P.R., while L.I. conducted the statistical analysis. D.S. collected data. M.E.G. and L.I. co-wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Beirut Arab University (No. 2019H-0063-HS-M-0318).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liang X., Chen X., Li J., Yan M., Yang Y. Study on body composition and its correlation with obesity: A Cohort Study in 5121 Chinese Han participants. Medicine (Baltim.) 2018;97:e10722. doi: 10.1097/MD.0000000000010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das S.K. Body composition measurement in severe obesity. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:602–606. doi: 10.1097/01.mco.0000171122.60665.5f. [DOI] [PubMed] [Google Scholar]
- 3.Silver H.J., Welch E.B., Avison M.J., Niswender K.D. Imaging body composition in obesity and weight loss: Challenges and opportunities. Diabetes Metab. Syndr. Obes. 2010;3:337–347. doi: 10.2147/DMSO.S9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Ghoch M., Rossi A.P., Calugi S., Rubele S., Soave F., Zamboni M., Chignola E., Mazzali G., Bazzani P.V., Dalle Grave R. Physical performance measures in screening for reduced lean body mass in adult females with obesity. Nutr. Metab. Cardiovasc. Dis. 2018;28:917–921. doi: 10.1016/j.numecd.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Nezameddin R., Itani L., Kreidieh D., El Masri D., Hana Tannir H., El Ghoch M. Understanding Sarcopenic Obesity in Terms of Definition and Health Consequences: A Clinical Review. Curr. Diabetes Rev. 2020;16:957–961. doi: 10.2174/1573399816666200109091449. [DOI] [PubMed] [Google Scholar]
- 6.Visser M., Deeg D.J., Lips P. Longitudinal Aging Study A: Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 7.Hirschfeld H.P., Kinsella R., Duque G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017;28:2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 9.Pichard C., Kyle U.G., Morabia A., Perrier A., Vermeulen B., Unger P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am. J. Clin. Nutr. 2004;79:613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 10.Szulc P., Munoz F., Marchand F., Chapurlat R., Delmas P.D. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: The prospective MINOS study. Am. J. Clin. Nutr. 2010;91:1227–1236. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- 11.Bachettini N.P., Bielemann R.M., Barbosa-Silva T.G., Menezes A.M.B., Tomasi E., Gonzalez M.C. Sarcopenia as a mortality predictor in community-dwelling older adults: A comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020;74:573–580. doi: 10.1038/s41430-019-0508-8. [DOI] [PubMed] [Google Scholar]
- 12.El Ghoch M., Fakhoury R. Challenges and New Directions in Obesity Management: Lifestyle Modification Programmes, Pharmacotherapy and Bariatric Surgery. J. Popul. Ther. Clin. Pharmacol. 2019;26:e1–e4. doi: 10.15586/jptcp.v26i2.599. [DOI] [PubMed] [Google Scholar]
- 13.Khadra D., Itani L., Tannir H., Kreidieh D., El Masri D., El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J. Diabetes. 2019;15:311–323. doi: 10.4239/wjd.v10.i5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khazem S., Itani L., Kreidieh D., El Masri D., Tannir H., Citarella R., El Ghoch M. Reduced Lean Body Mass and Cardiometabolic Diseases in Adult Males with Overweight and Obesity: A Pilot Study. Int. J. Environ. Res. Public Health. 2018;15:2754. doi: 10.3390/ijerph15122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu S.C., Khow K.S., Jadczak A.D., Visvanathan R. Clinical Screening Tools for Sarcopenia and Its Management. Curr. Gerontol. Geriatr. Res. 2016;2016:5978523. doi: 10.1155/2016/5978523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petroni M.L., Caletti M.T., Dalle Grave R., Bazzocchi A., Aparisi Gómez M.P., Marchesini G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients. 2019;11:1302. doi: 10.3390/nu11061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan L.F., Lim Z.Y., Choe R., Seetharaman S., Merchant R. Screening for Frailty and Sarcopenia Among Older Persons in Medical Outpatient Clinics and its Associations with Healthcare Burden. J. Am. Med. Dir. Assoc. 2017;18:583–587. doi: 10.1016/j.jamda.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Johnson Stoklossa C.A., Sharma A.M., Forhan M., Siervo M., Padwal R.S., Prado C.M. Prevalence of Sarcopenic Obesity in Adults with Class, II/III Obesity Using Different Diagnostic Criteria. J. Nutr. Metab. 2017;2017:7307618. doi: 10.1155/2017/7307618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh C., Jho S., No J.K., Kim H.S. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr. Res. 2015;35:1–6. doi: 10.1016/j.nutres.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Taing K.Y., Farkouh M.E., Moineddin R., Tu J.V., Jha P. Comparative associations between anthropometric and bioelectric impedance analysis derived adiposity measures with blood pressure and hypertension in India: A cross-sectional analysis. BMC Obes. 2017;1:37. doi: 10.1186/s40608-017-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton A., Balnave R., Adams R. Grip strength testing reliability. J. Hand Ther. 1994;7:163–170. doi: 10.1016/S0894-1130(12)80058-5. [DOI] [PubMed] [Google Scholar]
- 22.Maggio M., Ceda G.P., Ticinesi A., De Vita F., Gelmini G., Costantino C., Meschi T., Kressig R.W., Cesari M., Fabi M., et al. Instrumental and non-instrumental evaluation of 4-m walking speed in older individuals. PLoS ONE. 2016;11:e0153583. doi: 10.1371/journal.pone.0153583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashed A.M., Abdel-Wahab N., Moussa E.M.M., Hammam N. Association of hand grip strength with disease activity, disability and quality of life in children and adolescents with Juvenile Idiopathic Arthritis. Adv. Rheumatol. 2018;58:11. doi: 10.1186/s42358-018-0012-1. [DOI] [PubMed] [Google Scholar]
- 24.Tyrovolas S., Panagiotakos D., Georgousopoulou E., Chrysohoou C., Tousoulis D., Haro J.M., Pitsavos C. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: The ATTICA study. J. Epidemiol. Community Health. 2020;74:26–31. doi: 10.1136/jech-2019-212268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shou J., Chen P.J., Xiao W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020;12:14. doi: 10.1186/s13098-020-0523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuttle C.S.L., Thang L.A.N., Maier A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020;64:101185. doi: 10.1016/j.arr.2020.101185. [DOI] [PubMed] [Google Scholar]
- 27.Liao Y.S., Li H.C., Lu H.K., Lai C.L., Wang Y.S., Hsieh K.C. Comparison of Bioelectrical Impedance Analysis and Dual Energy X-ray Absorptiometry for Total and Segmental Bone Mineral Content with a Three-Compartment Model. Int. J. Environ. Res. Public Health. 2020;17:2595. doi: 10.3390/ijerph17072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H., Kim C.H., Kim D.W., Park M., Park H.S., Min S.S., Han S.H., Yee J.Y., Chung S., Kim C. External cross-validation of bioelectrical impedance analysis for the assessment of body composition in Korean adults. Nutr. Res. Pract. 2011;5:246–252. doi: 10.4162/nrp.2011.5.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boneva-Asiova Z., Boyanov M.A. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes. Metab. 2008;10:1012–1018. doi: 10.1111/j.1463-1326.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 30.Pateyjohns I.R., Brinkworth G.D., Buckley J.D., Noakes M., Clifton P.M. Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity (Silver Spring) 2006;14:2064–2070. doi: 10.1038/oby.2006.241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Are available from the corresponding author on reasonable request.