Abstract
BACKGROUND
Non-alcoholic fatty liver disease (NAFLD) is a global health issue that is correlated with obesity and oxidative stress.
AIM
To evaluate the anti-NAFLD effect of papaya in high fat diet induced obesity in rats.
METHODS
Four-week-old male Sprague-Dawley rats were divided into four groups after 1 wk of acclimatization: Group 1 was the rats fed a normal diet (C); group 2 was the rats fed a high fat diet (HFD); group 3 was the rats fed a HFD with 0.5 mL of papaya juice/100 g body weight (HFL), and group 4 was the rats fed a HFD with 1 mL of papaya juice/100 g body weight (HFH) for 12 wk. At the end of the treatment, blood and tissue samples were collected for biochemical analyses and histological assessment.
RESULTS
The results of the HFH group showed significantly reduced body weight (HFH vs HFD, P < 0.01), decreased NAFLD score (HFH vs HFD, P < 0.05), and reduced hepatic total cholesterol (HFL vs HFD, P < 0.01; HFH vs HFD, P < 0.001), hepatic triglyceride (HFH vs HFD, P < 0.05), malondialdehyde (HFL, HFH vs HFD, P < 0.001), tumour necrosis factor-α (HFH vs HFD, P < 0.05) and interleukin-6 (HFH vs HFD, P < 0.05) when compared to the HFD group. However, the liver weight showed no significant difference among the groups. The activities of catalase and superoxide dismutase significantly increased in HFH when compared with the HFD group (P < 0.05 and P < 0.001, respectively). The suppression of transcriptional factors of hepatic lipogenesis, including sterol regulatory element-binding protein 1c and fatty acid synthase, were observed in the papaya treated group (HFH vs HFD, P < 0.05). These beneficial effects of papaya against HFD-induced NAFLD are through lowering hepatic lipid accumulation, suppressing the lipogenic pathway, improving the balance of antioxidant status, and lowering systemic inflammation.
CONCLUSION
These current results provide experimental-based evidence suggesting papaya is an efficacious medicinal fruit for use in the prevention or treatment of NAFLD.
Keywords: High fat diet, Lipogenic gene expression, Non-alcoholic fatty liver disease, Obesity, Oxidative stress, Papaya
Core Tip: High fat diet consumption causes non-alcoholic fatty liver disease (NAFLD). This is one of the major liver diseases found worldwide. Liver fat accumulation leads to dysfunction of liver due to oxidative stress and inflammation. Papaya is an important export fruit from Asian and Latin America. It is a nutrient rich fruit with many medicinal properties. Our present study clearly demonstrated that the hepatoprotective mechanism of papaya against NAFLD was a result of the association of the hypolipidemic, anti-inflammatory, and antioxidant activities. This study provides evidence for the beneficial effects of papaya to reverse the progression of NAFLD in obese rats.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is of growing concern since its prevalence is increasing worldwide[1]. NAFLD is characterised by an accumulation of triglycerides and fatty acids in hepatocytes. The circulating pool of free fatty acids (FFAs) is increased in obese individuals and accounts for the majority of lipid accumulation in NAFLD. Excessive consumption of diets rich in fat is related to oxidative stress in various tissues including vessels, adipose tissues and liver and consequent to disease development[2]. Normally, oxidative stress such as reactive oxygen species (ROS) and reactive nitrogen species are continuously generated from inside the cells (e.g., electron transfer, cellular metabolism), but there is the counterbalance by the antioxidant system to defend the body from cellular or tissue damage[3]. In NAFLD, an imbalance of oxidant synthesis and antioxidants is the major contributor to the pathogenesis of the disease, leading to liver injury and hepatocyte deterioration[4]. Antioxidants have been suggested to be beneficial for health promotion and disease prevention. Therefore, we hypothesised that fruit rich in antioxidants may have potential benefit against NAFLD.
Carica papaya known as pawpaw or papaya is in the family of Caricaceae[5]. It is widely cultivated in many regions of the world, including Central and South America, Asia, and Africa, and its principal markets for consumption are the United States and Europe[6]. Papaya is a nutraceutical plant with many medicinal properties. Some studies have reported its health benefits including the treatment of gastrointestinal related disorders, diabetes, hypertension, hypercholesterolemia and hepatotoxicity, and its anti-microbial, anti-parasitic, and anti-viral properties[7,8]. Almost all parts of papaya can be used, especially the fruit of C. papaya. It is a nutritional source that is high in fibre, minerals and strong antioxidants including vitamin A, C and E. However, its health benefits in NAFLD are still the subject of research.
The purpose of this study was to evaluate the effect of papaya juice in the treatment of NAFLD. The doses of papaya juice used in this study can be practically applied to human use. Since papaya is low cost, easily available and widely marketed worldwide, the results from this study could be implemented in nutritional intervention that may be used in the prevention and treatment of NAFLD.
MATERIALS AND METHODS
Plant material and preparation of papaya
The Holland variety of papaya fruit (Carica papaya L.) was derived from a supermarket in Phitsanulok, Thailand. The fruit was harvested at a ripe stage, when papaya presents yellow areas on 50%-75% of the skin[9]. The juice was freshly prepared by extraction from the homogenised flesh of the Holland cultivar and separated from the pulp by squeezing it several times. The juice was then centrifuged at 1500 × g for 20 min. The papaya composition as shown in Table 1 was analysed by Food and Nutrition Laboratory, Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand.
Table 1.
Composition of papaya
|
Nutrients
|
Value
|
| Energy (kcal) | 26.62 ± 0.26 |
| Moisture (g) | 92.86 ± 0.06 |
| Protein (g) | 0.65 ± 0.02 |
| Total fat (g) | 0.00 ± 0.00 |
| Total carbohydrate (g) | 6.01 ± 0.09 |
| Soluble dietary fibre (g) | 0.87 ± 0.03 |
| Ash (g) | 0.49 ± 0.01 |
| Total sugar (g) | 3.97 ± 0.02 |
| Calcium (mg) | 25.95 ± 0.98 |
| Potassium (mg) | 14.50 ± 0.28 |
| Iron (mg) | 0.56 ± 0.01 |
| Total phenolic compounds (mg gallic acid/g papaya) | 0.56 ± 0.01 |
| Carotenoid profile: | |
| Beta-cryptoxanthin (μg) | 596.04 ± 15.27 |
| Lycopene (μg) | 1166.88 ± 11.24 |
| Beta-carotene (μg) | 78.96 ± 1.45 |
Animals and experimental design
The NAFLD animal model was developed as described previously[10]. Four-week-old male Sprague-Dawley rats weighing between 100 and 120 g were purchased from the National Laboratory Animal Centre at Salaya campus, Mahidol University (Nakon Pratom, Thailand). All animal experiments were carried out after getting approval from the Animal Ethics Committee at the Centre for Animal Research at Naresuan University, Phitsanulok, Thailand (Approval number NU-AE 580714). All procedures were performed in accordance with Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press)[11]. The animals were acclimatised for 1 wk and then randomised into four groups (n = 6-7). Group 1 was the control rats fed a commercial normal diet for 8 wk (C), while the three remaining groups (2-4) were fed a high fat diet (HFD) for 8 wk and oral gavage for 1 mo as follows; Group 1 was fed a normal diet for 8 wk and then treated with distilled water for an additional 4 wk, animals were maintained on a normal diet. After the first 8 wk period on HFD, animals in group 2 were fed a HFD for 4 wk, while those of groups 3 and 4 were kept on HFD and received 0.5 mL and 1 mL/100 g body weight/day of papaya juice, respectively.
The doses of papaya used in 0.5 mL of papaya juice/100 g body weight (HFL) and 1 mL of papaya juice/100 g body weight (HFH) were the equivalent of approximately 125 and 250 g of papaya consumed by a person, respectively. Diet composition of control and high fat diets were formulated according to AIN-93G as previously described with a slight modification[12]. Briefly, the high fat diets were composed of 1.5% cholesterol, 20% palm oil and 0.25% cholic acid. Body weights of rats were recorded weekly. At the end of the 12th week, the animals were euthanised by pentobarbital injection. The blood was drawn through cardiac puncture. Blood and tissue samples were collected and kept at -80 °C for further analysis.
Biochemical analyses
The serum was used to measure aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) by Bio Lab Medical Centre (Phitsanulok, Thailand).
Analysis of hepatic TAG and cholesterol content
Hepatic lipid was extracted according to a modified Folch method, as previously described[13]. Briefly, lipids were extracted from 0.5 g of liver with a mixture of chloroform/methanol (2:1, v/v) and dried under N2. The pellets were dissolved and used for the analysis of hepatic lipid contents. The hepatic contents of triglyceride and total cholesterol were determined using a colorimetric assay kit according to the instructions of the manufacturer (HUMAN Gesellschaft für Biochemica und Diagnostica mbH, Wiesbaden, Germany).
Histopathological analyses
To analyse the histopathology of the liver, the tissue was fixed immediately after removal in 10% formalin. The liver tissue was then embedded in paraffin, sectioned, and stained with haematoxylin and eosin. The histopathological features were scored for the liver lesions using NAFLD activity score (NAS) according to Xu et al[14]. NAS component represents the sum of score ranging from 0-8 for three histological features: Hepatocyte ballooning (0-2), lobular inflammation (0-3) and steatosis (0-3). The total NAS score of 0-3 was defined as not nonalcoholic steatohepatitis (NASH). The score greater than 5-8 was considered as NASH. The hepatic lipid accumulation assessment was modified from Malakul et al[12]. In brief, the frozen liver samples with optimal cutting temperature-embedded were cryosectioned at 5 μm with a cryostat, fixed in 4% v/v formalin for 10 min and then stained with Oil Red O working solution for triglycerides and free fatty acid staining.
Hepatic lipid peroxidation
The isolated rat livers were homogenised in phosphate buffered saline (PBS), and the total protein content of liver tissues was measured using a Bradford assay kit (Sigma-Aldrich, St. Louis, MO, United States). The lipid peroxidation of the hepatic tissue homogenate was determined by a thiobarbituric acid assay. The solutions were prepared according to Liu et al[15]. Briefly, the mixture of 15% trichloroacetic acid, 0.25 N HCl (Sigma-Aldrich) and 0.37% 2-thiobarbitulic acid (POCH, Sowinskiego, Poland) with 1:1:1 ratio was prepared. Then 200 μL of these reagents were added in each eppendorf tube and incubated in heat block at 95 °C for 15 min. The solutions were centrifuged at 3500 × g for 25 min and the supernatant in each tube was pipetted to 96 well plates. The samples were then measured at absorbance 535 nm with malondialdehyde as a standard, and the unit was expressed as μmoL/mg protein.
Catalase and superoxide dismutase activities
The livers were homogenised in ice cold PBS. The homogenate was centrifuged, and the supernatant were taken to measure the activities of catalase (CAT) and superoxide dismutase (SOD) by using commercial assay kits (Cayman Chemical Company, Ann Arbor, MI, United States). The final units for enzyme activities were normalised with protein concentration.
Determination of biomarkers of inflammation
The liver homogenates were used to determine the levels of tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6) by using commercial assay kits (Sigma-Aldrich). The final units for TNF-α and IL-6 were normalised with protein concentration.
Analysis of gene expression
Total ribonucleic acid (RNA) of the liver was isolated using RiboZol (Amresco, Dallas, TX, United States) according to the protocol provided by the manufacturer. The complementary deoxyribonucleic acid synthesis was performed in a reaction mixture containing 4 μL of reaction buffer, 2 μL of deoxyribonucleotide triphosphate, 1 μL of random primer, 1 μL of RNAse inhibitor, 1 μL of reverse transcriptase and 500 ng of total RNA. Polymerase chain reaction (PCR) was performed with PCR thermocycling. The PCR products were measured by agarose gel electrophoresis technique with 2% agarose gel and 1 × TBE running buffer (1M Tris, 0.9M boric acid and 1 mmoL/L EDTA). Deoxyribonucleic acid was stained with a fluorescent colour (Biotechnology, Daejeon, Korea). Each sample was assayed in triplicate, and β-actin was amplified in parallel to serve as an internal control for reverse transcription-PCR quantification. All mRNA gene expression data were normalised to the expression level of β-actin.
The sequences of the primers for genes used in this study were indicated as follows; SREBP-1c: forward 5’-TGGATTGCACATTTGAAGACAT-3’, reverse 5’-GCTCCTCTTTGATTCCAGGC-3’; ACC: forward 5’-GCCTCTTCCTGACAAACGAG-3’, reverse 5’-TCCATACGCCTGAAACATGA-3’; FAS: forward 5’-GGACATGGTCACAGACGATGAC-3’, reverse 5’-GTCGAACTTGGACAGATCCTTCA-3’. ACTB: forward5’-TGTCCACCTTCCAGCAGATGT-3’, reverse 5’- AGCTCAGTAACAGTCGA -3’.
Statistical analysis
Results are presented as the mean ± SE of the mean. Statistical analyses were performed using IBM SPSS version 23 (Armonk, NY, United States). Group difference was assessed by a one-way analysis of variance, followed by Tukey’s test for multiple comparisons. A P value < 0.05 was considered statistically significant.
RESULTS
Effects of papaya on liver weight, lipid contents and serum components in rats
The initial body weight and body weight at week 8 of all the experimental groups were not significantly different. However, at the end of treatment the HFD group showed significantly increased body weight when compared with the C group, while those parameters decreased in the HFH group. The result also showed that papaya improved hepatic lipid contents in HFD-fed rats. The HFD group showed significantly increased hepatic triglycerides (TG) and cholesterol levels when compared with the C. The TG levels were significantly decreased in the HFH (P < 0.05), while total cholesterol (TC) was significantly decreased in both the HFL (P < 0.01) and HFH (P < 0.001) when compared with the HFD group. This result indicated that papaya markedly reduced the hepatic TG and TC contents. The serum levels of AST, ALT and ALP were significantly increased in rats fed a HFD. Higher levels of those enzymes suggest that a HFD can induce liver inflammation or liver damage. Moreover, the liver damage indices also significantly decreased in the papaya treated group when compared to the HFD group (Table 2). This result suggests that papaya administration may improve liver injury found in NAFLD.
Table 2.
Effects of papaya on body weight, liver weight, hepatic lipid contents and liver damage indices in high fat diet induced obesity in rats
|
|
C
|
HFD
|
HFL
|
HFH
|
| Initial weight (g) | 218.0 ± 12.18 | 236.5 ± 13.58 | 215.5 ± 14.01 | 221.8 ± 14.29 |
| Body weight at week 8 (g) | 398.6 ± 9.462 | 457.6 ± 18.93a | 462.9 ± 17.11a | 454.9 ± 7.584a |
| Body weight at week 12 (g) | 465.83 ± 11.13 | 536 ± 33.24c | 509.33 ± 33.57a | 471.33 ± 15.04e |
| Liver weight (% of body weight) | 2.66 ± 0.13 | 4.46 ± 0.3c | 4.49 ± 0.51c | 4.33 ± 0.26c |
| Hepatic triglycerides (mg/dL) | 140.60 ± 13.95 | 211.00 ± 26.25a | 172.50 ± 7.89 | 152.20 ± 12.68d |
| Hepatic cholesterol (mg/dL) | 74.30 ± 5.58 | 152.60 ± 9.44c | 112.40 ± 7.96b,e | 92.38 ± 6.66f |
| Serum ALT (U/mL) | 38.80 ± 2.29 | 214.00 ± 48.95c | 109.30 ± 20.85d | 86.00 ± 7.57d |
| Serum AST (U/mL) | 126.40 ± 4.72 | 302.70 ± 51.72b | 211.30 ± 7.88 | 137.70 ± 21.42e |
| Serum ALP (U/mL) | 60.60 ± 1.80 | 86.20 ± 4.60b | 91.00 ± 4.16b | 88.00 ± 6.08b |
Data are expressed as the mean ± SE of the mean (n = 6-7).
P < 0.05.
P < 0.01.
P < 0.001 vs C.
P < 0.05.
P < 0.01.
P < 0.001 vs high fat diet group. ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate transaminase; C: Control; HFD: High fat diet; HFH: High fat diet treated with 1 mL of papaya juice/100 g body weight; HFL: High fat diet treated with 0.5 mL of papaya juice/100 g body weight.
Effects of papaya on lipid accumulation
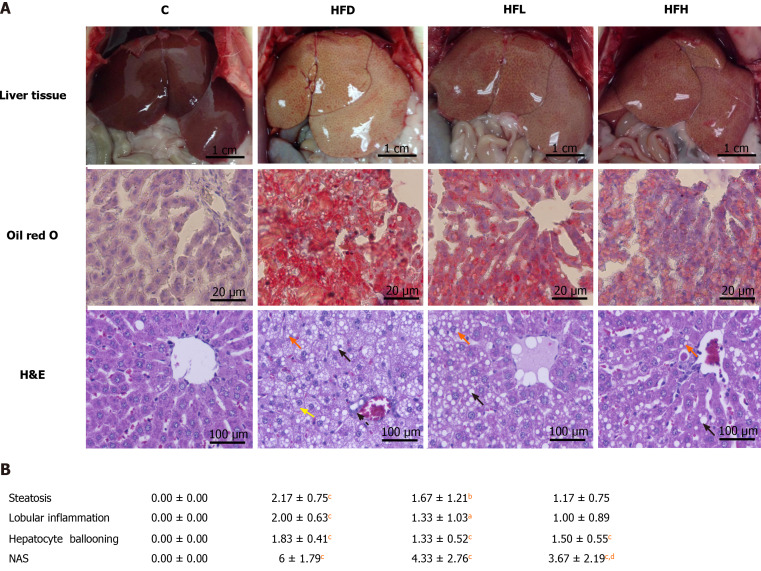
Oil Red O staining showed that hepatic lipid accumulation of HFD was significantly higher than that in the C group. The oral administration of papaya to HFD rats reduced steatosis and lipid droplet size as shown in Figure 1A. In addition, it showed that the liver samples from the HFD group showed significant fat deposition with the highest scores in steatosis, lobular inflammation and hepatocyte ballooning. The HFD group scores were significantly higher than those of the control group (P < 0.001), which strongly indicated the development of NAFLD. Interestingly, the significant reduction of steatosis, lobular inflammation and hepatocyte ballooning was observed after 4 wk of treatment with papaya (Figure 1B).
Figure 1.
Effect of papaya on non-alcoholic fatty liver disease. A: Macroscopic and microscopic appearance in rat hepatocytes. Macrovesicular steatosis (black arrow) are large lipid droplets that are present in the hepatocytes. Microvesicular steatosis (red arrow) are small lipid droplets that are present in the hepatocytes. Hepatocyte ballooning is recognised as cell swelling and enlargement within the cytoplasm (yellow arrow). Lobular inflammation in non-alcoholic steatohepatitis foci (dotted line arrow) are scattered in the hepatic lobule; B: Comparative analysis of non-alcoholic fatty liver disease activity score for all treatment groups. Data are expressed as mean ± SE of the mean (n = 6-7). aP < 0.05, bP < 0.01, cP < 0.001 vs C, and dP < 0.05 vs high fat diet group. C: Control; H&E: Hematoxylin and eosin; HFD: High fat diet; HFH: High fat diet treated with 1 mL of papaya juice/100 g body weight; HFL: High fat diet treated with 0.5 mL of papaya juice/100 g body weight; NAS: Non-alcoholic fatty liver disease activity score.
Effects of papaya on the oxidative status and antioxidant activities
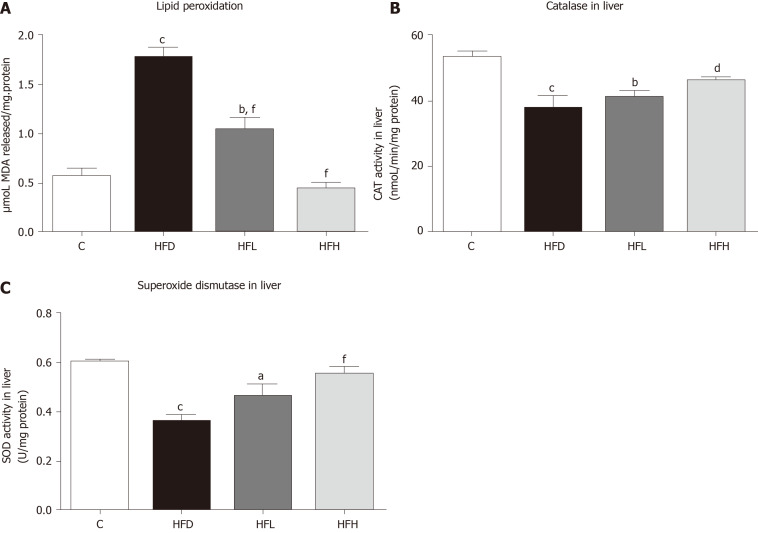
Papaya improved lipid peroxidation in HFD-fed rats. The HFD group showed significantly increased lipid peroxidation when compared with the C (P < 0.001). Furthermore, lipid peroxidation was significantly decreased in the HFD treated with papaya 0.5 and 1 mL/100 g body weight (P < 0.001) when compared with the HFD group (Figure 2A). In contrast, the CAT and SOD activities were found to decrease in the HFD group, whereas those significantly increased in HFH group (Figure 2B and C).
Figure 2.
Effects of papaya on antioxidant activities in liver tissue. A: Lipid peroxidation in the liver; B: Activity of catalase (CAT) in the liver; C: Activity of superoxide dismutase (SOD) in the liver. Data are expressed as mean ± SE of the mean (n = 6-7). aP < 0.05, bP < 0.01, cP < 0.001 vs control (C), and dP < 0.05, fP < 0.001 vs high fat diet (HFD) group. HFH: High fat diet treated with 1 mL of papaya juice/100 g body weight; HFL: High fat diet treated with 0.5 mL of papaya juice/100 g body weight; MDA: Malondialdehyde.
Effects of papaya on proinflammatory cytokines in liver tissue
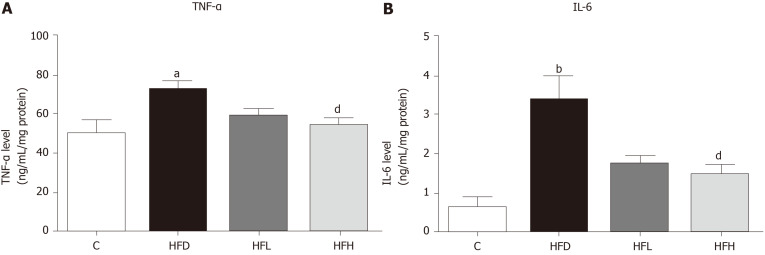
The results showed that HFD in rats significantly increased the serum levels of TNF-α (Figure 3A) and IL-6 (Figure 3B), while these two cytokine levels significantly decreased in the HFD treated with papaya 1 mL/100 g body weight (P < 0.05). Taken together, papaya administration can counterbalance lipid peroxidation and inflammation, which is normally found in NAFLD. Improvements in antioxidant activity were also observed.
Figure 3.
Effects of papaya on proinflammatory cytokines in liver tissue. A: Tumour necrosis factor-α (TNF-α) in the liver; B: Interleukin 6 (IL-6) in the liver. Data are expressed as mean ± SE of the mean (n = 6-7). aP < 0.05, bP < 0.01 vs control (C), and dP < 0.05 vs high fat diet (HFD) group. HFH: High fat diet treated with 1 mL of papaya juice/100 g body weight; HFL: High fat diet treated with 0.5 mL of papaya juice/100 g body weight.
Effects of papaya on the de novo lipogenic gene in liver tissue
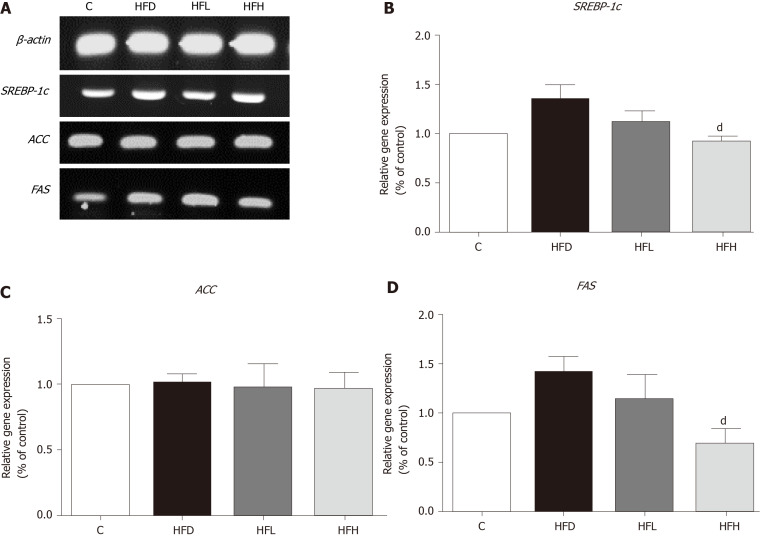
The mRNA expression of SREBP-1c and FAS had a tendency to increase in HFD rats as compared with the control. A significantly decreased expression of those genes were observed in HFH rats (P < 0.05) as shown in Figure 4A, B and D, respectively. In contrast, the expression of ACC was not different among the groups (Figure 4C). The data indicated that a possible involvement of lipogenesis in the papaya treated group is partially mediated through SREBP-1c, which down-regulates the expression of FAS. This event may account for the decreased fatty acid metabolism in the liver of rats treated with high doses of papaya juice.
Figure 4.
Effects of papaya on de novo lipogenic gene expression in liver tissue. A: Immunoblotting analysis of SREBP-1c, ACC, FAS and ACTB. ACTB was used as a normalization gene; B: Relative gene expression of SREBP-1; C: Relative gene expression of ACC; D: Relative gene expression of FAS. Data are expressed as mean ± SE of the mean (n = 5). dP < 0.05 vs high fat diet (HFD) group. C: Control; HFH: High fat diet treated with 1 mL of papaya juice/100 g body weight; HFL: High fat diet treated with 0.5 mL of papaya juice/100 g body weight.
DISCUSSION
Oxidative stress and inflammation are the main components that contributed to the pathogenesis of NAFLD. Many natural products rich in polyphenols, and strong antioxidant activity have been studied for their positive benefits in the treatment of NAFLD[16]. The presence of these bioactive compounds as well as the significant antioxidant activity in vitro has been observed in the pulp and fruit peel of papaya[9].
Our present study demonstrated that papaya attenuated lipid accumulation in HFD-induced obesity in rats. In this study the in vivo model of NAFLD was successfully established and developed to lipid accumulation in liver after feeding the rats an HFD. Those rats fed a HFD exhibited an increase in the weight of the liver and lipid contents, which is a feature of NAFLD[14]. The reverse alterations in hepatic lipid accumulation can be explained by the effects of papaya on lipid metabolism. The mechanism may be, in part, by the inhibition of pancreatic lipase by papaya[17]. Pancreatic lipase is an enzyme secreted from the pancreas and works in the small intestine to hydrolyse TG from diet to glycerol and free fatty acids. In this case, papaya juice hinders the digestion of TG, resulting in the reduction of lipid absorption and then promotion of the excretion of lipids outside the body. From previous studies, it has been shown that the excessive hepatic accumulation of TG and FFA induced hepatic steatosis[18]. From our study, it was demonstrated that the treatment with papaya ameliorates lipid accumulation in liver in HFD rats via the modulation of lipid metabolism-related molecules.
In NAFLD pathogenesis, imbalanced lipid metabolism leads to simple steatosis, oxidative damage and secretion of proinflammatory mediators. The liver serves as the major regulator for lipid metabolism that involves in several steps[19]. Hepatic lipid content is regulated by the cellular molecules that control the input and the output. The regulation depends on the metabolic status, the facilitation of hepatic fatty acid uptake, synthesis and storage in the liver, or the rapid metabolism to hepatic fatty acid oxidation as a source of energy may occur[20].
SREBP-1c exerts a significant control over the de novo synthesis of FAS[20]. It was further found that papaya eliminated hepatic steatosis in HFD rats. The latter effect might be partially mediated by the regulation of SREBP-1c. SREBP-1c is an important transcription factor of de novo lipogenesis in the liver, while its downstream gene-FAS is responsible for fatty acid catabolism[21]. In the livers of obese rats treated with papaya, SREBP-1c and FAS were remarkably decreased. This implies that papaya exerts its anti-lipogenic effect in consequence of the suppressed regulation of SREBP-1c and FAS, leading to decreased hepatic lipid accumulation.
Several studies have also demonstrated the antioxidant capacity of β-carotene and its act against oxidative stress in different models[22,23]. The significantly elevated hepatic content of TG, TC and malondialdehyde in NAFLD rats is a strong indicator of liver damage and oxidative stress[24]. The pathogenesis of NAFLD is widely accepted by the two-hit hypothesis; the first hit presents increasing levels of FAs and is a key part in the development of hepatic steatosis. Prolonging of hepatocellular damage and sensitised liver leads to the presence of oxidative stress and the release of cytokine or adipokine mediators, this situation is called a second hit[25]. More specifically, high fat consumption leads to increased FAs in liver and either enter β-oxidation or are stored as TG. The mitochondrial β-oxidation serves as energy sources and can generate numerous free radicals including ROS and lipid peroxidation from the electron transport chain though the mitochondrial respiration pathway[26]. Normally, the antioxidant defensive systems help to protect the organs against the deleterious substances[27]. Among these, SOD is a key antioxidant enzyme for the first defence reaction with the ROS-mediated cellular damage. SOD participates in the conversion of superoxide anions into less harmful H2O2 and oxygen. CAT is another antioxidant enzyme that can catalyse H2O2 into water and oxygen[28]. From our results, it clearly shows that SOD and CAT activities in the liver were significantly increased after papaya treatment. The mechanism is still unknown, but it might be because of the carotenoid compounds in papaya. Papaya is one of the important dietary sources for carotenoids including β-carotene and lycopene[29]. The liver is the main place for storage carotenoids, the powerful antioxidants from food, and this compound may help scavenge the results of oxidative stress produced in the liver[16].
High fat accumulation in the liver causes impairment of cellular homeostasis. ROS and lipid peroxidation generated in NAFLD are potent inducers of cytokine production and trigger the release of cytokine proinflammatory mediators such as TNF-α and IL-6[30]. TNF-α plays a crucial role in exert in a variety of biological effects including systemic inflammation and takes part in many stages of liver disease[31]. In contrast, IL-6 is secreted from various kinds of cells and is necessary to leukocyte recruitment and tissue homeostasis[32]. Recent studies have been reported that IL-6 enhances liver inflammation and related to insulin resistance in NAFLD[29]. Proinflammatory cytokine overproduction causes hepatocyte dysfunction and develops fibrosis later on.
We demonstrated from our results that papaya can reduce liver inflammation by the inhibiting the overproduction and activity of proinflammatory cytokines generated in high fat induced hepatic inflammation tissue. The mechanism may be from indirect action of papaya to reduce ROS and can modulate the overwhelming production of cytokines. In addition, papaya itself may play a direct role in the inflammation processes. As reported earlier, papaya possesses anti-inflammatory and immunomodulatory properties as stated both in vitro and in vivo studies[33]. Liver inflammation can aggravate liver damage, resulting in the progression of fibrosis, cirrhosis or liver failure. Reduced inflammatory secretion from cytokines may prevent steatosis and alleviate the progression of the disease[34].
CONCLUSION
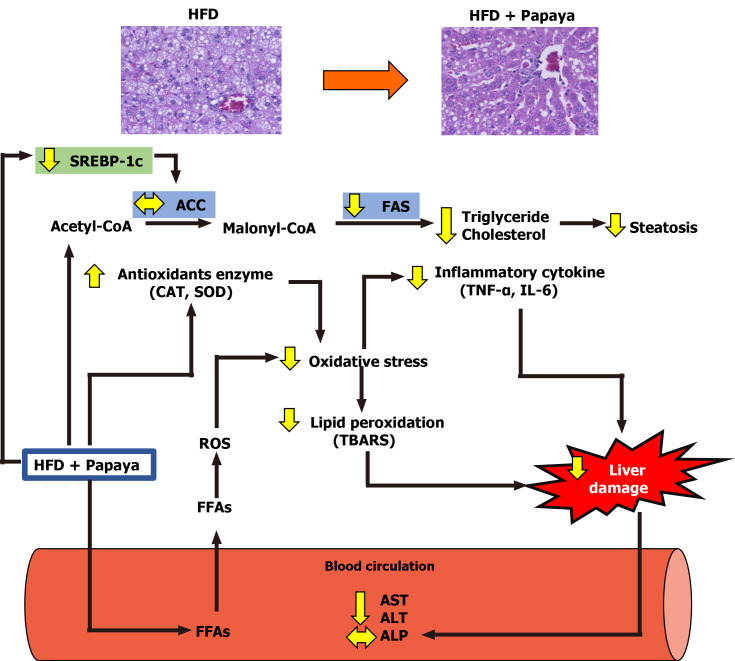
This study demonstrates for the first time the hepatoprotective capacity of the papaya fruit on the damage caused by HFD induced hepatic steatosis. From the obtained results, it can be suggested that the mechanism of action of the hepatoprotective effect of the papaya against the hepatic lipid accumulation in NAFLD was the combined result of the association of the anti-lipogenic, anti-inflammatory and antioxidant activities of papaya (Figure 5).
Figure 5.
Schematic diagram of possible mechanism of papaya juice on non-alcoholic fatty liver disease. The beneficial effect of papaya against hepatic steatosis in obese rats may occur through the inhibition of lipogenic pathways by reducing SREBP-1c and FAS gene expression, causing the reduction of hepatic fat accumulation. Papaya can improve enzymatic antioxidants [catalase (CAT) and superoxide dismutase (SOD)] and decrease lipid peroxidation in the liver. The administration of papaya significantly decreased proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6) to modulate liver damage. Papaya is therefore able to reduce the activities of aspartate transaminase (AST) and alanine transaminase (ALT) in serum. Overall, this study provides evidence for the beneficial effects of papaya to reverse the progression of non-alcoholic fatty liver disease in obese rats. ALP: Alkaline phosphatase; FFAs: Free fatty acids; HFD: High fat diet; ROS: Reactive oxygen species; TBARS: 2-Thiobarbituric acid reactive substances.
Moreover, the doses of papaya used in this study can be of practical use in human medicine. The results of this study provide experimental-based evidence suggesting papaya is an efficacious nutritional strategy for use in the prevention or treatment of NAFLD. However, future research should be performed using human trials to elucidate the intervention of papaya in clinical and public health implications.
ARTICLE HIGHLIGHTS
Research background
High fat diet consumption causes fat accumulation in liver [nonalcoholic fatty liver disease (NAFLD)], which leads to liver dysfunction due to oxidative stress and inflammation
Research motivation
Papaya is a nutritional, healthy and affordable fruit. It is available in all regions of the world and can be found year-round. Additional scientific evidence on the health and nutritional benefits of papaya are needed to promote health and papaya consumption.
Research objectives
To evaluate papaya’s health benefit against NAFLD in obese rats.
Research methods
Rats were fed with a high fat diet for 12 wk to induce obesity. Papaya juice at the implement doses were administered to the rats. Hepatic lipid contents, oxidative stress, inflammatory cytokines, lipogenic genes and liver pathology were assessed.
Research results
The hepatoprotective action of papaya against the accumulation of hepatic fat was a result of the association of the hypolipidemic effect partially through a suppression of SREBP-1c and FAS, anti-inflammatory and antioxidant activities.
Research conclusions
The results of this study provide experimental-based evidence that can contribute to the implement of papaya in the prevention and treatment of obesity and associated metabolic disorders.
Research perspectives
Our study offers an optimistic view of an anti-NAFLD effect of papaya; however, further evidence from human clinical studies is necessary.
ACKNOWLEDGEMENTS
We would like to thank Dr. Julintorn Samran, MD from the Department of Pathology, Faculty of Medicine, Naresuan University for assisting in technical advice for liver pathology evaluation. Thanks to Mr. Kevin Mark Roebl of the Division of International Affairs and Language Development for manuscript revision.
Footnotes
Institutional animal care and use committee statement: The experimental protocol was approved by the institutional animal care and committee of Naresuan University, Phitsanulok, Thailand. No. NU-AE 580714.
Conflict-of-interest statement: All other authors have nothing to disclose.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: October 23, 2020
First decision: November 25, 2020
Article in press: February 11, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ahmed M, Zhang X S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Wanwisa Deenin, Department of Physiology, Faculty of Medical Science, Naresuan University, Muang 65000, Phitsanulok, Thailand.
Wachirawadee Malakul, Department of Physiology, Faculty of Medical Science, Naresuan University, Muang 65000, Phitsanulok, Thailand.
Tantip Boonsong, Department of Biochemistry, Faculty of Medical Science, Naresuan University, Muang 65000, Phitsanulok, Thailand.
Ittipon Phoungpetchara, Department of Anatomy, Faculty of Medical Science, Naresuan University, Muang 65000, Phitsanulok, Thailand.
Sakara Tunsophon, Department of Physiology, Faculty of Medical Science, Naresuan University, Muang 65000, Phitsanulok, Thailand; Centre of Excellence for Innovation in Chemistry, Naresuan University, Muang 65000, Phitsanulok, Thailand. sakarat@nu.ac.th.
Data sharing statement
No additional data are available.
References
- 1.Kleiner DE, Berk PD, Hsu JY, Courcoulas AP, Flum D, Khandelwal S, Pender J, Pomp A, Roerig J, Machado LL, Wolfe BM, Belle SH LABS Consortium. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Semin Liver Dis. 2014;34:98–107. doi: 10.1055/s-0034-1371083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 2009;8:2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira JG, Vitória AP. Papaya: Nutritional and pharmacological characterization, and quality loss due to physiological disorders. An overview. Food Res Int . 2011;44:1306–1313. [Google Scholar]
- 7.O’Hare TJ, Williams DJ. Papaya as a Medicinal Plant. In: Ming R, Moore PH. Genetics and Genomics of Papaya. New York, NY: Springer New York, 2014: 391-407. [Google Scholar]
- 8.Santana LF, Inada AC, Espirito Santo BLSD, Filiú WFO, Pott A, Alves FM, Guimarães RCA, Freitas KC, Hiane PA. Nutraceutical Potential of Carica papaya in Metabolic Syndrome. Nutrients. 2019;11:1608. doi: 10.3390/nu11071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikram EHK, Stanley R, Netzel M, Fanning K. Phytochemicals of papaya and its traditional health and culinary uses – A review. J Food Compost Anal. 2015;41:201–211. [Google Scholar]
- 10.Al Zarzour RH, Ahmad M, Asmawi MZ, Kaur G, Saeed MAA, Al-Mansoub MA, Saghir SAM, Usman NS, Al-Dulaimi DW, Yam MF. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague-Dawley Rats. Nutrients. 2017;9:776. doi: 10.3390/nu9070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press, 2011. [Google Scholar]
- 12.Malakul W, Thirawarapan S, Suvitayavat W, Woodman OL. Type 1 diabetes and hypercholesterolaemia reveal the contribution of endothelium-derived hyperpolarizing factor to endothelium-dependent relaxation of the rat aorta. Clin Exp Pharmacol Physiol. 2008;35:192–200. doi: 10.1111/j.1440-1681.2007.04811.x. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Xu J, Rong S, Gao H, Chen C, Yang W, Deng Q, Huang Q, Xiao L, Huang F. A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats. Nutrients. 2017;9:271. doi: 10.3390/nu9030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Wang WX. Physiological and cellular responses of oysters (Crassostrea hongkongensis) in a multimetal-contaminated estuary. Environ Toxicol Chem. 2016;35:2577–2586. doi: 10.1002/etc.3426. [DOI] [PubMed] [Google Scholar]
- 16.Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J Gastroenterol. 2017;23:4146–4157. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trisat K, Limpeanchop N, Ounaroon A. Guava, Papaya, Pineapple, and Pomelo Juices Inhibit Pancreatic Lipase Activity and Cholesterol Micelle Solubility. Thai J Pharmacol. 2016:1–5. [Google Scholar]
- 18.Wu J, Zhang H, Zheng H, Jiang Y. Hepatic inflammation scores correlate with common carotid intima-media thickness in rats with NAFLD induced by a high-fat diet. BMC Vet Res. 2014;10:162. doi: 10.1186/1746-6148-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients. 2017;9:387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 21.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 22.Mueller L, Boehm V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules. 2011;16:1055–1069. doi: 10.3390/molecules16021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotondo Dottore G, Ionni I, Menconi F, Casini G, Sellari-Franceschini S, Nardi M, Vitti P, Marcocci C, Marinò M. Antioxidant effects of β-carotene, but not of retinol and vitamin E, in orbital fibroblasts from patients with Graves' orbitopathy (GO) J Endocrinol Invest. 2018;41:815–820. doi: 10.1007/s40618-017-0809-5. [DOI] [PubMed] [Google Scholar]
- 24.Xu P, Zhang XG, Li YM, Yu CH, Xu L, Xu GY. Research on the protection effect of pioglitazone for non-alcoholic fatty liver disease (NAFLD) in rats. J Zhejiang Univ Sci B. 2006;7:627–633. doi: 10.1631/jzus.2006.B0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–208. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- 26.Yokozawa T, Cho EJ, Sasaki S, Satoh A, Okamoto T, Sei Y. The protective role of Chinese prescription Kangen-karyu extract on diet-induced hypercholesterolemia in rats. Biol Pharm Bull. 2006;29:760–765. doi: 10.1248/bpb.29.760. [DOI] [PubMed] [Google Scholar]
- 27.Soylu AR, Altaner S, Aydodu N, Basaran UN, Tarcin O, Gedik N, Umit H, Tezel A, Ture M, Kutlu K, Kaymak K. Effects of vitamins E and C supplementation on hepatic glutathione peroxidase activity and tissue injury associated with ethanol ingestion in malnourished rats. Curr Ther Res Clin Exp. 2006;67:118–137. doi: 10.1016/j.curtheres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ucar F, Sezer S, Erdogan S, Akyol S, Armutcu F, Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013;18:127–133. doi: 10.1179/1351000213Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweiggert RM, Kopec RE, Villalobos-Gutierrez MG, Högel J, Quesada S, Esquivel P, Schwartz SJ, Carle R. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study. Br J Nutr. 2014;111:490–498. doi: 10.1017/S0007114513002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia. 2003;41:152–160. doi: 10.1002/glia.10168. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Xu W, Feng X, He Y, Liu X, Gao Y, Yang S, Shao Z, Yang C, Ye Z. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Pharmacol. 2015;28:351–361. doi: 10.1177/0394632015593228. [DOI] [PubMed] [Google Scholar]
- 32.Cronin JG, Kanamarlapudi V, Thornton CA, Sheldon IM. Signal transducer and activator of transcription-3 licenses Toll-like receptor 4-dependent interleukin (IL)-6 and IL-8 production via IL-6 receptor-positive feedback in endometrial cells. Mucosal Immunol. 2016;9:1125–1136. doi: 10.1038/mi.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey S, Cabot PJ, Shaw PN, Hewavitharana AK. Anti-inflammatory and immunomodulatory properties of Carica papaya. J Immunotoxicol. 2016;13:590–602. doi: 10.3109/1547691X.2016.1149528. [DOI] [PubMed] [Google Scholar]
- 34.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.