Abstract
Aims:
Congenital coronary artery anomalies are uncommon and may result in sudden death. Management of asymptomatic patients with anomalous aortic origin of the right coronary artery (AAORCA) remains controversial with lack of evidence to guide decision making. We hypothesized that patients with AAORCA may have exercise-inducible ischemia detectable as abnormalities in regional myocardial deformation on exercise stress echocardiography (ESE).
Methods:
We reviewed clinical data, computed tomography angiography and treadmill ESE from 33 AAORCA patients (21 unoperated, 12 operated) and 11 controls. Regional wall motion on ESE was visually assessed. Doppler tissue imaging was done pre and post exercise to evaluate regional myocardial wall deformation. The post- to pre-exercise time to peak systolic strain corrected for heart rate ratio (TPScR) for the left ventricular inferior and anterior walls of AAORCA patients was compared to controls.
Results:
No regional wall motion abnormalities were noted. The TPScR of the inferior wall was higher in unoperated (0.96 ± 0.41) but not operated (0.84 ± 0.28) AAORCA patients compared to controls (0.76 ± 0.18, P = .03 versus .23, respectively). There was no significant difference in TPScR of the anterior wall between unoperated patients and controls (P = .08).
Conclusion:
In some AAORCA patients undergoing ESE, TPScR of the left ventricular inferior wall is elevated, suggestive of ischemia induced by exercise in myocardium supplied by the right coronary artery. Further work is needed to understand the potential role of this finding in risk assessment.
Keywords: Coronary artery anomaly, congenital heart surgery, coronary artery surgery, echocardiography, ischemia, pediatric
Background
Congenital coronary artery anomalies are uncommon, though anomalous aortic origin of a coronary artery from the opposite sinus of Valsalva is an important cause of sudden cardiac death in childhood.1 The risk of death is higher during exercise and is thought to be related to anatomical characteristics of the anomalous coronary. These include the often slit-like shape of the coronary origin, the interarterial course between the aorta and pulmonary artery, the presence of an intramural course, and possibly the length of the intramural segment.2 Anomalous aortic origin of the right coronary artery (AAORCA) as compared to anomalous aortic origin of the left coronary artery (AAOLCA) is both more common and less often associated with sudden death.3,4 Symptoms may include chest pain or syncope with exercise, but sudden death may occur without prior symptoms. Patients are often identified incidentally by echocardiography performed for other reasons or in the evaluation of chest pain or syncope that may or may not be of cardiac origin. Surgery has focused on addressing one or more of the anatomical aspects described above, but surgical indications are unclear, especially for asymptomatic patients with AAORCA given the lower incidence of sudden death. Exercise stress imaging of the heart is sometimes used in these patients to determine if exercise can provoke coronary flow perturbation or ischemia detectable as either perfusion or wall motion abnormality (WMA), respectively. The clinical rationale for using these tests assumes that a positive test may mean a higher risk of sudden death and a negative test may help justify a more conservative, non-surgical approach. However, evidence to support this approach is lacking.2
In adults with atherosclerotic coronary artery disease (CAD), abnormalities in myocardial strain and strain rate have been shown to be sensitive indicators of myocardial ischemia and dysfunction.5,6 Peak systolic strain increases with exercise and has been shown to be reduced by ischemia.7 In addition, lower peak diastolic strain has been reported in exercise-induced ischemic segments.8 Onset and termination of systolic shortening is delayed by ischemia.9 Finally, a higher post to pre exercise time to peak systolic strain, corrected for heart rate, ratio (TPScR), specifically in the inferior wall for right coronary artery (RCA) stenosis, has been shown to be a highly sensitive marker of ischemia.10
In this study, we report the clinical characteristics, cardiac computed tomography angiography (CTA) findings, and results of exercise stress echocardiography in a series of patients with AAORCA. We hypothesized that patients with AAORCA may have exercise-inducible abnormalities in left ventricular (LV) inferior wall deformation as a marker of myocardial ischemia.
Methods
The study was approved by the institutional review board (IRB) of Johns Hopkins School of Medicine. Informed consent was waived by our institution’s IRB due to the retrospective nature of the study and minimal risk. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution’s human research committee. Sixty-three consecutive treadmill ESE from January 2014 to December 2017 performed on unique patients with either diagnosis of AAORCA or no heart disease were retrospectively reviewed. Of these, 44 (70%) had baseline and post exercise color Doppler tissue imaging (CDTI) of the LV inferior and anterior walls suitable for strain analysis. There were 33 patients with AAORCA (21 unoperated, 12 operated, only one paired) and 11 controls with no heart disease. The control group consisted of patients with a structurally normal heart who underwent ESE for evaluation of symptoms (chest pain or dizziness), or cardiac screening due to a family history of congenital heart disease.
Computed Tomography Angiography Evaluation of Coronary Anatomy
Cardiac CTA images were available for 14 (66%) of the patients with unoperated AAORCA. A single radiologist with experience in cardiovascular imaging (S.LZ.) reviewed each study. The RCA anatomy was evaluated for the following features: (1) ostial type (separate, shared or branch vessel), (2) proximal vessel morphology (normal, oval, or slit-like), (3) length of narrowing, (4) intramural vs. not intramural, (5) takeoff angle (acute vs. not acute), (5) takeoff level (above or below commissure), and (6) right versus left dominant coronary artery systems. Takeoff angle was considered acute if < 45°. The percentage of RCA narrowing was further quantified by measuring the smallest diameter of the narrowed portion (D1) and the diameter in the first frame after the end of narrowing (D2) to calculate percentage of narrowing as (D2-D1)/D2. The extent of RCA narrowing was calculated by multiplying percentage of narrowing by the length of narrowing.
Exercise Stress Echocardiography
All patients had treadmill exercise testing using the standard Bruce protocol. Echocardiographic images were obtained using GE Vivid E95 ultrasound machines (GE Health Medical). Echocardiographic B-mode images were obtained from the apical four-chamber, two-chamber, and long-axis views, and parasternal long- and short-axes views at baseline and immediately after termination of exercise. Regional wall motion was assessed by visual inspection by a single attending pediatric cardiologist with experience in reading ESEs (W.R.T.). Color Doppler tissue imaging, obtained at baseline pre-exercise and later in the post exercise period after allowing the heart rate to fall to ~50% peak levels, was used for myocardial wall deformation analysis to measure strain. Predicted maximum heart rate (beats per minute) was assumed to be 220 - age in years.
Strain Analysis
Left ventricular inferior and anterior wall longitudinal strain was measured using the CDTI clips from the apical two-chamber views using EchoPAC PC software (version 113, revision 1.0, GE Health Care). Mean heart and frame rates were 71 ±14 bpm and 95 ± 8 frames/second, respectively, at baseline and 115 ± 18 bpm and 95 ± 8 frames/second post exercise. The region of interest (oval in shape, measuring 20 mm x 6 mm) was placed in the middle third of the inferior wall, and the anterior wall and peak systolic strain was measured. End diastole, which served as the reference time point, was defined as occurring at the R-top on the electrocardiogram (ECG) tracing. Strain was expressed as a percentage and considered negative for shortening and positive for lengthening myocardium. Peak systolic strain was defined as the most negative strain value during systole. Time to peak strain (TPS) was defined as the time from the initial R-top to the time at peak systolic strain in seconds and was measured at baseline and post exercise. The TPS was corrected for heart rate using the Bazett formula such that TPSc (corrected) = TPS/(√RR), where RR interval was measured as time from the top of the initial R wave to the top of the second R wave (Figure 1). The TPSc ratio (TPScR) was calculated as the ratio of TPSc measured post exercise divided by TPSc pre exercise.
Figure 1.
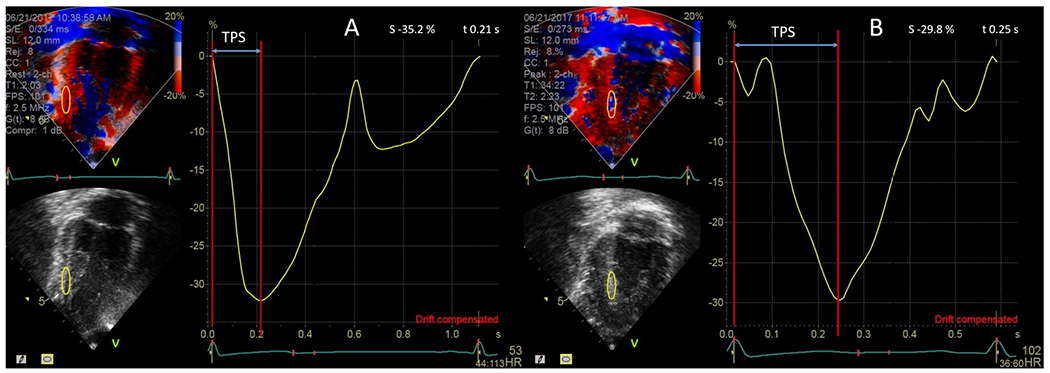
Color Doppler tissue imaging-derived strain curve of the inferior wall (yellow curve) at baseline (A) and during recovery (B) in a patient with unoperated AAORCA undergoing exercise stress echo. Time to peak strain is delayed post exercise compared to baseline.
Peak systolic strain ratio (PSSR) was defined as the ratio of the post- to pre-exercise peak systolic strain. Strain diastolic index (SDI) was calculated as (A-B)/A x 100%, where A indicates peak strain value at mitral valve opening, and B indicates peak strain at one-third of the diastolic interval. The SDI ratio (SDIR) was defined as SDI at post exercise compared to pre-exercise.
Statistical Analysis
Comparisons were made between patients with AAORCA and controls. The TPScR, PSSR and SDIR of the inferior and anterior walls were compared between these two groups. Categorical variables were reported as absolute values and percentages. Continuous variables were reported as the mean value ± SD. Univariate analysis and analysis of variance were performed to determine correlation between TPScR and the following variables: demographic data, symptoms, exercise stress test, procedural and physiological variables, management (surgery vs no surgery), and CTA descriptors of the anomalous RCA. Linear regression was used to determine correlation between TPScR and percentage and extent of RCA narrowing. Analyses were performed using R Statistical Software (Foundation for Statistical Computing) and Stata software (StataCorp). P value < .05 was considered statistically significant.
To assess inter- and intra-observer variability for TPScR, ten studies including five controls and five unoperated patients with AAORCA were randomly selected and analyzed by two trained observers. Variability was determined by percent error between readings calculated as (difference between readings/average of readings) x 100. Inter-observer variability between the two observers was calculated as the mean percentage error between the mean TPScR values of both observers. Intra- observer variability was determined by assessing the mean percentage error between readings for each observer.
Results
Overall, demographic and ESE procedural and physiologic data were not significantly different between groups (Table 1), except that the average percentage predicted maximum heart rate at post exercise strain assessment was significantly lower in the operated AAORCA patients compared to both unoperated AAORCA patients and controls. Of the 14 unoperated AAORCA patients that had CTA, all had a separate ostium of the RCA within the left coronary sinus, with most having slit-like orifice (12/14) and right dominant coronary artery systems (13/14; Table 2). All except one had an intramural course, with an average intramural segment length of 8.4 ± 4.4 mm.
Table 1:
Comparison of Demographic and Exercise Testing Procedural and Physiological Data Between Study Groups
| Study Groups |
P valuesa |
|||||
|---|---|---|---|---|---|---|
| Unoperated AAORCA | Operated AAORCA | Controls | Unoperated vs Control | Operated vs Control | Unoperated vs Operated | |
| Mean Age, y (SD) | 13.48 | 14.58 | 14.55 | 0.44 | 0.97 | 0.52 |
| (5.45) | (2.94) | (2.16) | ||||
| Male, % | 71 | 75 | 64 | 0.70 | 0.67 | 0.37 |
| Mean duration of exercise, min (SD) | 11.7 | 13.0 | 12.8 | 0.22 | 0.91 | 0.30 |
| (2.98) | (3.88) | (1.78) | ||||
| Mean METS at peak exercise (SD) | 11.90 | 13.33 | 13 | 0.14 | 0.76 | 0.17 |
| (2.47) | (3.39) | (1.41) | ||||
| Mean % predicted max HR achieved (SD) | 90.33 | 89.83 | 90.11 | 0.93 | 0.91 | 0.82 |
| (6.64) | (4.41) | (5.82) | ||||
| Mean % predicted max HR at post exercise strain assessment (SD) | 54.95 | 43.58 | 59.11 | 0.35 | 0.005 | <0.001 |
| (6.93) | (2.50) | (12.05) | ||||
Abbreviations: AAORCA, anomalous aortic origin of the right coronary artery; HR, heart rate; METS, metabolic equivalent of task.
Based on two-sided Student’s t-test.
Table 2:
Anatomic Features Seen on Computed Tomography Angiography in 14 Unoperated Patients With AAORCA
| Unoperated AAORCA Patient ID | Ostia Type | Proximal Vessel Morphology | Length of Narrowing (mm) | Intramural Location | Takeoff Anglea | Takeoff Levelb | Right vs Left Dominant |
|---|---|---|---|---|---|---|---|
| 4 | Separate | Slit-like | 9 | Intramural | Acute | At | Right |
| 6 | Separate | Slit-like | 7 | Intramural | Acute | At | Right |
| 7 | Separate | Slit-like | 8 | Intramural | Acute | At | Left |
| 9 | Separate | Slit-like | 11 | Intramural | Acute | Above | Right |
| 10 | Separate | Oval | 5 | Intramural | Acute | At | Right |
| 12 | Separate | Slit-like | 7 | Intramural | Acute | At | Right |
| 13 | Separate | Slit-like | 6 | Intramural | Acute | At | Right |
| 14 | Separate | Slit-like | 11 | Intramural | Acute | At | Right |
| 15 | Separate | Slit-like | 11 | Intramural | Acute | At | Right |
| 16 | Separate | Slit-like | 6 | Intramural | Acute | Above | Right |
| 17 | Separate | Slit-like | 20 | Intramural | Acute | At | Right |
| 18 | Separate | Slit-like | 8 | Intramural | Acute | At | Right |
| 19 | Separate | Normal | 0 | Not intramural | Acute | Above | Right |
| 20 | Separate | Slit-like | 9 | Intramural | Acute | At | Right |
Abbreviation: AAORCA, anomalous aortic origin of the right coronary artery.
Acute = <45%.
Relative to the aortic sino-tubular junction.
There were no exercise-induced regional wall motion or ECG abnormalities noted for any patient. There was no significant difference in PSSR or SDIR between groups (Table 3). However, TPScR of the inferior wall was significantly higher in unoperated but not operated AAORCA patients compared to controls, whereas there was no significant difference in TPScR of the anterior wall between these groups. The TPScR greater than 1.1 was found in five (24%) of the unoperated and two (17%) of the operated AAORCA patients, but in none of the controls (Figure 2).
Table 3:
Comparison of Strain Ratios (Post/Pre-exercise) From the Left Ventricular Inferior and Anterior Walls Between Unoperated and Operated AAORCA Patients Versus Controls.
| Study Groups |
P valuesa |
||||
|---|---|---|---|---|---|
| Unoperated AAORCA | Operated AAORCA | Controls | Unoperated vs Control | Operated vs Control | |
| Mean TPScR (inferior) | 0.96 (0.41) | 0.84 (0.28) | 0.76 (0.18) | 0.03 | 0.23 |
| Mean TPScR (anterior) | 0.98 (0.31) | 0.75 (0.24) | 0.84 (0.16) | 0.08 | 0.85 |
| Mean PSSR (inferior) | 0.97 (0.41) | 1.30 (0.69) | 1.23 (1.18) | 0.25 | 0.57 |
| Mean PSSR (anterior) | 1.02 (0.34) | 1.16 (0.54) | 0.86 (0.39) | 0.85 | 0.93 |
| Mean SDIR (inferior) | 1.09 (0.59) | 1.07 (0.90) | 0.84 (0.36) | 0.92 | 0.78 |
| Mean SDIR (anterior) | 0.86 (0.41) | 1.58 (1.60) | 1.08 (0.51) | 0.14 | 0.84 |
Abbreviations: AAORCA, anomalous aortic origin of the right coronary artery; PSSR, ratio of post- over pre exercise peak systolic strain; SDIR, ratio of post- over pre-exercise strain diastolic index; TPScR, ratio of post over pre-exercise time to peak systolic strain corrected for heart rate.
Based on one-sided Student’s t-test.
Figure 2.
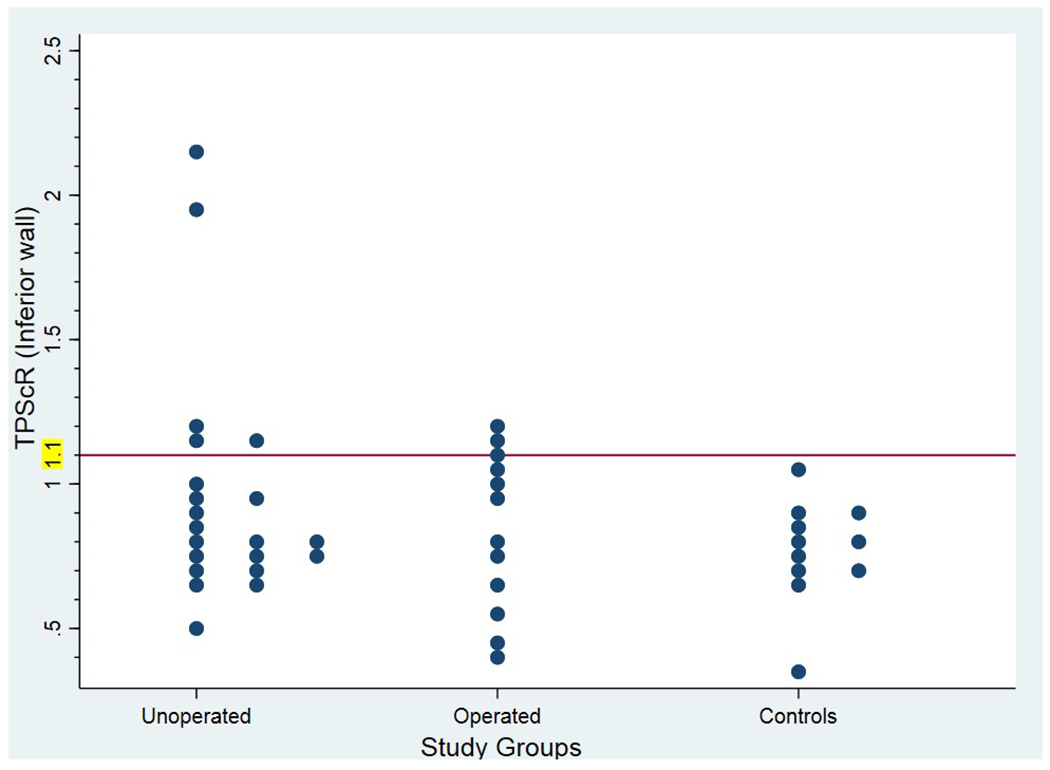
Dot plot showing all individual post- to pre-exercise time to peak systolic strain corrected for heart rate ratio (TPScR values in unoperated and operated anomalous aortic origin of the right coronary artery (AAORCA) patients and controls.
Intra- and inter-observer variabilities for TPScR measurements were 4.8% ± 5.8% and 15.0% ± 12.0%, respectively.
There was no association found between TPScR and demographic or exercise test procedural or physiologic data, symptoms or management. In addition, for the 14 unoperated AAORCA patients that had CTA, there was no significant correlation between TPScR and any anatomic feature of the RCA, including percentage or extent of slit-like narrowing. Symptoms and management decisions of unoperated AAORCA patients are shown in Table 4.
Table 4:
Symptoms and Management of Unoperated AAORCA Patients
| Unoperated AAORCA Patient ID | TPScR (inferior wall) | Chest pain at rest | Chest pain with exertion | Shortness of breath | Dizziness at rest | Dizziness with exertion | Syncope at rest | Syncope with exertion | Management decision |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.72 | no | no | no | no | no | no | no | No surgery |
| 2 | 1.14 | no | no | no | no | no | no | no | No surgery |
| 3 | 0.71 | unknown | yes | no | no | no | no | no | Surgery |
| 4 | 0.76 | unknown | yes | yes | no | no | no | no | No surgery |
| 5 | 1.21 | no | yes | no | no | no | no | no | Surgery |
| 6 | 0.94 | no | no | no | no | no | no | no | No surgery |
| 7 | 0.65 | yes | yes | no | no | no | no | no | No surgery |
| 8 | 0.77 | unknown | yes | no | no | yes | no | no | No surgery |
| 9 | 0.52 | yes | yes | no | no | no | no | no | Surgery |
| 10 | 2.17 | no | no | no | no | no | no | no | No surgery |
| 11 | 1.97 | no | no | no | no | yes | no | yes | No surgery |
| 12 | 0.80 | no | no | no | no | no | no | no | No surgery |
| 13 | 0.90 | unknown | yes | no | no | no | no | no | Surgery |
| 14 | 0.97 | no | no | no | no | yes | no | no | Surgery |
| 15 | 0.80 | unknown | yes | no | no | no | no | no | No surgery |
| 16 | 0.85 | yes | no | no | yes | no | no | no | No surgery |
| 17 | 0.81 | no | no | no | no | yes | no | yes | No surgery |
| 18 | 1.01 | yes | yes | no | no | no | no | no | Surgery |
| 19 | 0.73 | no | no | no | no | no | no | no | No surgery |
| 20 | 0.66 | yes | yes | no | no | no | no | no | No surgery |
| 21 | 1.15 | no | no | no | no | no | yes | no | No surgery |
Abbreviation: AAORCA, anomalous aortic origin of the right coronary artery; TPScR, ratio of post- over pre-exercise time to peak systolic strain corrected for heart rate.
The medical records of all participants were reviewed to document clinical outcomes since the ESE. The mean time of follow-up was 3.2 (SD: 1.0) and 5.1 (SD: 2.5) years for unoperated and operated AAORCA patients, respectively. Of the 21 initially unoperated AAORCA patients, 6 (29%) subsequently underwent surgery. The average TPScR of these patients was 0.9, with a range of 0.52 to 1.21. Five of these patients underwent surgery due to previously documented exertional chest pain and the sixth patient due to a desire to be a competitive athlete. None of these patients reported exertional symptoms nor had evidence of ischemia by other testing following ESE and surgery. One of the operated AAORCA patients was noted to have premature ventricular contractions and couplets on a follow-up ESE.
Discussion
Exercise stress echocardiography in adults with CAD has a sensitivity and specificity of 80% to 88% and 83% to 86%, respectively, to detect coronary luminal stenosis of at least 50%, which is considered the minimum degree of stenosis necessary to cause exercise-induced regional WMA observable by visual inspection.11 The gold standard test for determining the degree of coronary stenosis is angiography. Risk of a cardiovascular event is less than 1% if ESE is negative and classified as intermediate or high if positive, depending on clinical features.12 In distinction, no current gold standard test exists for AAOCA which correlates with higher risk of cardiac events, and anatomic imaging in this condition does not currently convey the same predictive information as 50% or greater luminal stenosis on angiography does for coronary atherosclerotic disease. Because of this lack of a gold standard surrogate endpoint, sensitivity, specificity and predictive value of stress imaging in this condition are unknown, and therefore, the results are necessarily difficult to interpret and use to guide decision making, whether positive or negative. Data from the Congenital Heart Surgeons’ Society Registry for anomalous aortic origin of the coronary artery on 560 patients with both AAORCA and AAOLCA were recently published.13 The results show that although there is less evidence of ischemia in patients with AAORCA, there is still a significant mortality risk associated with this lesion.13 However, despite the importance of the findings presented in this study, there are still no clear guidelines to use for risk stratifying patients with AAORCA. In addition, since stress imaging protocols and interpretation of results vary across contributing centers, the value of registry data for understanding the true incidence of exercise-induced ischemia in this condition may be limited. Indeed, well-documented observation of exercise-inducible WMA in AAORCA has not been widely reported even for symptomatic patients.14 It is unclear whether this indicates that stress imaging to detect WMA has not been fully examined in this patient population, lacks sensitivity to discriminate higher from lower risk anatomic configurations, or is possibly not appropriate for risk stratification given the low incidence of sudden death and thus presumably low pretest probability of stress-inducible ischemia sufficient to cause WMA.
In the present study, there were no exercise-induced WMA or ECG changes consistent with ischemia seen in patients with AAORCA. In the study by Romp et. al., nine patients with anomalous coronary artery origins (seven AAOLCA and two AAORCA) underwent postoperative ESE, with none showing stress-induced WMA.15 Similarly, Osaki et. al. reported dobutamine stress echocardiography results on 17 patients with unoperated anomalous coronary artery origins (13 AAOLCA and 18 AAORCA) and no stress-induced WMA were observed.16
Strain imaging has been studied extensively in adults with CAD.7 Two-dimensional speckle-tracking techniques are commonly used to assess regional and global peak strain especially in patients at rest. However, the higher heart rates associated with stress imaging make Doppler-based techniques, which can achieve higher frame rates, potentially more useful for strain assessment than speckle-tracking, especially when precise time-based measurements are required. Alhough Doppler strain imaging is angle-dependent, this limitation can be overcome by selectively interrogating myocardial segments in which the angle is close to zero, as is usually possible for the LV mid inferior and anterior walls imaged from the apical two-chamber view. In addition, CDTI is particularly well-suited for strain measurements during ESE since it can be acquired in the background during usual B-mode stress imaging protocols. In a study by Takagi T et. al., TPScR was shown to be sensitive and specific for detecting regional myocardial ischemia in adults with significant CAD (> 50% luminal diameter stenosis as measured by coronary angiography).10 Specifically, TPScR ≥ 1.1 in the LV inferior wall was 100% sensitive, 92% specific and 93% accurate in detecting significant CAD of the RCA.10 In the present study, a TPScR >1.1 in the LV inferior wall was found in 24% of unoperated patients with AAORCA but not in any controls. We speculate that this may correlate with ischemia in this myocardial segment, which is typically supplied by the RCA. In addition, 17% (2/12) of operated patients had TPScR >1.1, which may suggest risk of ischemia in this population even after surgical intervention. Although the average predicted maximum heart rate at post exercise strain assessment in operated patients was lower compared to unoperated patients and controls, it is not clear whether this had an effect on TPScR. Nees et. al. reported 53 AAOCA patients with symptoms at presentation, 36% of which continued to have symptoms following surgical repair.17 Of note, this population included both AAORCA and AAOLCA patients. Anatomic features such as a high (distal) origin, interarterial and intramural course, a slit-like orifice and an acute angle of takeoff may be associated with increased risk of ischemia in patients with anomalous aortic origin of the coronary.18 However, to our knowledge, no direct correlation between any specific anatomic feature and increased risk of cardiac events has been conclusively shown. In the present study, the majority of patients with unoperated AAORCA had a separate RCA ostium within the left sinus, a slit-like origin and an intramural course with a mean length of 8.4 ± 4.2 mm. Interestingly, there was no apparent association between TPScR and any of these anatomic features or a measure of the percentage or extent of slit-like narrowing. Importantly, however, only one of the five unoperated patients with TPScR > 1.1 had a CTA; thus, an association with CTA findings cannot be excluded. In addition to the aforementioned CTA features, there was also no correlation between symptoms such as exertional chest pain, dizziness or syncope with TPScR (Table 4).
Advances in imaging modalities have improved our ability to diagnose anomalous aortic origin of the coronary arteries and describe differences in anatomic features that may or may not be associated with increased risk of ischemia in this population. In addition to cardiac CTA, cardiac magnetic resonance imaging (MRI) has been used to assess for fibrosis, though the incidence and predictive value of this finding are not known.19,20 Nuclear perfusion imaging and stress cardiac MRI have also been used to evaluate myocardial perfusion, with stress cardiac MRI thought to be more reliable in identifying myocardial ischemia.21,22 Fractional flow reserve and intravascular ultrasound are additional modalities used to study coronary stenoses.23–25 However, these potentially informative techniques have not been validated for use in the assessment of AAOCA, and correlation of findings with outcomes is currently not available. Further investigation of these techniques to develop patient-specific risk assessment is warranted as suggested by Driesen et al.25 The potential role of TPScR in risk stratification deserves further investigation, including prospective evaluation in a larger cohort of patients with AAORCA.
Study Limitations
As discussed above, there is no gold standard test which correlates with higher risk of death and presumably with induced ischemia in AAORCA, and hence, validating TPScR as a risk stratification tool is challenging without longer term follow-up data. An additional limitation of this study is the small sample size and fewer control patients than those with AAORCA. The study group of this retrospective analysis reflects our institutional practice of performing ESE at the discretion of the referring cardiologists, not by standardized protocol per diagnosis, and ESE is not done frequently for patients without known heart disease, limiting the availability of control patients. Similarly, CTA was not performed in all patients in this study, again reflecting clinical practice variation, and may have limited our ability to find correlations between anatomic features and TPScR. In addition, while including patients with AAOLCA would have been of particular interest because of their increased risk of sudden death, these patients are typically referred for surgery without preoperative stress imaging at our institution, thus no studies were available for review.
Conclusions
More evidence-based measures are needed to aid in risk stratification of patients with AAORCA. This study confirms previous reports that exercise-induced regional WMA are uncommon in these patients; however, it suggests TPScR may be an indicator of higher risk for ischemia. Additional studies are needed to assess the reproducibility of this finding and compare this value to longer term outcomes. In addition, comparison to surrogate measures known to correlate with ischemia such as highly sensitive troponin or fibrosis burden as quantified by cardiac MRI may provide additional insights.
Acknowledgements
The authors are grateful to Dr. Shelby Kutty for reviewing this manuscript and providing comments that improved the quality of the finished product.
Disclosure Statement
Dr. Binka was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute [T32 HL125239] while conducting this study. The authors report no relationships that could be construed as a conflict of interest. The authors assume full responsibility for the control of the design of the study, methods used, outcome parameters, analysis of data, and production of the written report.
References
- 1.Cheezum MK, Liberthson RR, Shah NR, et al. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69(12):1592–1608. [DOI] [PubMed] [Google Scholar]
- 2.Friedman AH, Fogel MA, Stephens P, et al. Identification, imaging, functional assessment and management of congenital coronary arterial abnormalities in children. Cardiol Young. 2007;17 (suppl 2):56–67. [DOI] [PubMed] [Google Scholar]
- 3.Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998;29(7):689–695. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992;20(3):640–647. [DOI] [PubMed] [Google Scholar]
- 5.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351–369. [DOI] [PubMed] [Google Scholar]
- 6.Choi JO, Cho SW, Song YB, et al. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr. 2009;10(5):695–701. [DOI] [PubMed] [Google Scholar]
- 7.Hoit BD. Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging. 2011;4(2):179–190. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Hozumi T, Takemoto Y, et al. Estimation of myocardial ischemia by diastolic strain analysis in exercise stress echocardiography: Comparison with exercise thallium-201 single photon emission computed tomography [in Japanese]. J Cardiol. 2006;47(4). [PubMed] [Google Scholar]
- 9.Takagi T, Takagi A, Hozumi T, Yoshikawa J. Detection of significant stenotic lesions in the left anterior descending coronary artery using adenosine triphosphate stress strain imaging: comparison with coronary flow velocity reserve measurement using transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2006;19(8):1001–1011. [DOI] [PubMed] [Google Scholar]
- 10.Takagi T, Takagi A, Yoshikawa J. Detection of coronary artery disease using delayed strain imaging at 5 min after the termination of exercise stress: head to head comparison with conventional treadmill stress echocardiography. J Cardiol. 2010;55(1):41–48. [DOI] [PubMed] [Google Scholar]
- 11.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20(9):1021–1041. [DOI] [PubMed] [Google Scholar]
- 12.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2008;9(4):415–437. [DOI] [PubMed] [Google Scholar]
- 13.Jegatheeswaran A, Devlin PJ, McCrindle BW, et al. Features associated with myocardial ischemia in anomalous aortic origin of a coronary artery: A Congenital Heart Surgeons’ Society study. J Thorac Cardiovasc Surg. 2019. September;158(3):822–834.e3. [DOI] [PubMed] [Google Scholar]
- 14.Thompson WR. Stress echocardiography in paediatrics: implications for the evaluation of anomalous aortic origin of the coronary arteries. Cardiol Young. 2015;25(8):1524–1530. [DOI] [PubMed] [Google Scholar]
- 15.Romp RL, Herlong JR, Landolfo CK, et al. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg. 2003;76(2):589–596. [DOI] [PubMed] [Google Scholar]
- 16.Osaki M, McCrindle BW, Van Arsdell G, Dipchand AI. Anomalous origin of a coronary artery from the opposite sinus of Valsalva with an interarterial course: clinical profile and approach to management in the pediatric population. Pediatr Cardiol. 2008;29(1):24–30. [DOI] [PubMed] [Google Scholar]
- 17.Nees SN, Flyer JN, Chelliah A, et al. Patients with anomalous aortic origin of the coronary artery remain at risk after surgical repair. J Thorac Cardiovasc Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opolski MP, Pregowski J, Kruk M, et al. Prevalence and characteristics of coronary anomalies originating from the opposite sinus of Valsalva in 8,522 patients referred for coronary computed tomography angiography. Am J Cardiol. 2013;111(9):1361–1367. [DOI] [PubMed] [Google Scholar]
- 19.Gräni C, Buechel RR, Kaufmann PA, Kwong RY. Multimodality imaging in individuals with Anomalous Coronary Arteries. JACC Cardiovasc Imaging. 2017;10(4):471–481. [DOI] [PubMed] [Google Scholar]
- 20.Mavrogeni S, Spargias K, Karagiannis S, et al. Anomalous origin of right coronary artery: Magnetic resonance angiography and viability study. Int J Cardiol. 2006;109(2):195–200. [DOI] [PubMed] [Google Scholar]
- 21.Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD Jr, Tweddell JS. Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153(6): 1440–1457. [DOI] [PubMed] [Google Scholar]
- 22.Molossi S, Martínez-Bravo LE, Mery CM. Anomalous Aortic Origin of a Coronary Artery. Methodist Debakey Cardiovasc J. 2019. April-June;15(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelini P Novel imaging of coronary artery anomalies to assess their prevalence, the causes of clinical symptoms, and the risk of sudden cardiac death. Circ Cardiovasc Imaging. 2014;7(4):747–754. [DOI] [PubMed] [Google Scholar]
- 24.Angelini P Coronary artery anomalies: An entity in search of an identity. Circulation. 2007;115(10):1296–1305. [DOI] [PubMed] [Google Scholar]
- 25.Driesen BW, Warmerdam EG, Sieswerda GJT, et al. Anomalous coronary artery originating from the opposite sinus of Valsalva (ACAOS), fractional flow reserve- and intravascular ultrasound-guided management in adult patients. Catheter Cardiovasc Interv. 2018;92(1):68–75. [DOI] [PubMed] [Google Scholar]


