Abstract
The European Commission asked the European Food Safety Authority (EFSA) to prepare a statement on a framework for the environmental risk assessment (ERA) of transition metals (e.g. iron and copper) used as active substances in plant protection products (PPPs). Non‐degradability, essentiality and specific conditions affecting fate and behaviour as well as their toxicity are distinctive characteristics possibly not covered in current guidance for PPPs. The proposed risk assessment framework starts with a preliminary phase, in which monitoring data on transition metals in relevant environmental compartments are provided. They deliver the metal natural background and anthropogenic residue levels to be considered in the exposure calculations. A first assessment step is then performed assuming fully bioavailable residues. Should the first step fail, refined ERA can, in principle, consider bioavailability issues; however, non‐equilibrium conditions need to be taken into account. Simple models that are fit for purpose should be employed in order to avoid unnecessary complexity. Exposure models and scenarios would need to be adapted to address environmental processes and parameters relevant to the fate and behaviour of transition metals in water, sediment and soils (e.g. speciation). All developments should follow current EFSA guidance documents. If refined approaches have been used in the risk assessment of PPPs containing metals, post‐registration monitoring and controlled long‐term studies should be conducted and assessed. Utilisation of the same transition metal in other PPPs or for other uses will lead to accumulation in environmental compartments acting as sinks. In general, it has to be considered that the prospective risk assessment of metal‐containing PPPs can only cover a defined period as there are limitations in the long‐term hazard assessment due to issues of non‐degradability. It is therefore recommended to consider these aspects in any risk management decisions and to align the ERA with the goals of other overarching legislative frameworks.
Keywords: groundwater, modelling, monitoring, non‐target organisms, soil, surface water
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2021.EN-6501/full
Summary
The European Commission asked the European Food Safety Authority (EFSA) to prepare a statement on a framework for conducting the environmental exposure and risk assessment for transition metals and their compounds (e.g. salts) from the chemical periodic table of element groups 7–11 of period 4 (iron, cobalt, nickel and copper) when used as active substances in plant protection products (PPPs), as the current guidance documents were developed for synthetic organic chemicals. The non‐degradability of transition metals, the specific physico‐chemical conditions affecting their behaviour and toxicity and their essentiality are distinctive characteristics that are not covered in the current guidance documents. EFSA was also asked to explore the approaches used in other regulatory areas for this purpose – such as in the context of the REACH Regulation – and align when technically possible the pesticide assessment methodology to these. In particular EFSA was asked:
to liaise with European Chemicals Agency (ECHA) to gather information on available guidance for the assessment of transition metals;
to develop a methodology for the environmental risk assessment for transition metal compounds covering the relevant environmental compartments (terrestrial and aquatic compartments) and the concerned non‐target organisms. The methodology should include criteria on how to assess and consider the environmental concentration estimates, bioavailability, homoeostatic mechanisms and bioaccumulation of transition metal compounds;
to establish criteria for the evaluation of monitoring studies;
to provide guidance on the application of any uncertainty factors in the environmental risk assessments when insufficient evidence is available.
The statement outlines appropriate scientific methodology and limitations for assessing transition metals and their compounds used as active substances in PPPs and indicates possible solutions. The statement has considered guidance on metals from the ECHA, the European Commission (especially regarding the Water Framework Directive) and the Organisation for Economic Co‐operation and Development (OECD) as well as guidance developed for environmental risk assessment (ERA) of PPPs by EFSA and the European Commission.
The protection goals for the environment remain the same independent to whether a PPP contains as an active substance a transition metal, a transition metal compound or other compounds. Therefore, this statement does not elaborate specific protection goals or exposure assessment goals possibly needed, e.g. for non‐target organisms related to transition metals.
General risk assessment framework for transition metals
The following general framework for the ERA of transition metal compounds is proposed, consisting of the following steps:
A preliminary phase ahead of the first step of the risk assessment, in which the applicant gathers all relevant and reliable available occurrence information on residue levels in the environment and makes them available to the competent authority assessors. Monitoring data should inform risk assessors on the metal levels in the environment and deliver reliable levels related to the land uses to be included in exposure assessment steps.
In a first assessment step, relevant natural background data and all anthropogenic additions are included in the exposure assessment and all residue levels are considered bioavailable to non‐target organisms. This is independent from the physico‐chemical properties of the target compartment. Exposure assessment should focus on realistic worst‐case residue levels and distinction between different land uses might be implemented if information is available. Relevant guidance should be followed where applicable.
If higher assessment steps are performed, reduced bioavailability of residues from past applications could be considered. However, for freshly added transition metals such as PPP, equilibrium cannot be assumed.
For addressing speciation and bioavailability issues, models and scenarios including environmental processes and parameters relevant to the fate and behaviour of transition metals in water, sediment and soils would need to be adapted or developed. It is proposed that – if envisaged – dedicated modules are developed within current frameworks (i.e. FOCUS surface water, FOCUS groundwater and the EFSA soil exposure guidance). All developments need to follow the modelling cycle as described in the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a). The new modelling approaches need to be made fully available to the competent authorities for assessment and model outcomes should be checked for compliance with the current general regulatory framework.
Regarding the effect assessment, processes at the biotic ligand site might be considered, as suggested in principle in the ECHA guidance document (ECHA, 2008). The approaches proposed in the ECHA guidance document (e.g. for aquatic organisms) require equilibrium conditions and consider that only free metal ions contribute to toxicity (no oral/contact uptake). If appropriate, it is proposed to employ simple models considered fit for purpose to address these processes and to identify realistic worst‐case scenarios, to avoid unnecessary complexity. In addition, following EFSA guidance, also higher tier effect studies (e.g. species sensitivity distribution (SSD), semi‐field or field studies) can be taken into account. Due to the non‐degradability of metals in the environment, higher tier effects studies used for refinement should be initiated in the relevant compartments acting as sinks (e.g. soils and sediments) and be prolonged to relevant time frames of transition metal applications (one or more approval periods, see Section 2.3). As for lower assessment steps, latest developments on the assessment of direct and indirect effects following long‐term application of PPPs should be considered as for other PPPs (EFSA Scientific Opinions and Statements on Non‐Target Organisms, e.g. EFSA PPR Panel, 2014b, 2015a, 2017, 2018).
If the risk assessment concludes at higher assessment steps on low risk for the intended uses of transition metals as active substances in PPP for the time period that has been evaluated, then it is indicated that general protection goals as laid down in Regulation (EC) No 1107/2009 and specific protection goals of sectorial guidance (e.g. the Birds and Mammals EFSA guidance document, EFSA, 2010a) are met. Conclusions on low risk in the ERA should be taken based on sufficient certainty and the available scientific knowledge, to adequately cover the protection goals. Post‐registration monitoring needs to be implemented and should be targeted to the environmental compartments of concern and comprise chemical and biological/ecological assessments. Monitoring schemes (according to Art. 6(i) and Art. 67(2) of Regulation (EC) No 1107/2009) should be agreed with the competent authorities.
In general, it has to be considered that the prospective risk assessment and management of non‐degradable elements can only cover a defined period, as all PPP intended uses will increase the loads in the environment and uncertainties exist especially regarding the hazard assessment. In addition, utilisation of the same transition metal in another PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will result in further input to sink compartments and add to the total metal concentrations, possibly posing risks to non‐target organisms. It is therefore recommended to consider these aspects and uncertainties in any risk management decision on the approval of transition metals and their compounds as active substances or the authorisation of PPPs with transition metals (e.g. comparative assessment, cost–benefit analysis, reduced application rates, etc.) and accompanied focused monitoring. It is also recommended to align the prospective ERA with the goals of other overarching legislative framework (e.g. Water Framework Directive, Drinking Water Directive, Sustainable Use Directive).
Aquatic compartment
In principle, the aquatic hazard assessment can be based on the studies listed in the data requirement Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b) if details about water characteristics and equilibration of the test system are given. The OECD guidance No 259 (OECD, 2016) gives further guidance about the selection of reliable and relevant ecotoxicity data. The study type and duration define the appropriate use for and time frame of the assessment. Acclimatisation of species with time is not considered as a suitable refinement option and accumulation on the individual level is not expected for essential metals due to homoeostasis. The toxicity of transition metals is determined by the physico‐chemical characteristics of the compartment as this affects the speciation of transition metals. Depending on the type of metal and competing ligands, the bioavailability of transition metals may be increased or decreased with changing pH. A low organic carbon content and water hardness leads to a higher bioavailability and therefore potential effects. Bioavailability is further affected by biotic factors. Waterborne uptake via the gills as well as dietary uptake may occur and the relevance of either uptake route depends on the metal, exposure time and concentration (in both water and food), diet type and composition as well as species and life stage. Several reviews have addressed the challenges and possibilities to address this issue. Whereas for crustaceans exposed to Cu, the current knowledge indicates that only the waterborne uptake route is considered relevant is considered most relevant for sediment‐dwelling organisms due to the dissipation to and accumulation in the sediment acting as a sink, but also resuspension of the sediment. The bioavailable fraction in the sediment, pore water or overlaying water is dependent on the organisms and type of metal as well as the geochemistry of the sediment. Therefore, organisms covering the different positions in the aquatic trophic web with different metal exposure routes should be considered in the aquatic risk assessment. At present, the same tiered approach as described in the aquatic guidance document (EFSA PPR Panel, 2013c) is considered relevant for transition metals. However, to compare or combine the ecotoxicological studies, the metal speciation in the test system needs to be considered. To assess the interaction between metal ions and different ligands, speciation and bioavailability models have been developed. Furthermore, the assessment factors suggested in the aquatic guidance document (EFSA PPR Panel, 2013c) address the spatio‐temporal extrapolation from the model ecosystem to the field ecosystem and may not be sufficient to account for the differences in surface water chemistries relevant for transition metals. Due to the wide and repeated use of e.g. copper, exposure of surface waters is expected to be extended. Since additionally the metals in the PPP do not degrade and could be resuspended from the sediment, the recovery option (ERO) for the evaluation of mesocosm studies as described by EFSA (EFSA PPR Panel, 2013c) is not recommended.
The non‐degradability of transition metals, the physico‐chemical conditions of media affecting their fate and behaviour and toxicity of transition metals in the aquatic systems are distinctive characteristics that are not covered in the current guidance. The behaviour and the toxicity of the transition metals to non‐target aquatic organisms are affected by the form of metal present in the media (speciation). The speciation is mainly controlled by the properties of the environmental matrices (e.g. soil pH, OM, CEC, clay content…). The speciation of transition metals in aquatic systems can change with time and can reduce their availability. These processes lead to increased or decreased toxic forms concentrations in the aquatic system. For the time being, none of the currently used FOCUS models can simulate the fate and behaviour of metals in an appropriate way as speciation and availability as well abiotic factors affecting them are not included in these models and scenarios. However, it is agreed that the current risk assessment performed in terms of total transition metals according to EFSA procedure is considered conservative as the transition metals are considered fully bioavailable in the aquatic systems. The assumed conservativeness of the current risk assessment performed based on total transition metals needs to be critically assessed in relation to their characteristics (substance not degraded) and various uncertainties (e.g. variable bioavailability according to environmental conditions (definition of ‘realistic worst‐case’ scenarios), potential remobilisation of metal bound to particulates once ingested, very steep dose–response relationship between exposure concentrations and effects on populations). In addition, transition metals applied with PPP will add to the background residue levels (from previous applications or from natural background), increasing total concentrations in the environment, as these elements are not degradable, and could possibly be adding to the risk since they may be remobilised under certain conditions, therefore increasing their bioavailability. The exposure and effect assessments assume that transition metals present in the environment from past (accumulated) and present (freshly added) applications are bioavailable to non‐target organisms leading to a conservative risk assessment in the first step. The exposure assessment should consider concentrations from freshly added and background values together. Time being a driving factor affecting the speciation and the availability of metals in the aquatic systems, the freshly added metal and the background residues level should be considered distinctly (non‐equilibrium for freshly added metals – after direct application or entries due to run‐off and drainage into waterbodies – vs. equilibrium for accumulated residues in systems). To produce less conservative exposure concentrations in a refined exposure assessment for transition metals, adaptation of the models and existing scenarios or development of new models would be required to describe the fate of metals in an appropriate way (including additional processes e.g. speciation, bioavailability, equilibrium/non‐equilibrium conditions, ageing process and factors affecting these processes).
Regarding the risk assessment in aquatic environments, in the preliminary phase described in the general framework, monitoring data in surface waters including sediments need to be assessed. Soil background data are not considered a suitable surrogate for the sediment due to the differences in biogeochemistry. In the first tier, the risk assessment is based on the highest dissolved concentration over time, predicted environmental concentration (PEC)sw;max and compared with RACtotal dissolved for the water phase. Particles < 0.45 μm constitute those considered dissolved. For sediment organisms, the highest total concentration over time considering accumulation should be used. Equilibrium status in the test system should reflect the equilibrium of the exposure path to be assessed. Models for estimating PECs for transition metals should consider all entries to different waterbodies as well as the annual repeated application and the long‐term uses. Relevant spatial and temporal dimensions should be considered in the exposure calculations as was performed by FOCUS. In the higher tier, the risk assessment can be based on the highest bioavailable concentration over time and compared with the refined regulatory acceptable concentration (RAC) normalised for bioavailability. Models of different complexity are available to address the bioavailability, which consider the interaction of the metals with the physico‐chemical parameters of the water within a defined boundary as well as with the binding sites in the organisms. Each type of model has its underlying assumptions, which need to be fulfilled to be applicable. Biotic ligand models (BLM) assume that the biotic ligands and metal speciation are in equilibrium and that only the waterborne exposure is relevant for the toxicity, which may not be applicable for pulse exposures via drift or run‐off, or metals other than Cu. Several organism‐specific models exist for different species of algae, invertebrates and fish, which may be extrapolated to other species if sufficient justification is provided. For the sediment, depending on the type of organism, the use of partitioning to Fe‐Mn (oxy)hydroxides, speciation calculations, simultaneously extracted metals and acid‐volatile sulfides (SEM‐AVS) and/or organic carbon normalisation could be appropriate. For transition metals used as PPP, these prerequisites of the models may not be fulfilled for all situations of the prospective risk assessment (e.g. non‐equilibrium due to freshly added metal, diversity of sites for a national prospective assessment), therefore management (e.g. cost–benefit analysis, reduced application rates, etc.) and accompanied focused monitoring could be considered.
Terrestrial compartment
The hazard and risk assessment for terrestrial invertebrates and plants (in‐soil organisms, non‐target arthropods (NTAs), NTTPs and bees) exposed to transition metals as active substances in PPPs can be performed in principle as for other active substances and following up‐to‐date data requirements and agreed evaluation on recurring issues (EU No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b); EFSA, 2019). Problems arising from outdated guidance compared with newer legislation and data requirement (e.g. for in‐soil organisms) also apply to the assessment of transition metals. For some problems related to outdated guidance, interim solutions were found.
Regarding in‐soil organisms, one EFSA Scientific Opinion (EFSA PPR Panel, 2017) has evaluated the current state of the science and indicated gaps and possible improvements for hazard, exposure and risk assessment. As for other active substances, also for transition metals, both contact and oral exposure routes are considered important. In general, laboratory tests with soil organisms cover exposure routes only in different proportions and care needs to be taken, especially to assess the oral exposure of transition metal. SSDs are considered a suitable tool to take the sensitivity differences between soil organism species into account. Where sufficient evidence exists, endpoints from species belonging to different soil organism groups like arthropods and annelids might be jointly assessed. Because of different endpoint types and protection goals, including microorganisms and non‐target terrestrial plants in one SSD with in‐soil organisms is not favoured for the time being. At higher tier, long‐term field studies with e.g. earthworms or soil mesofauna are considered extremely useful to follow the fate, behaviour and toxicity of non‐degradable transition metals on communities in time. It is considered essential to measure effective concentrations of the tested compound in test soils, especially if refinement options on bioavailability are envisaged and/or pore water concentrations are evaluated. The OECD artificial soil employed in laboratory testing with soil organisms exposed to PPP is considered not to be a realistic worst case on transition metal bioavailability. The impact of soil properties on bioavailability and toxicity of metals to soil organisms in agricultural field soils should be investigated systematically for the different compounds. Models relating exposure to effects should be established by following EFSA Guidance on good modelling practice (EFSA PPR Panel, 2014a).
Exposure assessment in soil: Due to the long‐term uses of transition metal‐containing PPPs and their persistency, the agricultural background residues level in agricultural fields might be increased and are therefore relevant to be included in the exposure calculations. As a worst case, it is recommended to consider, in a similar way, freshly added copper and aged residues in soil (background/anthropogenic concentration) in the first step calculations. The assumptions in the current Persistence in Soil Analytical Model (PERSAM) tool and some input parameters in the PERSAM model are not adapted for transition metals. It was explored if soil scenarios for the three regulatory zones implemented in the PERSAM tool could be parameterised for total and pore water concentrations for different metals. The total concentration of freshly added metals depends primarily on the bulk density of the soil; however, the background concentration is affected by different properties such as the pH and clay content of the soil. This is because the concentration in the soil solution, and therefore also leaching of metals, depends on these properties. At Tier 1, crop interception and wash‐off are not considered in the PERSAM calculations, but the major factors affecting the sorption of transition metals (e.g. pH dependency and dependency on CEC and clay content) are not implemented in the PERSAM model in an appropriate way for metals. However, it is considered that Tier 1–Predicted Environmental Concentrations (PEC) for total concentration can be derived using the PERSAM model and used for the risk assessment, but Tier 1–PEC for pore water concentration is not appropriately estimated. Soil property‐dependent parameters (e.g. pH for soil sorption) can be considered at Tier 2. However, a default foliar wash‐off factor and a 10‐day degradation half‐life on leaves is assumed in the standard Tier 2 procedure for degrading substances. Therefore, Tier 2–PECsoil for both total and pore water concentrations is underestimated for metals, because they will persist on leaves. The speciation and the availability of transition metals and the environmental conditions affecting these processes are not considered in current EFSA exposure model calculations and the risk assessment for non‐target soil organisms. To better reflect the fate and behaviour of transition metals in the soil profile in an appropriate way, these processes affecting their availability in the soil profiles could be included in the modelling. It should be explored whether the available JRC database for agricultural soils is possibly appropriate to analyse and define ranges for other soil parameters relevant for metal sorption (e.g. pH, CEC).
When linking exposure to effect assessment in soils, endpoints expressed as soil pore water concentrations or total concentrations need to be compared. For pore water‐related endpoints and exposure, however, further developments are needed (e.g. how to deal with legacy studies, time course of pore water concentrations, delayed effects in soil organisms). The approach to compare test outcomes from soils with different properties based on total concentrations to the respective PEC as suggested in the ECHA guidance could also be followed for transition metals as active substances in PPP. It should be however avoided that to cover different regional situations, endpoints are scaled to soils with reduced bioavailability and, in addition, the calculated PEC considers the same influencing parameters (e.g. by considering specific metal pools as aged or not bioavailable).
The current risk assessment scheme for soil organisms concludes on acceptable risks if the toxicity to exposure ratio (TER) for chronic risk is ≥ 5. Before conducting the risk assessment, the background levels and the anthropogenic residues in soils need to be evaluated in a preliminary phase. At the first tier, endpoints expressed as total soil concentrations can be compared with PEC for soil for total soil concentrations assuming freshly added and long‐term simulated accumulation of metal compound as bioavailable. A refined assessment based on endpoints derived from SSD would be at present a non‐standard higher tier approach in the risk assessment of soil organisms. Since a number of uncertainties exist, it is proposed to apply an assessment factor of 2–3 to SSD‐derived endpoints for soil organisms exposed to transition metals as PPP. If it is envisaged to calculate the risk quotient based on endpoints related to pore water concentrations or resulting from SSD, an uncertainty analysis should be performed to conclude if the acceptability criterion needs to be further adapted.
Transition metals as PPP are freshly applied one or more times per season and possibly also in different products. Therefore, rather than applying an ageing factor to the effect endpoints, it is proposed to account for sorption and reduced availability of transition metals residues – if applicable at higher tier – when calculating the PEC in soils. Refining predicted concentrations in soil to account for bioavailability declining with time needs to be carefully evaluated, especially on the definition of bioavailable metal pools to soil organisms, as these are likely to be organisms and compound specific. Metal adsorbed to soil particles might become bioavailable again in the guts of soil organisms. As the calculation of PEC in soils is always linked to a definite time frame (choice of the number of years in which the compound is applied), the outcome of the risk assessment covers only the assessed time period.
Hazard characterisation for NTTP following current procedures as for other active substances in PPP is considered also applicable for transition metals and aligned with ECHA (ECHA, 2017c) guidance. However, as summarised in the EFSA Opinion on NTTPs (EFSA PPR Panel, 2014b), the available tests and endpoints do not cover the whole life cycle of plants. Phytotoxicity of the tested substances can also be considered for transition metals as well as endpoints derived from microcosms or field experiments with NTTP communities. Regarding the exposure assessment for NTTP, issues on the soil compartment have been discussed above. For deposition via spray drift, the spray drift percentage values as for other active substances are considered suitable for the spray applications of metal‐containing products. For dustable powder applications, it is suggested to use conservative drift percentage value in the absence of robust data. Linking exposure and effects for NTTP will consider whether a specific mechanism of action is known for the tested substance to separate the exposure routes via spray drift or soil uptake. Regarding transition metals, both routes of exposure will most probably be relevant. Soil concentrations of transition metals are to be linked to endpoints derived from seedling emergence tests. Both soil concentrations and sprayed deposits will be linked to endpoints from the vegetative vigour test and phytotoxicity endpoints. The risk assessment for NTTP can be performed for transition metals used as active substances in PPP in principle as for other active substances. As for in‐soil organisms, a Scientific Opinion from the PPR Panels and EFSA publication (EFSA PPR Panel, 2014b; EFSA, 2019) highlight the gaps of the current guidance and also propose options for higher tier assessment steps.
There are at present no indications that for NTA and bees specific provisions for transition metals as active substances in PPP need to be developed. For synthetic organic chemicals as active substances in PPP, newest developments should be implemented also for transition metals. Under the current ECHA framework, invertebrates living above ground are not specifically addressed. Guidance on the risk assessment for arthropod pollinators is under development at ECHA. The PPR Panel recommends the use of the EFSA bee guidance (EFSA, 2013b) also for assessment of transition metals. The revised EFSA bee guidance – when available – should be considered for the assessment of transition metals. According to the guidance in place, only contact exposure is fully covered in the hazard characterisation of NTA. Oral exposure (e.g. for herbivores) or overspray is currently not assessed, but deemed relevant for NTA (EFSA PPR Panel, 2015a; EFSA, 2019). Considerable uncertainties exist on the currently performed field tests at higher tier, as well as on the consequences of the year‐on‐year application of pesticides in landscapes with a high proportion of agricultural land (species loss, insufficient recovery).
Generally speaking, the toxicity of transition metals to terrestrial vertebrates is related to the availability of the free metal ion (e.g. copper). In toxicity tests, the toxicity of metal compounds is therefore expressed in metal concentration units. In acute toxicity tests, the salts tested displayed very similar toxicity values, and it was concluded that all ions of a transition metal such as Cu should be considered as a single entity. Current guidelines for toxicity testing are acceptable to conduct toxicity tests for transition metals used as PPP. Current guidance documents (both EFSA, 2010a and ECHA guidance document on metals, ECHA, 2008) only consider the oral route of exposure. Bioavailability of metal ions by the oral route is generally low. The low pH of the gastric content will favour the dissolution of many metal salts. The mineral compounds used as PPPs are made of transition metal ions. These metals are not metabolised, therefore half‐lives, bioaccumulation and bioconcentration factors may not be appropriate as these factors have been designed for organic compounds. To overcome this issue, ECHA (ECHA guidance document on metals, ECHA, 2008) developed higher tier risk assessment schemes to evaluate the bioaccumulation of these metals in terrestrial, as well as aquatic, food webs. EFSA also developed a weight‐of‐evidence approach, based on several scientific papers. Among the limitations of this approach is the fact that it is limited to small rodents in a polluted environment. It is also recommended to provide proper residue data (including background data) to develop higher tier scenarios, taking into account the real amount provided by the applied PPP. Among terrestrial vertebrates, it is also considered that amphibians may be more at risk from dermal exposure.
Groundwater
Due to the long‐term uses of transition metals containing PPPs, leaching of transition metals to groundwater cannot be excluded. For the time being, the current hydrological parameterisation of the FOCUS models is considered to be suitable to estimate the groundwater recharge (EFSA, 2018). However, this does not necessarily mean that the FOCUS groundwater scenarios represent realistic worst‐case conditions for transition metals because the sorption, and therefore the leaching potential, depends on multiple soil factors such as pH, clay content, CEC and redox potential that have not been included in the FOCUS scenario selection procedure. Therefore, dedicated scenarios need to be developed for many transition metals.
The risk to groundwater contamination with the agreed European FOCUS models (European commission, 2014) should be performed for a period sufficiently long to cover the long‐term groundwater exposure. For transition metals used in PPPs, the agricultural background residues level from agricultural fields is relevant and should be included in the groundwater modelling. A methodology on how to consider it in modelling is proposed in this document. The speciation and availability of transition metals being affected by soil properties, contrasting conditions for soil properties (e.g. soil pH, clay content) should be therefore considered for deriving a representative Kd value to be used in modelling to produce a conservative risk assessment. As an example, low pH and low OC content should be selected for copper as worst‐case conditions for deriving Kd modelling endpoints while low potential redox and low soil pH should be considered for iron. To produce a more realistic worst‐case risk assessment, there is the possibility to adapt the existing models and the corresponding scenarios. The use of additional submodels to take e.g. speciation and ageing process into account could be an alternative. In addition, soil properties in the existing scenarios could be adapted for the main soil properties affecting the speciation and availability of transition metals in soil. When soil properties implemented in the FOCUS groundwater scenarios have been adapted for groundwater modelling purpose of transition metals, the recommendations of the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed to facilitate the assessment of such scenarios. The parametric drinking water limits are the threshold values specified for different transition metals set in the European Drinking Water Directive (Council Directive 98/83/EC) as specified in the Regulation 2018/676 (European Commission, 2018). The drinking water limit of 0.1 μg/L that applies to organic pesticides and their relevant metabolites is only relevant for organometal compounds.
Post‐registration groundwater monitoring could be proposed in a higher tier assessment, in accordance with the tiered approach European Commission (2014). The recommendations by Gimsing et al. (2019) for assessing such data could be followed. Care should be taken to ensure that the potential origins of metals in groundwater are considered through an inventory of the background values.
Uncertainty analysis
All the uncertainties that affect the assessment when a standardised procedure is being developed need to be identified and described according to the EFSA Scientific Committee (EFSA Scientific Committee, 2018). The uncertainties are potentially greater for transition metals than for synthetic organic chemicals used as PPPs owing to the consideration of persistency and bioavailability. The essentiality of some transition metals limits the size of the assessment factor from a scientific perspective. Conclusion on low risk in the ERA needs to provide a sufficient certainty based on the scientific knowledge available to adequately cover the protection goals and cannot be compensated by increasing the assessment factor or by post‐registration monitoring.
Monitoring
Within the aim of this statement, three different levels of environmental monitoring were described, serving different purposes in the ERA of transition metals (see General Framework): general monitoring data evaluation before dossier submission, post‐registration monitoring following eventual active substance approval and PPP authorisation and targeted long‐term studies accompanying authorisation and investigating specific relationships between e.g. application rates and biological responses. It is recommended that all available and reliable occurrence information on transition metals in relevant environmental matrices are provided ahead of the start of the scientific risk assessment of transition metals as active substances in PPP together with the dossier in addition to the systematic literature review (EFSA, 2011). It is proposed that, if conclusions on low risk for non‐target organisms exposed to transition metals following PPP intended uses for a given time frame have been reached at higher tier level, post‐registration chemical and biological/ecological monitoring is implemented, to follow long‐term trends in exposure and effects and detect possible upcoming risks. Such data will provide useful information for possible renewal applications. Especially the non‐degradability of transition metals in the environment and the related uncertainties in long‐term effects underpin the post‐registration monitoring requirements. Monitoring of PPPs should be proportionate, and therefore, monitoring efforts should focus on those environmental compartments and non‐target organism groups identified as vulnerable. Controlled long‐term studies bridge the prospective risk assessment approaches with the effective impact in the field after year‐on‐year application of PPP with transition metals. They can deliver very relevant information on: (i) the state of environmental media; (ii) the risk assessment accurateness; and (iii) necessary risk management decisions.
General remark on the development of risk assessment methodologies for transition metals used as PPPs
Ideally, the parameterisation of adapted and/or new exposure scenarios and newly developed higher tier approaches needs to be agreed at European level. It is recommended to establish scientific working groups that deal with model parameterisation and development. All developments need to follow the modelling cycle as described in the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a).
1. Introduction
In the letter dated 13 February 2019 from the European Commission (EC) to the European Food Safety Authority (EFSA), a request was made for a statement on a framework for conducting the environmental exposure and risk assessment for transition metals and their compounds when used as active substances in plant protection products (PPP).1 As stated in the Terms of Reference by the European Commission (please see chapter below), the non‐degradability of transition metals, the specific physico‐chemical conditions affecting their behaviour and toxicity and their partial essentiality are distinctive characteristics in comparison with organic chemicals used as active substances in PPP.
The environmental risk assessment (ERA) performed in the context of the evaluation of transition metals as active substances in PPP is perceived by many stakeholders as not appropriate, considering the specificities of these substances, e.g. copper compounds. The EFSA ‘Conclusions on the peer review of the pesticide risk assessment of copper compounds’ of 20 December 2017 stated expressly that ‘the available guidance in the area of pesticide active substance ERA does not specifically cover metal compounds’.
It is however necessary to have an appropriate methodology in place to reduce uncertainties as regards the risks for the environment resulting from the intended uses of transition metals as PPPs.
EFSA was requested not only to provide a statement on a framework for the ERA of transition metals but also to explore the approaches used in other regulatory areas for this purpose – such as in the context of the REACH Regulation2 – and align the pesticide assessment methodology to these as much as technically possible.
1.1. Background and Terms of Reference as provided by the requestor
Some transition metals in the chemical periodic table of elements groups 7–11 of period 4 have compounds that can be of interest in plant protection (e.g. copper compounds). However, the guidance documents available on ERA of pesticides containing organic compounds as active substances are not always directly applicable to metal compounds due to several reasons. Some biogeochemical processes can be different for metal compounds compared with organic compounds. Physico‐chemical conditions of the environmental matrices (e.g. pH, free ions,…) that affect speciation and bioavailability can be much more influential on the effects of metal compounds on non‐target organisms than for organic chemicals.
Additionally, metal compounds are already present in the environment at variable levels, either appearing naturally or added via different sources. Some metals are, in addition, nutritionally essential, which is the case for copper compounds.
The ECHA guidance for the implementation of the REACH and Biocidal Products Regulations3 on safety assessment of chemicals includes specific chapters which are focused on environmental risk characterisation for transition4 metals and their compounds; additional activities in this area are ongoing, including the assessment of new testing proposals developed under REACH. Some of these elements are also relevant for the assessment of copper and copper compounds as PPP and therefore need to be considered to maintain a consistent approach across EU legislation, requiring collaboration and alignment between ECHA and EFSA.
EFSA is requested to provide a statement outlining an appropriate scientific methodology for the ERA for transition metal compounds used as active substances in PPP. This statement should provide guidance to applicants, competent authorities of the Member States and EFSA experts when assessing certain transition metal compounds, e.g. copper compounds.
The framework methodology developed should be consistent with the regulatory framework and data requirements for PPPs under Regulation (EC) No 1107/20095 and address the specific properties of transition metal compounds, including metals that are also nutritionally essential elements.
The framework methodology should identify the key principles for the assessment and describe how such principles shall be implemented in the context of risk assessment. It should aim to deliver the same level of confidence in the risk characterisation of transition metal compounds, such as copper compounds, as the current levels achieved for organic chemicals.
If applicable, cross‐reference to available relevant guidance should be made in order not to duplicate the development of guidance. For instance, the considerations already laid down for transition metals in the relevant ECHA guidance should be taken into account (both, specific approaches already developed and under development),6 any other EFSA Scientific Opinion or any pertinent EU or internationally recognised guidance. Experience gained in the context of risk assessments carried out on copper and its compounds used as PPP and in other sectors of EU legislation (e.g. Biocides, Water Framework Directive) should also be considered. The framework methodology is expected to explain which data are necessary and which assessment shall be carried out to address the lack of specific approaches in the environmental risk assessments highlighted in the EFSA conclusion on copper compounds.
In particular, EFSA is requested:
to liaise with ECHA to gather information on available guidance for the assessment of transition metals, as well as ongoing developments related to risk assessment and testing strategies for metals;
to develop a methodology for the environmental risk assessment for transition metal compounds covering the relevant environmental compartments (terrestrial and aquatic compartments) and the concerned non‐target organisms. The methodology should include criteria on how to assess and consider the environmental concentration estimates, bioavailability, homeostatic mechanisms and bioaccumulation of transition metal compounds. Additionally, consideration should be given on how to integrate specific approaches in the risk assessment methodology (e.g. BLMs);
to establish criteria for the evaluation of environmental exposure assessments, i.e. monitoring studies;
to provide guidance on the application of any uncertainty factors in the ERAs when insufficient evidence is available, for instance with respect to the speciation of transition metal compounds in the environment.
Member States, stakeholders and the general public shall be consulted.
1.2. Interpretation of the Terms of Reference
The request from European Commission to EFSA was made for a statement on a framework for ERA of certain transition metals (namely iron, cobalt, nickel and copper). Although the mandate mentions that the statement should provide guidance to applicants, competent authorities of the Member States and EFSA experts when assessing certain transition metal compounds, this is interpreted as advice and recommendations. As the mandate is very broad covering the complete environmental risk assessment, it was deemed not possible to provide specific detailed guidance for all compartments, as also general existing guidance for several non‐target organism groups is currently under revision (e.g. terrestrial ecotoxicology for in‐soil organisms, non‐target terrestrial plants, non‐target arthropods (NTA), bees, birds and mammals). The development of adapted models and specific scenarios for all environmental compartments will also need extensive dedicated work. Within the time frame and the resources available for this mandate, guidance is given on which approaches are considered appropriate to be further developed and which approaches are not fully applicable to transition metals and their compounds as active substances in PPP. EFSA defines as ‘transition metal compounds’ any complexes of transition metals that become registered as active substances in PPP (current examples are e.g. copper salts or ferric phosphate).
Furthermore, EFSA defines a statement of a scientific panel as a concise document that does not go into the same level of detail as an Opinion.
The mandate from the European Commission is also very ambitious by asking for the complete ERA to deliver the same level of confidence in the risk characterisation of transition metal compounds as the current levels achieved for organic chemicals. The confidence in the ERA will depend on the characteristics of the transition metal and relevance and the reliability of the data and RA approaches provided by the applicant.
Regarding a request for guidance on the application of any uncertainty factors in the environmental risk assessments when insufficient evidence is available, indications are given if generally applicable. Specific uncertainty will depend on the information provided and nature of the transition metal assessed and will be specific for each metal assessed.
2. The Framework
2.1. Transition metals and their compounds as active substances in PPPs
2.1.1. Use of transition metals and their compounds as pesticides
In some EU Member States, transition metals e.g. iron and copper compounds are present in PPPs used in conventional, as well as organic farming. Transition metal compounds can be natural compounds, which allow them to be used in organic farming when contained in authorised PPPs. Transition metals e.g. copper compounds are also used as fertilisers in agriculture to ensure that crops grown on soils with e.g. low copper content receive sufficient substance for optimising crop growth and development. Transition metals may also end up on agricultural fields when manure containing transition metals is applied as a fertiliser on agricultural fields. When transition metals (e.g. copper compounds) are used as feed supplements for farm animals, the compounds and metal ions are excreted and end up in composted manure.
For the most recent EU evaluation of copper compounds for use in plant protection, the conclusions were reached based on the evaluation of the representative uses as a fungicide on grapes, tomatoes and cucurbits (EFSA, 2018). For the latest EU PPP evaluations, the representative use of iron sulfate was as a herbicide on amenity and sports turf for the control of moss (EFSA, 2012a). The EU evaluation of the representative uses of ferric phosphate was assessed, considering the compound is used as a molluscicide in agriculture and horticulture for control of harmful slug and snail species in all edible and non‐edible crops (EFSA, 2015).
As transition metals can originate from many sources (e.g. mineral weathering from bedrock, natural (geogenic) atmospheric deposition and anthropogenic atmospheric deposition, mineral fertiliser additions, transition metals‐containing products applications), it is not always possible to determine the concentrations of transition metals in soil, surface water and groundwater that originate from use of the compounds as a PPP. Land‐use and agricultural management practices are major factors determining variation in soil content of transition metals. In addition, concentrations of transition metals are related to soil properties like pH, texture, organic content and geology. For copper, the mean total concentration of copper in croplands in the EU is 19 mg/kg, and for vineyards, the mean total concentration of copper is 49 mg/kg, with a very high variability between countries, climatic, geological and pedological factors (Ballabio et al., 2018). The authors indicated that the accumulation of copper in soils is ‘correlated mostly to soil properties, environmental factors and lithology’ (Ballabio et al., 2018), the agricultural practices and the other potential sources cited above (Table 1).
Table 1.
Comparison of exposure of the aquatic and terrestrial environmental through different uses of transition metals by way of examples
| PPP | Manure | Fertiliser | Rain gutters | Anti‐fouling paint (boats) | Industrial production | |
|---|---|---|---|---|---|---|
| Application to the environment | Intended, direct | Non‐intended, direct | Intended, direct | Non‐intended, indirect | Non‐intended, direct | Non‐intended, indirect |
| Aquatic environment | ||||||
| Emission path | Drift, run‐off, drainage (and point sources) | Run‐off | Drift, run‐off, drainage | Rain events via sewage treatment plants or direct emission to surface waters | Solubilisation | Smokestack emission, atmospheric long‐range transport and deposition |
| Timescale | Repeated pulse application, short‐ and long‐term exposure | Repeated pulse application, short‐ and long‐term exposure | Repeated pulse application, short‐ and long‐term exposure | Continuous input, long‐term exposure | Continuous input, long‐term exposure | Continuous input, long‐term exposure |
| Spatial scale | (Small) waterbodies in agricultural areas (edge of field) | (Small) waterbodies in agricultural areas (edge of field) | (Small) waterbodies in agricultural areas (edge of field) | Discharge from sewage treatment plants | Waterbodies | Global |
| Terrestrial environment | ||||||
| Emission path | Direct application, wash‐off from plants (in‐field); drift, run‐off (off‐field) | Direct application | Direct application | Disposal of sewage sludge contaminated via rain gutters on soils | Smokestack emission, atmospheric long‐range transport and deposition | |
| Timescale | Repeated pulse application, short‐ and long‐term exposure | Repeated pulse application, short‐ and long‐term exposure | Repeated pulse application, short‐ and long‐term exposure | Repeated pulse, short and long‐term exposure | Continuous input, long‐term exposure | |
| Spatial‐scale | In‐field; off‐field | In‐field | In‐field; off‐field | Mainly in‐field | Global | |
2.1.2. Chemical properties and fate of transition metals
Transition metals are metals that have more than one oxidation state. The occurrence of a specific state will depend on its stability and the oxidation reduction potential of the medium in which it is present (soil, water and sediment). The term ‘speciation’ refers to the relative proportion of different forms or phases in which an element (e.g. copper) is present. For metals, free ions and organic and inorganic forms are most relevant.
Because transition metals are elements, they cannot break down any further via hydrolysis, metabolism or any other degradation processes. They are therefore considered as persistent in environmental matrices (soil, water and sediment). After application, short‐term and long‐term processes (ageing), due to precipitation/nucleation, occlusion within organic matter and micropore/mesopore diffusion set‐up, occur (Zeng et al., 2017). These processes contribute to the decline of the bioavailability over time in the environmental matrices and especially for soil and sediment. After application on soil, the transition metals will be partitioned between various forms in both the soil solution and soil solids. In soils, the main factors driving speciation and availability of transition metals are pH, CEC, clay content and organic matter content (ECHA guidance document on metals; ECHA, 2008). As an example, a higher proportion of dissolved free copper in the pore water would be only expected in acidic soils, because the availability of free copper ions increases with pH below 5 (Vol. 3 B.8 (CP), RAR Copper Compounds, 2017).
When introduced into the environment as salts, metals can be present in the free ionic form or be bound to different types of inorganic ligands (such as clay and iron, manganese and aluminium oxides, sulfates, carbonates) and organic ligands (such as humic or fulvic acids), depending on the pH, the redox potential and presence of other competing ions (Ca2+, Mg2+). At low pH, more free metal ions are available, but may not be as bioavailable as might be expected due to competition with protons at the binding sites in the organisms; at high pH, the bioavailability may be increased as less H3O+ is competing for binding sites of cationic metals or decreased as more OH– is competing for binding sites of anionic metals. The binding to these ligands will impact the uptake of metals by biota and so the ecological effects. It is therefore important to take the water, sediment and soil chemistry into consideration when using the results of standard ecotoxicity tests to predict effects under field conditions.
As usually organic and inorganic ligand concentrations greatly exceed free metal ion concentrations in the water column and in soils, a large buffering capacity exists. For instance, for copper in natural environments, typically, well in excess of 90% of the copper is complexed. Therefore, for the evaluation of toxicity, the speed of complexation with ligands is crucial. Metals in equilibrium show a different toxicity than metal freshly added to a system. This was observed in several studies and differences in equilibration times were observed for inorganic and organic ligands. Whereas e.g. in water columns equilibrium is reached within seconds or minutes with dissolved inorganic ligands, the equilibration with dissolved organic carbon (DOC) can take hours to days and depends furthermore on the concentration of DOC, being slower (30 h vs. 0.1 to 4 h) at higher (> 5 mg/L DOC) than at lower (< 1 mg/L DOC) concentrations (Mebane et al., 2020). Another kinetic process is the phase transfer from the water to the sediment phase due to e.g. deposition of algae or faecal pellets, or binding to sediment. Unlike for organic chemicals in PPP where the partition between the water and sediment phase mainly depends on the lipophilicity of the active substance and the level of organic carbon content of the environmental matrix, the partitioning of metals depends on the water and sediment chemistry (e.g. pH, organic content, sulfur and iron compounds and redox status). The redox status of the sediment affects the level of AVS, which in turn, together with the pH, affects the speciation, solubility and therefore toxicity of metals in the sediment phase.
Under certain conditions, the concentration of bioavailable copper is also influenced by the location in the water column. It may be significantly higher in the bottom waters, as organic ligands are degraded in the oxic sediment layer (0–2 cm) and bound copper is therefore released into the pore water, which is then resuspended into the aquatic system (Kiaune and Singhasemanon, 2011). However, other studies report that although possible the release of metals from sediment is rather limited. Oxidation of anoxic sediments or resuspension events do not lead to the remobilisation of metals and rather indicate an irreversible transfer of metals from water to the sediment (Rader et al., 2019). However, resuspended metals strongly bound to particulate matter may be taken up by filter feeders and remobilised in the gastrointestinal tract (Weltens et al., 2000). Sediment‐based food chains might be an important pathway for dietborne‐metal exposure of benthic organisms (Luoma and Rainbow, 2008; see DeForest and Meyer, 2015).
2.1.3. (Eco)Toxicological particularities of transition metals
Transition metals (in particular iron, cobalt, nickel and copper) are naturally occurring in the environment and many are essential for the biological function of organisms. However, if in excess, these metals are potentially toxic and, to maintain metal homoeostasis, organisms must tightly coordinate metal acquisition and excretion (Bury et al., 2003) and in this way limit accumulation. It has been demonstrated for copper and other metals that homoeostatic mechanisms will depend on the external concentration of metals in the environment. At low concentrations, organisms are actively accumulating metals, while at high concentrations, they will excrete more or reduce the uptake of these metals (e.g. copper compounds, VRAR, 2008).
However, homoeostasis controls internal metal concentrations within a narrow range. Homoeostasis cannot mitigate the effects of additions above these ranges (accumulation). The fact that transition metals are naturally occurring elements and that some of the transition metals are essential elements (e.g. copper) should not be understood as indication of low risks for non‐target organisms. Especially if the applied amount exceeds natural background environmental loads by order of magnitudes, and the transition metal has a high efficacy and rather unspecific mode of action, side effects on non‐target organisms are likely (e.g. in soils and sediment; Lamichhane et al., 2018). Therefore, as for all PPP, the optimal application rate is the minimum effective dose to protect the specific crop against the target pest, which needs to be carefully established before the risk assessment.
The diet is the main source for essential metals, but in aquatic organisms an alternative uptake route is available from the water, i.e. via gills.
According to the definition by the ECHA guidance document on metals (ECHA, 2008), the bioavailable fraction depends on the metal forms that prevail under the specific environmental conditions (abiotic factors) and on the biological receptors (biotic factors) and can be defined as the metal fraction that can be taken up and that can interact with the organism's specific metabolic machinery. Bioavailability is therefore organism specific. The bioavailable fraction is smaller than the total fraction, therefore the calculation of the bioavailable fraction leads to a refined (reduced) exposure concentration. The consideration of the (bio)availability concepts pertains both to the estimated environmental exposure and effect levels.
In aqueous systems, bioavailability is often correlated with the free metal ion concentration, because the free ion is often the most bioavailable form of a dissolved metal (Peakall and Burger, 2003). Organic matter decreases the metal uptake by fish gills. Metals entering the aquatic environment will distribute over different metal species depending on the water chemistry. All variants of Cu as active ingredient were discussed together for their toxicity (in the Cu RAR Copper Compounds, 2017), although different forms of Cu exist as active substance in PPP (copper(II) hydroxide, copper(I) oxide, Bordeaux mixture (traditional mixture of copper(II) sulfate and calcium hydroxide), copper oxychloride and tribasic copper sulfate).
In mammalian toxicity, it is generally agreed that the toxic form of transition metals is the free ion form (e.g. Cu2+ and Zn2+). Studies reported in the EFSA conclusion on copper indicate a general bioequivalence between different copper salts in the Bordeaux mixture consisting in tribasic copper sulfate and copper oxide. EFSA therefore concluded that all copper compounds have a similar order of magnitude of toxicity towards mammals. It is also known that copper salts in an acidic environment, such as the gastric content, are mostly present in its dissociated Cu2+ form. However, depending on the duration of stay in this environment, dissociation may not be complete, therefore some differences in bioavailability and toxicity of copper salts exist. There is a general agreement to consider copper sulfate as the most bioavailable copper salt in mammals and a worst case for toxicity testing (EFSA, 2018). In animal poisoning, the form of copper is generally not considered and toxic doses are usually expressed in mg per kg body weight of copper (Gupta, 2018).
In mammals, dietary exposure is the major route of exposure for all metals and absorption of the free metal ion is the main driver of bioavailability of metal elements (Cu, Fe, Zn). Overall digestive absorption of copper salts, for instance, is 40–49% via both active and passive uptakes by the brush border cell of the small intestine. The EFSA Scientific Opinion on dietary reference values for copper considers an oral absorption of 50% of copper in humans (EFSA, 2018). Rats have very similar figures. It also appears that, as a result of homoeostasis, increasing copper concentrations in the diet will result in a lower bioavailability (EFSA, 2018). In ruminants, the main drivers of bioavailability are the presence of sulfur, molybdenum and soil in the gastric content (Thompson, 2007).
Copper is bound to two proteins (albumin and transcuprein) and transported to the liver. From the liver, copper is distributed in the bloodstream to other organs primarily bound to another protein (ceruloplasmin up to 70%). Excess copper induces the production of metallothioneins that will bind the metal and render it unavailable. The cells will store the excess copper. Natural sloughing off of brush border cells plays a major role in the elimination of copper, especially for copper accumulation. Copper is not metabolised. It is excreted via the bile. Minor amounts can be detected in urine, skin or hair. Bioaccumulation normally does not occur. There are two major exceptions to this rule:
very high daily doses in chronic exposure
genetic disease (Wilson's disease) or genetic deficiencies in copper elimination.
Some species are known to be more sensitive to copper or copper accumulation. Dogs are known to have a lower ability than humans to excrete copper. Among ruminant species, it is also known that sheep (some breeds like Texel) and, to a lesser extent, goat and cattle may accumulate copper from their diet and require low‐concentration mineral supplements (Thompson, 2007).
In terrestrial vertebrates, with the exceptions mentioned above, there is evidence of homoeostatic mechanisms in most species of birds and mammals for all essential metals (including Cu, Fe and Zn for instance). This is a result of decreased accumulation when dietary concentrations increase, but also of increased elimination of brush border cells with accumulated copper residues bound to metallothioneins (EFSA, 2018).
In invertebrate species, the variation of toxicity across species is a result of both external factors (bioavailability of copper from the environment) and internal factors (homoeostatic mechanisms and the detoxification in ‘metallothioneins like proteins’). In both aquatic and terrestrial invertebrates, copper homoeostasis is related to the existence of P‐Type ATPases (pumping copper across biological membranes in both directions) and copper chaperones responsible for intracellular copper homoeostasis. These proteins are highly conserved across species, suggesting their universal role (including vertebrates). It appears that species sensitivity is generally a matter of detoxification mechanisms, including the induction of metallothioneins, rather than a result of the position in the trophic web (e.g. copper compounds; VRAR, 2008).
2.2. Consequences for the assessment of transition metals when used as active substances in PPPs
Due to the non‐degradable nature of transition metals, a continuous application of PPP containing transition metals requires to link the regulatory acceptable concentration of the active substance to a period of time. Once metals are added to the environment, they remain there, enter a biogeochemical cycle and accumulate in sink compartments (e.g. soils and sediments). Depending on the physico‐chemical properties of the surface water, sediment or soil and on the species present, a different amount of added metal will be taken up and tolerated before potentially irreversible changes occur with time. With continuous addition of metals, the individual organisms will show sublethal or lethal effects or acclimate, and eventually the species composition of the community will change, i.e. some populations will adapt whereas others may perish (Soldo and Behra, 2000). Depending on the pre‐exposure, species and communities may be more or less tolerant as a result of metal acclimation at background concentrations. This accounts for the variability in effects observed in different field studies and renders the correlation between laboratory studies and field/monitoring studies more difficult. Metal‐tolerant microorganisms in natural populations (bacteria and algae) may accumulate metals, and therefore facilitate metal transfer to grazing species in higher trophic levels (Wikfors and Ukeles, 1982).
In general, and independently from the outcome of the risk characterisation for different non‐target organisms, it has to be considered that the prospective risk assessment of non‐degradable compounds can only cover a defined period linked to risk management decisions due to the limitations in the long‐term hazard assessment and to the time frame assessed.
In principle, according to Commission Regulation (EU) 546/20117 (European Commission, 2011) (point 2.5.1. Fate and distribution in the environment):
‘No authorisation shall be granted if the active substance…after use of the PPP under the proposed conditions of use: – during tests in the field, persist in soil for more than 1 year (i.e. DT90 > 1 year and DT50 > 3 months), or – during laboratory tests, form non‐extractable residues in amounts exceeding 70% of the initial dose after 100 days with a mineralisation rate of less than 5% in 100 days.’
These criteria apply to transition metals in the environment on the absence of degradation in soil.
In the following, (EU) 546/2011 includes an opening clause, stating that authorisation cannot be granted:
‘…unless it is scientifically demonstrated that under field conditions there is no accumulation in soil at such levels that unacceptable residues in succeeding crops occur and/or that unacceptable phytotoxic effects on succeeding crops occur and/or that there is an unacceptable impact on the environment’.
Metals have been considered as persistent in the European Union and several compounds are additionally toxic to very toxic to non‐target organisms, meeting therefore the properties of being ‘candidates for substitution’ according to Regulation (EC) No 1107/2009. Up‐to‐date information on different transition metal compounds can be retrieved e.g. from the ECHA Brief Profiles for classifications and from the updated database of the EU Commission8 on candidates for substitution.
If the evaluated transition metal is classified as candidate for substitution, comparative assessment might identify alternative products with other active substances.
The precautionary principle is to be implemented also through the ecological risk assessment, which therefore needs to adequately address the toxicity and exposure of the intended uses in relevant time frames. Transition metals represent a special case in this context as their toxicity is determined by their site‐specific and species‐specific bioavailability influenced by several environmental factor combinations and their fate determined by their persistency.
Despite the inherent limitation of scientific knowledge in the risk assessment and having the goals of a sustainable use of PPP in mind, conclusions on low risk in the ERA of transition metals should nevertheless provide a sufficient certainty to adequately cover the protection goals based on the current state of science and technology and for the time frame assessed. Following intended uses of PPP with transition metals, however, the loads of these compounds will inevitably increase in the environment. In addition, utilisation of the same transition metal for other uses than PPP and in other PPPs (e.g. as additive in animal feeds or in fertilisers) will result in inputs to a common compartment (e.g. soil) and add to the total concentrations possibly posing risks to non‐target organisms and groundwater.
It is therefore recommended to consider the possible additive effect of different PPPs and other uses in any risk management decision on the approval of transition metal compounds as active substances or the authorisation of plant protection products with transition metals. It is further recommended to align the prospective risks characterisation of all intended uses with the goals of other overarching legislative frameworks (e.g. the Sustainable Use Directive,9 Water Framework Directive,10 Habitat Directive,11 Drinking Water Directive12 and Soil protection legislations at national level).
2.3. Proposed framework for ERA of transition metals
2.3.1. Overall approach and problem formulation
In principle, the protection goals for the environment do not differ depending on whether a PPP with defined intended uses contains as active substance a transition metal or other compounds. Therefore, this statement does not elaborate specific protection goals or exposure targets for e.g. non‐target organisms related to transition metals. The PPR Panel is however aware that recent EFSA guidance and EFSA PPR Scientific Opinions of the last years have proposed specific protection goal (SPG) options for several non‐target organisms related to the protection of biodiversity and ecosystem services. Since this statement deals in principle with all environmental compartments, the proposals currently under discussion, future agreements on SPG and possible consequences for risk assessment schemes would apply also to the framework for assessing transition metals as active substances in PPP.
The steps followed in the preparation of this framework are the following:
consideration of hazard characterisation for non‐target organisms under EFSA guidance;
consideration of exposure assessment for different compartments under EFSA guidance;
identification of specific issues related to the assessment of transition metals employed as active substance in PPP;
identification of metal specific consideration and approaches according to the guidance on the ‘Environmental risk assessment for metals and metal compounds’ (ECHA, 2008) and other relevant guidance;
assessment of the applicability of proposed methodologies in the context of transition metals specifically used as active substances in PPP;
conclusions and recommendation for the different compartments and respective non‐target organisms.
This PPR statement has considered guidance on metals from ECHA, the European Commission (especially on the Water Framework Directive, WFD) and OECD as well as guidance developed for environmental exposure and risk assessment of PPPs by EFSA and the European Commission.
The intention of the framework is to provide advice and recommendations under which circumstances guidance on metals developed for other regulatory frameworks (e.g. under REACH and the WFD) can be used. For an overview of the different assessment frameworks, please see Appendix A. The framework also considers under which circumstances guidance developed for plant protection products can be used also for the environmental risk assessment of transition metals as active substances in PPP.
2.3.2. General Framework for ERA of transition metals in PPP
The following general framework for the ERA of transition metals is proposed.
The General Framework for the ERA of transition metals used as active substances in PPPs consists of several steps. A preliminary phase is proposed ahead of the first step of the risk assessment, which intends to gather all available information on residue levels in the environment and make them available to the assessors. In a first assessment step, relevant natural background data and anthropogenic additions are included in the exposure assessment and residue levels are considered to be bioavailable to non‐target organisms. If higher assessment steps are performed, reduced bioavailability of residues from past applications can be considered. However, for freshly added transition metals such as PPP, equilibrium condition cannot be assumed. If conclusion on low risk can be reached, then post‐registration monitoring needs to be implemented to follow up on long‐term application of non‐degradable compounds and results need to feed back to risk assessment and management processes.
For the temporal scale of the risk assessment, the working group defines ‘relevant time frames’ as those time periods for which the application of the product containing transition metals can be realistically assumed, should the transition metal compound be repeatedly approved as active substance in future. As transition metal compounds are one of the oldest employed active substances for PPPs and still widely used, the relevant time frames for exposure calculations should realistically not cover only one approval period but also longer time frames.
In the exposure assessment, it is therefore suggested to cover single as well as several approval periods (e.g. 5 and 10 years) but also longer time frames (e.g. > 50 years). For synthetic compounds, the time frame is set by the outcome of field studies, but for metals this is not a realistic option.
For effect characterisation in controlled field tests (e.g. soil organisms), studies should be planned to cover more than one approval period, so that they can be prolonged without sampling problems. There is in principle no maximum time frame that can be defined a priori for all transition metal compounds and intended uses. Before stopping a long‐time trial, it should be agreed with competent authorities that no further changes in the observed communities can be expected.
If the accumulation of transition metals due to the application with PPP will result in environmental levels above the background without use in agricultural areas, the availability and duration of long‐term studies will be part of the uncertainty assessment in the ERA and will determine the height of the assessment factor.
Preliminary phase
All relevant and reliable available occurrence information on transition metals in relevant environmental compartments are provided ahead of the start of the scientific risk assessment of transition metals as active substances in PPP by the applicant together with the dossier to the competent authority assessors. This should include public literature data as requested in the systematic literature review (EFSA, 2011) and monitoring data gathered and assessed by independent scientific bodies (e.g. JRC).
Monitoring data should be representative in terms of spatial distribution and characteristics for all environmental matrices (soil, sediment, surface water and groundwater) across the European Member States.
Monitoring data should allow if possible for the discrimination between natural background levels and anthropogenic residues in relevant matrices and areas.
Monitoring data should inform risk assessors on the metal levels in the environment and deliver reliable levels to be included in exposure assessment steps relevant for the land uses to be assessed.
The monitoring data should be reproducible, publicly available and consist of an agreed data set. It is recommended that post‐registration monitoring data possibly existing from previous authorisations are included in the publicly available data set.
First assessment step
Exposure and effect assessment
The first assessment step is performed following general EFSA approaches and scenarios with realistic worst‐case assumptions.
All background and anthropogenic residue levels are included in the calculation of predicted exposure quantities. Standard model scenario parameterisations may need to be adjusted to meet realistic worst‐case assumptions, distinction between different land uses might be implemented if information is available.
The exposure and effect assessments assume at this step that transition metals present in the environment from past (accumulated) and present (freshly added) applications are bioavailable to non‐target organisms.
Latest developments on the assessment of direct and indirect effects following long‐term application of pesticides should be considered as for other PPPs (EFSA Scientific Opinions and Statements on Non‐Target Organisms, e.g. EFSA PPR Panel, 2014b, 2015a, 2017, 2018).
Risk assessment outcome and consequences in the first step
If the risk assessment concludes at the first step on low risk for the intended uses of transition metals as active substances in PPP, then it is indicated that for the assessed time period general protection goals as laid down in Regulation (EC) No 1107/2009 and specific protection goals of sectorial guidance are met (e.g. Birds and Mammals EFSA guidance document, EFSA, 2010a).
In general, it has to be considered that the prospective risk assessment and management of non‐degradable compounds can only cover a defined period, as all PPP intended uses will inevitably increase the loads in the environment and also due to temporal limitations in the long‐term hazard assessment, unlike in the exposure assessment where models and monitoring data are available. In addition, utilisation of the same transition metal in another PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will result in inputs to a common compartment (e.g. soil) and add to the total concentrations possibly posing risks to non‐target organisms and groundwater.
It is therefore recommended to consider these aspects and uncertainties in any risk management decision on the approval of transition metals as active substances or the authorisation of PPPs with transition metals (e.g. comparative assessment, cost–benefit analysis, optimised application rates, etc.); it is also recommended to align the prospective ERA with the goals of other overarching legislative framework (e.g. Water Framework Directive, Drinking Water Directive and Sustainable Use Directive).
Higher tier assessment step
Exposure assessment
Higher tier assessment might include some refinement options following the agreed overarching EFSA tiered approaches for groundwater, surface water and soils.
Regarding the exposure assessment, the estimation of reduced bioavailability of transition metal residues from past PPP applications might be considered, as suggested in principle in the ECHA guidance document (2008).
It needs to be kept in mind, though, that intended uses of PPPs will result in freshly added transition metals to the environment (e.g. water bodies and soils) through drift and direct application. Equilibrium conditions in the different media cannot be assumed for these metal fractions when performing the ERA of intended uses of PPPs.
Transition metals applied as PPP will add to the residue levels (from previous applications or from natural background), increasing total concentrations in the environment.
As these elements are not degradable, they will accumulate in sinks (e.g. soils and sediments) and could potentially be adding to the risk and be remobilised under certain conditions, for example after land‐use changes.
For addressing speciation and bioavailability issues, models and scenarios including environmental processes and parameters relevant to the fate and behaviour of transition metals in water, sediment and soils would need to be adapted or developed.
It is proposed that – if envisaged – dedicated modules are developed within the current framework (e.g. in the FOCUS surface water, FOCUS groundwater, PERSAM framework, EFSA soil exposure guidance).
All developments need to follow the modelling cycle as in the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a).
The new modelling approaches need to be made fully available to the competent authorities for assessment and model outcome should be checked for compliance with the current general regulatory framework (see also EFSA PPR Panel, 2014a).
Effect assessment
Regarding the effect assessment, processes at the biotic ligand site for transition metals might be considered, as suggested in principle in the ECHA guidance document (ECHA, 2008).
It needs to be kept in mind that current approaches (e.g. for aquatic organisms) assume equilibrium conditions and that only free metal ions contribute to toxicity (no oral/contact uptake). Therefore, reciprocity of exposure and effects and the exclusion of possible delayed effects from initial exposure peaks are pre‐requisites for the implementation of the current approaches according to ECHA guidance document (ECHA, 2008).
Environmental conditions in Europe might lead to a considerable set of possible combinations of parameters relevant for modelling. If appropriate, it is therefore proposed to employ simple available models considered fit for purpose to address these processes and to identify realistic worst‐case scenarios, to avoid unnecessary complexity.
In addition, following EFSA guidance, also higher tier effect studies (e.g. SSD, semi‐field or field studies) can be taken into account.
Due to the non‐degradability of metals in the environment, higher tier effect studies used for refinement should be targeted at the relevant compartments acting as sinks (e.g. soils and sediments) and be prolonged to relevant time frames of transition metal applications.
Finally, as in lower assessment steps, latest developments on the assessment of direct and indirect effects following long‐term application of pesticides should be considered as for other PPPs (EFSA Scientific Opinions and Statements on Non‐Target Organisms; EFSA PPR Panel, 2014b, 2015a, 2017, 2018)
Risk assessment outcome and consequences at higher tier risk assessment steps
If the risk assessment concludes at higher tier on low risk for the intended uses of transition metals as active substances in PPR:
then it is indicated that for the assessed time period general protection goals as laid down in Regulation (EC) No 1107/2009 and specific protection goals in sectorial guidance (e.g. Birds and Mammals EFSA guidance document, EFSA, 2010a) are met.
Because of the non‐degradability of metals – to follow up exposure and effect time trends and to indicate possible upcoming risks – post‐registration monitoring for the intended uses needs to be implemented
Post‐registration monitoring should be targeted to the environmental compartments of concern and comprise chemical and biological/ecological assessment. Monitoring schemes (according to Art. 6(i) and Art. 67(2) of Regulation (EC) No 1107/2009) should be agreed with the competent authorities.
Conclusions on low risk in the ERA should be taken based on sufficient certainty and the available scientific knowledge, to adequately cover the protection goals.
In general, it has to be considered that the prospective risk assessment and management of non‐degradable compounds can only cover a defined period, since all PPP intended uses will inevitably increase the loads in the environment and also due to temporal limitations in the long‐term hazard assessment, unlike in the exposure assessment where models and monitoring data are available.
In addition, utilisation of the same transition metal in another PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will result in inputs to a common sink compartment (e.g. soil) and add to the total metal concentrations possibly posing risks to non‐target organisms and groundwater.
It is therefore recommended to consider these aspects and uncertainties in any risk management decision on the approval of transition metals as active substances or the authorisation of PPPs with transition metals (e.g. comparative assessment, cost–benefit analysis, optimised application rates, etc.) and accompanied focused monitoring; it is also recommended to align the prospective ERA with the goals of other overarching legislative framework (e.g. Water Framework Directive, Drinking Water Directive, Sustainable Use Directive).
Risk assessment for groundwater
Leaching of transition metals to groundwater due to the long‐term uses of transition metal‐containing PPPs cannot be excluded.
Factors affecting leaching into the groundwater should be considered in modelling. The selection of input parameters should allow reaching the exposure target. For the time being, neither guidance document, nor a specific model exists to assess the leaching of transition metals to groundwater in the context of the PPP.
Following an amendment to the uniform principles, existing drinking water limits set by European Drinking Water Directive (98/83/EC) should be considered if available for the metal species.
General remark on the development of risk assessment methodologies for metals as PPPs
Ideally, the parameterisation of adapted and/or new exposure scenarios and models needs to be agreed at European level. It is recommended to establish scientific working groups that deal with model parameterisation and development.
3. Environmental risk assessment
3.1. Aquatic environment (surface waters and sediment)
3.1.1. Effect assessment and ecotoxicologically relevant exposure quantities
Hazard characterisation
In the first tier of the ERA for aquatic organisms exposed to pesticides, the hazard characterisation, is currently based on results of standardised OECD tests required by the Commission Regulation (EU) No 283/201313 (European Commission, 2013a) and No 284/201314 (European Commission, 2013b). The toxicity of metals is primarily driven by pH, temperature and the presence of organic and inorganic complexes in the medium. The obligatory standardised laboratory studies are based on OECD guidelines and are conducted under the optimal conditions to raise the test organisms.
In the risk assessment of PPPs the standard test organism for fish is Oncorhynchus mykiss, for which the ideal water temperature is 13–17°C, whereas for most other fish the tests should be conducted at 20–25°C according to the OECD guidelines Nos. 203 (2019) and 210 (2013). For aquatic invertebrates mainly Daphnia magna is tested, according to OECD Nos. 202 (2004a) and 211 (2012). As sediment species Chironomus riparius (OECD Nos. 218 or 219; 2004b,c) or Lumbriculus sp. (OECD No. 225; 2007) should be tested. For aquatic plants algae (OECD No. 201; 2011) and Lemna sp. (OECD No. 221; 2006a) are tested. Additionally, Myriophyllum sp. (OECD No. 238; 2014) or Glyceria maxima should be tested for herbicides. The artificial sediment should consist of 4–5% peat, 20% kaolin clay and 75–76% quartz sand with a pH of 7.0–7.5. No information is given about the AVS content in sediment (but considered to be low in artificial sediments, see below). Although wider pH ranges are partially permitted, most aquatic toxicity studies are conducted between pH 7 and 8 (Table 2).
Table 2.
Overview of parameters in OECD guidelines
| OECD no. | Species | pH | Temperature (°C) | TOC | CaCO3 (mg/L) | Hardness |
|---|---|---|---|---|---|---|
| 203, 210 | Fish, e.g. Oncorhynchus mykiss | 6.0–8.5 | 20–25, resp. 13–17 | < 2 mg/L | ||
| 202, 211 | Aquatic invertebrates, e.g. Daphnia magna | 6.0–9.0 | 18–22 | < 2 mg/L | 140–250 | |
| 218, 219 | Chironomus riparius | 6.0–9.0 | 20 ± 2°C | |||
| 225 | Lumbriculus sp. | 6.0–9.0 | 20 ± 2°C | |||
| 201 | Algae | 7.5–8.0 | 21–24 ± 2°C | < 0.6 mg/L | ||
| 211 | Lemna sp. | 6.5/7.5 | 24 ± 2°C | |||
| 238 | Myriophyllum sp. | 6.0–9.0 | 23 ± 2°C | |||
| 239 | Myriophyllum sp. (with sediment) | 7.5–8.0 | 20 ± 2°C |
In OECD guidance No. 259 (OECD, 2016) practical guidance is given for the selection of reliable and relevant ecotoxicity data. It is imperative to consider the natural background and the physico‐chemical conditions of the test medium in comparison with the environment under investigation (pH, hardness, DOC). The essentiality of the metals needs to be accounted for in the culture medium to not affect the sensitivity of the test organisms. The addition of chelators (e.g. EDTA) for sparingly soluble metals should be avoided as they may decrease the bioavailability and therefore toxicity. OECD advises to conduct the tests with high‐purity soluble salts and allow for proper equilibration time after spiking to achieve a stable bioavailable fraction. This is especially of relevance for flow‐through systems, which are generally used to maintain a stable concentration. However, it has been observed that if the metal stock solution is mixed with the medium in the diluter head tank just 45 min before entering the test system, equilibrium conditions have not been reached and free Cu was elevated leading to higher toxicity than in static systems (Erickson et al., 1996; see Mebane et al., 2020). ‘Equilibration in a water‐only system will be reached within hours or days, while for sediments and soils full chemical equilibrium may only be obtained after several months or even years. Sediment spiked with Cu took 15 days to reach equilibration and 40 days for Zn (OECD No. 259, OECD, 2016). Furthermore, the addition of metals to the sediment may decrease the pH and increase the redox potential leading to the diffusional loss of metals from the sediment. It should be considered that if sediment tests are conducted in a static manner the spiked sediment may act as a source of metals to the overlying water (OECD No. 259, OECD, 2016). A semi‐static or flow‐through system is recommended to maintain the concentration in the water column, which needs to be measured.
The reliability criteria according to OECD No. 259 (OECD, 2016) states:
‘Considering the strong influence of water physico‐chemistry on metal toxicity, the physico‐chemical conditions (metal concentrations abiotic factors and biotic factors) should be adequately described and reported, especially if it is the intention to carry out corrections for bioavailability. The aquatic medium should be characterised by DOC concentration, hardness, pH, alkalinity and any other specific parameter of importance to the metal in question. With regards to metal concentrations, the current state of the science dictates that only test results where metal concentrations have been measured should be used. Absence of measured exposure concentration data is a clear reason to reject a data point. Measured data should reflect the dissolved fraction (< 0.45 μm) and/or total metal concentrations. Measurements of dissolved metal concentrations are critical to the assessment of sparingly soluble metals (particles and precipitation may occur) and in the use of natural waters as test media (adsorption to suspended solids may occur). A description of the filter methodology and its efficiency is therefore most relevant.’
Due to the essentiality of metals the dose–response curve may be U‐shaped rather than linear, as at low concentrations deficiency may be observed and in a range before toxic effects optimal growth occurs. ‘This phenomenon is often referred to as the hormesis effect. If non‐monotonic dose–response curves are observed the conventional log‐logistic dose–response model is less useful to fit the toxicity data and adaptations need to be made’. (OECD No. 259, OECD, 2016). To address this point a larger concentration range could be tested.
In order for the (simple) first tier to be sufficiently protective, the test set‐up needs to be worst case regarding the bioavailability, so that the total dissolved concentration does not overestimate the available fraction.
In the higher tier approach described in the aquatic guidance document (EFSA PPR Panel, 2013c), the geometric mean (Tier 2a), SSD (Tier 2b) or mesocosm studies (Tier 3) may be used. Whereas the former two are still based on standardised laboratory studies using different species of the relevant taxonomic groups, the later reflects a freshwater community under (semi‐)field conditions. In principle, if the comparability and physico‐chemical conditions of the studies are considered, the same approach is applicable for transition metals.
The time frame of the evaluation has an impact for repeatedly applied and non‐degradable, persistent metals regarding accumulation and acclimatisation. On the individual level, metals applied as PPP are not expected to significantly accumulate if the concentrations are within the levels that can be regulated by homoeostasis. At elevated ambient levels, shifts in biodiversity on a community level may occur. After a 12‐week exposure to 0.3 mg Cu/L changes in the distribution of algal classes from a community dominated by Cyanophyceae to one dominated by Chlorophyta and changes in the photosynthesis rate occurred. Significant increases in tolerance to copper and co‐tolerance to zinc, nickel and silver were found in communities previously exposed to ≥ 0.006 mg Cu/L (Soldo and Behra, 2000).
Therefore, as for other PPP, the interplay of different metals and the duration of the mesocosm studies are a challenge to also be addressed for transition metals.
Ecotoxicologically relevant exposure quantities
Surface waters
As described above, physico‐chemical parameters determine the metal speciation and therefore form the basis for the consideration of bioavailability in the risk assessment. The test parameters in the standardised studies do not cover the wide range of physico‐chemical parameters of natural EU surface waters (pH between 6.67 and 8.2; hardness between 27.8 and 260 mg/L CaCO3, DOC between 2.5 and 27.5 mg/L according to ECHA guidance document, ECHA, 2008) and may therefore not reflect the speciation and bioavailability in all natural systems. A low organic carbon content and water hardness leads to a higher bioavailability and therefore toxicity. As many aquatic toxicity tests are conducted in artificial waters (low DOC, low suspended solids) ECHA assumed in a step 1 derivation of a generic predicted no effect concentration (PNEC) that these studies tend to maximise bioavailability and, in those cases, total concentrations can be considered equal to dissolved concentration (ECHA, 2008, 2017a). The results from aquatic toxicity tests are usually expressed as total and dissolved measured concentrations, and the bioavailable free ionic metal concentration is not determined, rendering a comparison of endpoints from different studies difficult. For this reason, bioavailability models have been developed that incorporate chemical interactions between metal ions and dissolved organic ligands and inorganic ligands as well as interactions between metal and other cations (e.g. Ca2+, H3O+, Na+) and biological binding sites.
Metal uptake depends next to the abiotic factors discussed above also on biotic factors such as tolerance, size and life stages, species and nutrition related to the test organisms (Wang, 1987). In fish and invertebrates, the gill (waterborne exposure) and the gut tissue (dietary exposure) are commonly considered to be the primary target for metal uptake and/or toxicity (Paquin et al., 2002; see VRAR, 2008). In the acute studies, no feed is added and even in the chronic studies where feed is added, the feed is uncontaminated unlike in natural ecosystems. Although relevant for organic PPP as well, this is especially of concern for metals as they do not degrade, but may bioaccumulate over time depending on acclimatisation and homoeostasis, and may be remobilised in the gastrointestinal tract. The relevance of both exposure routes towards uptake and toxicity of cationic metals seems still unclear and may depend on the metal, exposure time and concentration (level and relative in both medium), diet type and composition as well as species and life stage. Several reviews are addressing the relevance of the dietary toxicity of metals (Clearwater et al., 2002; DeForest and Meyer, 2015).
Acute toxicity is linked to the binding of metals to the surface of fish gills and waterborne Cu caused considerably higher mortality than dietary Cu at a given influx rate, although the metal concentration in the intestine and liver were comparable for dietary and waterborne uptake with the same influx rate (Dang et al., 2012). Overall the gills and gill‐like surfaces seem to be the key target organs for acute as well as chronic copper toxicities in fish and invertebrates. For iron, due to the low solubility and hydrophilic nature the uptake via gills is limited and it has been suggested that the diet meets the daily iron requirements (Watanabe et al., 1997; see Bury et al., 2003).
As dietary studies have resulted in contradictory results, the toxicity of dietary metals to fish seems to be better expressed by referring to the dietary dose rather than to the concentration in the diet (Clearwater et al., 2002). This way the relative efficiency of Cu uptake from the water and diet is rather similar. Dietary studies are hampered due to the poor replication of contaminated feed in the laboratory; even contaminated live feed in the laboratory did not truly mimic the bioavailability of field collected metal‐contaminated feed (Clearwater et al., 2002), which shows a seasonal dynamic (DeForest and Meyer, 2015). Furthermore, dietary studies are non‐standardised and the feeding rate and time of metal accumulation to reach steady state needs to be better considered (DeForest and Meyer, 2015). Depending on the exposure route different organs are targeted, possibly resulting in different effects. In dietary Cu exposure studies (mainly with artificial feed), effects on survival and growth were investigated whereas effects on reproduction were not investigated in the reviewed studies (Clearwater et al., 2002). Negative effects on reproduction of C. dubia due to dietary exposure to Cu may have been caused by direct dietary toxicity or possibly indirect due to reduced feeding rate (DeForest and Meyer, 2015). Sublethal effects that might affect the fitness of wild fish populations such as negative effects on digestive enzymes, intestinal morphology or swimming performance due to the metabolic costs may be observed through dietary uptake (Clearwater et al., 2002).
Feeding had an important effect on waterborne metal uptake and both the feeding status of the fish and the relative metal exposure through water and food should therefore be considered in prediction of the metal bioaccumulation (Guo et al., 2016). Over time, uptake of waterborne Cu per unit body weight decreased with increasing fish size and was greater in fed fish than in starved fish (Kamunde and Wood, 2003).
In comparative toxicity studies of waterborne and dietary Cu exposures, the chronic toxicity (reproduction and growth) of D. magna was driven by the waterborne copper uptake and not by the dietary copper uptake (De Schamphelaere and Janssen, 2004 see DeForest and Meyer, 2015). However, the need for further dietary studies with different live diets for herbivorous invertebrates was highlighted (DeForest and Meyer, 2015). Dietborne Zn may selectively accumulate in reproductive tissue of D. magna and both exposure routes were considered relevant for the risk assessment, also as alterations of the algal diet may have been caused indirectly due to Zn exposure (Evens et al., 2012 see DeForest and Meyer, 2015).
The relevance of the different exposure routes also depends on the speciation and concentration as increased bioavailability of dietary Cu and Zn was detectable when the metal was both organically chelated and provided in very low daily doses (Clearwater et al., 2002). Contaminated feed may also be avoided by fish. At elevated Cu concentrations (282 μg Cu/g) in the diet, 99% of the accumulated Cu in fish was taken up by the diet, whereas at lower concentration (0.8 μg Cu/g), the waterborne uptake was more relevant (Kamunde et al., 2002).
The uptake of copper through the diet influences the uptake of waterborne copper, suggesting a homoeostatic interaction between the two routes of uptake (Kamunde et al., 2001, 2005; see VRAR, 2008). At normal copper exposure levels, dietary copper plays an important role in total copper uptake and turnover rates in rainbow trout, O. mykiss. Exposure to high dietary copper resulted in an induction in copper regulation mechanisms, whereas it had no effect on growth, condition factor or food consumption efficiency (Kamunde et al., 2001, 2005; see VRAR, 2008). Dietary copper induced copper regulation via the gut, bile and gills, but had no effect on growth, condition factor or food consumption efficiency. Excessive dietary Cu levels may be retained in the gut and excreted into the faeces (Clearwater et al., 2000; see Bury et al., 2003).
According to Clearwater et al. (2002), ‘Both dietborne and waterborne Cu or Zn exposure can contribute to metal uptake and toxicity’ in fish. DeForest and Meyer (2015) stated that ‘Ag, As, Cd, Cu, Ni and Zn have caused dietborne toxicity in laboratory exposures when the dietborne concentrations resulted from the exposure of the food to waterborne concentrations near toxicity thresholds’. Adding, although ‘in general, adverse effects to organisms simultaneously exposed to waterborne and dietborne metal were greater than when exposed to water or diet alone’, and ‘three studies indicating that waterborne Cu toxicity is more significant than dietborne‐Cu toxicity for three sensitive crustaceans suggest that waterborne Cu guidelines might be protective against dietborne‐Cu toxicity’. (DeForest and Meyer, 2015).
Sediment
For sediment organisms, metal toxicity depends on the concentration in sediment, pore water and overlaying water. Which compartment is most relevant depends on the organisms and type of metal as well as the geochemistry (AVS, TOC, as well as Fe‐ or Mn‐oxides) of the sediment (Méndez‐Fernández et al., 2014; Zhang et al., 2014). The comparability of toxicity endpoints is determined by the organic content and the pH of the sediment. Whereas total metals (Cu, Pb) in sediments were the best predictors of bioaccumulation in Chironomus riparius according to Roulier et al. (2008), suggesting that the ingestion of particles needs to be considered, Vink (2009) found that total concentrations of Cu, Zn, Pb, Cd and Ni in the sediment did not reflect the magnitude of total body concentrations. Concentrations of free metal ions in the overlying surface water proved to be the best predictor for uptake in oligochaete Limnodrilus spp. and the midge Chironomus riparius (Vink, 2009). For Tubifex tubifex, a benthic infaunal species that feeds on the fine‐sediment fraction (< 63 μm), sediment total metal concentration, and for some metals pore water (Cu, Ni and Zn), was found to be a better predictor for tissue residues (Méndez‐Fernández et al., 2014). Simulations of different conditions showed that both dissolved and sediment‐associated metal can be important sources of metal exposure for the worms and that the relative importance strongly depends on the metal and exposure conditions including the lability of the metals in the sediment phase (Redeker et al., 2004) and the feeding behaviour and ecology (De Jonge et al., 2010).
Benthic organisms, such as deposit or detritus feeders, live in the sediment surface and are mainly exposed via particle sediment. Sediment‐based food chains might be an important pathway for diet borne‐metal exposure of benthic organisms (Luoma and Rainbow, 2008; see DeForest and Meyer, 2015). Bivalves filter particles from the water–sediment interface and can keep the particles for a long time in their digestive tracts (high uptake and low efflux rates). The efficiency of the metal assimilation from different sediment phases therefore depends on the physiology of the organisms (e.g. residence time and chemistry of the gut) and is independent of AVS (De Jonge et al., 2010). Metal toxicity in benthic feeders has been attributed to the dietary uptake of (resuspended) sediment‐bound metal and to a lesser extent to metals in the pore water (Zhang et al., 2014). However, selective feeding due to different food quality of the particles may affect ingestion rate and contribute to the preferential uptake of (metal‐enriched) algae (Lee et al., 2016). Metals associated with sediments had lower bioavailabilities than metals bound with diatoms (Ke and Wang, 2019). Although AVS bind metals and affect the bioavailability, metals bound to AVS have also been found to be bioavailable to deposit feeders (Lee et al., 2000), but bioavailability of sediment‐bound metals was higher from oxic sediments (Chong and Wang, 2000).
Also planktonic organisms, such as Daphnia magna, may be exposed to sediment‐bound metals. Metals may be released from the sediment into the pore water and then diffuse in the overlying waters or resuspended sediments may be ingested by filter feeders although the preferred food are algae. D. magna may also browse on the sediment surface if food is scarce. Once ingested, metals may be desorbed in the digestive tract. Different toxicity of sediment‐bound metals to filter feeders was observed in different studies, possibly depending on the desorption behaviour of different metals (Weltens et al., 2000; Gillis et al., 2006).
The assessment of different exposures, dissolved vs. particulate, is confounded by the apparent uncoupling of bioaccumulation and metal toxicity.
The toxicological effects due the accumulation of a metal depends on the detoxification mechanisms present in a species. In general, when detoxification systems are in place toxicity does not depend on total accumulated metal concentration but is related to a threshold concentration of internal metabolically available metal (Rainbow, 2007). Regarding accumulation for sediment dwellers, such as chironomids, the time to reach the internal plateau is metal dependent and may take 2–3 days for Zn, but 7 days for Cu (Roulier et al., 2008).
The relationship between sediment geochemistry and metal accumulation by aquatic invertebrates varies between taxonomic groups and is highly dependent on feeding behaviour and ecology (De Jonge et al., 2010). Therefore, according to Méndez‐Fernández et al. (2014) sound ecological risk assessment of metals requires the study of various organisms with different positions in the aquatic trophic web and with different metal exposure routes. In OECD No. 218 guideline (OECD, 2004b) with Chironomus sp., an artificial sediment with low concentrations of metals is used, potentially underestimating the added effect value. However, using artificial sediments under controlled laboratory conditions probably does reflect a worst‐case scenario, as these conditions tend to promote the bioavailability of copper (Sahlin and Ågerstrand, 2018).
As sediments function as a sink for transition metals, this compartment is of special concern for the aquatic risk assessment. The accumulation of the non‐degradable transition metals needs to be adequately addressed by expanding the number of surrogate sediment test species. To cover all trophic levels and to achieve a more reliable risk assessment, the following sediment species should be tested for the higher tier assessment: e.g. Hyalella azteca, Gammarus pulex, Tubifex, Lumbriculus variegatus, Chironomus riparius, Hexagenia sp., Ephoron virgo, mollusc and benthic microalgae.
Endpoints derived from higher tier studies
In a Tier 2 approach, depending on the number of available species tested, the data can be pooled in an SSD to derive a RAC by applying an assessment factor. If more than one comparable study is available for one species, the geometric mean of the endpoints is usually calculated and the resulting endpoint for that species is then used in the SSD. However, for metal toxicity the test media may have a large influence on the result; therefore the lowest endpoint per species should be used (ECHA, 2008) if the data cannot be normalised (e.g. by BLM).
As highlighted in the aquatic guidance document (EFSA PPR Panel, 2013c), SSD should be based on studies performed under comparable conditions, i.e. delivering similar effect endpoints (e.g. reproduction, growth, etc.), covering sensitive life stages and conducted under appropriate exposure duration.
In the tiered approach, the assessment factors used to derive RACs usually decrease, suggesting that the uncertainty decreases. However, the specificity of the higher tier studies such as mesocosm studies increases as they are site specific and cannot be considered as representative for all situations. The assessment factors suggested in the aquatic guidance document (EFSA PPR Panel, 2013c) address the spatio‐temporal extrapolation from the model ecosystem to the field ecosystem. However, for metals the bioavailability may vary 100‐fold (Adams et al., 2020) and the intraspecies sensitivity > 30‐fold (Schlekat et al., 2020) due to different naturally occurring water chemistry parameters in surface waters. Lentic mesocosm studies may have a lower DOC content than lotic systems, which affects the bioavailability and therefore the toxic effects and the endpoint delivered. Mesocosm studies are considered useful though to investigate direct and long‐term exposure and indirect effects by mimicking the repeated fresh addition of metals on a whole assemblage of species representing a specific field community. Due to the wide and repeated use of copper, exposure of surface waters is expected to be extended, therefore recovery observed in a mesocosm study may not be representative for natural waters since these concerns about time and space add to the concerns already highlighted in the aquatic guidance document (EFSA PPR Panel, 2013c) and in the report of EFSA on general recurring issues (EFSA, 2019) about the ecological recovery option (ERO) (e.g. relevance of ERO‐RAC in mesocosms depends on the representativity of the community in terms of traits compared with the field). Therefore, using the no observed effect concentration (NOEC) as the endpoint and the ecological threshold option for deriving a RAC is most appropriate.
The protectiveness of the risk assessment based on a Tier 2 SSD approach may be assessed by comparing RAC from SSD with RACs from Tier 3 mesocosm study. This calibration of the tiered approach can be made if the laboratory studies used to derive endpoints for the SSD are normalised to the physico‐chemistry of the mesocosm study.
Conclusion for the aquatic hazard assessment
The hazard assessment can be based on the studies listed in the data requirement Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b) if details about water characteristic and equilibration of the test system are given.
Whereas flow‐through test systems are generally considered to provide a constant exposure, for metals equilibrium may not have been reached depending on the residence time in the diluter head tank. This is of relevance when linking exposure and effect concentration.
A test system that is not in equilibrium is considered worst case, as the free metal ion concentration is elevated causing higher toxicity than in equilibrium condition.
Depending on the renewal period and the equilibration time in the test solution, also semi‐static test systems are not expected to be in equilibrium.
Depending on the type of metal only the waterborne uptake via the gills is relevant for the toxicity (e.g. Cu), whereas for other metals also the dietary uptake needs to be considered (e.g. Zn).
The ecotoxicological endpoint of the effect assessment should be expressed in the total (sum of all species) and dissolved (< 0.45 μm) concentration.
Surrogate test species need to cover all relevant exposure routes and trophic levels for accumulating transition metals.
It needs to be made clear to what the RAC refers (worst case, regional, specific condition). The conditions covered by the specific set‐up of the mesocosm study need to be stated as the toxicity of transition metals is determined by a set of parameters resulting in a greater variability; this extent of variability is most likely to be not covered by the standard assessment factors for organic PPP.
3.1.2. Exposure assessment, fate and behaviour of transition metals in surface waters and sediment
As part of the Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b), the exposure calculations for the active substance and its relevant transformation products are required for aquatic compartments (surface water and sediment).
FOCUS approach
To address this issue, the models of the FOCUS package (FOCUS STEP 1–415) are used according to a stepwise approach proposed in the FOCUS surface water guidance document (2001, 2015). Further refinements are proposed (e.g. development of specific scenarios for FOCUS models or other, monitoring study).
Soil properties including soil pH and clay content are indeed defined for each FOCUS surface water scenario (refer to annexes C and D of FOCUS, 2001). These properties are not given for the water and sediment compartments. Therefore, it should be kept in mind that with the exception of the organic matter content and soil texture (soil structure), soil properties have not been taken into account in the scenario selection procedure of the FOCUS surface water scenarios (FOCUS, 2001). Water and sediment properties have been completely ignored. Therefore, properties such as pH in soil, water or sediment are meaningless with respect to the vulnerability of the current FOCUS surface water scenarios (EFSA, 2020). Therefore, for surface water scenario selection development relevant for transition metals soil, sediment and water properties like pH should be taken into account. As outlined in EFSA (2020), the panel recommends developing new scenarios that can address substances whose fate and behaviour depend on other soil properties than texture and organic matter content.
At the first tier, the FOCUS STEP 1–2 tool that is a user‐friendly application based on conservative and simple assumptions, is used for deriving predictive environmental concentration in water and sediment (PECsw and PECsediment). Although it was developed for organic compounds, this tool has been also considered as useful in the framework of the risk assessment of some inorganic compounds.
For the active substance iron sulfate (EFSA, 2012a), exposure calculations for the water compartment are needed for iron(III) ion, produced from the dissociation in water of the active substance iron sulfate. This tool has been considered as applicable for estimating predictable environmental concentration of iron(III) ion due to the high solubility in water of iron sulfate. In the absence of reliable data, a Kdoc value of 0 mL/g OC was considered for iron to maximise the exposure of non‐target organisms in the water column and for estimating maximum concentration in water column. No estimation of the concentration in sediment was needed for the risk assessment (no Kdoc value for sediment compartment needed).
In contrast, no exposure calculation for iron(III) ion is required due to the very low water solubility of the active substance ferric phosphate (EFSA, 2015). This is in line with the recommendations of the ECHA guidance document. For the metal compound that will be only poorly soluble and sufficiently stable to not rapidly transform into a water‐soluble form, the ECHA guidance document on metals (ECHA, 2008, 2018a) indicates that
‘the substance itself should be assessed taking into account its specific partitioning characteristics. For the aquatic environment, it can be assumed as a first estimate that the substance will dissolve up to its water solubility limit, and that this fraction will be the bioavailable form’.
For ionisable inorganic compounds already reviewed at the EU level (e.g. disodium phosphonates (EFSA, 2013a), potassium phosphonates (EFSA, 2012b)), it was recommended to perform the risk assessment considering two sets of simulations using a Koc value of 0 L/kg to maximise the concentration in the water column and a Kdoc of 10,000 L/kg OC to maximise the concentration in the sediment. As another example, it was agreed in EFSA (2018) to derive a pseudo‐Kdoc from the effective Kd normalised to the organic carbon content of 5% defined in the FOCUS scenario for estimating the FOCUS STEP 1–2 PEC concentration in both the water column and in the sediment.
For copper, the FOCUS STEP 1–2 tool may be used with relevant modelling endpoints for deriving total concentration in surface water and sediment compartments.
However, as indicated above, the toxicity of transition metals can be time dependent due to the equilibrium process of transition metals in the aquatic systems. From the assumptions in FOCUS STEP 1–2 tool, it could be therefore considered as a conservative approach to derive the maximum PEC in the water column due to the drift with FOCUS STEP 1–2 tool, as no equilibrium is reached in the sediment. At step 2, the maximum PEC in the water column cannot be considered as conservative enough as the entries via run‐off and drainage occur several days after application and partitioning between water column and sediment based on K doc is considered. For the sediment, the maximum FOCUS STEP 1–2 PECsediment is underestimated as FOCUS STEP 1–2 assumes an equilibrium of transition metals between water column and sediment based on a K doc value.
As the adsorption of transition metals is not controlled only by organic matter, but also by other solid‐phase constituents like clay minerals and oxides, a Koc value as input parameter is not appropriate. Therefore, the distribution of metals between the aqueous phase and soil/sediment/suspended matter should be described based on measured soil/water, sediment/water and suspended matter/water equilibrium distribution coefficients. These Kd values for transition metal are not true adsorption constants and can vary as a function of the metal loading and of environmental characteristics such as pH, ionic strength, redox conditions or the composition of the liquid phase (i.e. DOC and concentration other complexing ions) and solid phase (organic matter, clay, oxides, sulfides (only for sediment)). Therefore, Kd values may differ from site to site and can change over time. This explains the wide range in Kd values observed for metals (ECHA, 2008).
The relative importance of all these factors varies from metal to metal and depends on environmental conditions. However, pH is generally regarded as the most important factor in soils and in aquatic systems. The choice of the Kd values has important consequences for the outcome of the exposure assessment. Small Kd values will predict a larger PEC and higher risk in water, and large Kd values will lead to a large PEC in sediments. As a Kd value cannot be used directly in FOCUS STEP 1–2, the equation should be adapted or Kd value should be recalculated with OC% (5% in sediment).
In some cases, default values for Kd (10,000 for sediment and 0 for the water column) have been used for some inorganic compounds in FOCUSsw STEP 1 and 2 calculation. The WG considers that this approach might not always be appropriate for transition metal compounds and ions. At STEP 1, all entries of contamination to waterbodies occurs at the same day, but only run‐off/drainage (corrected for run‐off) is considered for partitioning between water and sediment. Therefore, not all entries of contamination contribute to the transfer to the sediment. At STEP 1, the use of a default Kd value of 0 is, however, a conservative approach for the water column, but the use of a Kd value of 10,000 is not conservative for the sediment compartment. At STEP 2, all entries of contamination to waterbodies do not occur at the same day and partitioning between water and sediment is considered separately for each entry. Therefore, a default Kd of 0 for the water column is only appropriate to calculate PECsw for drift for one single application. A distribution coefficient between ‘available’ and ‘unavailable’ fractions derived for organic compounds is applied for each application. A default Kd value of 10,000 is not seen as a conservative approach for transition metals for estimating the concentration in sediment. Considering the above, the use of default Kd values is not considered appropriated in the exposure assessment for all transition metals, but measured data should be made available.
The establishing of the modelling endpoints should be considered with caution. Indeed, the dissipation of transition metals, e.g. copper compounds in modelling, must be taken into account with caution and the input parameters should be selected in a way that avoid double counting of simultaneous processes (e.g. DissT50 and Kd/Kdoc).
Adaptation of the FOCUS STEP 1–2 tool is also needed to take the long‐term accumulation in sediment from repeated uses over the years into account. In the absence of other models already agreed in the context of PPPs, the accumulation in sediment for total copper over a period of 10 years via spray drift and run‐off/drainage considering the FOCUS STEP 1–2 tool with a Kdoc worst‐case default value of 10,000 L/kg, and adding at the end a value of 17 mg/kg (median) as background level of copper in sediment were considered for the EU assessment (EFSA, 2017). The choice of the median was determined by the poor reporting. However, the relevance of the median concentrations of copper in European stream sediment is questioned regarding the potential accumulation of copper in sediment (data gap on this issue identified in the EFSA conclusion on copper compounds; EFSA, 2017). Information on the potential accumulation in sediment are still needed in the absence of sufficient data in the literature. In addition, the concentration can be extremely variable in space and in time. Please also refer to Part 4 on Criteria for performing and evaluating environmental monitoring studies.
As for soil compartment, the background level in sediment should be implemented before the application of copper as PPPs in modelling. A realistic background concentration in sediment should be selected following the recommendations as proposed for the soil compartment. Background levels can be derived from available monitoring/surveying data. Please also refer to the General Framework under Part 2.3.2 and to chapter 4 on Criteria for performing and evaluating environmental monitoring studies. Monitoring/surveying data should be representative in terms of spatial distribution and characteristics for soils across the European Member States. Natural and anthropogenic realistic worst‐case residue levels should be included in the exposure assessment, distinction between different land uses might be implemented if information is available.
Furthermore, time affecting the soil availability of transition metals (ageing process), the freshly added sediment and the aged sediment residues (previous transition metals application and natural concentration) should not be considered in the same way in modelling.
In addition, as described in the Scientific Opinion on the assessment of the sediment organism (EFSA, 2015), two types of sediment exposure scenarios are recommended, a worst‐case scenario for the pore water and one for the total sediment content. The selection of the worst‐case conditions should be related to the properties of the assessed compounds (e.g. low pH to maximise the concentration of transition metal in the pore water).
Therefore, adaptation of the existing agreed EU models or development of new models and the corresponding scenarios could be needed to take into account the abiotic processes (e.g. speciation, bioavailability, ageing process) and environmental factors affecting them.
The higher tier calculations using FOCUS SWASH model, which includes PRZM and MACRO submodels cannot be used to refine the exposure assessment. Indeed, some factors affecting the speciation of transition metals in soil, sediment and water column are not taken into account in the PEC calculations (e.g. pH, organic carbon, cation exchange capacity, clay content in soil compartment; acid‐volatile sulfate, organic carbon, pH, oxidation potential (Eh) in sediment compartment; major ions (Ca, Mg, Na, K,…), pH, alkalinity and DOC in water column as proposed in the ECHA guidance document). In addition, the mobility of compounds in the soil profiles as implemented in the FOCUS scenarios is only due to the K foc value. In STEP 3, an instantaneous equilibrium with the sediment is assumed based on a Koc value, which is not considered sufficiently worst case for transition metals. New parameterisations are seen needed to take factors affecting speciation/bioavailability of metals in aquatic systems into account. Scenario definition should be updated accordingly. In addition, not only instantaneous equilibrium with the sediment should be considered to derive realistic worst‐case concentration in aquatic systems at STEP 3 (please also refer to the General Framework under Part 2.3.2 and to chapter 4 on Criteria for performing and evaluating environmental monitoring studies). Should specific scenarios be developed, the selected parameters should be representative of the parameters measured across European countries.
An issue on the use of Federal Research Centre for Agriculture and Forestry (BBA) drift values table (Rautmann, 2000) in exposure calculation for dustable powder product is identified. Please refer to Part 3.3.2 for more details.
The ecotoxicological relevant concentration (i.e. the free metal concentration) should be estimated and be used to conduct the risk assessment. The ECHA guidance (ECHA, 2008) proposes the use of speciation model when relevant physico‐chemical parameters are available. Different speciation models are available, and an option could be to link a speciation model to the FOCUS surface water models. It should be demonstrated that the selected speciation model is sufficiently robust and verified according the PPR Scientific Opinion on good modelling (EFSA PPR Panel, 2014a) before used for assessment of a transition metal used as a pesticide.
Intermediate dynamic model for metals (IDMM)
The IDMM tool has been addressed in this statement as it had been proposed (but not accepted) for the assessment of copper compounds used as pesticides and as feed additives. For this reason, the IDMM tool is described as an example regarding the issues that need to be considered before using a model designed for assessing metal exposure to surface water and sediments.
For the time being, this model was not considered suitable to derive reliable PEC for water column and sediment compartments by the experts during the peer review of the active substance copper. The soil compartment is implemented in the model to assess processes resulting in the exposure of water systems, but does not deliver soil exposure concentrations. A part of this statement, the experts would not recommend the use of the IDMM tool for the risk assessment purpose of transition metals used as PPPs. Some major deviations in the IDMM assumptions have been identified (see below).
Information on the IDMM tool is presented later in Appendix B.
Table 3 briefly summarised both approaches (FOCUS model versus IDMM tool).
Table 3.
Comparison of FOCUS approach and IDMM approach
| FOCUS approach | IDMM approach | |
|---|---|---|
| Models | FOCUS STEP 1.2 version 3.2 | IDMM model; version proposed in the context of the renewal approval of copper (EFSA, 2017) |
| Goal | Estimation of max. conc. in surface water and sediment | Long‐term accumulation in sediment |
| Item | Total concentration | Total concentration, dissolved concentration, free metal, complexed metal, labile metal, adsorbed labile metal, aged adsorbed metals |
| Entries routes to water bodies | Drift, run‐off/drainage/erosion |
Run‐off, drainage Drift not considered |
| Entries to soil | PPP application | Natural (geogenic) atmospheric deposition and anthropogenic atmospheric deposition, mineral fertiliser additions, PPP application |
| Loss of transition metals from soil compartment | Run‐off/drainage (transfers to surface water) |
Run‐off/drainage (transfers to surface water) Crops: copper removed from soil by uptake into harvestable parts of crops (annual time step) Leaching to soil deeper |
| Soil background concentration | Not considered | Estimated by model |
| Sediment background concentration | Manually implemented | Estimated by model |
| Modelling period | One year | Several years |
| Soil properties | – | FOCUS surface water (SW) scenarios, except for some parameters (pore water pH, dissolved organic carbon and major ion concentration, % organic matter content, water saturation) |
| Surface water definitions |
Static waterbodies water depth (cm): 30 Sediment depth(cm): 5 Effective sediment depth (cm): 1 Sediment OC (%): 5 Sediment bulk density (kg/L): 0.8 Surface water divided into two compartments: fractions ‘available’/‘unavailable’ for sorption |
Ditch and stream (pond not considered) FOCUS SW scenarios (scenarios D and R) Drainage‐based scenario: 2 soil layers (0–30 cm and 30 cm‐drain) Run‐off based scenario: 3 soil layers (0–5 cm, 5–30 cm, 30‐base of the soil profile); only the two‐first layers considered for run‐off FOCUS SW scenarios. Specific surface water characteristics were developed (pH, DOC, major cations) and AVS for sediment compartment |
| % run‐off/drainage | Default value (2–10%) | Annual fluxes of water considered from each soil layer to surface water or layer below |
| Drift value | Rautmann values (BBA) | – |
| DT50 in soil, surface water and sediment |
– Default value |
– Default value |
| Partitioning between water/sediment |
Based on Koc value 1/n not needed |
Speciation (WHAM model/Model VI) Based on Kd value Daily process |
| Partitioning between soil/pore water | – |
Speciation (WHAM model/Model VI) and ageing Daily process |
| Plant uptake factor | – | Once a year |
| Crop interception | 0% | – |
| Processes considered in soil compartment |
Degradation Daily process |
Degradation Speciation (WHAM model/Model VI) and ageing Soil erosion Daily process |
| Processes considered in sediment compartment |
Degradation Daily process |
Degradation Speciation (WHAM model/Model VI) Sediment erosion |
| Mitigation measures | – | Landscape and mitigation (2007) developed for organic compounds |
Whatever the model developed for assessment of transition metals used as pesticides, the following specific information need to be provided:
Different sources of contamination implemented in the model (natural and anthropogenic entries).
Loss in soil by plant uptake. Plant uptake factor not only considered at the harvest of the crop. Detailed information on the origin of the copper content in plants (uptake from soils, atmospheric deposition on plants and/or absorption following foliar applications of copper fungicide on crops). These values could be not conservative enough for all intended uses (e.g. arable crops). Consideration on the impact the dynamic of metal pool in soil over the time (transfer from soils to plants is not a dynamic process in IDMM tool as for the plant uptake factor via roots implemented in FOCUS models).
Different types of water bodies (pond, ditch and stream).
The representativeness of the selected parameters in relation to the characteristics of the European soils.
Selection of representative abiotic factors (surface water and sediment scenarios) to take into account the speciation/bioavailability of transition metals and to estimate the long‐term accumulation of copper in sediment.
Time frame in aquatic systems (sediment and water column) of dynamic processes (equilibrium, non‐equilibrium conditions).
The information on the selection of kinetic constants to determine aged residues in soil and sediment.
Outcome of the model has to be compared with measured data in similar conditions.
Use of the mitigation measures proposed in the landscape and mitigation for transition metals.
Time frames of applications (freshly applied several times every year).
The recommendations of the EFSA PPR Panel Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed to ease the assessment of models and the parameters implemented within the model.
Conclusions and recommendations for exposure assessment, fate and behaviour of transition metals in surface waters and sediment
The current risk assessment according to EFSA performed in terms of total transition metals is deemed conservative as the transition metals are considered dissolved and fully bioavailable. The assumed conservativeness of the current risk assessment performed based on total transition metals needs to be critically assessed in relation to their characteristics (substance not metabolised) and various uncertainties (e.g. variable bioavailability according to environmental conditions (definition of ‘realistic worst‐case’ scenarios), potential remobilisation of metal bound to particulates once ingested, very steep dose–response relationship between exposure concentrations and effects on populations).
The FOCUS scenarios STEP 1–2 assume that no equilibrium condition is reached with the sediment and therefore allow the worst‐case assessment of the concentration in the water phase due to the drift input only, but not for the sediment. For the other entries (run‐off and drainage/erosion), the concentration in the water and sediment are not conservative since equilibrium conditions between column and sediment is implemented in the tool. Current FOCUS STEP 1–2 tool can be used to conduct a worst‐case assessment of the concentration in the water phase due to the drift. Given the assumptions in the tool (e.g. equilibrium process based on a K doc value), the other PEC values should not be considered for the risk assessment of the non‐target aquatic organisms.
The sorption parameters should be derived in an appropriate way.
Due to the persistency of transition metals in the environmental compartments (soil and sediment), the accumulation of transition metals in sediment has to be addressed.
For sediment risk assessment, two worst‐case scenarios should be considered, one for the pore water and one for the total sediment concentration according to the EFSA Scientific Opinion (EFSA PPR Panel, 2015b). The properties of the worst‐case sediment scenarios should be derived to maximise the concentration of transition metals in each fraction.
A background level in sediment at least should be included in the exposure calculations to reflect the persistency of transition metals in the environmental compartments.
To produce more realistic but less conservative exposure concentrations for transition metals, there is a possibility to consider the speciation and the availability of transition metals in the environment and factors affecting it should be taken into account.
All relevant information justifying the selected physico‐chemical parameters that affect the processes mentioned above should be provided.
The current version of IDMM tool is not seen as applicable due to several uncertainties identified in the model assumptions (e.g. all route of contamination not considered,…).
For the time being, none of the currently used FOCUS models can simulate the fate of metals in an appropriate way. Adaptation of these models and existing scenarios or development of new models would be required to describe the fate of metals. Assessment of surface water models for transition metals and whether they are sufficiently robust and reliable for use in a regulatory context is a complex task and would need to be dealt with under a separate mandate and submitted into a version control group before being used in risk assessment.
The recommendations of the EFSA PPR Panel Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed to ease the assessment of models and the parameters implemented within the model.
3.1.3. Linking exposure and effects
The exposure scenarios are intended to predict realistic worst‐case exposure concentrations in edge‐of‐field surface waters. In the first Tier, the risk assessment is based on the highest dissolved concentration over time, PECsw;max and compared with RACtotal dissolved for the water phase. For sediment organisms the highest total concentration over time considering accumulation should be used. In the higher tier, the risk assessment can be based on the highest bioavailable concentration over time and compared with the refined RAC normalised for bioavailability.
Bioavailability of metal compounds in the water phase
Given that observed toxicity is not related to the total metal concentration the bioavailable fraction has been equated to the dissolved fraction, which is the approach currently applied in the peer review of copper compounds (EFSA, 2018). However, the fraction causing toxicity correlates generally speaking with the free metal ion, which depends on the water chemistry. Physico‐chemical parameters such as DOC, pH and hardness can modify the toxicity of metals by several orders of magnitude (Rüdel et al., 2015). Therefore, simplified approaches have been developed, which assess the bioavailability by considering organic matter or water hardness. However, hardness‐dependent metal toxicity varies under different conditions of pH and alkalinity (Paquin et al., 2002).
Transition metals used as PPP are mainly inorganic complexes. Therefore, as the PPP is mixed in the water tank and then enters the environment via spray drift, the speciation will change. The initially bioavailable free metal ion is expected to decrease due to complexation with DOC leading to a decrease in toxicity, which may occur over extended time periods due to slow ligand exchange rates. Binding to organic or inorganic particles may lead to the precipitation of metals and a shift from the dissolved to the particulate phase. Furthermore, biota can influence the metal chemistry by surface reaction, uptake, synthesis of intracellular chelator and secretion of low molar mass exudates, as well as production of extracellular polymeric substances with complexing properties by reducing the bioavailability during the day (Tercier‐Waeber et al., 2012). The timescale of these various processes determines the relevant concentration for ERA and needs to be matched with the dynamics in the effect study.
Expanding our knowledge in the aquatic chemistry of metals, the physiology of aquatic organisms and aquatic toxicology, led to the understanding that the bioavailability also depends on the interaction of the dissolved metal ions with the binding sites (i.e. proteins or organelles) in the organisms and on the competition with other cations or ligands (Paquin et al., 2002). This complexity has been addressed by the development of BLM or alternatives such as the so‐called generalised bioavailability model (gBAM; e.g. Nys et al., 2020).The toxic effect depends on the metal speciation under different environmental conditions (spatial and temporal) and on the aquatic organism. BLMs have been developed to predict acute and chronic toxicity to algae, invertebrates and fish for a range of physico‐chemical water parameters. Outside these verified boundaries, e.g. waters with extreme hardness (Ca < 5 mg/L) or acidity (pH < 6.5), the bioavailability may be underestimated. The effect of different freshwater chemistry (pH 5.5–8.3, DOC: 1.6–18.2 mg/L and water hardness: 8–225 mg CaCO3/L) may affect the bioavailability by ~ 4, 2 and 2 orders of magnitude for Cu, Ni and Zn (Gandhi et al., 2010). The models assume that the biotic ligands and metal speciation are in equilibrium and that only the waterborne exposure is relevant for the toxicity. For copper it is assumed that the gills of fish and invertebrates and cell surfaces of algae are the key target organs for acute as well as chronic copper toxicities, which justifies the use of BLM for the assessment of equilibrated conditions by ECHA. The metal uptake in the BLMs is assumed to be slow in comparison with the rate of binding to the biotic ligand (Bjerregaard et al., 2015). Therefore, the equilibrium constants for the metal‐target site interaction need to be known for each species (Slaveykova and Wilkinson, 2005) as well as the level of accumulation which causes the toxic response. A description of the models and possible validation procedure can be found in the ECHA guidance (ECHA, 2008). Furthermore, the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed for model validation. In the last years these BLM models have been considered suitable for the prospective risk assessment of the pelagic community under REACH and in the retrospective risk assessment under the WFD for the site‐specific evaluation of water quality. The drawback of the BLMs is that most of them are proprietary software and require expert knowledge (Rüdel et al., 2015). Furthermore, a large set of parameters are required (about 10 parameters) to calculate the bioavailable fraction. Therefore, more user‐friendly BLM‐based models have been developed (mainly for Cu, Ni, Zn), which only require pH, DOC, hardness and dissolved metal concentration as minimum data input (Rüdel et al., 2015). As a further simplification multiple linear regression (MLR) models have been developed, which are based on statistical relationships between toxicity endpoints (e.g. growth impairment, reproduction) and water chemistry parameters (e.g. hardness, DOC, pH) and therefore do not require the extensive water chemistry data (Brix et al., 2017; Schlekat et al., 2020).
According to Rüdel et al. (2015) there is still potential for further refinements of the BLMs to:
‘improve characterisation of the relationship between bioaccumulation and toxicity and better coverage of the toxicodynamic processes, as well as improved modelling of metal complexation to natural organic matter and an optimised derivation of binding constants of the covered metals to the biotic ligands’.
Especially at low metal concentrations in surface water the rate of uptake may not be slower than the binding to gill tissue and equilibrium for metals with slow reaction kinetics may not be reached (Bjerregaard et al., 2015). Toxicokinetic processes such as uptake, distribution, storage and elimination of metals in single organisms need to be further investigated (Bjerregaard et al., 2015). Furthermore, currently not considered is the influence of temperature, Fe, Al and nutrients on the model outcome (Schlekat et al., 2020) as well as the influence of colloid–metal complexes on the bioavailability (Tercier‐Waeber et al., 2012).
The use of BLMs in the risk assessment is linked to uncertainties, which would need to be considered in the choice of assessment factors, such as: restricted boundaries of water characteristics, extrapolation to other species and extent of model validation. BLMs adequately address toxic effects in certain aquatic organisms. Changes in community structures, species richness and diversity are outside the area of BLMs. Furthermore, as the basic assumption of BLMs is equilibrium, pulse exposures from drift or run‐off cannot be adequately addressed (Mebane et al., 2020).
The ECHA guidance (ECHA, 2008) gives explanation what needs to be considered in developing the speciation and competition module and how the BLMs can be validated. It is recommended that organism‐specific bioavailability models should be used as much as possible. Depending on the number of BLMs available, two options are available to correct for bioavailability:
Baseline bioavailability correction limited to those species (algae, invertebrates and fish) for which an actual chronic BLM has been developed. The effect concentration (e.g. EC10, NOEC) for algae, invertebrates and fish can be normalised for particular water‐quality conditions.
Full bioavailability correction in cases where there is justification for using the originally developed chronic BLM for those species within the same trophic level for which no specific bioavailability model has been developed (e.g. insects, amphibians, molluscs). Based on e.g. a similar mode of action or similarity of species, existing BLMs may be used to read across to other species by conducting specifically designed toxicity tests for selected organisms and carefully considering the uncertainties in drawing conclusions.
To conclude, the use of BLMs to consider the bioavailability of metals in a refined risk assessment requires that:
The model is freely available and well documented to check the validity of the model.
The model has been developed for the species of concern or can be extrapolated to another species if a similar mechanism of action between species can be assumed (ECHA, 2008).
-
The underlying assumptions cover the areas of concern, i.e.
-
–
as the underlying assumption of the model assumes equilibrium, the model is not suitable to address short‐term exposure via drift or run‐off for those scenario's when a significant binding to ligands is considered, or
-
–
as the underlying assumption is that only the waterborne fraction is relevant for the toxicity, the model is not suitable to address dietary exposure for those species or metals where it might be considered relevant.
-
–
The aquatic systems to be assessed show water characteristics within the validated boundaries.
The uncertainties of the model have been identified and can be considered in the assessment factor.
The water‐quality characteristics, which are required as parameters for the model, have been documented in the bioassay report.
The speciation module of the BLM corresponds to the exposure model for the PEC calculation.
Under these circumstances BLMs can be used to identify areas where the risk addressed by the model can be considered acceptable or not.
Bioavailability of metal compounds in the sediment
As mentioned in the ECHA guidance, metal availability in sediments is governed by various ligands/processes (e.g. organic carbon, sulfides, iron and manganese oxy‐hydroxide and redox potential), and the relative importance of these binding phases may differ depending on the metals binding capacity and general behaviour. Various approaches can be used to take (bio)availability into account.
The use of partitioning to Fe‐Mn (oxy)hydroxides, speciation calculations (reduced forms under anoxic conditions) and organic carbon normalisation can be used if evidence is at hand that these factors do mitigate metal toxicity. For those metals/metal compounds that are susceptible to binding with sulfides or with organic carbon, the use of the SEM‐AVS and/or organic carbon normalisation could be appropriate. The ECHA guidance gives further explanation and examples how these factors could be used to refine the effect assessment for sediment‐dwelling organisms. However, it needs to be considered that the relationship of SEM‐AVS may not be appropriate for benthic organisms living in oxygenated burrows (Fairbrother et al., 2007). If no data are available, toxicity has to be tested in a reasonable worst‐case scenario, i.e. a sediment with high bioavailability of the metal substance tested. This ensures that results are protective for most sediments:
‘For metals that have a high affinity to bind with organic carbon, it is recommended to explore whether a linear relationship can be established between the observed toxicity levels and the presence of organic carbon. If so, the toxicity value could be normalised to a standard condition. In the EU, a standard sediment has a default OC value of 5%. In a similar way, the normalisation could be performed with other sediment ligands such as Fe/Mn oxy hydroxides when it can be shown that a relationship exists between the observed toxicity and the ligand’ (ECHA, 2008).
The toxicity of metals in the sediment depends on the pH, AVS and redox status of the sediment, which may change with depth and time. Depending on the relevant exposure route of sediment dwellers (pore water or sediment), the relevant concentration for ERA may differ. Although metals may be released from sediments over time via diagenetic or biological processes, their non‐degradability requires the assessment of the accumulated concentration over years.
3.1.4. Risk assessment
Before conducting the risk assessment, the background levels in surface waters and sediments need to be evaluated in a preliminary phase. The lack of monitoring data for the sediment compartment cannot be compensated by using background metal data in soil as surrogate data due to differences in the biogeochemical processes between these two compartments (Fairbrother et al., 2007). In addition, a literature search performed according to the EFSA guidance document (2011) should be conducted to gain sufficient information on the fate and behaviour of metals in the aquatic systems, especially on factors affecting their speciation/availability.
In the first tier, the risk assessment for aquatic organisms is based on the total dissolved fraction, which is the approach currently applied in the peer review of copper compounds (EFSA, 2018) when comparing endpoints from the ecotoxicological studies and the estimated PEC with models. The combination of substance‐specific data, scenario‐specific data, crop‐specific data, spatial and weather data results in the estimated realistic worst‐case PEC in surface water and related sediments that is used in the risk assessment process (FOCUS, 2001). If models are used for estimating PECs for transition metals relevant spatial and temporal dimensions should be taken into account as was performed by FOCUS. Most aquatic toxicity tests conducted in artificial waters (low DOC, low suspended solids) tend to maximise bioavailability and in those cases total concentrations can be considered equal to dissolved (ECHA, 2008). However, if abiotic factors could have mitigated bioavailability or natural water was used bioavailability correction should be used.
If the first tier fails, the risk may be refined considering the bioavailability in the aquatic system without losing its protectiveness. However, the consideration of the bioavailability requires tools to calculate the bioavailability and knowledge about appropriate physico‐chemical (abiotic) parameters. The abiotic factors need to be defined, i.e. by using environmental scenarios describing different agricultural conditions, and address a range of relevant physico‐chemical properties e.g. pH, hardness, DOC and organic matter while making sure that realistic worst‐case scenarios are included. To compare them, both exposure and effect concentrations should be expressed at the same level of (bio)availability (ECHA, 2008) by considering the same set of water‐quality conditions (e.g. pH, major cations, DOC). All relevant information justifying the selected physico‐chemical parameters that affect the (bio)availability should be provided.
Options to consider the bioavailability are preferably the use of the simplified tools (physico‐chemical speciation tools) or alternatively appropriate BLM.
The full BLM procedure requires the determination of the metal speciation in aquatic studies using a chemical speciation model such as the Windermere Humic Aqueous Model (WHAM). Then the endpoint may be normalised to physico‐chemical parameters of different environmental scenarios using appropriate BLM (if available), which can then be compared with the PEC for the different scenarios. However, there is a defined range of physico‐chemical conditions over which a BLM has been validated, which are limited by the water chemistry parameters (e.g. water hardness, pH, DOC) of the ecotoxicity testing used to develop the BLM (draft technical guidance on implementing Environmental Quality Standards (EQS) for metals, European Commission, 2019). Furthermore, the BLM are metal and organism specific. Acute and chronic BLMs for the same metal may also not be interchangeable. Chronic BLMs exist mostly for only a limited number of species representing various trophic levels (algae, fish, invertebrates) (ECHA, 2008). These BLMs can be applied to other species if sufficient justification has been provided that these species possess a similar mechanism of actions (e.g. similar stability constants between the cations (Ca, Mg, H) and the biotic ligands, similar site of action).
For transition metals used as PPP, these pre‐requisites of the models may not be fulfilled for all situations of the prospective risk assessment (e.g. non‐equilibrium due to freshly added metal, diversity of sites for a national prospective assessment), therefore management (e.g. cost–benefit analysis, optimised application rates, etc.) and accompanied focused monitoring should be considered.
The risk assessment for sediment dwellers should be based on mg/kg dry weight (dw) or mg/L in pore water. To consider bioavailability in the refinement step, the chronic endpoint from sediment studies for metals that have a high affinity to bind to organic carbon can be normalised in a simple way by the organic carbon fraction if a linear relationship exists between the observed toxicity and the ligand (ECHA, 2008). The sediment toxicity of divalent metals may be normalised by considering the SEM‐AVS fraction while considering competitive displacement kinetics between different metals (ECHA, 2008, p. 48). Currently no BLM models are available for the sediment.
Conclusions for the aquatic risk assessment
The aquatic risk assessment of transition metals differs from organic substances with regards to the consideration of the bioavailability of the substances due to the physico‐chemical water qualities.
The persistency of transition metals is considered of relevance for the sediment risk assessment and the impact on sediment‐dwelling organisms due to accumulation needs to be addressed.
The approach described in the aquatic guidance document (EFSA PPR Panel, 2013c) is considered applicable for the hazard assessment. However, more details on the physico‐chemical parameters in the studies, especially for mesocosm studies need to be given (OECD, 2016).
For the ERA of transition metals all tiers as described in the aquatic guidance document (EFSA PPR Panel, 2013c) are considered relevant for refinement.
The risk assessment should consider the uncertainties due to the physico‐chemical conditions addressed in the studies and the scenarios to be assessed.
The approach to assess the bioavailability as described by ECHA (ECHA, 2008) is considered scientifically appropriate. However, for transition metals used as PPP the applicability of the approach differs in some important aspects such as the equilibrium of the transition metals in the aquatic phase and the relevant exposure routes for transition metals other than Cu. The applicability of the bioavailability models for the use in the prospective risk assessment needs to be specifically checked case by case according to the Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a).
Unlike for the retrospective – site specific – risk assessment of metals, the use of transition metals as PPP leads to non‐equilibrated conditions due to for instance the entry via drift in an unknown diversity of sites.
3.1.5. General conclusions and recommendations for the aquatic environment
Hazard assessment
Toxicity tests, which have been standardised for organic PPP can be used for transition metals. However, more information needs to be submitted as routinely done describing the water chemistry. Furthermore, it needs to be addressed if the metal is in equilibrium or not in order to appropriately use the endpoints in the risk assessment (OECD, 2016). At present, the same tiered approach as described in the aquatic guidance document (EFSA PPR Panel, 2013c) is considered relevant for transition metals.
Exposure assessment
For estimating the environmental concentration for the aquatic compartments, the FOCUS STEP 1–2 model has been considered as suitable for the transition metals. The current version of FOCUS STEP 1–2 model can be used for deriving the initial PEC in the water column due to the drift, but it is not recommended for the other entries to waterbodies (run‐off and draining) and for estimating maximum PEC in sediment. Indeed, equilibrium process based on Koc value is considered leading to underestimate the exposure concentration due to the uncertainties on the use of a K doc value for transition metals for distribute transition metals between water column and sediment. Furthermore, as described in the Scientific Opinion on the effect assessment for sediment organisms (EFSA PPR Panel, 2015b) two types of sediment exposure scenarios are recommended, a worst‐case scenario for the pore water and one for the total concentration.
Therefore, adaptation of the existing agreed EU models or development of new models and the corresponding scenarios could be seen as needed to take into account the abiotic processes (e.g. speciation, bioavailability, ageing process) and environmental factors affecting them.
Whatever the adapted/developed model, the EFSA guidance document on good modelling practice (EFSA PPR Panel, 2014a) should be followed.
All entries to different waterbodies should be considered in model as well as the annual repeated application and the long‐term uses. Spatial and temporal considerations are recommended for exposure calculations.
Taking the total concentration as bioavailable constitutes a conservative approach for the exposure assessment of transition metals. In the refined risk assessment bioavailability may be considered using simple fit‐for‐purpose bioavailability models considering the assumptions hold for the case to evaluate.
Specific recommendation for aquatic risk assessment
Detailed recommendations about specific issues to be considered in the aquatic risk assessment of transition metals are listed:
The total and dissolved measured concentrations in filtered (0.45 μm) water samples needs to be given, including the background concentration in the test media or sediment. For sediments the fraction after aqua regia digestion should be given (ECHA, 2008). The concentrations in sediments, pore water and overlying water should be provided.
-
To calculate the bioavailable fraction, the following abiotic factors need to be reported for each study according to the ECHA guidance document (ECHA, 2008) and EQS draft (European Commission, 2019):
-
–
In water: pH; hardness or dissolved Ca concentrations in filtered (0.45 μm) water samples; alkalinity; DOC fraction in filtered (0.45 μm) water samples; presence of complexing agents such as humic acids and EDTA.
-
–
In sediments: acid‐volatile sulfide (AVS) levels in sediments (at a sampling depth of 0–5 cm); organic matter (OC) determined as a minimum by loss on ignition; pH in 0.01 mol/L CaCl2; redox potential (Eh) in situ; Fe‐Mn oxyhydroxides; particle size; other ions when considered relevant in the BLM ammonium content of pore water; nitrogen content; SEM (= simultaneously extracted metals).
-
–
Water/sediment studies should be conducted using sediments with low AVS levels (e.g. artificial sediments or natural sediments with low OC and high sand fraction) as a worst case. The amount of AVS in sediment should be < 0.15 mmol/kg dry weight. The endpoint for sediment‐dwelling organisms needs to be normalised to the organic carbon content of 2.5%.
To cover all trophic levels, the following sediment species should be tested in the higher tier risk assessment: e.g. Hyalella azteca, Gammarus pulex, Tubifex tubifex, Lumbriculus variegatus, Chironomus riparius, Hexagenia sp., Ephoron virgo, mollusc, benthic microalgae.
Equilibrium status of the metal concentration over time in the toxicity studies (water and sediment) needs to be known to assess the time to onset of effects. The time to reach equilibrium depends on the test system and metal. Pre‐equilibration (of at least 12 h for water, resp. days to months for sediments) of the added metal before exposure of the test organisms is suggested in case long‐term effects other than reproduction are being investigated, as reproduction may be affected by the concentration over a small time window.
Equilibrium status in the test system (water and sediment) should reflect the equilibrium of the exposure path to be assessed. Input via drift and event‐driven inputs such as run‐off are not expected to have reached equilibrium in the waterbody for the acute risk assessment.
The endpoints for the water compartment can be normalised in a refinement step to the physico‐chemical parameters either using speciation models or if available BLMs. Concentrations in sediment can be corrected by considering binding to OC, SEM‐AVS or Fe‐Mn (oxy)hydroxides.
At present, the same tiered approach for water and sediment as described in the aquatic guidance document (EFSA PPR Panel, 2013c) or the Scientific Opinion on sediment organisms (EFSA PPR Panel, 2015b) is considered relevant for transition metals. However, in order to compare or combine the ecotoxicological studies, the metal speciation in the test system needs to be considered.
It is recommended to conduct laboratory studies including all sensitive species to derive an HC5. However, only studies with comparable study designs based on the same endpoint may be combined. The geometric mean for one species can be calculated for normalised endpoints in case multiple studies are available, otherwise the lowest endpoint should be used. The guidance given in the aquatic guidance document (EFSA PPR Panel, 2013c) with regards to the number of species needed and the assessment of SSD is considered suitable for transition metals.
Mesocosm studies are considered suitable refinement since they are semi‐field studies conducted with a natural assemblage of species exposed under more realistic conditions. They address species sensitivities, species interactions and indirect effects as well as the fate in a (semi‐)natural environment. The physico‐chemical conditions in the study needs to be compared with the conditions to be assessed. They are considered useful to assess the protectiveness of the SSD based on endpoints normalised to the conditions in the mesocosm study.
The consideration of recovery in a water–sediment system for the derivation of a RAC is not considered acceptable as the metals do not degrade and could be resuspended from the sediment.
Species sensitivity distributions and mesocosm studies are addressing different levels of refinements; they are both considered as relevant for a refined risk assessment of PPP with metals.
It was agreed in the peer review of copper compounds that the concepts of bioconcentration (BCF), bioaccumulation (BAF) and biomagnification (BMF) values have no meaning for homoeostatically regulated essential metals as there is an inverse relation between the copper bioaccumulation and copper concentrations in the environment.
The development of tolerance in communities should not be considered in a refined risk assessment as it is site specific.
The environmental scenario should represent the level of conservativeness (e.g. 90th percentile exposure concentration) defined in the problem definition (EFSA PPR Panel, 2014a).
All relevant exposure routes must be taken into account in the model (e.g. direct overspray, spray drift, atmospheric deposition, run‐off (both solute and eroded soil inputs) and drainage) (EFSA PPR Panel, 2014a).
All relevant information justifying the selected physico‐chemical parameters that affects speciation and availability of metals in aquatic systems should be provided. Spatio‐temporal dimension of the exposure regime should match the specific protection goal. In exposure modelling, usually a 90th percentile is used (EFSA PPR Panel, 2014a).
Models should be documented in a transparent way and include all information needed for the risk assessor to evaluate the model. All models should be accompanied by a detailed user manual. The procedures for obtaining the pesticide properties and the uses being assessed should be clearly described and wherever possible harmonised to avoid user subjectivity. A qualitative assessment of the uncertainties should be provided (EFSA PPR Panel, 2014a).
It should be demonstrated by the applicant that the specific protection goal is satisfied as part of the Regulation (EC) 1107/2009 with the assumptions implemented in model and the characteristics of the developed scenarios (EFSA PPR Panel, 2014a).
The development of exposure scenarios for specific transition metals needs to be backed up by representative data on their distribution in relevant European agricultural landscapes.
3.2. Terrestrial in‐soil environment
3.2.1. Effect assessment and ecotoxicologically relevant exposure quantities
Hazard characterisation
In the first step of the ERA for soil organisms exposed to pesticides, the hazard characterisation is currently based on results of tests performed according to OECD guidelines and following the data requirement currently in place (Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b) for active substances and PPPs, respectively). Sublethal effects on soil macrofauna (earthworms) and soil mesofauna (soil Collembola and mites) shall be investigated where the active substance can contaminate soil.
For soil macrofauna and mesofauna (earthworm, Collembola and soil mites), tests are performed in most cases with a standard substrate (‘OECD artificial soil’) composed of 10% or 5% organic matter (peat or coco peat for tropical conditions; OECD 222 (OECD, 2004a, 2004b, 2004c, 2004d), 232 (OECD, 2009), 226 (OECD, 2008); Mangala et al., 2009), 70–75% fine sand and 20% kaolin clay, adjusted to a pH value of 6.0 ± 0.5. Uncontaminated food is provided on the soil surface.
For transition metals, the laboratory tests can be in principle performed as for organic chemicals (ECHA, 2008, 2017c, chapter R.7c: Endpoint specific guidance; R.7.11 Effects on terrestrial organisms). More results are however available from test set‐ups with soil organisms exposed to transition metals, especially for Cu, where the organic matter content of the artificial soils was varied, or natural soils were employed in the lab trials (e.g. RAR Copper Compounds, 2017, Vol. 3, B9 and references therein, see also chapter ‘Linking exposure and effects’).
In general, laboratory tests with soil organisms cover only in different proportions the overall hazard characterisation for the tested species exposed to the assessed compound. While for endogeic earthworms the laboratory tests with Eisenia fetida covers contact exposure and part of oral exposure through some food mixed into the soil before contamination, tests with microarthropods all provide ‘clean food’ during the test.
Regarding the testing of earthworms at higher tier, guidance for field tests according to ISO standards (ISO 11268‐3 in ISO, 1999) is available as well as an extensive knowledge based on the performance of such tests in the field. Experience with transition metals has also been gained in long‐term field testing with earthworms (e.g. studies on grassland soils; Klein, 2015; RAR Copper Compounds, 2017; EFSA, 2018, conclusion). de Jong et al. (2006) have outlined how to report the outcomes of earthworm field tests and recent publications have addressed the appropriate evaluation of the results of such tests (EFSA General Meeting (EFSA, 2019), OECD Guideline Development). However, guidance for performing field testing with other soil organisms than earthworms (e.g. soil mesofauna) is lacking. Such field studies are currently set up similarly to earthworm field studies, but with amended sampling design (e.g. Römbke et al., 2009; ISO 23611‐2 in ISO, 2006). In addition, terrestrial model ecosystems (TME) with complex communities are considered to deliver relevant endpoints for soil mesofauna, and if sufficiently large and replicated, also for earthworm species (Schaeffer et al., 2010; Toschki et al., 2015).
There are for the time being no agreed test set‐ups available for soil organisms that can be considered ‘intermediate tier’ between standard laboratory testing and field trials. However, the testing of several soil organism species belonging to different groups (e.g. collembolans and mites as microarthropods; earthworms and enchytraeids as Annelida) can deliver very relevant information on the sensitivity distribution between species. The so‐called SSD approach is considered in general ‘a powerful tool’ (EFSA, 2019) also in the effect characterisation for soil organisms, even if not often applied for pesticide assessments (Frampton et al., 2006).
The use of the SSD approach in the pesticide effect characterisation in terrestrial environments is currently applied for non‐target terrestrial plants (Boutin et al., 2014; EFSA PPR Panel, 2014b). Here, a hazard concentration/rate for 5% of the species tested is calculated, in which a fraction of 95% are considered to be covered, having an endpoint (ECx) higher than the calculated HC/R5. Note that the median HC/R5 value is the concentration that with 50% certainty is lower than the toxicity values (e.g. EC50s or NOECs) for 95% of the species tested.
For soil organisms, the use of SSD has also been promoted in the Scientific Opinion of EFSA on the science behind the risk assessment for in‐soil organisms (EFSA PPR Panel, 2017). No conclusion had been drawn at that time, as ‘standard SSD methodology cannot yet be applied to in‐soil organisms until further guidance on how toxicity data can be combined (e.g. toxicity data for different taxonomic groups of in‐soil organisms, etc.) will become available’ (EFSA PPR Panel, 2017). To advance in this respect, information on the amount of toxicity data that might be needed for a reliable SSD and advice on the possible combination of endpoints from different species can be found in the aquatic guidance document (EFSA PPR Panel, 2013c), the Scientific Opinion on sediment organisms (EFSA PPR Panel, 2015b) and in the ECHA guidance documents (ECHA, 2008, 2017c).
In general, the aquatic guidance document (EFSA PPR Panel, 2017) suggests that a minimum of eight data points should be available to compute a reliable SSD. The data should represent toxicity endpoints from sensitive species. Assessing pesticides with a clear mode of action makes the choice of the sensitive group of organisms to be tested more straightforward then for compounds like transition metals, where a mode of action (MoA) cannot be determined according to standard criteria (e.g. Kienzler et al., 2017). Possibly, the hazard characterisation and the amount of available toxicity data with different species and groups of soil organisms is fairly comparable with the situation for sediment organisms, considered that for soil organisms only chronic data are required for submission. In EFSA PPR Panel (2015a,b), chronic SSDs are computed based on chronic EC10/NOEC values, and the selection of the sensitive species to be tested is suggested to follow the decision scheme along which Tier 1 test species were chosen, ‘e.g. arthropods for pesticides with insecticidal properties and different taxonomic groups for fungicides with biocidal properties’. This indicates that when sufficient evidence exists that no specific taxon is particularly affected compared with others, endpoints from species belonging to different soil organism groups like arthropods and annelids could be merged in an SSD curve to calculate HC5‐values. However, in the framework of a general statement on transition metals within the current guidance for soil organisms, no specific definition of ‘sufficient evidence’ can be given: the distribution of data should be evaluated case by case, possibly excluding extreme data points belonging to specific groups to check for variability reduction. Given that for microorganisms and non‐target terrestrial plants exposed to PPPs different type of endpoints will be delivered according to data requirements (e.g. functional vs. structural; NOEC vs. EC50; EU No 283/2013 (European Commission, 2013a); EU No 284/2013 (European Commission, 2013b)) and/or different protection goals in pesticide guidance exists (see chapter 3.3), including these organisms in one SSD with in‐soil organisms is not favoured for the time being. It is however acknowledged that the type of exposure for in‐soil mesofauna and macrofauna, microorganisms and plant can be comparable (ECHA 2008, 2017c), e.g. for metals affecting plant roots and seedlings’ emergence or juvenile earthworm development in different soils. Therefore, approaches joining different organism groups can also be appropriate, e.g. if the protection goals are similar (no in/off‐crop differentiation) and/or the endpoints address similar effect magnitudes as in other legislations (e.g. ECHA, 2008).
If not enough data are available to construct an SSD, EFSA PPR Panel (2013c, 2015a,b) suggest to apply a so‐called geomean approach, to make good use of all available endpoints for the same species exposed to the same chemical into account (or species of one ‘taxa’ e.g. ‘insecta’ or ‘crustacea’). It should be noted, though, that the calculation of geomean values for chronic endpoints is currently not supported (EFSA PPR Panel, 2013c; EFSA, 2019), so that this approach is not suggested for in‐soil organisms.
It should also be noted that jointly assessing different endpoints from tests with soil meso‐ and macrofauna always implies merging non‐constant exposure regimes. During the tests, compound concentrations are not maintained constant, adsorption processes are ongoing and also degradation processes (not relevant for metals). As development – and therefore test duration – is different for different species, availability of compounds can be different for different species in relevant time windows (e.g. sensitive stages in reproductive cycle or development of juveniles). This is not the case in the field, where population structure is not homogeneous through synchronisation as in the laboratory. These implications are of general nature and not specific for transition metal compounds, but should be considered when firstly implementing approaches like SSD for defining endpoints for in‐soil organisms.
Additionally, Tier 1 tests with soil organisms might be carried out also in different (natural) soils. Here, important information inherent in the data (e.g. higher toxicity in soils with less binding capacity) should not be disregarded and mistaken for natural variability. Scaling for soil parameters known to consistently affect the availability of the specific compound to be assessed could be an option, if sufficiently backed up by evidence (as for ECHA 2008, 2017c, see also chapter ‘linking exposure and effects’).
All discussed, methodologies to assess jointly the outcome of several tests on species or soil organism groups would be non‐standard higher tier approaches and need to be part of a weight‐of‐evidence approach until new guidance for in‐soil organisms is being developed.
Regarding microorganisms, currently only data on effects on soil nitrogen transformation are required for active substances in PPP (OECD 2000b; Commission Regulation (EU) No 283/2013 (European Commission, 2013a)). The test is intended to provide sufficient data to evaluate the impact of active substances on soil microbial activity. Tests should be performed in freshly sampled agricultural soils not contaminated with the substance to be assessed. EFSA PPR Panel (2017) has indicated that adaptations are needed for the test battery for in‐soil organisms. For microorganisms, the panel proposed retaining and advancing the N‐transformation test, and adding a test with mycorrhizal fungi to the data requirements and risk assessment (EFSA PPR Panel, 2017; Mallmann et al., 2018).
Ecotoxicologically relevant exposure quantities for soil organisms
In the Scientific Opinion of EFSA on in‐soil organisms (EFSA PPR Panel, 2017), a dedicated chapter addresses how ecotoxicologically relevant exposure quantities for soil organisms can be identified. Ecotoxicologically relevant exposure concentration (EREC) help to best link exposure and effects and the respective factors influencing availability of pesticides to soil organisms (chapter 7.10, EFSA PPR Panel, 2017).
Regarding exposure routes, it is stated that:
‘…in‐soil organisms are exposed to PPPs via a variety of pathways. This is modulated mainly by their morphology (e.g. their body form or the structure of the epidermis), physiology (e.g. the way they take up water, food and oxygen) and behaviour (where they live and move in soil) (Peijnenburg et al., 2012). Moreover, these pathways may vary during the life cycles of some species. The relative relevance of these uptake routes for the body burdens is also dependent on the properties of the chemical (e.g. hydrophobicity) and environmental conditions like soil properties and climate. The major uptake routes considered for soil organism are:
-
–
contact with soil, soil pore water and litter (so diffusion into the body via the ‘skin’);
-
–
ingestion of food (soil organic matter, litter, bacteria, fungi, prey), of soil particles and soil water …’ (EFSA PPR Panel, 2017).
A major uptake route for soil organisms is the uptake from the so‐called ‘soil pore water’, which should indicate the available amount of the assessed compound in the soil solution. The concentration in pore water is considered to be the driving factor for uptake and toxicity of pesticides for microorganisms.
Regarding soil fauna, there is a debate on the relative importance of the pore water concentrations compared with total bulk soil concentrations in explaining the observed toxicity values. This debate is often not fully straightforward, since discussions on relative quantitative relevance of different exposure routes intermingle with discussion on the availability of compounds in the different matrices. If separately analysed, the combination toxicity of the different exposure routes is considered to be additive, even if the relative contribution is different and depends massively on the specific metal and species investigated (e.g. Bruus Pedersen et al., 2000; Vijver et al., 2003). Species behaviour and feeding mode do not allow for the derivation of generalised patterns, internal burdens are reported to depend mostly from pore water concentrations or extractable fractions, but other investigation see by contrast better correlation with total soil concentrations (e.g. Bruus Pedersen et al., 1997; Vijver et al., 2001; de Vaufleury and Pihan, 2002; Frische et al., 2003; and references therein; Smolders et al., 2009). Interestingly, if normalised to the extractable amount from soil and matrices, the uptake by soil organisms from food and soil might be comparable (e.g. Bruus Pedersen et al., 2000).
The importance of uptake via food cannot be disregarded: soil organisms digest the organic matter in the soil (e.g. endogeic earthworms), feed predominantly on fungi and bacteria (e.g. Collembola) or are predator (e.g. predatory mites). The calculation of exposure quantities in these matrices is difficult, as well as their contribution to body burdens. Oral uptake route has high relative relevance for compounds with high sorptivity. Also plant litter debris on the soil surface are an important food source for anecic earthworms (e.g. L. terrestris) and litter dwelling organisms also in non‐permanent crops. Uptake of metals via food does impact reproduction of soil organisms (e.g. Bengtsson et al., 1985). As demonstrated by Natal‐da‐Luz Gevaert et al. (2019), current tests tend to underestimate the situation in the field by providing clean food to animals exposed to contaminated soils.
For the single exposure routes, ecotoxicologically relevant exposure concentrations (EREC) can be identified. They should be based, as proposed for other pesticides, on the knowledge about the major exposure routes and the temporal and spatial exposure profile for in‐soil organisms (EFSA PPR Panel, 2017).
Most soil organisms live in the upper soil layers or feed there (e.g. EFSA, 2017) and will be exposed to the highest concentration of the freshly applied PPP after spraying. For these organism groups, the possible uncontaminated layers deeper in the soil are not always a shelter, since food attract them to the surface and not all soil organisms can avoid different toxic compounds. For metals, there is indication that different species avoid contaminated substrates in the lab and in the field, but sensitivity varies greatly between species and groups (e.g. Tranvik and Eijsackers, 1989; Lukkari and Haimi, 2005). According to EFSA PPR Panel (2017), ‘averaging of exposure between high concentrations in the top layer and the low concentrations in deeper soil layers would deliver a space weighted average’, which, as the ‘time weighted average’ concentration, could be used only under specific preconditions (e.g. reciprocity) and only for specific measurement endpoints (e.g. growth)’.
If after application the resulting concentrations of the compounds in the uppermost soil layer are high, the permanence there can elicit acute or chronic effects. For organisms living in permanent burrows (e.g. anecic worms) and feeding on the soil surface, also the concentrations in the upper soil centimetre(s) will be relevant. The EREC calculated by choosing 5 cm soil depth was in some cases not sufficiently high to explain the observed effects on soil organisms (e.g. for earthworms and Collembola; Evans, 2003; Toschki et al., 2015). Please refer to EFSA PPR Panel (2017) for further information on the identified EREC for in‐soil organisms.
For endogeic worms, TK/TD models have been suggested as possibly relevant for exploring specific situations combining short‐term permanence in contaminated substrates with fast compound degradations and no delayed effects related to initial exposure. Metals are reported to be taken up very fast and – depending on the compound – excreted in uncontaminated substrates (Fisker et al., 2013; Kilpi‐Koski et al., 2019). Some metals can be regulated through metallothionein or metallothionein‐like proteins (e.g. in earthworms and collembolans; Hensbergen et al., 1999; Sturzenbaum et al., 2001; Sizmur and Hodson, 2009; Maria et al., 2014), with different pathways for essential and non‐essential compounds, but the excretion capacity is limited and uncontaminated habitat not always accessible.
Summarising, regarding the full coverage of the exposure route via food for soil organisms, there is a gap in the current risk assessment schemes for the adaptation of existing tests to include contaminated food as well as for the further development of tests for litter feeding organisms (e.g. isopods), but the gap is not specific for transition metals as active substances in PPP. Regarding contact exposure, EFSA PPR Panel (2017) concludes that ‘since both exposure routes via total soil or pore water concentration are considered to be relevant, and will have different relative importance for different substances and species, it is recommended to assess both exposure routes for in‐soil organisms’.
Conclusion for the hazard assessment in soils
For transition metals, the laboratory tests can be in principle performed as for organic chemicals.
Contact and oral exposure routes are both considered important for soil organisms. In general, laboratory tests with soil organisms cover only partly the overall hazard characterisation for the tested species exposed to the assessed compound. Tests with microarthropods all provide ‘clean food’ during the test, adding to uncertainties.
SSD are considered a suitable tool to take the sensitivity differences between soil organism species into account. Where sufficient evidence exists that no specific taxon is particularly affected compared with others, endpoints from species belonging to different soil organism groups like arthropods and annelids could be merged in an SSD curve to calculate HC5‐values.
Given that for microorganisms and non‐target terrestrial plants exposed to PPPs different type of endpoints are submitted and/or different protection goals in pesticide guidance exists, including these organisms in one SSD with in‐soil organisms is not favoured for the time being. It is however acknowledged that the exposure is similar and therefore approaches joining different organism groups can also be appropriate under other legislations (e.g. ECHA, 2008), when endpoints are similar as well as protection goals.
A test guideline for soil mesofauna studies in the field is lacking, but experiences from earthworm field studies have been successfully transferred to these groups.
Regarding the testing of earthworms at higher tier, experience with transition metals shows that field tests are extremely important for understanding the long‐term impact of transition metals.
3.2.2. Exposure assessment, fate and behaviour of transition metals in soils
As part of Commission Regulation (EU) 546/201116, 283/2013 (European Commission, 2013a) and 284/2013 (European Commission, 2013b), a risk assessment for the soil organisms is required for the active substance and the relevant transformation products observed in soil. The environmental conditions affecting the relevant processes involved in the fate and behaviour in soil of active substance and their transformation products should be also identified.
Until the entry into force of EFSA guidance document (2017), the methodology for estimating exposure concentration in soil (PECsoil) is presented in the FOCUS document (1997). To provide a conservative risk assessment for the soil compartment, calculations of the predicted environmental concentration in soil (PECsoil) are based on a simple relationship. Therefore, the amount of compound (active substance and relevant transformation products) reaching soil (corrected for the crop interception) is homogenised on a soil having a standard soil depth (e.g. 5 and 20 cm) and a defined soil density (historically e.g. 1.5 g/cm3). For transition metals the exposure assessment should cover the long‐term exposure following repeated application either by estimating exposure calculations or by providing accumulation field study. It is well known that ‘the application of Cu has created an issue with the accumulation of Cu in vineyard soils and groundwater’ (Jacobson et al., 2005; Komárek et al., 2010; cited in Ballabio et al., 2018). It should therefore be considered that due to its persistence and its low mobility in soil Cu will accumulate in soil.
To reflect the persistence of metals in soil a DegT50 value of 1,000,000 days should be used. Due to their persistency in soil and the long history of use for some metals, realistic agricultural background concentration in soil should also be considered in the PEC calculation based on recent topsoil surveys (Ballabio et al., 2018; Orgiazzi et al., 2018). Methodology based on the recommendations of the ECHA guidance document (ECHA, 2008) could be followed to derive an agricultural background concentration for different types of crops (Frequency distributions of copper concentration exposure concentrations across EU countries). For deriving a representative background concentration, the 10th and the 90th percentile of soil concentration should be considered to take the variability of their level in soil into account. Regarding the data selected for estimating the background concentration, the highest concentration in agricultural application observed in the monitoring data should not be discarded from the data sets without any scientific justification since these values could be representative of agricultural practices. The raw data supporting the background concentration in the soil should be made available. Please also refer to Part 4 on Criteria for performing and evaluating environmental monitoring studies.
Such data represent the preliminary phase in the risk assessment and needs to be considered in the exposure calculations in all steps of the risk assessment. While in the first assessment step natural background and anthropogenic residues are considered bioavailable to non‐target organisms, it could be considered in refinement steps how the bioavailability of residues can be reduced in time.
Different approaches were considered at the EU level to take the background concentration in the risk assessment into account. For transition metals showing important level in soil due to their long history of use (e.g. copper), it was concluded that the representative agricultural background concentration should be included in the exposure calculation for soil compartment. For other transition metals (e.g. iron), the estimated PECsoil should be compared with and comprised in the range of the background concentration in soil.
The Ecotoxicological Relevant Exposure Quantity/Concentrations should be estimated and used to conduct the risk assessment. For some transition metals (e.g. ferric phosphate; EFSA, 2015), a risk assessment is required for the complexed form only due to its low solubility in water. In contrast, exposure calculation for metal ion is needed (e.g. iron sulfate; EFSA, 2012a) due to its solubility and dissociation in water of the complexed form. For these active substances, no degradation in soil was considered. The soil background level was not included in calculation.
Persistence in Soil Analytical Model (PERSAM)
The simple analytical model as implemented in the PERSAM software tool has been developed for estimating the predicted environmental concentration in soils of organic compounds for regulatory risk assessment purpose (EFSA, 2017). The goal is to assess the 90th percentile concentration considering all agricultural fields within a regulatory zone (north–centre–south) where a PPP is intended to be used.
A detailed description of the PERSAM model, the soil scenarios and the input parameters required for running the model is available in the EFSA guidance document (EFSA, 2017). This model and the corresponding soil scenarios have been developed for organic compounds and need to be adapted to take bioavailability of transition metals into account. Some relevant soil characteristics as CEC are missing in available simple analytical model and numeric models for transition metals. Development of representative soil scenarios specific to each transition metal including the main factors affecting their fate and behaviour in soil (e.g. pH, OC, CEC, clay content) would be needed.
Some parameters in the PERSAM model – as the defaults half‐life for the decline of the dislodgeable residue of 10 days and a wash‐off factor of 0.1 mm−1 on plant – are not conservative enough for transition metals, but it would be recommended to not include a FOCUS crop interception in modelling for transition metals. The methodology for selecting representative soil scenarios into PERSAM model could not allow reaching the specific exposure target, since the estimation of 90th percentile concentration is developed for organic compounds (based on degradation and mobility in soil only, scenario adjustment factors).
It is noticeable that the background concentration cannot be handled in the PERSAM model. The inclusion of an additional submodel within the current PERSAM tool could be an option to take the agricultural background level from all sources before the first application of transition metals used as PPPs into account.
It should be explored if soil scenarios for the three regulatory zones implemented in PERSAM tool could be parameterised for total and pore water concentrations for different metals. The total concentration of freshly added metals depends primarily on the bulk densities of the soil; however, the background concentration is affected by different properties such as the pH and clay content of the soil. This is because the concentration in the soil solution and therefore also the leaching of metals depends on these properties.
At Tier 1, crop interception and wash‐off factor are not considered in the PERSAM calculations. The major factors affecting the sorption of transition metals (e.g. pH dependency) are not implemented in PERSAM model. Although the concentration of metals in pore water at Tier 1 is not deemed appropriately estimated and cannot be used for the risk assessment of the soil organisms, Tier 1–PEC for total concentration can be derived using PERSAM model and used for the risk assessment. Soil properties dependent parameters (e.g. pH and soil sorption) can be considered at Tier 2. However, the canopy processes e.g. standard default values for wash‐off and foliar decline cannot be considered appropriate for inorganic compounds. Therefore, Tier 2–PECsoil for both total and pore water concentrations are underestimated by the tool without changing these defaults. Regarding these concerns, adaptation of the tool is needed to produce realistic worst‐case concentration in soil.
Pesticide leaching models (e.g. FOCUS PELMO/PEARL models)
Pesticide leaching models (e.g. FOCUS PELMO/PEARL models as in EFSA guidance document, 2017) deliver similar outcome than the PERSAM model, especially on the soil characteristics selection in soil scenarios. It is noticeable that a Kd value can be used as modelling endpoint in FOCUS models. See Part 3.6 where considerations for the selection of Kd values have been discussed.
Intermediate dynamic model for metals (IDMM)
See Part 3.1.2. where considerations for the soil column have been discussed.
Conclusions for the exposure assessment in soils
The approach proposed in the ECHA guidance (ECHA, 2008) for industrial chemicals cannot be strictly applied in the context of PPPs risk assessment purpose. Two distinct approaches were identified in the risk assessment provided by EFSA and ECHA. While the risk assessment for the soil organisms is performed in the ECHA approach (ECHA, 2008) from the estimated background (natural and anthropogenic) concentration of transition metals in soil, the risk assessment in the EFSA approach is based on an estimation of soil concentration derived from agricultural background in soil and the long‐term use of transition metals as PPPs.
For the time being, the current methodology (SANCO document, FOCUS, 1997) based on simple assumptions gives a good estimation of the soil total concentration of total transition metals in soil. However, this approach does not make it possible to take into account the availability of transition metals in soil and the environmental factors and time affecting it.
Although simple analytical models and numerical models (e.g. PERSAM and FOCUS models) exist, it has been identified that the existing soil scenarios are not fully adapted for assessing transition metals.
For the time being, Tier 1 PERSAM–PECsoil for total metal can be used for the risk assessment but not Tier 2. Tier 1–2 PERSAM–PECsoil for pore water are not considered reliable for the risk assessment of the soil organisms.
The development of specific soil scenarios for transition metals seems to be needed. It would be recommended to include the soil characteristics (pH, OC, CEC and clay content) in such scenarios.
To better reflect the fate and behaviour of transition metals in the soil profile, other processes (e.g. speciation/availability) affecting their leaching in the soil profile could be included in the modelling.
The recommendations of the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed to ease the assessment of both such a model and the parameters implemented within the model.
3.2.3. Linking exposure and effects
In Part 3.2.1, Ecotoxicologically relevant exposure quantities (EREQ) for in‐soil organisms have been identified. For soil organisms, all identified quantities are ‘concentrations’ (EREC), that should be compared with PEC in soils (see Part 3.2.2 above) for the calculation of risk quotients.
EFSA PPR Panel (2017) has indicated that both total and pore water concentrations are relevant for soil organisms. At present, however, ecotoxicological endpoints are reported from tests with soil organisms for pesticide evaluation as total nominal initial concentrations. In some cases – and according to older guidance – compounds are sprayed on the soil surface, delivering effect rates that are converted to concentrations by taking the amount of soil and the area of the soil surface in the test vessels into consideration. No measurements of the compounds at the beginning or during the test/at test end are performed, even if this has been repeatedly required (EFSA, 2009; EFSA PPR Panel, 2017).
Total metal concentrations in soils
According to guidance in place (European Commission, 2002), the ecotoxicological endpoints based on total nominal concentrations are to be related to total predicted soil concentrations. In contrast to the assessment of sediment organisms (EFSA, 2015), the ecotoxicological endpoints are not normalised to the organic carbon content of the soil matrix.
However, the OECD artificial soil largely employed in laboratory testing with soil organisms does not resemble typical field conditions, since agricultural soil diverge from the laboratory test substrate by content and quality of organic matter or pH values. The specific combination of high peat, high fine sand and high clay content is very unlikely in the field (please see figures below; and EFSA scientific report, 2010b; Xu et al., 2019). In addition, the OECD artificial soil is not a worst case regarding the availability of the test compound for organisms, independently whether organic or inorganic substances are investigated. Especially the binding sites in the organic matter and clay fraction lead to a reduced bioavailability. Also, the adjusted pH does not, in many cases, represent a worst case for metal adsorption (Figures 1 and 2).
Figure 1.
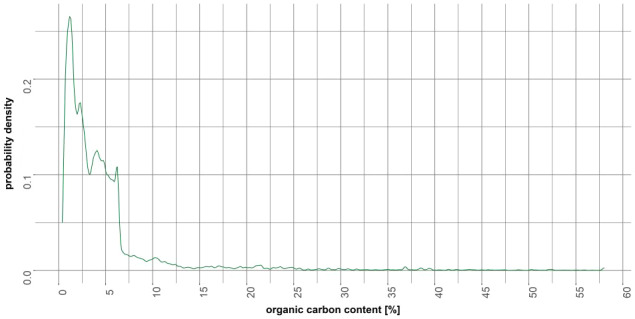
Probability density distribution of organic carbon in agricultural soils in Europe (database PERSAM/JRC; EFSA PPR Panel, 2012; Hiederer, 2012. Visualisation © darwinstatistics)
Figure 2.
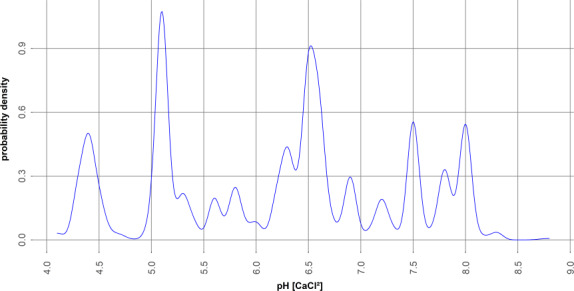
Probability density distribution of pH values in agricultural soils in Europe (database PERSAM/JRC; EFSA PPR Panel, 2012; Hiederer, 2012. Visualisation © darwinstatistics)
To take such bioavailability issues at least roughly for the appropriate hazard characterisation into account, test endpoints for pesticides with a log Pow > 2 are currently divided by a factor of 2 (EFSA, 2015). EFSA reconfirms that the correction factor of 2 is applied also if a lower organic matter content was used in the test (i.e. 5% instead of 10%), until better guidance is available. Some MS accept that outcomes from tests performed with a reduced organic matter content in the standard soil (e.g. 5%) are not divided by 2. However, further investigations are needed to better describe the relationships between sorption, availability and toxicity of tested compound for different soil organism groups, since the application of a fixed factor related to a trigger value does not allow for scaled responses.
Available metal concentrations in soils
From the exposure assessment side, the calculation of pore water concentrations for pesticides is suggested in previous EFSA outputs (e.g. EFSA PPR Panel, 2012) and implemented in latest guidance (EFSA, 2017). The adaptations needed to calculate worst‐case scenarios for the three regulatory zones accordingly to the concept applied for other pesticides than metals is described in chapter 3.22 above.
However, in the guidance currently still implemented for ecotoxicological assessment (European Commission, 2002), no indication is given on how to derive endpoints related to pore water concentrations nor how to handle such endpoints – if delivered – in a risk assessment scheme.
One possibility to calculate pore water concentrations from legacy studies where no exposure measurements were performed was outlined in EFSA PPR Panel (2017), where it was suggested to scale the end‐point – depending on the coefficient of equilibrium sorption on organic matter (Kom) of the substance and the organic matter content in the study soil – to the properties of the soils in the three regulatory zones. Since other soil parameters are relevant for the sorption and desorption of metals, such approaches would need to be specifically developed for transition metals as active substances in PPPs.
It should be considered, though, that pore water concentrations will not follow an easily predictable course over the exposure time of soil organisms in the test and also in the field soils, where drying and rewetting might lead to rise and fall of concentrations in soil. For the time being, not enough information is available on the time to onset of effects and on delayed effects in soil organisms in general to understand sensitive periods and their relation to the time course of exposure. Therefore, initial concentrations including accumulation were identified as relevant for applied pesticides according to their intended uses (EFSA, 2017; EFSA PPR Panel, 2017).
The bioavailability of metal residues in soils resulting from application in previous years is deemed to depend on soil properties and to decrease over time. As summarised in the ECHA guidance, ‘the bioavailability of the test compound, and therefore the toxicity observed, is influenced by…soil properties’ (ECHA, 2008).
The approach suggested by ECHA diverges from the pesticide assessment under the remit of EFSA in so far as ECHA proposes in the first place that ‘toxicity has to be tested in a reasonable worst‐case scenario, i.e. a soil with high bioavailability of the metal substance tested which is then compared with the PEC’ (ECHA, 2008). This approach could be a stricter worst‐case approach that could be also investigated for metals as active substances in PPPs, under the premise that the explanatory toxicity model linking exposure and effects and describing the possible toxicity changes observed in natural soils compared with the standard artificial soils is developed according to EFSA PPR Panel (2014a,b). Following all steps of the modelling cycle could help identifying those uncertainties that have been addressed in the additional tests and their share in the assessment factor. Such models would need to be developed for different species and different compounds separately.
In a second step, if sufficient information is available to correlate the toxicity to relevant soil parameters, the endpoints (e.g. NOEC/EC10) could be normalised to conditions that are considered relevant for the risk assessment and compared with the calculated PECsoil (e.g. for the different regulatory zones). Care should be taken that the conditions are sufficiently worst case and that the chosen ‘abiotic parameters fall within the geochemical boundaries of the bioavailability model (e.g. range of CEC, organic matter, pH)’ correction applied (ECHA, 2008). Following the rationale above, an explanatory toxicity model developed according to EFSA PPR Panel (2014a,b) would be based on several tests in soils with different parameter ranges. It is essential to reiterate the indications by ECHA guidance document (ECHA, 2008) that the conditions assessed should match the bioavailability correction investigated. In addition, the parameters employed in exposure models to calculate the relevant PEC should be addressing the same EREC as the ecotoxicological tests and care should be taken not to double account for bioavailability corrections. It should be avoided that e.g. to cover different regional situations, endpoints are scaled to soils with reduced bioavailability (leading to higher endpoint values) and in addition the calculated PEC in these soils is reduced by taking the same influencing parameters into account (e.g. by considering specific metal pools as not bioavailable).
ECHA (2008) suggests in some steps that when sufficient taxonomic groups are available, an HC5 based on normalised data could be used. This approach could be implemented in future when bioavailability models for soil to normalise endpoints to worst‐case conditions might become available following development according to EFSA PPR Panel (2014a,b) modelling cycle.
3.2.4. Risk assessment
The risk assessment scheme for soil organisms exposed to pesticides currently in place states that the TER for chronic risk should be calculated by comparing the NOEC/EC10 from chronic reproduction tests with the PECsoil (European Commission, 2002, SANCO/10329/2002).
The chronic risk for soil organisms is considered acceptable if TER ≥ 5 (EC 546/2011 (European Commission, 2011)). Unfortunately, the guidance document dates back to 2002 and does not fully reflect the new general regulation (EC No 1107/2009) as well as new data requirements (EU No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b)). Therefore, some inconsistencies exist on the risk assessment for soil organisms other than earthworms (soil mesofauna). EFSA has adopted the procedure described in the updated data requirements, so that risk indicated at lower tier for soil mesofauna (structural endpoints) cannot always be addressed by the outcome of litter bag studies (functional endpoints), as laid down in the guidance document (European Commission, 2002).
Before conducting the risk assessment, the background levels and the anthropogenic residues in soils need to be evaluated in a preliminary phase.
At the first tier, endpoints expressed as total soil concentrations can be compared with PECsoil for total soil concentrations assuming that the long‐term simulated accumulation of metal compound as bioavailable fraction.
TER risk quotient could also be calculated by comparing the EREC pore water concentrations, if such values had been measured in the laboratory trials. However, at present, no guidance is available that describes how the ‘pore water’ fraction should be practically measured (e.g. use of solvents) and if one fraction would be relevant for several in‐soil organism groups. Possibly, the pore water concentration in the test could be calculated (EFSA, 2017), but the applied model needs to be validated for transition metals according to EFSA PPR Panel (2014a,b), including external data sets to predict and corroborate toxicity estimates. Regarding the risk assessment based on pore water concentrations, it is unclear whether the uncertainties in the extrapolation from laboratory to the reference Tier in the field are comparable with those arising from the comparison of total concentrations (e.g. if the acceptability criterion would be the same).
Ecotoxicological endpoints can be refined by further testing additional soil organism species, possibly allowing for the definition of an HC5 covering 95% of species’ endpoints from an SSD curve. Investigating the sensitivity distribution of toxic responses addresses part of the uncertainties in the risk assessment. It is suggested that for the time being if a robust SSD of the sensitive species/taxonomic group(s) can be computed, the calculated HC5 is not used in the risk assessment of transition metals as active substances in PPP without an assessment factor. This is because jointly assessing several endpoints for soil organism species/groups would follow at present a non‐standard higher tier procedure for PPP assessment and could be only part of a weight‐of‐evidence approach until new EFSA guidance for in‐soil organisms is being developed. As the acceptability criterion TER for chronic risks is set to 5 for standard worst‐case assessment, and a number of uncertainties on coverage of exposure routes and effects on community remains, it is recommended that an assessment factor (AF) of 2–3 is chosen to be applied to the calculated HC5 for soil organisms exposed to transition metals as PPP. The quality of the retrieved or newly submitted data by the applicant will be part of the assessment by the authorities in order to decide on the AF to be applied. The availability of raw data to check for the derivation of ecotoxicological endpoints and the description of study details will be decisive for the inclusion of the data points in the SSD, the derivation of e.g. HC5 and the decision on the AF to be applied (see also EFSA PPR Panel, 2013c, chapter 8.4).
For the endpoints determined in the laboratory, ECHA guidance document (ECHA, 2008) proposes a generic lab‐to‐field (L/F) factor to correct for higher toxicity observed in standard tests with laboratory‐spiked soils compared with tests in soils affected by long‐term use of e.g. Cu‐based PPPs. ECHA guidance document (ECHA, 2008) proposed to apply the L/F factors to ecotoxicological endpoints to account for the ageing of metals after long‐term equilibration in field soils. Transition metals as PPP are however freshly applied one or more times per season, and the suggested ageing is not taking place in relevant time frames for the risk assessment of intended PPP uses. It is therefore proposed to account for sorption and reduced availability of transition metals residues – if applicable – when calculating the predicted environmental concentrations (PEC) in soils.
Refining exposure assessment would need the definition of exposure scenarios specific for transition metals (see Section 3.2.2) but the principles of the tiered approach would follow the actual guidance (EFSA, 2017). Refining predicted concentrations in soil to account for bioavailability declines with time needs to be carefully evaluated. It is suggested that such approaches are discussed to begin with for the definition of the bioavailable metal fraction resulting from the residues accumulating in soils from applications in previous growing seasons and following continuous sorption and desorption processes in the soil environment. Metal adsorbed to soil particle might become bioavailable again in the guts of soil organisms.
The ECHA guidance document (ECHA, 2008) proposes to perform a risk assessment along a so‐called total risk approach (TRA) or to choose the added risk approach (ARA). The ARA considers that the natural background has no toxic effects on non‐target organisms. The separation of natural background from anthropogenic residues and their further consideration is however not fully clear in the ARA. For transition metals applied in PPP, however, anthropogenic residues might have the highest share in the total concentrations in soils compared with natural background and need to be considered in the risk assessment, additionally to the freshly applied amount when using PPP with transition metals. It is therefore concluded that the TRA is relevant to transition metals applied as active substance in PPP. If the ARA should be developed further, natural background level mapping data should be available to be considered in future.
Further developments are however needed to decide on the definition of bioavailable metal pools to soil organisms, since these will likely be organisms and compound specific and might change with time. Soil organisms are known to modulate the bioavailability of metals in soils themselves, through mixing, feeding on soils and extracting organic matter by digestion, storing and excreting complexed metals, sorbing metals to mucus and changing the pH of burrows (Sturzenbaum et al., 2001; Sizmur and Hodson, 2009; Sizmur et al., 2010, 2011; Ruiz et al., 2011). The impact of soil organisms on metal bioavailability can be seen also after leaching of freshly added metals (e.g. Udovic et al., 2007). This may cause changes in the different metal pools compared with predictions not including in‐soil organisms’ activities.
Higher tier studies with soil organisms in the field, better accompanied by long‐term biological and chemical monitoring, will deliver the best approximation to impact at the reference tier, being the communities of soil organisms in the field. Effect on community composition might be close to the no‐observed concentration in the laboratory, as species interactions and additional stressors might impact competitiveness of ecotoxicologically sensitive and/or ecologically vulnerable species (e.g. Klepper et al., 1999; Peijnenburg and Jager, 2003; long‐term study, Klein, 2015).
Conclusions for the risk assessment in soils
The risk assessment for soil organisms exposed to transition metals as active substances in PPP can be performed in principle as for other active substances (European Commission, 2002). As the calculation of PECs in soils is always linked to a definite time frame (choice of the number of years in which the compound is applied), the outcome of the risk assessment covers only the assessed time period.
Other soil parameters than only organic carbon content may additionally determine the fate and behaviour of transition metals compared with synthetic chemical substances. Determining the relationships between sorption, desorption, bioavailability and toxicity is central for defining appropriate exposure and assessment scenarios. Bioavailability and ageing correction approaches as proposed in ECHA guidance (ECHA, 2008) cannot be fully applied, as transition metals as PPP are freshly applied once or repeatedly in a season. Bioavailability corrections may be considered e.g. for fractions of the residues accumulating in the soils after intended uses in previous years. It is however at present not possible to generally determine whether fractions considered not bioavailable for soil organisms might be stable over years or become bioavailable again.
Following intended uses of PPP with transition metals, the loads of these compounds will inevitably increase in the environment and transition metals will accumulate in environmental sinks as soil. In addition, utilisation of the same transition metal in another PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will result in further input to the soil compartment and add to the total concentrations possibly posing risks to soil organisms. Chemical and biological/ecological monitoring is therefore needed to follow exposure and effect in the long time.
3.2.5. General conclusions and recommendations for the in‐soil compartment
The hazard and risk assessment for soil organisms exposed to transition metals as active substances in PPP can be performed in principle as for other active substances (European Commission, 2002). Problems arising from outdated guidance compared with newer legislation and data requirement apply also to transition metals and can be dealt with by applying interim solutions (EFSA PPR Panel, 2017; EFSA, 2019).
Bioavailability and ageing correction approaches as proposed in ECHA guidance (ECHA, 2008) cannot be fully applied, as transition metals as PPP are freshly applied once or repeatedly in a season. Bioavailability corrections may be considered e.g. for fractions of the residues accumulating in the soils after intended uses in previous years.
As for other compartments, also the prospective risk assessment of non‐degradable compounds in soils can only cover a defined period and is therefore strictly linked to risk management decisions. All PPP intended uses will increase the loads in the environment and uncertainties exist especially regarding the hazard assessment. In addition, utilisation of the same transition metal in another PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will result in further input to sink compartments and add to the total metal concentrations, possibly posing risks to non‐target organisms. It is therefore recommended to consider these aspects and uncertainties in any risk management decision on the approval of transition metals as active substances or the authorisation of PPPs with transition metals (e.g. comparative assessment, cost–benefit analysis, optimised application rates, etc.) and accompanied focused monitoring; it is also recommended to align the prospective ERA with the goals of other overarching legislative framework (e.g. Sustainable Use Directive, Soil protection legislations, Water Framework Directive, Drinking Water Directive).
Specific conclusions and recommendations for the soil compartment are:
As for transition metals (e.g. for copper) several endpoints exist with different organisms, soils and metal speciation, the applicability of an SSD approach should be investigated. As no guidance is currently available for soil organisms, these methodologies would be non‐standard higher tier assessments to be included in a weight‐of-evidence approach.
Long‐term field studies with e.g. earthworms are extremely useful to follow the fate, behaviour and toxicity of non‐degradable transition metals on communities in time. They should be included if available not only for risk assessment purposes but also to inform risk management decisions.
In (semi)field higher tier approaches effects on soil mesofauna (microarthropods), should be also evaluated in indicated, even if specific guidance on surrogate reference tier is lacking. Results can be evaluated similarly to earthworm field studies and following interim solutions till guidance is prepared.
Effective concentrations are not measured in tests with soil organisms. This needs to be addressed if refinement options on bioavailability are envisaged: if no exposure quantities are determined, it is difficult to related endpoints other than to PECs addressing total (nominal) metal contents.
The impact of soil properties on bioavailability and following toxicity of metals to soil organisms in agricultural field soils should be investigated systematically for the different compounds. Models relating exposure to effects should be established by following EFSA guidance on good modelling practice (EFSA PPR Panel, 2014a).
Availability/speciation of transition metals and environmental conditions affecting these processes are not considered in current EFSA exposure model calculations and the risk assessment for non‐target soil organisms. Availability/speciation highly depends on environmental conditions and chemistry in particular pH, OC, CEC and clay content. The timescale is another parameter affecting their availability/speciation.
Assumptions in current PERSAM tool and some input parameters in PERSAM model are not adapted for transition metals. For the time being, Tier 1 PERSAM–PECsoil for total metal can be used for the risk assessment of the non‐target soil organisms, but not Tier 2 calculations. Tier 1–2 PERSAM–PECsoil for pore water are not considered suitable for the risk assessment of the non‐target soil organisms.
It is concluded that no distinction between freshly added copper and aged residues in soil (background/anthropogenic concentration) in first step calculations should be performed (all considered bioavailable).
It is not sure that the specific exposure target on pore water concentrations is fulfilled with the current PERSAM tool if used for transition metals. The improvement of the assessment should be able to address the risk assessment question and the specific protection goals.
It should be explored whether the soil pore water concentration from legacy studies can be scaled e.g. as proposed in the Scientific Opinion of EFSA PPR Panel (2017) for in‐soil organisms for organic pesticides but adapted to metals.
It is not sure that the specific exposure target is fulfilled with the current model for transition metals. The improvement of the assessment should be able to address the risk assessment question and the specific protection goals.
It should be explored whether the available JRC data base for agricultural soils is possibly appropriate to analyse and define ranges for other soil parameters relevant for metal sorption (e.g. pH, CEC) and to provide natural background and anthropogenic residues data.
It should be explored if soil scenarios for the three regulatory zones could be parameterised for total and pore water concentrations for different metals. Total content might still be depending on soil densities (organic matter contents), but for pore water other parameters would need to be included. At Tier 1, crop interception and wash‐off factor are not considered in the PERSAM calculations, but the major factors affecting the sorption of transition metals (e.g. pH dependency) are not implemented in PERSAM model. Therefore, Tier 1–PEC for total concentration can be derived using PERSAM model and used for the risk assessment, but Tier 1–PEC for pore water concentration is therefore not appropriately estimated. The dependency to soil parameters e.g. pH dependency for soil sorption of transition metals can be performed at Tier 2, but crop interception is considered by model and wash‐off factor and a 10‐day degradation on leaves is assumed. Therefore, Tier 2‐PECsoil for both total and pore water concentrations are underestimated.
Spatio‐temporal dimension of the exposure regime should match the specific protection goal. In exposure modelling, usually a 90th percentile is used (EFSA PPR Panel, 2014a).
3.3. Terrestrial environment: non‐target terrestrial plants
3.3.1. Effect assessment and ecotoxicologically relevant exposure quantities
Hazard characterisation
Standard tests currently required for organic chemicals as active substances in PPP (EU No 283/2013 (European Commission, 2013a); EU No 284/2013 (European Commission, 2013b)) are also applicable to testing transition metal compounds.
Hazard characterisation for NTTP is in this respect fully in line with ECHA guidance (ECHA, 2008; and ECHA endpoint specific guidance, R.7.11 Effects on terrestrial organisms, ECHA 2017c). In addition to the data on seedling emergence (OECD, 2006b; test No. 208) and the vegetative vigour test (OECD, 2006c; test No.227) also screening data might be submitted. Screening data shall establish whether test substances exhibit herbicidal or plant growth regulatory activity.
According to EFSA PPR Panel (2014b) and ECHA (2017c), however, the available tests do not cover the whole life cycle of the plants. It has been indicated that gaps exist as tests are conducted at the seedling/juvenile stage using mostly annual crops. Moreover, effects are recorded at the vegetative stage under greenhouse conditions with plants grown individually or in monoculture. Especially seed production and germinability are not evaluated (EFSA PPR Panel, 2014b). An open point regards the sensitivity of crop plants vs. wild plants and whether fern, mosses and woody plants are covered by the current set of studies (Boutin and Rogers, 2000, Boutin et al., 2012, 2014; EFSA PPR Panel, 2014b). At an expert meeting in 2019 (EFSA, 2019), it was concluded that also endpoints describing phytotoxicity of the tested substances for plants shall be reported and might be used for risk assessment. When enough data points are available, SSD endpoints might be derived for non‐target terrestrial plants. Uncertainties on the covered species should be investigated and included in the final assessment. At higher tier, it has been criticised that monocultures are tested instead of communities; assessing multispecies interactions in microcosms or field experiments should be pursued.
Ecotoxicologically relevant exposure quantities
For NTTP, different exposure routes can be characterised, related to specific exposure quantities:
NTTP pesticidal action via leaves or translocation from leaves to roots and/or to whole plant: this exposure route is related to deposited spray/dust deposition in the in‐field and off‐field areas. EFSA PPR Panel (2014b) considers droplet drift to be the most important factor for off‐field emissions to non‐target areas and exposure of NTTP.
NTTP pesticidal action via roots or translocation from roots to plants: this exposure route is related to soil total and pore water concentrations. Please refer to Section 3.2 for discussions on the impact of soil properties on the availability and toxicity of transition metals in the soil environment. Surface run‐off may contribute to the contamination of non‐target terrestrial ecosystems in the neighbourhood of agricultural areas as well as deposition of drift to soil if not intercepted by plants (EFSA PPR Panel, 2014b).
3.3.2. Exposure assessment, fate and behaviour of transition metals above ground
In the ECHA guidance for metals (ECHA, 2008) and ECHA endpoint specific guidance (ECHA, 2017c), NTTP are grouped under soil organisms when considering the exposure route through soil. Please refer to the consideration on possible bioavailability corrections for residues in soils from previous metal intended uses applications in Section 3.2.
Pesticide ERA considers mainly the exposure via drift, and for some specific mechanisms of action also via soils.
Regarding the exposure assessment of drift droplets, please refer to the following chapter addressing the exposure in the above‐ground environment (Section 3.4).
3.3.3. Linking exposure and effects
As discussed above, different routes of exposure might affect NTTP. If a specific mechanism of action is known for the tested substance, then exposure routes might be separated (e.g. effects via translocation from the leaves). In most cases regarding transition metals, both routes of exposure will be relevant, as sprayed PPP will be deposited in different proportions on leaves and on the soil. However, available tests do not cover both exposure routes equally.
Soil concentrations of transition metals are to be linked to endpoints derived from seedling emergence tests. Both soil concentrations and sprayed deposits will be linked to endpoints from the vegetative vigour test. Soil and spray deposit can relate to phytotoxicity endpoints.
3.3.4. Risk assessment
The risk assessment for non‐target terrestrial plants can be performed for transition metals used as active substances in PPP in principle as for other pesticides. Currently, the guidance by SANCO (European Commission, 2002) is however being updated because of newer legislation in place (EC No 1107/2009). Scientific Opinion of the PPR Panels (EFSA PPR Panel, 2014b) highlights the gaps of the current guidance and proposes also options for higher tier assessment steps.
3.3.5. General conclusions and recommendations for NTTP
Standard tests currently performed for organic chemicals as active substances in PPP are also applicable to testing transition metal compounds (in line with ECHA guidance document). Phytotoxicity endpoints might be used for risk assessment.
As is the case also for other PPP, the available tests do however not cover the whole life cycle of the plants. Especially seed production and germinability are not evaluated. The sensitivity of crop plants vs. wild plants and the coverage of fern, mosses and woody plants is not fully understood (EFSA, 2014b).
When enough data points are available, SSD endpoints might be derived for non‐target terrestrial plants.
Exposure routes of NTTP are via soil and via spray/drift droplets. Please refer to the consideration regarding possible bioavailability corrections for residues in soils and to the timescale of the assessment to Section 3.2. Regarding the exposure assessment of drift droplets, please refer to Section 3.4.
3.4. Terrestrial above‐ground environment: invertebrates (Non‐Target Arthropods and bees)
Regarding above‐ground environments, there are no indications for the time being that specific provisions for transition metals as active substances in PPP need to be developed for NTA and bees.
As for organic chemicals as active substances in PPP, newest developments should be implemented also for transition metals (e.g. current update of the Bee Guidance Document EFSA, 2013b; EFSA Scientific Opinion on NTA, EFSA PPR Panel, 2015b). Under the ECHA framework, invertebrates living above ground are not specifically addressed. However, the European Commission has mandated ECHA to develop a guidance for assessing the risks to arthropod pollinators (including bees) from biocides exposure to ensure a high and harmonised level of protection of the environment, taking into account EFSA's Guidance Document on the Risk Assessment of PPPs on Bees (currently under review). The ECHA guidance is expected to be finished by the end of 2021.
3.4.1. Effect assessment and ecotoxicologically relevant exposure quantities
Hazard characterisation
Bees
The PPR Panel recommends the use of the EFSA bee guidance (EFSA, 2013b) also for assessment of transition metals. As this guidance is under update, the revised EFSA bee guidance when available should be considered for transition metals.
There are currently no references to managed bees and wild pollinators in the ECHA guidance (ECHA, 2008) nor in the ECHA endpoint specific guidance (ECHA, 2017c). However, in the ECHA guidance on the Biocidal Products Regulation (ECHA, 2018a,b) reference is made to a guidance under development (see above).
Non‐target arthropods (NTA)
According to current data requirements for NTA (Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b)), effects on non‐target terrestrial arthropods shall be investigated for all active substances if NTA are exposed. In a first step, two indicator species, the cereal aphid parasitoid Aphidius rhopalosiphi (Hymenoptera: Braconidae) and the predatory mite Typhlodromus pyri (Acari: Phytoseiidae) are tested for effects resulting from dry residues on glass plates. Refinement options include the testing on natural substrates and/or in three‐dimensional test set‐ups and/or with additional NTA species. An overview of available test guidelines is reported in EFSA PPR Panel (2015a).
Main criticisms to the selected species and exposure routes are that only so‐called beneficial NTA are tested, representing the guild of predators and exposing them only via contact. Oral exposure (e.g. for herbivores) or overspray are currently not assessed, but deemed relevant for NTA (EFSA PPR Panel, 2015a). Until further guidance is developed and adopted, interim agreements have been reached by EFSA in a recurring issue meeting (EFSA, 2019). For instance, data for herbivorous species are not requested but ‘in cases where a concern is raised (e.g. based on the mode of action of the active substance), then this should be highlighted in the risk assessment and acknowledged in the EFSA conclusion’.
Regarding higher tier testing, community composition is important, including herbivorous species and pollinators. Considerable uncertainties exist on the currently performed field tests, as the set‐up does not allow for the proper assessment of effects on very mobile species. Some aspects of De Jonge et al. (2010) as guidance for EU‐level assessments can be considered, until further guidance for the evaluation of NTA field studies is available. Concerns on the minimum time considered acceptable for an in‐field recolonisation were raised (currently 1 year).
Consequences of the year‐on‐year application of pesticides in landscapes with a high proportion of agricultural land are currently not addressed nor at lower tier nor in the field tests. Loss of NTA diversity and biomass without possibility of recovery due to poor and insufficient off‐field habitat features result also in indirect effects on the terrestrial food web and need to be considered as soon as possible according to current regulation (EC 1007/2009; EFSA PPR Panel, 2015b; Topping et al., 2015).
Ecotoxicologically relevant exposure quantities
Exposure routes relevant for NTA and bees are contact and oral exposure. While for bees the oral exposure route is covered by dedicated studies, this is not the case for NTA. The ecotoxicologically relevant exposure quantities for contact differ between bees and NTA, because of traditionally different assessment, e.g. for NTA, toxicity data are related to deposition rates while, for bees, the endpoints are expressed as doses per animal. Unfortunately, no tests are implemented covering the oral route for NTA, a data gap indicated by the EFSA PPR Panel in the Scientific Opinion (EFSA PPR Panel, 2015b).
3.4.2. Exposure assessment, fate and behaviour of transition metals above ground
For the risk assessment of the NTA (and also NTTP), the off‐field residues can be calculated using the BBA drift values table (Rautmann, 2000) except for dustable powder formulation as recommended in guidance document on regulatory testing and risk assessment procedures for PPPs with NTA (2000).
For dustable powder formulation, several concerns (crop interception and spray drift % value BBA) have been identified. The crop interception factors are designed for spray drift applications (van Beinum and Beulke, 2010; Olesen and Jensen, 2013). The use of these values in exposure calculations for dustable powder application is therefore questionable and might not be appropriate for regulatory purposes without any further detailed evidence or additional data.
The EFSA guidance document on DegT50 values (2014) underlines that ‘It is important that the crop interception factors used in the regulatory risk assessment are based on well documented data and therefore act as robust and representative values’. As a worst‐case, exposure calculations considering no crop interception in the corresponding risk assessment should be proposed.
The spray drift % values BBA (Rautmann, 2000) are designed for spray applications, the use of these values in exposure calculations for dustable powder application is therefore questionable and cannot be used for the renewal approval of the active substance without any further detailed evidence or additional data. The Scientific Opinion addressing the state of the science on risk assessment of PPPs for NTA (EFSA PPR Panel, 2015b) underlines that:
‘particulate drift can occur due to application of dust from dustable powder formulations (e.g. sulfur dusting in vineyards). Particulate drift happens generally over a short range and in short periods after application and is therefore comparable to droplet drift. The main driving force is the particle size/weight of the dust particles’.
There is a need for more information on the extrapolation of the BBA drift % value (Rautmann, 2000) to foliar dust applications before their use in further calculations.
For the bee's chronic risk assessment following the bee guidance document (EFSA, 2013b), TWA values based on the default DT50 of 10 days set in the birds and mammals guidance document are available (EFSA, 2010a,b). For transition metals these values would not be applicable as long as new data on residue measurements in pollen and nectar are lacking. The guidance on the risk assessment for bees is currently being updated.
3.4.3. Linking exposure and effects
For bees, the methodology for linking exposure and effects has been agreed under the guidance document (EFSA, 2013b). As the bee guidance is being updated, attention should be paid to possible amendments.
For NTA, deposited rates in in‐field and off‐field areas are related to test endpoints derived in tests where the substances are sprayed. If oral exposure routes to transition metals should be considered particularly relevant for NTA in future, then dedicated tests might need to be performed and endpoints possibly also be related to deposited rates.
3.4.4. Risk assessment
The PPR Panel recommends the use of the EFSA bee guidance also for assessment of transition metals. As this guidance is under update, the revised EFSA bee guidance when available should be considered for transition metals.
The first tier risk assessment for NTA can, in principle, be conducted according to ESCORT 2 guidance. EFSA (2019) includes recommendations for the use of the vegetation distribution factor (VDF, reduced to 5). During the last EU review of copper compounds, the experts did not consider the use of the Multiple Application Factor (MAFsoil) for BBCH < 20 as appropriate for NTA considering the available evidence showing that copper accumulates in soil. The PPR Panel recommends the use of a MAFsoil of 0 for BBCH < 20 and for NTA.
In the absence of robust information on the crop interception and the particulate drift for dustable powder product, worst‐case values should be used for the risk assessment of NTA.
Considerable uncertainties exist on the higher tier assessment steps for NTA. The set‐up of field studies does not allow for the assessment of effects on very mobile species. Moreover, the consequences of the year‐on‐year application of pesticides in landscapes with a high proportion of agricultural land are currently not addressed, nor at lower tier or in the field tests.
3.4.5. General conclusions and recommendations for NTA and Bees
In principle, no specific requirements for assessing the risk to NTA and bees exposed to transition metals as active substances in PPP are deemed necessary for the time being. Necessary amendment to the risk assessment procedures as for other active substances in PPP, however also apply for transition metals.
It is concluded that the spray drift % values used for other active substances are considered suitable for the spray applications of metal‐containing products. For dustable powder applications, it is recommended to use conservative drift % value in the absence of robust data.
3.5. Terrestrial above‐ground environment: Vertebrates (Birds, Mammals, Amphibians, Reptiles)
3.5.1. Effect assessment and ecotoxicologically relevant exposure quantities
Effect assessment of copper salts has been conducted following the current EFSA guidance documents for birds and mammals (EFSA, 2010a).
For birds, the acute toxicity values of transition metals are determined for the different salts used as PPPs. For copper salts, the values determined are quite similar and EFSA suggested to define copper salts as a unique entity and use the lowest value from the whole data set available. Chronic toxicity tests are available and standard toxicity testing may be used as well, although different copper salts may have different toxicity values (subchronic and reproduction no observed effect level (NOEL)). Therefore, copper salts may not be assessed as a single entity with respect to chronic toxicity testing (EFSA, 2017). It is likely that the same is true for other trace elements (for instance: Zn, ATSDR document) generally as a result of differences in bioavailability of salts via the oral route.
Effect assessment of copper and copper salts in other terrestrial species is based on mammalian toxicity studies. As for bird species, all toxicity tests are considered appropriate for the determination of toxicity endpoints according to the current EFSA Bird and Mammal Guidance (EFSA, 2010a). All toxicological endpoints from mammalian acute, short‐term or chronic toxicity studies are also considered acceptable (LD50, NOAEL).
In the exposure assessment, only oral exposure is considered (drinking water and food intake).
Because of the mineral nature of transition metals, there is no metabolism or biodegradation of the active substances used in PPPs. As a consequence, the standard models for Tier 1 repeated exposure assessment including TWA, MAF cannot be readily applied to repeated exposure assessment for copper and other transition metals risk assessment. The default DT50 of 10 days for dissipation of transition metals cannot be used. For copper, all scenarios fail as a result of the absence of degradation of their metal salts and the lack of proper dissipation studies of metal salt residues on food items. Therefore, EFSA suggested to conduct the current risk assessment based on a weight‐of‐evidence approach. Several published papers and studies were provided. In highly exposed environments (with environmental concentrations superior to concentrations resulting from the use of copper as a fungicide), it is shown that terrestrial vertebrates (voles, shrews, mice) will maintain a homoeostatic equilibrium for copper. Some field data in cattle do not indicate any evidence of increased liver concentrations of either Cu, Zn or Fe in animals grazing near mining and industrial areas in Spain (Miranda et al., 2005). There is no information on other mammalian species (medium and large herbivores for instance). There are some exceptions to the homeostasis rule, mostly sheep and other ruminant species. A genetic defect can result in Cu accumulation by these species when exposed to excessive Cu in their diet, including from soil/plant transfer (Grace et al., 1998) or consumption of forage harvested after copper fungicide application (Oruc et al., 2009; Gupta, 2018). There is no documented evidence for Cu accumulation in wild species, but wild ruminants may potentially be exposed (e.g. cervids foraging in orchards). It is therefore suggested that the potential exposure of ruminants be discussed/documented for copper compounds. Genetic defects may also affect other species. In dogs, the Wilson's disease (genetic deficiency in copper elimination) will result in chronic hepatitis in several breeds (Labrador and other). Increased Cu and Fe may occur at the same time (Fieten et al., 2013; Cedeño et al., 2016). It is not known whether other mammalian or bird species may also present this genetic trait.
Generally speaking, very similar conclusions may be drawn for other transition metals used as PPPs, such as iron or zinc. Both are trace elements and may accumulate in many species (domestic species such as cattle and dogs, see for instance Allen et al., 1983; Plumlee, 2004; Cedeño et al., 2016). Overall, Zn toxicity from environmental exposure is documented in terrestrial vertebrates (mostly in domestic species), while iron overload is only described after massive accidental intake of iron‐products (Plumlee, 2004; Buhl et al., 2013). As for copper, residue data should be provided (background levels, before pesticide application, residues after pesticide application at different time points) to develop proper Tier 1 scenarios. In the absence of residue data, or if Tier 1 scenarios fail, a weight‐of‐evidence approach should also be considered for the Tier 1 ERA of these transition metals.
For higher tier RA, EFSA considered field studies (EFSA, 2017). A drawback in these studies is that residue levels of Cu are not measured systematically. It appears necessary to have residue studies conducted according to guidelines and in accordance with the GAP with levels of copper measured before/after each treatment for correct exposure assessment (i.e. taking into account background Cu residue concentrations).
Exposure assessment via drinking water based on the puddle scenario under the current guidance document is acceptable.
Secondary poisoning is only assessed via a weight‐of‐evidence approach for earthworm‐eating birds (extrapolated to mammals) and fish‐eating vertebrates. In the current EFSA guidance document, to assess secondary poisoning in earthworm or fish‐eating birds and mammals, appropriate BAF and BCF need to be established. As stated in the Environmental Risk Assessment of 2018 (EFSA, 2018) there is generally accepted information about bioaccumulation of Cu in aquatic invertebrates and vertebrates, confirming the general homeostasis of Cu in living organism. As a consequence, BAF, BCF do not really make sense for well‐regulated essential elements such as Cu.
A specific guidance has been developed by ECHA guidance document (ECHA, 2008) for metals and metal products and may be recommended as an appropriate approach to assess secondary poisoning of transition metals such as Cu, Fe or Zn for fish‐eating birds and mammals, with recommendations to establish BAF, relative absorption factor (RAF) (ECHA, 2008, Appendix R.7.13‐2). In the same document, a general recommendation to evaluate the risk of secondary poisoning of earthworm‐eating vertebrates is also suggested, especially to estimate properly the concentration of minerals and their bioavailability from earthworms including gut residues due to the ingestion of contaminated soil by earthworms (see below).
In the 2017 assessment of Cu compounds (EFSA, 2017), the weight‐of‐evidence approach provided some information with respect to the lack of evidence of higher risk for earthworm‐eating birds living in orchards sprayed with copper compounds. Some evidence was also provided with respect to the lack of risk of bioaccumulation in the aquatic food chain, although the studies provided did not focus on the organisms of concern (mollusc) in the aquatic food chain. ECHA guidance provides a scientifically sound basis to estimate secondary poisoning from trace elements in general in relevant food chains.
The Tier 1 approach uses the lowest NOEC oral divided by a default AF and no specific adjustment is made for species differences in diet composition, body weight of Food Intake Rate (FIR).
The Tier 2 approach includes relevant diets and FIR adapted to the relevant species (i.e. species more relevant to assess food chains based on the contaminated prey items). To derive species‐specific FIR, ECHA guidance (ECHA, 2008, Appendix R.7.13‐2) suggests allometric scaling and an AF of 10.
As a refinement and to take into account the relative bioavailability of metal compounds the ECHA guidance recommends to establish a RAF based on the dietary item investigated (prey, soil…) compared with the highly soluble salts generally used in toxicity testing. This RAF is obtained from bioavailability studies and a weight‐of‐evidence approach. To refine the RAF, data on food consumption is essential but rarely available for most species. Furthermore, RAF may vary depending on the dietary item.
Ecotoxicologically relevant exposure quantities for terrestrial vertebrates via oral and dermal exposure
Currently only the oral exposure is being considered for risk assessment in birds and mammals for both biocidal products and PPPs.
The available information on copper absorption through the skin (EFSA, 2017) considers a 1% absorption for copper concentrated solutions and 9% for diluted solutions.
Dermal toxicity tests of several copper salts have been reviewed. All acute toxicity values were > 2,000 mg/kg body weight.
At that stage, dermal absorption does not appear to play a significant role in the overall exposure of birds and mammals. High risk of dermal exposure and absorption has been identified, however, for amphibians and, to a lesser extent, reptiles as a result of direct overspray or contact with sprayed crops. Currently, there is no regulation or guideline to conduct appropriate risk assessment via dermal uptake for these species (EFSA, 2018).
3.5.2. Exposure assessment, fate and behaviour of transition metals above ground
Drinking water risk assessment for terrestrial vertebrates
For the active substance having an estimated Koc value being > 30,000 (assuming 5% organic carbon in soil), the effective application rate is calculated by multiplying the proposed application rates by MAF values based on the DT50 in soil (EFSA, 2009); for copper a soil DT50 is not available, therefore as a worst‐case approach the maximum yearly application rate for is assumed to be the maximum effective application rate.
MAF value to be used in the assessment: See Point 3.5.1.
3.5.3. General conclusions and recommendations
The recommendation for birds and mammals is to:
conduct acute/repeated toxicity studies as requested by the current EFSA recommendations to establish proper toxicity endpoints;
it is suggested to document residue levels of transition metals used as PPPs to perform exposure assessment and risk assessment with appropriate data;
whenever needed, potential exposure of domestic or wild ruminant species should be discussed;
water exposure scenarios can be used to estimate drinking water exposure;
secondary poisoning for earthworm‐ or fish‐eating birds or mammals should be considered either via a weight‐of-evidence approach or using the current ECHA guidance for chemical safety assessment; ECHA, 2008, Appendix R.7.13‐2;
dermal exposure is not taken into consideration but may have to be considered for amphibians.
3.6. Leaching to groundwater
3.6.1. Assessment
The leaching of transition metals to groundwater due to the long‐term uses of transition metals‐containing PPPs cannot be excluded.
The authors Ballabio et al. (2018) underlined that ‘Over the past decades, Cu has been extensively used as a fungicide, especially in vineyards to combat mildew. The application of Cu has created an issue with the accumulation of Cu in vineyard soils and groundwater (Jacobson et al., 2005; Komárek et al., 2010)’. From the existing data reviewed at the EC level, copper concentrations are usually below the legal limit of 2 mg/L set by the European Drinking Water Directive (Council Directive 98/83/EC17) for groundwater (Vol. 3 B8 (CP), RAR Copper Compounds, 2017). For transition metals, an exceedance of the limit set by the European Drinking Water Directive (Council Directive 98/83/EC) for groundwater leads to the exclusion at the local/regional/national level. It is noticeable that these concentrations reflect the contribution of all potential sources of contamination (natural and anthropogenic entries) and the effect of the environmental conditions.
For the anthropogenic entries, a gradient of transition metals concentration along the soil profiles is usually observed suggesting a low mobility in soil, especially for copper compounds. The transfer of transition metals to deeper soil by leaching can occur either associated to fine soil particles and/or in free form.
The major parameters affecting the leaching of transition metals in the soil are related to the soil characteristics (e.g. soil pH, soil texture, CEC, OM, clay content, concentration of metals in soils,…). As an example, a higher proportion of dissolved copper fraction in the liquid soil phase and leachate would be only expected in strong acidic soils, because the solubility of free copper ions increases with pH below 5 (Vol. 3 B.8 (CP), RAR Copper Compounds, 2017). Highest copper concentrations were found in aquifers with lowest pH, related to infiltration of acidic surface waters or for volcanic aquifers (Vol. 3 B8 (CP), RAR Copper Compounds, 2017). For transition metals, the capacity to leach can be also due to other significant processes, many of which are irreversible (Vol. 3 B.8 (CP), RAR Copper Compounds, 2017). Adsorption to clay and mineral oxides can occur at all depths in the soil column and not just at the surface layer as is the case for organic matter interactions (Vol. 3 B.8 (CP), RAR Copper Compounds, 2017). Increased sorption with time (aged sorption) is a relevant process for transition metals but no guideline on how to derive reliable input parameters for transition metals exists. Other parameters can affect their distribution in the soil profiles: the agricultural practices (e.g. long‐term uses, mechanical disturbance of the soil profiles, man‐made mechanical mixing of topsoil with uncontaminated or lesser contaminated subsoil, …) (Vol. 3 B.8 (CP), RAR Copper Compounds, 2017; Ballabio et al., 2018) and the climatic conditions.
As part of the Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b), the risk to groundwater contamination of active substances and its relevant soil metabolites (SANCO/221/2000 rev. 10 – final; European Commission, 2003) has to be assessed.
For the time being, the current FOCUS models (European Commission, 2014) have been considered suitable and used to estimate the potential leaching of transition metal in groundwater compartment.
Selection of input parameters to be used in modelling:
To reflect the persistence of metals in soil a DegT50 value of 1,000,000 days should be used. For persistent organic compound recently reviewed at the European level (Draft assessment report (DAR) of pydiflumetofen, 2018), it has been requested to perform PECgw calculations for a longer period than the recommended period and to provide material balance in the soil column to cover the long‐term groundwater exposure for a persistent compound. For this reason, the FOCUS simulations for transition metals should be performed for a period sufficiently long to cover the long‐term groundwater exposure. For the time being, there is a possibility to run the FOCUS groundwater models for a period of 66 years from the existing climatic files. Application(s) every year and the agricultural background concentration can be considered in the modelling. If longer period is recommended, the existing climate files in FOCUS tools can be adapted without changing the model code; climatic series of hundreds of years can be created manually and then implemented into the FOCUS tools. Changing the climate files results in an adapted FOCUS tool (please see the recommendations on the development of models).
Vapour pressure and Henry constant should be fixed to a minimal value.
Regarding the soil mobility, the OECD guideline No. 106 (OECD, 2000a) is recommended for deriving Kd/Kf values normalised organic carbon content of soil to characterise the soil mobility of compounds. The empiric Freundlich model is used to describe the sorption isotherm with two parameters (Kf and 1/n); these parameters can be used in the FOCUS modelling. Usually, the measured concentrations in solution is interpreted as total metal concentrations in solution. However, ‘the total metal concentration in solution can be divided into free, inorganically complexed and organically complexed metal concentrations’ (Elzinga et al., 1999). Furthermore, metal that is naturally present in the natural soil before spiking of the item test, could affect the estimation of the sorption parameters. Therefore, the approach by Degryse et al. (2009) could be considered to derive reliable Kd values (initial transition metals concentration in soil before spiking should be taken into account in the sorption test).
It is noticeable as concluded by the authors Elzinga et al. (1999) that ‘field application of the isotherms for Cu (and for Cd and Zn at the larger concentrations) requires corrections for discrepancies between laboratory and field partitioning. The Freundlich adsorption models underestimated metal contents determined from 1 mol/L HNO3 digestion on field samples, up to a factor of 6 (Cd and Cu) or 10 (Zn)’.
The concept in which the coefficient for sorption on soil organic matter, Kom, is used to calculate the coefficient Kf for sorption on the whole soil does not hold for metals as soil constituents like clay minerals or iron and aluminium hydroxides and other soil properties (e.g. soil pH, CEC) also may play a major role in the sorption of metals. Therefore, a more general provision is needed in the model. In analogy to Kom, a coefficient Ksc for sorption on a soil constituent could be defined. The content of the soil constituent, msc (kg per kg), is then specified as a function of depth in the soil. The sorption coefficient Kd for pesticide sorption on whole soil in the various horizons could then be calculated by: Kf = msc × Ksc.
In cases in which a metal is sorbed on two soil constituents, e.g. organic matter and clay, the user should specify the equivalent values of Ksc and msc, using the information on the relative strength of sorption of the pesticide to the two soil constituents (ECHA, 2008).
The dependency of sorption parameters to soil properties can be taken into account for selecting modelling endpoint to be used in FOCUS modelling with the nine FOCUS standard scenarios whether it is demonstrated correlation with soil parameters (EFSA PPR Panel, 2013a). However, as acknowledged in the EFSA Scientific Opinion on lower tiers (EFSA PPR Panel, 2013b) the vulnerability of the FOCUS standard scenarios with respect to leaching is determined by the organic matter content and texture only. Therefore, using soil properties other than organic matter and texture may significantly change the target vulnerability of the scenario. As stated in EFSA Scientific Opinion (EFSA PPR Panel, 2013a) it is not recommended to use scenario‐specific properties. Instead it is recommended to perform calculations with two sets of parameters for contrasting conditions. Contrasting conditions have to be defined in agreement with the major soil properties affecting the soil mobility of transition metals. Selection of such conditions can be metal specific. As an example, low pH, low OC content and low clay content should be selected for copper as worst‐case conditions for deriving modelling endpoints, while low potential redox and low soil pH should be considered for iron. The soil data set in the PERSAM tool could be used for selecting worst‐case topsoil conditions for developing scenarios for groundwater exposure modelling.
Kd value should be representative to agricultural soils at country/EU level. If a Kd distribution is available, a low‐end value (e.g. 10th percentile) and a high‐end value (e.g. 90th percentile) are selected for the sensitivity analysis. For a limited data set, the minimum and maximum must be used as lower and upper bounds as worst‐case scenarios. The results of the uncertainty analysis can be used to check the robustness of the risk evaluation and could trigger further refinements when needed. The representativity of the data available for the sites under assessment must also be discussed (ECHA, 2008).
A detailed statistical analysis of data should be given. All individual Kd values and the characteristics of the soils should be made available.
As specified in the EFSA guidance document to obtain DegT50 values (2014), the FOCUS group recommends using the arithmetic mean of the Freundlich coefficient (1/n) from the available reliable adsorption studies in modelling calculations. In the absence of reliable data, a default value of 1 for 1/n should be considered.
For transition metals used as PPPs the agricultural background level from agricultural fields would be relevant. A representative agricultural background concentration in soil related to the crop should be included in modelling. The monitoring data should not be discarded without robust scientific evidence. The higher results due to the use of copper in agricultural practices should not be considered as an outlier. Please also refer to Part 4 on Criteria for performing and evaluating environmental monitoring studies.
It should be kept in mind that the background concentration in groundwater estimated from the monitoring data and/or surveying data cannot be dealt in modelling purpose (Gimsing et al., 2019). Please also refer to Part 4 on Criteria for performing and evaluating environmental monitoring studies. Firstly, the monitoring/surveying data do not refer specifically to agricultural practices only (contribution of the different potential sources and upstream catchment). Secondly, the FOCUS modelling gives concentrations of transition metals at the bottom of the soil profiles (defined at 1 m), whereas the monitoring data are from the aquifer. Both types of results cannot be combined together without introducing uncertainties.
Time affecting the soil availability of transition metals (ageing process), the freshly added soils and the aged soil residues (agricultural background concentration) should not be considered in the same way in modelling.
To take into account these abiotic processes, inclusion of speciation and ageing submodels in the existing FOCUS models could be needed to produce a realistic worst‐case risk assessment.
The uniform principles decision‐making criteria 2.5.1.2 (i) as last amended refer to Council Directive 98/83/EC. So, the parametric drinking water limit is the threshold values specified for different transition metals set by European Drinking Water Directive (Council Directive 98/83/EC) as specify in the Regulation 2018/67618. The drinking water limit of 0.1 μg/L that applies to organic pesticides would only be relevant for organometal compounds.
A brief summary of the risk assessment conducted at the European level for some transition metals
Regarding potential for groundwater exposure, due to the very low water solubility of ferric phosphate (EFSA, 2015), the experts agreed that there was no need to derive predictive environmental concentration in groundwater (PECgw) for this compound. Should any ferric phosphate be physically moved into deeper soil layers via preferential flow it could never be present in groundwater above the relevant parametric drinking water limit due to its very low water solubility (1.86 × 10−6 μg/L at 25°C). The dissociation products (Fe3+, HPO4 2− and or H2PO4 −) will only ever be present in the soil solution in very low amounts. HPO4 2− and H2PO4 − can be considered to be ‘transformation products of no concern’ (SANCO/221/2000 rev. 10 – final; European Commission, 2003). Regarding the iron ions, the concentration of the two possible iron ions in groundwater originating from the uses evaluated could not be above the indicator parameter of 200 μg/L set for iron by Council Directive 98/83/EC.
For iron sulfate (EFSA, 2012a), the ‘Flux method’ based on the FOCUS scenarios (water volume percolated at 1 m depth) and the annual applied rate was considered for estimating PECgw of iron and sulfate ions. The resulting PECgw for iron is approximately 730 times the maximum allowed concentration in drinking water, indicating a potential for groundwater contamination of iron following the representative use of iron sulfate. The relevant EU drinking water indicator parameters set by the drinking water directive are 250 mg/L (sulfate) and 200 μg/L (iron). It is noted that these calculations are overestimates, based on worst‐case assumptions. In addition, this method is no longer recommended at the European level.
For copper compounds, the potential for groundwater exposure of copper compounds has to be addressed as they are not considered as ‘products of no concern’ (SANCO/221/2000 rev. 10 – final; European Commission, 2003). The leaching of copper compounds in soil was expected to be limited due to their relatively high soil adsorption coefficient (Kd). However, Ballabio et al. (2018) cited some references indicating that:
‘Over the past decades, Cu has been extensively used as a fungicide, especially in vineyards to combat mildew. The application of Cu has created an issue with the accumulation of Cu in vineyard soils and groundwater’ (Jacobson et al., 2005; Komárek et al., 2010).
As part of the Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b), the current FOCUS models have been considered suitable and used to estimate the potential leaching of ‘total copper’ in groundwater compartment. Experts’ discussion on the selection of modelling endpoints conclude that a DT50 value of 1,000,000 days should be used to reflect the persistence of copper in soil. As there were indications that adsorption might be pH dependent, it was agreed to use a geometric mean K doc value calculated from soils in the pH (CaCl2) range 4–5. Simulations were carried out using the standard approach for implementing adsorption reduction with depth based on organic carbon as defined for the FOCUS scenarios. Please also refer to the previous recommendations for deriving the K doc values in the sorption test with natural soils. The estimated agricultural background concentration has been included as input parameters to reflect the influence of the long‐term use of copper. The established agricultural background concentration of copper in soil before the first application of the assessed formulated product as fungicide is implemented in the FOCUS groundwater modelling. The default depth factor (that indicates the relative transformation rate in the soil layer) is kept. For copper compounds, FOCUS groundwater modelling was performed for 26 years (EFSA, 2017). The potential for groundwater exposure from the representative uses by the copper compounds above the parametric drinking water limit (European Drinking Water Directive (98/83/EC) for pesticides was concluded to be low in geoclimatic situations that are represented by all nine FOCUS groundwater scenarios.
3.6.2. Conclusions and recommendations for groundwater
The leaching of transition metals to groundwater due to the long‐term uses of transition metals‐containing PPPs cannot be excluded.
Factors affecting the leaching of transition metals into the soil should be considered in modelling.
The selection of input parameters should allow reaching the exposure target.
For the time being, neither guidance document nor a specific model exists to assess leaching of transition metals to groundwater in the context of the PPP.
Following an amendment to the uniform principles, existing drinking water limit set by European Drinking Water Directive (Council Directive 98/83/EC) should be considered for transition metals when available for the transition metal assessed (Table 4).
Table 4.
The following should be considered
| Exposure target: | 90th‐percentile concentration in the intended area of use |
|---|---|
| Models to be considered: | FOCUS models/FOCUS scenarios |
| Selection of parameters: | Transition metals |
| Crop interception | Fixed to default value; no degradation on crops |
| DT 50 | Fixed to default value (DT50 should be aligned with the modelling period; no degradation) |
| K f |
Should reflect the dependency to several soil properties when needed Should be representative of the environmental conditions encountered in the region of interest Selected in line with the recommendations of the FOCUS/EFSA documentation |
| 1/n | Arithmetic mean of reliable data; 1 in the absence of data |
| Henry constant | Fixed to default value; no volatilisation |
| Water solubility: | Fixed to a minimal value |
| Plant uptake factor | Fixed to default value |
| Agricultural background concentration: | Should be established in accordance to the intended use |
| Period to assess: | Sufficiently long to cover the long‐term groundwater exposure |
For transition metals considered as relevant in the SANCO document (SANCO/221/2000 rev. 10 – final; European Commission, 2003), the PPR Panel recommends:
To consider the FOCUS models that are already agreed at the EU level.
To use modelling endpoints adapted for the transition metals (especially for DT50 and Kd values). To produce a conservative risk assessment, the modelling endpoints (especially the Kd value) should be derived for worst‐case conditions, maximising their mobility into the soil profile. The modelling endpoints should be selected to reach the defined exposure target (90th‐percentile concentration in the intended area of use).
To assess the risk to groundwater contamination with the agreed European FOCUS models for a period sufficiently long to cover the long‐term groundwater exposure to reflect the influence of the long‐term use of transition metals as PPPs. For the time being, there is a possibility to run the FOCUS groundwater models for a period of 66 years from the existing climatic files. Application(s) every year and the agricultural background concentration can be considered in the modelling. If longer period is recommended, the existing climate files in the FOCUS tools can be adapted.
Due to the soil persistence of transition metals, the agricultural background concentration in soil has to be included in the groundwater modelling to reflect the influence of the long‐term use of transition metals as PPPs. The estimation of this agricultural background concentration in soil should be established from relevant scientific data.
The major abiotic parameters affecting the soil mobility of transition metals and therefore the leaching to groundwater should be included in the modelling.
To better reflect the fate and behaviour of transition metals in the soil profile, other processes (e.g. speciation/availability) affecting their leaching in the soil profile could be included in the modelling.
Should soil properties implemented in the FOCUS groundwater scenarios be adapted for groundwater modelling purpose of transition metals, the recommendations of the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed to ease the assessment of such scenarios (please also refer to recommendations identified under Part 3.1.5 on the development of updated scenarios).
To ease the risk assessment of transition metals in the context of PPPs, guidance on how to conduct the risk assessment for groundwater is needed.
Models should be documented in a transparent way and include all information needed for the risk assessor to evaluate the model. All models should be accompanied by a detailed user manual. The procedures for obtaining the pesticide properties and uses should be clearly described and wherever possible harmonised procedures must be used to avoid user subjectivity. A qualitative assessment of the uncertainties should be provided (EFSA PPR Panel, 2014a).
It should be demonstrated by the applicant that the specific protection goal is satisfied as part of the Regulation (EC) 1107/2009 with the assumptions implemented in model and the characteristics of the developed scenarios (EFSA PPR Panel, 2014a).
The development of exposure scenarios for specific transition metals needs to be backed up by representative data on their distribution in relevant European agricultural landscapes.
Ideally, the parameterisation of adapted and/or new exposure scenarios needs to be agreed at European level. If it should not be possible to deal with it in a dedicated working group, it is suggested to discuss the available data and the possible choice of parameters for scenario parameterisation with respect to higher tier approaches with competent authorities in the framework of active substance evaluation, so that a peer review can take place.
3.7. Addressing uncertainty in the risk assessment
The EFSA Scientific Committee (2018) ‘Guidance on Uncertainty in EFSA Scientific Assessment’ provides specific guidance on the treatment of uncertainty when standardised assessment procedures are being developed. All the uncertainties that affect assessments for which a standardised procedure is being developed need to be identified and described. As many sources of uncertainty as is feasible should be considered.
The uncertainties are potentially greater for transition metals than for synthetic organic chemicals used as PPPs owing to the consideration of persistency and bioavailability. The question is therefore whether increasing the AF can cover the uncertainties. However, the essentiality of some transition metals limits the size of the AF from a scientific perspective. General AFs are not suggested as the uncertainty assessment is compound and compartment specific. Furthermore, specific uncertainty will depend on the information provided and will therefore be distinctive for each assessment.
In the following Table 5, sources of uncertainties in the risk assessment of transition metals are identified and evaluated for their potential impact on the risk assessment also in comparison with the risk assessment of organic compounds used as PPP.
Table 5.
Relevant issues to be considered, sources of uncertainties and their effect on the environmental risk assessment of transition metals in aquatic and terrestrial environments
| Relevant issues, sources of uncertainty | Potential to underestimate the risk in the field | Potential to overestimate the risk in the field | Impact on risk assessment | Difference to ERA of synthetic organic PPP |
|---|---|---|---|---|
| Importance of oral uptake of feed for overall body burden | In standard ecotoxicology studies feed is not contaminated (only partly in earthworm tests), but may contribute to toxicity | Feed may reduce the uptake of waterborne copper by fish | Impact considered to be more relevant for the chronic risk assessment | Similar uncertainty; it should be however be considered that metals bound to food might be remobilised in the gastrointestinal tract due to low pH values and are then present as free metal ions |
| Organisms acclimate to metals | Laboratory organisms may be less sensitive than natural communities | Natural communities may be less sensitive than laboratory organisms | Organisms should be cultivated at concentrations normally used in culture media and should not be pre‐exposed to elevated metal concentrations. Control/background concentrations needs to be measured and kept very low in test | Same uncertainty |
| Consideration of homoeostasis | High environmental concentrations may overcome the homoeostasis mechanisms in acute and chronic exposure scenarios | Applying assessment factors (AF) may lead to concentrations below the natural background concentration. Applying AF may indicate a risk even though homoeostasis keeps internal concentrations low | Added risk approach might be considered | Not relevant |
| Sensitivity of life stages | Certain life stages could be more sensitive than the ones tested | Certain life stages could be less sensitive than the ones tested | A range of life stages needs to be tested | Same uncertainty |
| Hormesis (enhanced performance at low levels of induced stress) | EC10 extrapolated below lowest tested concentration may be overestimated | Same uncertainty | ||
| Study duration | Effects due to repeated and long‐term exposure of non‐degradable metals not addressed in standardised studies | Ageing and pre‐equilibration of metals leads to lower bioavailability; dissipation of accumulated metal may occur with time | Accumulation to be considered in PECplateau as PPP are repeatedly freshly added; ageing may be considered in refinement for PECplateau | Same uncertainty, limited study duration fails to address risk to future generations |
| Equilibrium between water and sediment | Metal may transfer to the sediment leading to lower exposure | Metal may be released from sediment leading to higher exposure | Concentration needs to be measured in both water and sediment | Same uncertainty for strongly adsorbing organic PPP |
| Influence of bioavailability on toxicity | Physico‐chemical parameters may increase bioavailability | Physico‐chemical parameters may decrease bioavailability | Normalisation required for comparability of studies, if influencing parameters are known and models linking exposure and effects are validated | Relevant for certain organic PPP (e.g. pyrethroids) |
| Bioavailability depends on physico‐chemical parameters of the water, sediment or soil | Bioavailability may be higher in natural environments | Bioavailability may be lower in natural environments | Scenarios need to include a realistic range of physico‐chemical parameters to identify worst‐case combinations (especially pH and organic matter) | Bioavailability depends only on physico‐chemical characteristics of active ingredient |
| Type of sediment/type of soil | Sediment in study may have a high AVS content, soils used in studies might have higher binding capacities, decreasing bioavailability | If soil with low AVS/low organic matter/low pH/low CEC is used in the studies, bioavailability is increased, but might be lower in the field | AVS content in sediment/soil parameters need to be known | Type of sediment/soil will also influence the toxicity of organic PPP |
| Timescale | Modelling period too short (according to the soil persistence of metals) | Period considered in modelling should be sufficiently long to cover soil persistence of transition metals | Same uncertainty for persistent PPP | |
| Ageing process | Bioavailable fraction may decrease in soils over the time | Same uncertainty | ||
| Temporal and spatial variation of AVS in sediment | AVS only relates to the uptake via pore water and has no impact on the uptake via diet, chronic exposure for certain sediment species may be underestimated | Different sediment species need to be tested to cover difference in uptake | Not relevant | |
| Bioavailable metal fraction changes with time | Metal pools are assumed not to be bioavailable, but they become available (again) with time | Metal pools are assumed to be bioavailable, but they are not | Time dependency of adsorption and desorption of metals to soil component need to be investigated | Not relevant |
| Range of Kp values |
Water column: high Kp: more metal sorbed Sediment: low Kp: more metal dissolved |
Water column: low Kp: less metal sorbed Sediment: high Kp: more metal sorbed |
Sensitivity analysis for exposure assessment needed | Not relevant |
| Use of the chelating agent EDTA in the dilution medium | Chelating agent may reduce the bioavailability | The use of chelating agent should be avoided when feasible | Same uncertainty depending on PPP | |
| Exposure routes | Relevant exposure route not taken into account | Relevant exposure route not taken into account | Exposure models need to be developed which adequately address all relevant exposure routes for metals | Same uncertainty |
| Type of waterbodies considered in exposure calculations | Waterbodies may be very shallow and temporary | Waterbodies may be larger than assumed or the water residence time lower | Different types of waterbodies (e.g. stream, ditch, pond) should be considered | Same uncertainty |
| Soil properties in the existing FOCUS models | Major factors affecting the soil mobility of metals not taken into account | Adaptation of groundwater scenarios needed | Not relevant | |
| Agricultural background concentration in soil | Background concentration considered as freshly added residues | Inclusion of agricultural background level related to the target areas needed | Not relevant | |
| Background concentration in sediment | Background concentration considered as freshly added residues | Inclusion of agricultural background level related to the type of water bodies and target areas needed | Not relevant | |
| Non‐constant exposure in Tier 1 tests with soil organisms | Exposure profile in the field might result in higher exposure quantities (area under the curve) | Exposure profile in the field might result in lower exposure quantities (area under the curve) | Concentrations needs to be measured over the tests with soil organisms | Same uncertainty |
| Risk mitigation measures (RMM, e.g. vegetative buffer strips) | Exposure reduction calculated for RMM as for organic compounds (FOCUS, 2007) could lead to an underestimation of the risk, e.g. due to run‐off/soil erosion of metals |
Appropriateness of the existing mitigation measures to transition metals. Safety precautions can be proposed (e.g. SPE 3 to protect groundwater/aquatic organisms do not apply to (soil type or situation to be specified) soils) |
RMM were developed with organic PPP in mind |
4. Consideration of environmental monitoring study results in the ERA of transition metals
4.1. Aims and purposes of environmental monitoring
Environmental monitoring investigates in recurrent studies the environmental media loads with e.g. particular chemicals and might include also the appraisal of the state of biological communities in the probed compartment. The specific aims of monitoring studies greatly diverge and, depending on their purpose and the requirements to the set‐up, performance and evaluation of monitoring results are different.
Within the aim of this statement, three different levels of environmental monitoring can be described, serving different purposes in the ERA of transition metals. It is proposed to differentiate between general monitoring data evaluation before dossier submission, post‐registration monitoring following eventual active substance approval and PPP authorisation and targeted long‐term studies accompanying authorisation and investigating specific relationships between e.g. application rates and biological responses. These recurrent investigations of the state of the environment are conceptually not fully separated, insofar as e.g. targeted long‐term studies might aid the pool of post‐registration monitoring activities or post‐registration monitoring data might help understanding overall environmental loads. Therefore, the same data can be allocated to different data pools and used for different purposes (e.g. as supporting information, to address remaining uncertainties or to clarify causalities in compartment exposed to multiple residues). The separation was chosen to allocate the different activities to different stages of ERA of transition metals.
4.1.1. Monitoring of the actual environmental transition metals loads
Monitoring the actual contents (e.g. concentrations) in the different environmental media serves the purpose of giving an overview of the presently reached chemical loads. In the purpose of this statement, such data will be needed in the preliminary phase to the assessment of transition metals as active substances in PPP (see further below). If performed in areas with different and known land‐use histories, monitored contents of transition metals in abiotic and biotic matrices can help to distinguish natural from anthropogenic backgrounds. Metals are traditionally well measured in international and national monitoring campaigns, for which the measurement of metal contents in abiotic matrices is far more extensive (e.g. in soils; Ballabio et al., 2018) compared with the evaluation of biotic matrices (e.g. Fliedner et al., 2014).
The published scientific literature contains information on transition metals monitored in the environment. This should be performed in the context of the systematic review using the EFSA guidance (EFSA, 2011) to be provided by applicants. Moreover, national and international monitoring databases should be mined for data on metals. Especially, monitored data from areas and regions where the transition metal is intended to be (or has been) used are considered to be very useful, as they may be most representative in terms of spatial distribution and characteristics of the environmental matrices. National and international monitoring databases should be made accessible to the public.
The discrimination between natural background and added anthropogenic residues due e.g. to application of pesticides or contaminated manure can help to evaluate the consequences of repeated past applications (e.g. Panagos et al., 2018).
The data mined in the preliminary phase of the General Framework proposed will be used to inform the first step of the risk assessment by indicating the level of the natural or pre‐existing contamination. Background levels or accumulated residues should be considered in the calculation of PEC, especially in compartments known as sinks (i.e. soils and sediments).
To inform risk assessors and risk managers, it is proposed that data on environmental monitoring of transition metals are carefully collected, evaluated and submitted with dossiers aiming at supporting the (renewal of) approval or the authorisation of transition metals in PPP. As it might be very challenging to obtain suitable monitoring data representative in terms of spatial distribution (e.g. consider patchiness) and characteristics for all environmental matrices (soil, sediment, surface water and groundwater) across the European Member States, it is recommended to develop and agree on appropriate frameworks. Such needs have been already put forward for specific matrices (e.g. for sediment, EFSA PPR Panel, 2015b).
In the Scientific Opinion on sediment organisms (EFSA PPR Panel, 2015b), it has been identified that further monitoring data on pesticide concentrations in sediments are needed. It is recommended to monitor sediment concentrations of transition metals especially in edge‐of‐field small water bodies not considered in wider monitoring schemes (e.g. WFD).
4.1.2. Post‐registration monitoring
For PPPs, post‐registration monitoring differs from general monitoring in that active substances have already been authorised in formulated products applied according to intended uses. Post‐registration monitoring is currently implemented in the ERA procedures for groundwater. There it is foreseen that, if sampling programmes detect active substance residues above regulated limits (e.g. 0.1 μg active substance/L in groundwater), then dedicated post‐registration monitoring programmes need to be performed to clarify findings and possibly lead to regulatory actions (e.g. Aden et al., 2002; European Commission, 2014; Gimsing et al., 2019). Furthermore, post‐registration monitoring has already been suggested in the Scientific Opinion for the assessment of exposure of organisms to substances in soil (EFSA, 2010b) for substances that build up residues on a timescale of at least 5 years. Although it is acknowledged that it is ‘very difficult to design monitoring programmes such that results can be linked to a specific use of a single a.s. in a specific crop while also excluding other confounding factors’, it has also been suggested in the aquatic guidance document (EFSA, 2013c).
Only seldomly post‐registration monitoring has been implemented for other environmental areas. In the first approval of copper as active substance in PPP, the European Commission has defined under:
‘Particular conditions to be taken into account on short‐term basis by Member States in relation to the granting of authorisations of PPPs containing copper compounds’ that ‘Member States shall initiate monitoring programmes in vulnerable areas where the contamination of the soil compartment by copper is of concern, to set, where appropriate, limitations such as maximum application rates (European Commission, 2009a,b,c).’
This provision has triggered monitoring programmes in several Member States (e.g. Riepert et al., 2010, 2012). By contrast, the monitoring of communities and of biological probes is very rarely performed, even if it has been identified as a very relevant tool to follow up the long‐term effects of pesticide applications (e.g. Frische et al., 2016; Vijver et al., 2017; Topping et al., 2020).
Post‐registration monitoring studies can provide relevant data on concentrations and trends over time in the environmental compartments such as soil sediment and water in agricultural areas where transition metals as active substances in PPP are applied.
The EU Directive to achieve a sustainable use of pesticides (European Parliament (EC) Directive 2009/128/EC) indicates that ‘it is necessary to measure the progress achieved in the reduction of risks and adverse impacts from pesticide use for human health and the environment’. A strategic guidance document on monitoring and surveying the impacts of pesticide use has been developed by the European Commission (European Commission, 2017). The guidance indicates that the main objectives for monitoring of PPPs are to follow up on decisions concluding on the absence of unacceptable effects on, among other things, the environment when PPP were authorised under Regulation (EC) No 1107/2009. Monitoring is envisaged for specific active substances in cases it is required according to Art. 6(i) and Art. 67(2) of Regulation (EC) No 1107/2009. These articles state that:
‘approval may be subject to conditions and restrictions including: (i) the need to impose risk mitigation measures and monitoring after use’ (Art. 6)
and that, for control and record‐keeping provisions:
‘producers of PPPs shall undertake post‐authorisation monitoring on the request of the competent authorities’ (Art. 67(2)).
It is proposed that, if conclusions on low risk for non‐target organisms exposed to transition metals following PPP intended uses have been reached at higher tier level by implementing novel risk assessment refinements regarding bioavailability (e.g. bioavailability correction of transition metal residues and/or to binding sites in the organisms), they should be backed by strong risk management decision and post‐registration monitoring of possible long‐term effects should be implemented.
Especially the non‐degradability of transition metals in the environment and the related uncertainties to long‐term effects underpin the post‐registration monitoring requirements. To keep monitoring of PPPs proportionate, it should focus on those environmental compartments and non‐target organism groups identified as being vulnerable.
As the authorisation holders of a PPP has the responsibility to immediately notify ‘any new information concerning the PPP which could suggest it does not comply anymore with the criteria set out in the Regulation (Art. 56 of Regulation (EC) No 1107/2009)’ (European Commission, 2017) monitoring outcomes will help to identify possible adverse effects on the environment in the long term.
This proposal is in line with the authorisation and use cycle of PPP at EU level, as described by the guidance document on monitoring (European Commission, 2017) and shown in the following Figure 3.
Figure 3.
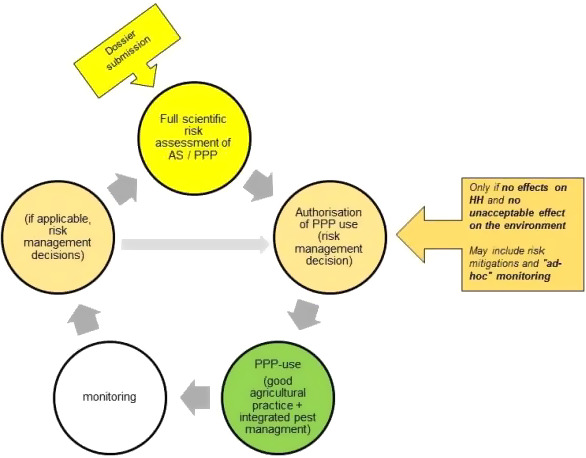
Monitoring and surveillance in the context of the authorisation and use cycle of PPPs in the EU (Regulation (EC) No 1107/2009 and Regulations (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b) Source: European Commission, 2017. Guidance on monitoring and surveying of impacts of pesticide use on human health and the environment under Article 7(3) of Directive 2009/128/EC (European Commission, 2009b)
Active substance monitoring requested for specific authorisations (Art. 6(i) and/or Art. 67(2) of Regulation (EC) 1107/2009) will be mostly designed case by case depending on the specific active substance of interest, and no specific guidance is provided in the EU document (European Commission, 2017).
Some relevant information for performing monitoring can be found in PPR and EFSA guidance (e.g. EFSA PPR Panel, 2013b; EFSA, 2017); ECHA guidance (ECHA, 2008; or in e.g. WFD, 2000; Fründ et al., 2011; VDI, 2014;ISO, 2018; Gimsing et al., 2019). Additional recommendations are given further below.
4.1.3. Dedicated long‐term studies
To address risks identified for non‐target organisms or groundwater at lower tier level, higher tier studies (e.g. effect studies or accumulation studies) might be performed.
Especially for non‐degrading chemicals as transition metals, it is essential that the temporal scales of the studies are adapted to longer time frames. Evidence of long‐term effects might emerge after decades of investigation (e.g. Klein, 2015). Controlled studies with different treatment levels are extremely relevant for the higher tier risk assessment of transition metals as active substances in PPP.
Dedicated long‐term studies are also relevant in the context of any renewal, withdrawal or amendment of authorisation as requested by Regulations (EC) No 1107/2009 (Art. 43 and Art. 44), No 283/2013 and No 284/2013 or to trigger a review of an active substance approval pursuant to Article 21 of Regulation (EC) No 1107/2009. Risk management action will follow on a rationale built on a:
‘causality link between the PPP use and the observed unexpected effect: the plausibility of a causal relationship between a PPP use and the observed effect needs to be assessed. The effects observed may refer either to direct measurements of residues in matrices (e.g. biomonitoring) or indirect effects on, e.g. populations of organisms’ (European Commission, 2017).
Controlled studies can deliver causality links otherwise difficult to demonstrate under general monitoring programmes.
Controlled long‐term studies bridge the prospective risk assessment approaches with the effective impact in the field after year‐on‐year application of PPP with transition metals. They can deliver very relevant information on: (i) the state of environmental media; (ii) the risk assessment accurateness; and (iii) necessary risk management decisions.
It needs to be pointed out that such type of controlled studies are difficult to implement in aquatic environments, because of spatial scales of assessment (e.g. catchments).
Comparison of monitoring sites with different level of contaminations, due to e.g. different land‐use history, can help identifying level of contamination and site conditions leading to shift in community composition in aquatic as well as in terrestrial environments.
4.1.4. Conclusions and recommendations for monitoring
-
It is recommended that all available information on transition metals in relevant environmental matrices are provided ahead of the start of the scientific risk assessment of transition metals as active substances in PPP together with the dossier. this includes:
-
–
Provision of representative monitoring data (in terms of spatial distribution and matrix characteristics) across European Member States for all environmental matrices (soil, sediment, surface water and groundwater).
-
–
Provision of monitoring data to investigate if anthropogenic residue levels of transition metals increased in soil and sediment over time. Information on the potential contamination sources for each site (potential atmospheric deposition, agricultural practices…).
-
–
Survey of relevant ecotoxicology concentrations of transition metals (e.g. total, dissolved, bioavailable) in the environmental matrices. Factors affecting the speciation and availability of transition metals in environmental matrices on the same sampling sites (e.g. pH, OC, CEC, clay content, soil texture, climatic conditions, agricultural practices, …) should be additionally measured.
-
–
Information on analytical methods/on the sampling methods of the environmental matrices.
-
–
Provision of the raw data and statistical data analysis. Data should not be discarded without robust scientific evidence. Consideration of potential impact of hot spot near to the studied site on the outcome of monitoring data.
-
–
The full soil profiles should be considered to derive reliable soil background concentrations.
-
–
For accumulation studies and to provide the appropriate baseline, background concentrations should be measured on the monitoring site some weeks before the study starts. The potential accumulation of transition metals in soil and sediment should be considered in the estimation of the baseline background concentrations.
-
–
Historical data on farmers’ use of the transition metal on the monitoring site and the adjacent fields should be provided.
-
–
Agricultural use of the transition metal in the area around the monitoring site and in the catchment area should be reported.
-
–
Mined monitoring data from different sources need to be assessed in more details to check their representativity and their suitability to be implemented e.g. in the exposure calculations, scenario development or effect assessment.
-
If conclusions on low risk for non‐target organisms exposed to transition metals in PPP have been reached at higher tier level, it is proposed that they should be backed by strong management decisions and post‐registration monitoring of possible long‐term effects is implemented:
-
–
To keep the efforts proportionate, post‐registration monitoring should focus on those environmental compartments and non‐target organism groups and communities identified as being vulnerable.
-
–
Survey of the abiotic and biotic targets should be performed according to agreed standards (e.g. aligned to EFSA guidance or of related agencies).
-
–
It is recommended that the applicant contacts the competent authority for agreement on the vulnerable sites and organism groups and the study design for post‐registration monitoring.
5. General conclusions and recommendations
While the hazard assessment can in principle be performed as for synthetic organic chemicals, there is a need for the development of suitable exposure models and scenarios for transition metals as active substance in PPP.
If bioavailability is to be considered in higher tier assessments, specific models and guidance for the risk assessment of transition metals used as PPP are needed.
The recommendations of the EFSA Scientific Opinion on good modelling practice (EFSA PPR Panel, 2014a) should be followed when models are adapted or developed for transition metals as active substances in PPP, including the descriptions of environmental scenarios.
Ideally, the parameterisation of adapted and/or new exposure scenarios and newly developed higher tier approaches needs to be agreed at European level. It is recommended to establish scientific working groups that deals with model parameterisation and development.
The uncertainty assessment should specifically consider the physico‐chemical conditions affecting speciation and bioavailability, homoeostasis, accumulation and background concentrations due to the persistency of transition metals.
Following intended uses of PPP with transition metals, the loads of these compounds will inevitably increase in the environment and transition metals will accumulate in environmental sinks. In addition, utilisation of the same transition metal in other PPP or for other uses than PPP (e.g. as additive in animal feeds or in fertilisers) will add to the total concentrations possibly posing risks to non‐target organisms.
The risk assessment outcome of transition metal compounds as active substances in PPP is always linked to the time frame assessed.
If higher tier assessment steps are implemented, chemical and biological/ecological monitoring is needed to follow exposure and effects in the long time.
It is recommended to consider all the above aspects and uncertainties in any risk management decision on the approval of transition metals as active substances or the authorisation of PPPs with transition metals (e.g. comparative assessment, cost–benefit analysis, optimised application rates, etc.) and accompanied focused monitoring. It is proposed that prospective risks characterisation of transition metals is aligned with the goals of other overarching legislative frameworks (e.g. Sustainable Use Directive, Water Framework Directive, Soil protection legislations, etc.).
6. Conclusions and recommendation for the specific compartments
6.1. Aquatic compartment
6.1.1. Hazard assessment
The hazard assessment can be based on the studies listed in the data requirement Commission Regulation (EU) No 283/2013 (European Commission, 2013a) and No 284/2013 (European Commission, 2013b) if details about water characteristic (especially for mesocosm studies) and equilibration of the test system are given.
The total and dissolved measured concentrations in filtered (0.45 μm) water samples needs to be given, including the background concentration and the physico‐chemical parameters of the test media or sediment. For sediments the fraction after aqua regia digestion should be given (ECHA, 2008).
Equilibrium status of the metal concentration over time in the toxicity studies needs to be known to assess the time to onset of effects. Input via drift and event‐driven inputs such as run‐off are not expected to have reached equilibrium in the waterbody for the acute risk assessment.
Surrogate test species need to cover all relevant exposure routes (gills, diet) and trophic levels for accumulating transition metals. To cover all trophic levels, the following sediment species should be tested in the higher tier risk assessment: e.g. Hyalella azteca, Gammarus pulex, Tubifex tubifex, Lumbriculus variegatus, Chironomus riparius, Hexagenia sp., Ephoron virgo, mollusc, benthic microalgae.
The consideration of recovery in a water–sediment system for the derivation of a RAC is not considered acceptable as the metals do not degrade and could be resuspended from the sediment.
6.1.2. Exposure assessment, fate and behaviour of transition metals
For estimating the environmental concentration for the aquatic compartments, the current version of FOCUS STEP 1–2 tool can be used for deriving the initial PEC in the water column due to the drift, but it is not recommended for the other entries to waterbodies (run‐off and drainage) and for estimating maximum PEC in sediment.
To produce more realistic, but less conservative, exposure concentrations for transition metals, there is a possibility to consider the speciation and the availability of transition metals in the environment and factors affecting it should be taken into account.
6.1.3. Risk assessment
Taking the total concentration as bioavailable constitutes a conservative approach for the exposure assessment of transition metals. In the refined risk assessment bioavailability may be considered using simple fit‐for-purpose bioavailability models considering the assumptions hold for the case to evaluate (see ECHA, 2008).
At present, for the ERA of transition metals the same tiered approach as described in the aquatic guidance document (EFSA PPR Panel, 2013c) is considered relevant for transition metals. However, to compare or combine the ecotoxicological studies the metal speciation in the test system needs to be considered.
The endpoints for the water compartment should be expressed at the same level of (bio)availability. This can be achieved by normalisation in a refinement step to the same set of physico‐chemical parameters for exposure and effect concentrations either using simple speciation models or, if available and considered suitable, BLMs. Concentrations in sediment can be corrected by considering binding to OC, SEM‐AVS or Fe‐Mn (oxy)hydroxides.
The development of tolerance in communities should not be considered in a refined risk assessment as it is site specific.
6.2. Terrestrial compartment
6.2.1. Hazard assessment (all organism groups)
The hazard and risk assessment for in‐soil organisms, NTA, NTTP and bees exposed to transition metals as active substances in PPP can be performed in principle as for other active substances. Problems arising from outdated guidance compared with newer legislation and data requirement apply also to transition metals. For some problems, interim solutions were found.
The applicability of an SSD approach should be investigated for soil organisms. As no guidance is currently available for soil organisms, these methodologies would be non‐standard higher tier assessments to be included in a weight‐of-evidence approach.
Long‐term field studies with e.g. earthworms or mesofauna are extremely useful to follow the fate, behaviour and toxicity of non‐degradable transition metals on communities in time. They should be included if available not only for risk assessment purposes but also to inform risk management decisions.
Effective concentrations are not measured in tests with soil organisms. Needs to be addressed if refinement options regarding bioavailability are envisaged: if no exposure quantities are determined, it is difficult to related endpoints other than to PECs addressing total (nominal) metal contents.
The impact of soil properties on bioavailability and following toxicity of metals to soil organisms in agricultural field soils should be investigated systematically for the different compounds.
For birds and mammals, it is recommended to conduct acute/repeated toxicity studies as requested by the current EFSA recommendations to establish proper toxicity endpoints.
It is suggested to document residue levels of transition metals used as PPPs to perform exposure assessment and risk assessment for birds and mammals with appropriate data.
Whenever needed, potential exposure of domestic or wild ruminant species should be discussed.
Secondary poisoning for earthworm‐ or fish‐eating birds or mammals should be considered either via a weight‐of-evidence approach or using the current ECHA guidance for chemical safety assessment (ECHA, 2008, Appendix R.7.13‐2).
6.2.2. Exposure assessment, fate and behaviour of transition metals
Availability/speciation of transition metals and environmental conditions affecting these processes are not considered in current EFSA exposure model calculations and the risk assessment. Availability/speciation highly depends on environmental conditions and chemistry in particular pH, OC, CEC and clay content.
Although simple analytical models and numerical models (e.g. PERSAM and FOCUS models) exist, it has been identified that the existing soil scenarios are not fully adapted for assessing transition metals.
The development of specific soil scenarios for transition metals is needed to use pore water concentrations in the risk characterisation. It would be recommended to include the soil characteristics (pH, OC, CEC and clay content) in such scenarios.
To better reflect the fate and behaviour of transition metals in the soil profile, other processes (e.g. speciation/availability) affecting their leaching in the soil profile could be included in the modelling.
6.2.3. Risk assessment
The risk assessment for in‐soil organisms, NTA, NTTP and bees exposed to transition metals as active substances in PPP can be performed in principle as for other active substances.
Bioavailability and ageing correction approaches as proposed in the ECHA guidance (ECHA, 2008) cannot be fully applied, as transition metals as PPP are freshly applied to terrestrial compartments once or repeatedly in a season. Bioavailability corrections may be considered, e.g. for fractions of the residues accumulating in the soils after intended uses in previous years. Metals adsorbed to soil particles can be remobilised in the gut of soil organisms.
6.3. Groundwater
The risk for groundwater contamination performed with the agreed European FOCUS models should be assessed for a period sufficiently long to cover the long‐term groundwater exposure over multiple years.
Due to the soil persistency of transition metals, the agricultural background concentration in soil has to be included in the groundwater modelling to reflect the influence of the long‐term use of transition metals as PPPs.
To produce a conservative risk assessment, the modelling endpoints (especially Kd value) should be derived for worst‐case conditions maximising the mobility of transition metals into the soil profile.
To better reflect the fate and behaviour of transition metals in the soil profile, there is the possibility to include other processes affecting their leaching in the soil profile (e.g. speciation/availability, ageing processes) in the model.
6.4. Monitoring
Within the aim of this statement, three different levels of environmental monitoring are described, serving different purposes in the ERA of transition metals. It is proposed to differentiate between general monitoring data evaluation before dossier submission, post‐registration monitoring following eventual active substance approval and PPP authorisation and targeted long‐term studies accompanying authorisation and investigating specific relationships between e.g. application rates and biological responses.
It is recommended that all relevant and reliable available occurrence information on transition metals in relevant environmental compartments are provided and analysed ahead of the start of the scientific risk assessment of transition metals as active substances in PPP by the applicant together with the dossier to the competent authority assessors.
It is proposed that, if conclusions on low risk for non‐target organisms exposed to transition metals following PPP intended uses have been reached at higher tier level, they should be backed by strong risk management decisions and post‐registration monitoring of possible long‐term effects should be implemented.
Especially the non‐degradability of transition metals in the environment and the related uncertainties to long‐term effects underpin the post‐registration monitoring requirements.
To keep monitoring of PPPs proportionate, it should focus on those environmental compartments and non‐target organism groups identified as being vulnerable.
Controlled long‐term field studies, i.e. over multiple years bridge the prospective risk assessment approaches with the effective impact in the field after year‐on-year application of PPP with transition metals. They can deliver very relevant information on: (i) the state of environmental media; (ii) the risk assessment accurateness; and (iii) the necessary risk management decisions.
Abbreviations
- a.s.
Active substance
- AF
Assessment factor
- ARA
Added risk approach
- AVS
Acid Volatiles Sulfides
- BAF
Bioaccumulation factor
- BCF
Bioconcentration factor
- BLM
Biotic Ligand Model
- CEC
Cation Exchange Capacity
- DAR
Draft assessment report
- DegT50
Degradation half‐life for the total system (day)
- DissT50
Dissipation half‐life for the total system (day)
- DOC
Dissolved Organic Carbon
- DT50
Generic term to define both DegT50 or DissT50
- dw
dry weight
- ECx
Concentration where × % effect was observed/calculated
- ECHA
European Chemical Agency
- Eh
Oxidation Potential
- ERA
Environmental Risk Assessment
- EREC
Ecotoxicologically relevant exposure concentration
- EREQ
Ecotoxicologically relevant exposure quantities
- ERO
Ecological recovery option
- EQS
Environmental Quality Standard
- EU
European Union
- FIR
Food Intake Rate
- FOCUS
FOrum for the Co‐ordination of pesticide fate models and their USe
- FOREGS
Forum of European Geological Surveys (now EuroGeoSurveys)
- GAP
Good Agricultural Practice
- gBAM
generalised Bioavailability Model
- GD
Guidance Document
- GW/gw
Groundwater
- HCx
Hazardous concentration for x % of the species of an SSD
- IDMM
Intermediate Dynamic Model for Metals
- JRC
Joint Research Centre
- Kd, Kf
Sorption coefficient for sorption on soil (mL/g)
- Koc
Sorption coefficient for sorption on soil organic carbon (mL/g organic carbon)
- Kom
Sorption coefficient for sorption on soil organic matter (mL/g organic matter)
- Ksc
Sorption coefficient for one soil constituent (mL/g)
- MACRO
Leaching model, specifically developed for addressing macroporous water flow in soils
- MLR
Multiple linear regression
- MOA
Mode of action
- msc
content of the soil constituent
- NOEC
No observed effect concentrations
- NTA
Non‐Target Arthropods
- NTTP
Non‐Target Terrestrial Plants
- OC
Organic carbon
- OECD
Organisation for Economic Co‐operation and Development
- OM
Organic matter
- PEARL
Pesticide Emission Assessment at Regional and Local scales
- PEC
Predicted environmental concentration
- PELMO
Pesticide Leaching Model
- PERSAM
Persistence in Soil Analytical Model
- PNEC
Predicted no effect concentration
- PPP
Plant Protection Product
- PPR Panel
Panel of the Plant Protection Products and their Residues
- PRZM
Pesticide Root Zone Model for calculating fate and behaviour of substances in the unsaturated zone of the soil
- RA
Risk Assessment
- RAC
Regulatory Acceptable Concentration
- RAF
Relative Absorption Factor
- RAR
Renewal Assessment Report
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RMM
Risk mitigation measures
- SEM
Simultaneously Extracted Metals
- SPG
Specific protection goal
- SSD
Species Sensitivity Distribution
- SW
Surface water
- TER
Toxicity to exposure ratio
- TK/TD
Toxicokinetic/Toxicodynamic
- TME
Terrestrial model ecosystems
- TOC
Total organic carbon
- TRA
Total risk approach
- US EPA
United States Environmental Protection Agency
- VDF
Vegetation distribution factor
- VRAR
Voluntary Risk Assessment Report
- WFD
Water Framework Directive
- WG
Working Group
- WHAM
Windermere Humic Aqueous Model
- 1/n
Freundlich coefficient
Appendix A – Overview of the different assessment frameworks
1.
Table A.1.
Framework for metals risk assessment under different regulations in different countries. Please refer to the glossary for abbreviations
| EFSA (1107/2009) Cu RAR Copper Compounds, 2017 | OECD (No 259, OECD, 2016) | US EPA (Fairbrother et al., 2007; EPA 120/R‐07/001, March, 2007) | ECHA (REACH, App R.7.13‐2; ECHA, 2008, 2017a,b,c, 2018b) | WFD (2000/60/EC; 2008/105/EC) European Commission (2019) | |
|---|---|---|---|---|---|
| Scope | Active substance approval, national authorisation and management of PPP | Incorporation of bioavailability concepts for risk assessment and threshold values | Site‐specific assessment, national level assessment, national ranking | Registration of substances | Water management of river basins |
| Protection goal | No unacceptable effects | Protecting and maintaining the physical, chemical and biological integrity of US waters | Safe handling, manufacture, place on the market or use should not adversely affect human health or the environment | Protection of most sensitive waters; No effects on reproduction, growth or health | |
| Exposure | |||||
| Background | Considered for sediment and soil | Considered as metallo‐regions (several databases for sediment, water, biota) | Considered for water, soil, sediment to differentiate between added and total risk (measured, FOREGS database, geochemical modelling) | Considered for water (NBCa close to undisturbed conditions) | |
| Spatial‐scale | Realistic worst‐case (covers 90% of all cases), edge‐of‐field, in‐field | Local differences | No exposure target defined | Local, site specific, river basin | |
| Timescale | Acute (hours) and long‐term (weeks/year) | Rapid changes | Long‐term (20–100 years) | Short‐term (days) and long‐term (annual) | |
| Models | No specific model to address metals | No specific model to address metals | Monitoring data, ecoregion approach | Monitoring data | |
| Toxicity | |||||
| Bioavailability |
Water: dissolved concentration Sediment: total concentration for 2.5% OC Soil: total concentration No concept for tiered approach regarding bioavailability |
Water: BLM, simplified models (water hardness), dissolved fraction. Sediment: Fe/Mn, org C or SEM‐AVS normalisation Soil: Pore water, lab‐to‐field factor, normalisation models |
Water: water hardness adjustment, speciation models, BLM. Sediment: SEM‐AVS, normalisation Soil: Pore water, bulk soil, ageing |
Water: dissolved concentration, speciation models, BLM. Sediment: Fe/Mn, OC or SEM‐AVS normalisation Soil: worst‐case scenario for soil testing, leaching‐ageing factor, normalisation |
Water: Dissolved concentration, simplified tools, BLM |
| Essentiality | Considered in assessment factor (AF) | Considered in ecotox tests, consider hormesis | Consider in AF, site‐specific bioassays | Consider hormesis | Not explicitly considered |
| Bioaccumulation | Not considered relevant for secondary poisoning | Not addressed | Site specific: regression model for BCF/BAF, kinetic models | Site specific: regression model for BCF/BAF | Not addressed |
| Uptake route causing toxicity | Not specified |
Water: Mainly waterborne ingestion Soil: dermal |
Inhalation, ingestion, absorption, contact |
Water: waterborne ingestion Soil: contact |
Not specified |
Natural Background Concentration.
Appendix B – Description of the IDMM tool
1.
Exposure calculation for aquatic systems using IDMM tool
The IDMM is a dynamic model of intermediate complexity developed to allow calculation of long‐term metal accumulation in soil and sediment and leaching from the topsoil. An early version of this model was considered for the assessment of environmental impact of zinc and copper used in animal nutrition (Monteiro et al., 2010). The description of the model can be found in the latter document. As described in the scientific/technical report submitted to EFSA on Pre‐Assessment of Environmental Impact of Zinc and Copper Used in Animal Nutrition (Monteiro et al., 2010):
‘The model comprises a single soil layer and runs on an annual time step. On each time step, deposited or applied metal and metal derived from mineral weathering, is added to the pool already present and the metal pool is partitioned between the soil solids and pore water. Metal leaves the soil in drainage water and/or run‐off, in either dissolved form (as calculated by soil‐solution partitioning) or bound to soil particles eroded into the drainage water. The annual volume of water draining the soil is implicitly replenished from precipitation such that the pore water volume in each horizon remains constant. Partitioning of metal between the pore water and soil solids is dependent upon the pore water pH and the concentrations of dissolved organic matter and other ions present, as well as the soil organic matter concentration. The model computes initial pore water and soil metal concentrations by assuming a balance of all input and output fluxes (steady state). Key model variables, including metal input rates, pore water pH and pore water suspended solids concentration, are specified on an annual basis. The FOCUS scenarios comprise two types: drainage‐dominated soils and run‐off dominated soils. To consider the special features of each of these types, a specific version of the IDMM has been constructed for each.
The soil column is represented by three horizons representing surface soil, topsoil and deep soil, respectively. The surface soil layer has a depth of 5 cm, the topsoil layer has a depth of 25 cm and the deep soil layer extends from the bottom of the topsoil layer to the depth of artificial drainage. Vertical transport of metals (in dissolved and particulate form) from each soil layer to the layer below, and from the deep soil to groundwater, is modelled. Additionally, lateral drainage (also in dissolved and particulate form) from each horizon to surface water is simulated. Therefore, for example, it is possible to simulate the transport of suspended particulate matter (and associated metal) to surface waters in surface soil run‐off. The removal of metal from the system by uptake into crop plant and subsequent harvesting can be simulated.’
The IDMM also takes into account the hydrology, metal ageing, metal weathering, soil erosion and metal transport, metal removal by crops and organic matter inputs to the soil.
As an example, for the exposure assessment of copper used as PPP, the use of the IDMM has been proposed by the notifier for estimating the long‐term accumulation in sediment only. The equilibrium speciation of labile metal pool in soils was taken into account using a chemical speciation model (e.g. the Windermere Humic Aqueous Model, WHAM/VI model; daily time step) for metals in soil and water systems. Factors affecting the speciation are from the soil characteristics implemented in the existing FOCUS surface water scenarios soil columns and from additional parameters when missing (e.g. major ions soil concentrations) (sodium, calcium, nitrate, sulfate, carbonate, aluminium, iron(III); values of binding constants for metal binding to dissolved organic matter).
The range of the characteristics of the 10 FOCUS scenarios soil columns are: Soil bulk density 1.2–1.56 g/cm3; Topsoil pH: 5.4–8.7; Topsoil OM: 1.2–6.8; Topsoil clay content: 3–55%.
Different forms of copper within a soil layer were modelled by either equilibrium or kinetics in IDMM tool (labile copper in the pore water including free copper, metal bound to ligands such as carbonate and organic matter, and dissolved copper and aged soil residues).
Also ageing was considered as a process that transfer metal from the labile pool (i.e. that dissolved and adsorbed reversibly to soil particle surfaces) to a ‘fixed’ pool that exchanges with the labile pool over relatively long timescales (at least a year). The importance of ageing processes for metal behaviour in soils is their influence on the labile pool over time; the labile pool controls the dissolved concentration and so is important in determining metal losses from the soil to surface water and groundwater. The ageing is simulated as a two‐stage kinetic process which computes copper transfers among three solid‐phase pools: the adsorbed labile pool and two subpools of the aged pool: the ‘weakly aged’ pool and the ‘strongly aged’ pool.
The dimensions of water bodies defined in the surface water scenarios in FOCUS surface water model (FOCUS surface water guidance document, 2015) are considered in IDMM tool. Ditch and stream water bodies were considered, but the pond was not included.
The water column characteristics of the 10 aquatic scenarios implemented are: pH: 6.4–8.3; DOC: 0.8–19.0 mg; Sum of the concentrations of the major cations Na, Mg, K: 0.55–6.86 μequ./L; Organic carbon content in sediment fixed to 5% for all scenarios.
In addition, to estimate the speciation of copper in sediment, the concentration of AVS is included. The presence of AVS reduces the concentration of labile metals transition in the anoxic sediment. In IDMM tool, AVS in sediment ranges from 0 to 0.63 μmol/g.
To estimate the concentration of transition metals into surface water and sediments, the FOCUS surface water scenarios are considered and when missing, some properties are amended to take into account the speciation and the bioavailability of transition metals in such aquatic systems.
Due to soil erosion, soil depth and organic matter content are adjusted by model. This process has been included due to its impact on the pool of metal remaining in soil. Representativeness of the soil erosion process as implemented in model should be checked.
Suggested citation: EFSA PPR Panel (EFSA Panel of the Plant Protection Products and their Residues) , Hernandez‐Jerez A, Adriaanse P, Aldrich A, Berny P, Coja T, Duquesne S, Focks A, Marina M, Millet M, Pelkonen O, Tiktak A, Topping C, Widenfalk A, Wilks M, Wolterink G, Conrad A and Pieper S, 2021. Statement of the PPR Panel on a framework for conducting the environmental exposure and risk assessment for transition metals when used as active substances in plant protection products (PPP). EFSA Journal 2021;19(3):6498, 88 pp. 10.2903/j.efsa.2021.6498
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00374
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The PPR Panel wishes to thank the following for the support provided to this scientific output: Silvia Pieper, Annette Aldrich, Philippe Berny, Arnaud Conrad, Joop de Knecht (up to June 2020) and the EFSA staff Laura Padovani, Alessio Ippolito, Laima Abromaityte (up to May 2020), Olga Kulikova, Chris Lythgo and Mark Egsmose.
Adopted: 24 February 2021
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2021.EN-6501/full
Notes
SANTE. E4/EL/gb(2018)/7009529.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products.
ECHA guidance covers all metals and not only transition metals.
European Commission, 2009a, 2009b, 2009c. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.OJ L 309/1, 24.11.2009, pp. 1–50.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products.
Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides.
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy.
Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market.
FOCUS Surface Water package: STEP 1–2 v 3.2; SPIN v.2.2; SWASH v.5.3 (including submodels PRZM v.4.3.1, MACRO v.5.5.4 and TOXSWA v.5.5.3); SWAN v.5.00.
Commission Regulation (EU) No 546/2011 specifies that ‘2.5.1.1. No authorisation shall be granted if the active substance and, where they are of significance from the toxicological, ecotoxicological or environmental point of view, metabolites and breakdown or reaction products, after use of the plant protection product under the proposed conditions of use: during tests in the field, persist in soil for more than 1 year (i.e. DT90 > 1 year and DT50 > 3 months), or …unless it is scientifically demonstrated that under field conditions there is no accumulation in soil at such levels … and/or that there is an unacceptable impact on the environment, in accordance with the relevant requirements provided for in points 2.5.1.2, 2.5.1.3, 2.5.1.4 and 2.5.2’.
European Council, 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption.
Commission Regulation (EU) 2018/676 of 3 May 2018 correcting Commission Regulation (EU) No 546/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products.
References
- Adams W, Blust R, Dwyer R, Mount D, Nordheim E, Rodriguez PH and Spryg D, 2020. Bioavailability assessment of metals in freshwater environments: a historical review. Environmental Toxicology and Chemistry, 39, 48–59. 10.1002/etc.4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aden K, Binner R, Fischer R, Gottschild D, Klowskowski R, Schnikel K and Michalski B, 2002. Protection of groundwater from entry of plant protection products: guidance on how to clarify findings and implement post‐registration monitoring studies [official English translation]. Nachrichtenbl Deutsch Pflanzenschutzd, 54, 125–129. [Google Scholar]
- Allen JG, Masters HG, Peet RL, Mullins KR, Lewis RD, Skirrow SZ and Fry J, 1983. Zinc toxicity in ruminants. Journal of Comparative Pathology, 93, 363–377. [DOI] [PubMed] [Google Scholar]
- Ballabio C, Panagos P, Lugato E, Huang J, Orgiazzi A, Jones A, Fernández‐Ugalde O, Borrelli P and Montanarella L, 2018. Copper distribution in European topsoils: an assessment based on LUCAS soil survey. Science of The Total Environment, 636, 282–298. 10.1016/j.scitotenv.2018.04.268 [DOI] [PubMed] [Google Scholar]
- van Beinum W and Beulke S, 2010. Collection and evaluation of relevant information on crop interception for the revision of the Guidance Document on Persistence in Soil. Report FERA, Sand Hutton, UK. 41 pp. [Google Scholar]
- Bengtsson G, Gunnarsson T and Rundgren S, 1985. Influence of Metals on reproduction, mortality and population growth in Onychiurus armatus (Collembola). Journal of Applied Ecology, 22, 967–978. 10.2307/2403244 [DOI] [Google Scholar]
- Bjerregaard P, Andersen CBI and Andersen O, 2015. Ecotoxicology of Metals ‐ Sources, Transport, and Effects on the Ecosystem. [Google Scholar]
- Boutin C and Rogers CA, 2000. Pattern of sensitivity of plant species to various herbicides—an analysis with two databases. Ecotoxicology, 9, 255–271. [Google Scholar]
- Boutin C, Aya KL, Carpenter D, Thomas PJ and Rowland O, 2012. Phytotoxicity testing for herbicide regulation: shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Science of the Total Environment, 415, 79–92. [DOI] [PubMed] [Google Scholar]
- Boutin C, Strandberg B, Carpenter D, Mathiassen SK and Thomas P, 2014. Herbicide impact on non‐target plant reproduction: what are the toxicological and ecological implications? Environmental Pollution, 185, 295–306. [DOI] [PubMed] [Google Scholar]
- Brix KV, DeForest DK, Tear L, Grosell M and Adams WJ, 2017. Use of multiple linear regression models for setting water quality criteria for copper: a complementary approach to the biotic ligand model. Environmental Science and Technology, 51, 5182–5192. [DOI] [PubMed] [Google Scholar]
- Bruus Pedersen M, Temminghoff EJM, Marinussen MPJC, Elmegaard N and van Gestel CAM, 1997. Copper accumulation and fitness of Folsomia candida Willem in a copper contaminated sandy soil as affected by pH and soil moisture. Applied Soil Ecology, 6, 135–146. [Google Scholar]
- Bruus Pedersen M, van Gestel CAM and Elmegaard N, 2000. Effects of copper on reproduction of two collembolan species exposed through soil, food and water. Environmental Toxicology and Chemistry, 19, 2579–2588. [Google Scholar]
- Buhl KJ, Berman FW and Stone DL, 2013. Reports of metaldehyde and iron phosphate exposures in animals and characterisation of suspected iron toxicosis in dogs. Journal of the American Veterinary Medical Association, 242, 1244–1248. [DOI] [PubMed] [Google Scholar]
- Bury NR, Walker PA and Glover CN, 2003. Nutritive metal uptake in teleost fish. Journal of Experimental Biology, 206, 11–23. [DOI] [PubMed] [Google Scholar]
- Cedeño Y, Lopez‐Alfonso M and Miranda M, 2016. Hepatic concentrations of copper and other metals in dogs with and without chronic hepatitis. Journal of Small Animal Practice, 57, 703–709. [DOI] [PubMed] [Google Scholar]
- Chong K and Wang WX, 2000. Bioavailability of sediment‐bound Cd, Cr and Zn to the green mussel Perna viridis and the Manila clam Ruditapes philippinarum . Journal of Experimental Marine Biology and Ecology, 255, 75–92. [DOI] [PubMed] [Google Scholar]
- Clearwater SJ, Baskin SJ, Wood CM and McDonald DG, 2000. Gastrointestinal uptake and distribution of copper in rainbow trout. Journal of Experimental Biology, 203, 2433–2466. [DOI] [PubMed] [Google Scholar]
- Clearwater SJ, Farag AM and Meyer JS, 2002. Bioavailability and toxicity of dietborne copper and zinc to fish. Comparative Biochemistry and Physiology – C Toxicology and Pharmacology, 132, 269–313. [DOI] [PubMed] [Google Scholar]
- Dang F, Wang W‐X and Rainbow PS, 2012. Unifying prolonged copper exposure, accumulation, and toxicity from food and water in a marine fish. Environmental Science and Technology, 46, 3465–3471. 10.1021/es203951z [DOI] [PubMed] [Google Scholar]
- DAR Pydiflumetofen , 2018. Draft Assessment Report prepared according to the Commission Regulation (EC No 1007/2009) of RMS France; Co‐RMS Austria. Vol. 3 B.8 (plant pesticide product). Available online: http://registerofquestions.efsa.europa.eu/roqFrontend/outputLoader?output=ON-5821 as sanitised version
- De Jonge M, Blust R and Bervoets L, 2010. The relation between acid volatile sulfides (AVS) and metal accumulation in aquatic invertebrates: implications of feeding behavior and ecology. Environmental Pollution, 158, 1381–1391. [DOI] [PubMed] [Google Scholar]
- De Schamphelaere KAC and Janssen CR, 2004. Effects of chronic dietary copper exposure on growth and reproduction of Daphnia magna . Environmental Toxicology and Chemistry, 23, 2038–2047. [DOI] [PubMed] [Google Scholar]
- De Vaufleury AG and Pihan F, 2002. Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails: metal bioavailability and bioaccumulation. Environmental Toxicology and Chemistry, 21, 820–827. 10.1002/etc.5620210419 [DOI] [PubMed] [Google Scholar]
- DeForest DK and Meyer JS, 2015. Critical Review: toxicity of dietborne metals to aquatic organisms. Critical Reviews in Environmental Science and Technology, 45, 1176–1241. 10.1080/10643389.2014.955626 [DOI] [Google Scholar]
- Degryse F, Smolders E and Parker DR, 2009. Partitioning of metals (Cd Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications – a review. European Journal of Soil Science, 60, 590–612. 10.1111/j.1365-2389.2009.01142.x [DOI] [Google Scholar]
- ECHA (European Chemicals Agency), 2008. Guidance on information requirements and chemical safety assessment – Appendix R.7.13‐2: Environmental risk assessment for metals and metal compounds, 78 pp.
- ECHA (European Chemicals Agency), 2017a. Guidance on information requirements and chemical safety assessment. Chapter R.7b Endpoint specific guidance. R.7.8 Aquatic toxicity; long‐term toxicity to sediment organisms. 10.2823/43472 [DOI]
- ECHA (European Chemicals Agency), 2017b. Guidance on information requirements and chemical safety assessment. Chapter R.7c Endpoint specific guidance. R.7.10 Bioconcentration and bioaccumulation; long‐term toxicity to birds. 10.2823/43472 [DOI]
- ECHA (European Chemicals Agency), 2017c. Guidance on information requirements and chemical safety assessment. Chapter R.7c Endpoint specific guidance. R.7.11 Effects on terrestrial organisms. 10.2823/43472 [DOI]
- ECHA (European Chemicals Agency), 2018a. Guidance on the biocidal products regulation: Volume IV Environment – Part A: Information requirements (Version 1.2). 10.2823/49865 [DOI]
- ECHA (European Chemicals Agency), 2018b. Guidance on the biocidal products regulation: Volume IV Environment – Assessment and Evaluation (Parts B + C). Chapter 4.5.1 Risk characterisations for metals and metal compounds (Version 3.0. 10.2823/033935 [DOI]
- EFSA (European Food Safety Authority), 2009. Scientific Opinion of the Panel on Plant Protection Products and their Residues (PPR). The usefulness of total concentrations and pore water concentrations of pesticides in soil as metrics for the assessment of ecotoxicological effects. EFSA Journal 2009;7(1):922, 90 pp. 10.2903/j.efsa.2009.922 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Guidance Document on risk assessment for birds and mammals on request from EFSA. EFSA Journal 2010;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Scientific Report of EFSA: selection of scenarios for exposure of soil organisms to plant protection products. In support of Revision of the Guidance Document on Persistence in Soil under Council Directive 91/414/EEC and Council Regulation 11/07/2009 (SANCO/9188/VI/97 rev. 8, 12.07.2000). EFSA Journal 2010;8(6):1642, 82 pp. 10.2903/j.efsa.2010.1642 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Submission of scientific peer‐reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009, pp. 1–50. EFSA Journal 2011;9(2):2092, 49 pp. 10.2903/j.efsa.2011.2092 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012a. Conclusion on the peer review of the pesticide risk assessment of the active substance iron sulfate. EFSA Journal 2012;10(1):2521. 10.2903/j.efsa.2012.2521 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012b. Conclusion on the peer review of the pesticide risk assessment of the active substance potassium phosphonates. EFSA Journal 2012;10(12):2963, 43 pp. 10.2903/j.efsa.2012.2963 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. Conclusion on the peer review of the pesticide risk assessment of the active substance disodium phosphonate. EFSA Journal 2013;11(5):3213, 50 pp. 10.2903/j.efsa.2013.3213 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance ferric phosphate. EFSA Journal 2015;13(1):3973, 50 pp. 10.2903/j.efsa.2015.3973 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. EFSA Guidance Document for predicting environmental concentrations of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2017;15(10):4982, 115 pp. 10.2903/j.efsa.2017.4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Peer review of the pesticide risk assessment of the active substance copper compounds copper(I), copper(II) variants namely copper hydroxide, copper oxychloride, tribasic copper sulfate, copper(I) oxide, Bordeaux mixture. EFSA Journal 2018;16(1):5152, 25 pp. 10.2903/j.efsa.2018.5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. Technical report on the outcome of the Pesticides Peer Review Meeting on general recurring issues in ecotoxicology. EFSA supporting publication 2019;EN‐1673, 117 pp. 10.2903/sp.efsa.2019.EN-1673 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Scientific report of EFSA on the ‘repair action’ of the FOCUS surface water scenarios. EFSA Journal 2020;18(6):6119, 301 pp. doi.org/10.2903/j.efsa.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Scientific Opinion on the science behind the guidance for scenario selection and scenario parameterisation for predicting environmental concentrations in soil. EFSA Journal 2012;10(2):2562, 76 pp. [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013a. Scientific Opinion on the report of the FOCUS groundwater working group (FOCUS, 2009): assessment of higher tiers. EFSA Journal 2013;11(6):3291. doi.org/10.2903/j.efsa.2013.3291 [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013b. Scientific Opinion on the report of the FOCUS groundwater working group (FOCUS, 2009): assessment of lower tiers. EFSA Journal 2013;11(2):3114, 29 pp. 10.2903/j.efsa.2013.3114 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013c. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(6):3268. 10.2903/j.efsa.2013.3268 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2014a. Scientific Opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA Journal 2014;12(3):3589. 10.2903/j.efsa.2014.3589 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2014b. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for non‐target terrestrial plants. EFSA Journal 2014;12(7):3800, 163 pp. 10.2903/j.efsa.2014.3800 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2015a. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for non‐target arthropods. EFSA Journal 2015;13(2):3996, 3212 pp. 10.2903/j.efsa.2015.3996 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2015b. Scientific Opinion on the effect assessment for pesticides on sediment organisms in edge‐of‐field surface water. EFSA Journal 2015;13(7):4176, 145 pp. 10.2903/j.efsa.2015.4176 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2017. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for in‐soil organisms. EFSA Journal 2017;15(2):4690, 225 pp. 10.2903/j.efsa.2017.4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (Panel on Plant Protection Products and their Residues), 2018. Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA Journal 2018;16(2):5125. 10.2903/j.efsa.2018.5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga EJ, Vangrinsven JJM and Swartjes FA, 1999. General purpose Freundlich isotherms for cadmium, copper and zinc in soils. European Journal of Soil Science, 50, 139–149. [Google Scholar]
- Erickson RJ, Benoit DA, Mattson VR, Nelson HP and Leonard EN, 1996. The effects of water chemistry on the toxicity of copper to fathead minnows. Environmental Toxicology and Chemistry, 15, 181–193. 10.1002/etc.5620150217 [DOI] [Google Scholar]
- European Commission , 2002. Guidance document on terrestrial ecotoxicology under Council Directive 91/414/EEC. (SANCO/10329/2002) revision 2, final. 1–39. SANCO/10329/2002 rev.2‐final 17 October 2002.
- European Commission , 2003. Guidance document on the assessment of the relevance of metabolites in groundwater of substances regulated under Council directive 91/414/EEC. SANCO/221/2000‐rev.10‐final, 25 February 2003.
- European Commission , 2009a. Copper compounds. Review report for the active substance copper compounds finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 23 January 2009 in view of the inclusion of copper compounds in Annex I of Directive 91/414/EEC. SANCO/150/08 final, 26 May 2009.
- European Commission , 2009b. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309/1, 24.11.2009, pp. 1–50.
- European Commission , 2009c. EC Document Reference SANCO/13144/2010 version 3. European Parliament and Council (EC), 2009. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for community action to achieve the sustainable use of pesticides. 24.11.2009 Official Journal of the European Union L 309/71.
- European Commission , 2011. Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
- European Commission , 2013a. Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–94.
- European Commission , 2013b. Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 85–152.
- European Commission , 2014. Assessing Potential for Movement of Active Substances and their Metabolites to Ground Water in the EU. Report of the FOCUS Ground Water Work Group, EC Document Reference Sanco/13144/2010 version 3, 613 pp.
- European Commission , 2017. Guidance on monitoring and surveying of impacts of pesticide use on human health and the environment under Article 7(3) of Directive 2009/128/EC establishing a framework for community action to achieve the sustainable use of pesticides. Commission Notice. Brussels, 10.10.2017 C(2017) 6766 final.
- European Commission , 2018. Commission regulation (EU) 2018/676 of 3 May 2018 correcting Commission Regulation (EU) No 546/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products, OJ L 114, 4.5.2018, p. 2. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 32.
- European Commission , 2019. Draft Technical Guidance on Implementing Environmental Quality Standards (EQS) for Metals. Version 1, 5.11.2019.
- Evans PG, 2003. Study report no. RJ3219B. Company confidential.
- Evens RE, De Schamphelaere KAC, De Laender F and Janssen C, 2012. The effects of Zn‐contaminated diets on Daphnia magna reproduction may be related to Zn‐induced changes of the dietary P content rather than to the dietary Zn content itself. Aquatic Toxicology, 110–111, 9–16. [DOI] [PubMed] [Google Scholar]
- Fairbrother A, Wenstel R, Sappington K and Wood W, 2007. Framework for Metals Risk Assessment. Ecotoxicology and Environmental Safety, 68, 145–227. 10.1016/j.ecoenv.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Fieten H, Dirksen K, Van Den Ingh TSGAM, Winter EA, Watson AL, Leegwater PAJ and Rothuizen J, 2013. d‐Penicillamine treatment of copper‐associated hepatitis in Labrador retrievers. The Veterinary Journal, 196, 522–527. [DOI] [PubMed] [Google Scholar]
- Fisker K, Holmstrup M and Givskov Sørensen J, 2013. Variation in metallothionein gene expression is associated with adaptation to copper in the earthworm Dendrobaena octaedra . Comparative Biochemistry and Physiology, 157, 220–226. 10.1016/j.cbpc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Fliedner A, Rüdel H, Knopf B, Weinfurtner K, Paulus M, Ricking M and Koschorreck J, 2014. Spatial and temporal trends of metals and arsenic in German freshwater compartments. Environmental Science and Pollution Research, 21, 5521–5536. 10.1007/s11356-013-2487-y [DOI] [PubMed] [Google Scholar]
- FOCUS , 1997. Soil persistence models and EU registration, Document 7617/VI/96, 29.2.97.
- FOCUS , 2001. “FOCUS Surface Water Scenarios in the EU Evaluation Process under 91/414/EEC”. Report of the FOCUS Working Group on Surface Water Scenarios, EC Document Reference SANCO/4802/2001‐rev.2. 245 pp.
- FOCUS , 2007. Landscape and Mitigation Factors in Ecological Risk Assessment. SANCO/10422/2005, version 2.0, September 2007.
- FOCUS , 2015. Generic guidance for FOCUS surface water scenarios, version 1.4, May 2015.
- Frampton GK, Jänsch S, Scott‐Fordsmand JJ, Römbke J and van den Brink PJ, 2006. Effects of pesticides on soil invertebrates in laboratory studies: a review and analysis using species sensitivity distributions. Environmental Toxicology and Chemistry, 25, 2480–2489. 10.1897/05-438R.1 [DOI] [PubMed] [Google Scholar]
- Frische T, Mebes KH and Filser J, 2003. Assessing the bioavailability of contaminants in soils: a review on recent concepts. Federal Ministry of the Environment, Nature Conservation and Nuclear Safety. Research Report 201 64 214, UBA‐FB 000405. Available online: https://www.umweltbundesamt.de/publikationen/assessing-bioavailability-of-contaminants-in-soils
- Frische T, Egerer S, Matezki S, Pickl C and Wogram J, 2016. Five‐point program for sustainable plant protection. Position paper German Environment Agency. Available online: https://www.umweltbundesamt.de/publikationen/5-point-programme-for-sustainable-plant-protection [Google Scholar]
- Fründ HC, Graefe U and Tischer S, 2011. Earthworms as Bioindicators of Soil Quality. In: Karaca A (eds.). Biology of Earthworms. Soil Biology, vol. 24. Springer, Berlin, Heidelberg. pp. 261–278. [Google Scholar]
- Gandhi N, Diamond ML, van de Meent D, Huijbregts MAJ, Peijnenburg WJGM and Guinée J, 2010. New method for calculating comparative toxicity potential of cationic metals in freshwater: application to copper, nickel, and zinc. Environmental Science and Technology, 44, 5195–5201. [DOI] [PubMed] [Google Scholar]
- Gillis PL, Wood CM, Ranville JF and Chow‐Fraser P, 2006. Bioavailability of sediment‐associated Cu and Zn to Daphnia magna . Aquatic Toxicology, 77, 402–411. [DOI] [PubMed] [Google Scholar]
- Gimsing AL, Agert J, Baran N, Boivin A, Ferrari F, Gibson R, Hammond L, Hegler F, Jones RL, König W, Kreuger J, van der Linden T, Liss D, Loiseau L, Massey A, Miles B, Monrozies L, Newcombe A, Poot A, Reeves GL, Reichenberger S, Rosenbom AE, Staudenmaier H, Sur R, Schwen A, Stemmer M, Tüting W and Ulrich U, 2019. Conducting groundwater monitoring studies in Europe for pesticide active substances and their metabolites in the context of Regulation (EC) 1107/2009. Journal of Consumer Protection and Food Safety, 14, 1–93. 10.1007/s00003-019-01211-x [DOI] [Google Scholar]
- Grace ND, Knowles SO, Rounce JR, West DM and Lee J, 1998. Effect of increasing pasture copper concentrations on the copper status of grazing Romney sheep. New Zealand Journal of Agricultural Research, 41, 377–386. [Google Scholar]
- Guo Z, Zhang W, Du S, Zhou Y, Gao N, Zhang L and Green I, 2016. Feeding reduces waterborne Cu bioaccumulation in a marine rabbitfish Siganus oramin . Environmental Pollution, 208, 580–589. [DOI] [PubMed] [Google Scholar]
- Gupta RK, 2018. A review of copper poisoning in animals: sheep, goat and cattle. International Journal of Veterinary Sciences and Animal Husbandry, 3, 1–4. [Google Scholar]
- Hensbergen PJ, Donker MH, Van Velzen MJ, Roelof D, Van Der Schors RC, Hunziker E and Van Straalen NM, 1999. Primary structure of a cadmium‐induced metallothionein from the insect Orchesella cincta (Collembola). European Journal of Biochemistry, 259, 197–203. [DOI] [PubMed] [Google Scholar]
- Hiederer R, 2012. EFSA Spatial Data Version 1.1. Data Properties and Processing. Publications Office of the European Union. EUR, 25546, ISBN 978‐92-79‐27004‐8. 10.2788/54453 [DOI] [Google Scholar]
- ISO (International Organisation for Standardization), 1999. Soil quality: effects of pollutants on earthworms. Part 3: Guidance on the determination of effects in field situations (ISO 11268‐3). Revised in, 2014, 1–8. [Google Scholar]
- ISO (International Organisation for Standardization), 2006. Soil quality – sampling of soil invertebrates. Part 2: Sampling and extraction of micro‐arthropods (Collembola and Acarina) (ISO 23611‐2:2006). Revised in 2018. 12 pp.
- ISO (International Organisation for Standardization), 2018. Soil quality – sampling of soil invertebrates. Part 1: Hand‐sorting and extraction of earthworms. ISO 23611‐1:2018.
- Jacobson AR, Dousset S, Guichard N, Baveye P and Andreux F, 2005. Diuron mobility through vineyard soils contaminated with copper. Environmental Pollution, 138, 250–259. [DOI] [PubMed] [Google Scholar]
- de Jong FMW, van Beelen P, Smit CE and Montforts MHMM, 2006. Guidance for summarising earthworm field studies. A guidance document of the Dutch Platform for the Assessment of Higher Tier Studies. National Institute for Public Health and the Environment., Bilthoven, The Netherlands: RIVM report number 601506006/2006. Available online: www.rivm.nl/bibliotheek/601506006.pdf [Google Scholar]
- Kamunde C and Wood CM, 2003. The influence of ration size on copper homeostasis during sublethal dietary copper exposure in juvenile rainbow trout, Oncorhynchus mykiss . Aquatic Toxicology, 62, 235–254. [DOI] [PubMed] [Google Scholar]
- Kamunde CN, Grosell M, Lott JNA and Wood CM, 2001. Copper metabolism and gut morphology in rainbow trout (Oncorhynchus mykiss) during chronic sublethal dietary copper exposure. Canadian Journal of Fisheries and Aquatic Sciences, 58, 293–305. [Google Scholar]
- Kamunde C, Grosell M, Higgs D and Wood CM, 2002. Copper metabolism in actively growing rainbow trout (Oncorhynchus mykiss): interactions between dietary and waterborne copper uptake. The Journal of Experimental Biology, 205, 279–290. [DOI] [PubMed] [Google Scholar]
- Kamunde CN, Niyogi S and Wood CM, 2005. Interaction of dietary sodium chloride and waterborne copper in rainbow trout (Oncorhynchus mykiss): copper toxicity and sodium and chloride homeostasis. Canadian Journal of Fisheries and Aquatic 62: page 390–399. 10.1139/F04-169 [DOI] [Google Scholar]
- Ke Y and Wang WX, 2019. Dietary metal bioavailability in razor clam Sinonovacula constricta under fluctuating seston environments. Science of the Total Environment, 653, 131–139. [DOI] [PubMed] [Google Scholar]
- Kiaune L and Singhasemanon N, 2011. Pesticidal copper (I) oxide: environmental fate and aquatic toxicity. Reviews of Environmental Contamination and Toxicology, 213, 1–26. [DOI] [PubMed] [Google Scholar]
- Kienzler A, Barron MG, Belanger SE, Beasley A and Embry MR, 2017. Mode of action (MOA) assignment classifications for ecotoxicology: an evaluation of approaches. Environmental Science and Technology, 51, 10203–10211. 10.1021/acs.est.7b02337 [DOI] [PubMed] [Google Scholar]
- Kilpi‐Koski J, Penttinen OP, Väisänen AO and van Gestel CAM, 2019. An uptake and elimination kinetics approach to assess the bioavailability of chromium, copper, and arsenic to earthworms (Eisenia andrei) in contaminated field soils. Environmental Science and Pollution Research, 26, 15095–15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein O, 2015. A field study to evaluate the effects of copper on the earthworm fauna in central Europe. Final Report 2015. Report No.: 20031343/G1-NFEw. Summary in RAR Copper Compounds (2017) Vol. 3 B. 9 CA 8.4.1/02.
- Klepper O, Traas TP, Schouten AJ, Korthals GW and De Zwart D, 1999. Estimating the effect on soil organisms of exceeding no‐observed effect concentrations (NOECs) of persistent toxicants. Ecotoxicology, 8, 9–21. 10.1023/A:1008845226732 [DOI] [Google Scholar]
- Komárek M, Čadková E, Chrastný V, Bordas F and Bollinger J‐C, 2010. Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environment International, 36, 138–151. 10.1016/j.envint.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Lamichhane JR, Osdaghi E, Behlau F, Köhl J, Jones JB and Aubertot J‐N, 2018. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 28, 18 pp. 10.1007/s13593-018-0503-9 [DOI] [Google Scholar]
- Lee JH, Birch GF and Simpson SL, 2016. Metal‐contaminated resuspended sediment particles are a minormetal‐uptake route for the Sydney rock oyster (Saccostrea glomerata) ‐ A mesocosm study, Sydney Harbour estuary, Australia. Marine Pollution Bulletin, 104, 190–197. [DOI] [PubMed] [Google Scholar]
- Lee BG, Griscom SB, Lee JS, Choi HJ, Koh CH, Luoma SN and Fischer NS, 2000. Influences of dietary uptake and reactive sulfides on metal bioavailability from aquatic sediments. Science, 287, page 282–284. [DOI] [PubMed] [Google Scholar]
- Lukkari T and Haimi J, 2005. Avoidance of Cu‐ and Zn‐contaminated soil by three ecologically different earthworm species. Ecotoxicology and Environmental Safety, 62, 35–41. 10.1016/j.ecoenv.2004.11.012 [DOI] [PubMed] [Google Scholar]
- Luoma SN and Rainbow PS, 2008. Metal contamination in aquatic environments: Science and lateral management. Cambridge University Press, Cambridge. [Google Scholar]
- Mallmann GC, Sousa JP, Sundh I, Pieper S, Arena M, Purin da Cruz S and Klauberg‐Filho O, 2018. Placing arbuscular mycorrhizal fungi on the risk assessment test battery of plant protection products (PPPs). Ecotoxicology, 27, 809–818. 10.1007/s10646-018-1946-0 [DOI] [PubMed] [Google Scholar]
- Mangala P, De Silva CS and van Gestel CAM, 2009. Development of an alternative artificial soil for earthworm toxicity testing in tropical countries. Applied Soil Ecology, 43, 170–174. 10.1016/j.apsoil.2009.07.002 [DOI] [Google Scholar]
- Maria VL, Ribeiro MJ and Amorim MJB, 2014. Oxidative stress biomarkers and metallothionein in Folsomia candida – responses to Cu and Cd. Environmental Research, 133, 164–169. 10.1016/j.envres.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Mebane CA, Chowdhury MJ, De Schamphelaere KA, Lofts S, Paquin PR, Santore RC and Wood CM, 2020. Metal bioavailability models: current status, lessons learned, considerations for regulatory use and the path forward. Environmental Toxicology and Chemistry, 39, 60–84. 10.1002/etc.4560 [DOI] [PubMed] [Google Scholar]
- Méndez‐Fernández L, De Jonge M and Bervoets L, 2014. Influences of sediment geochemistry on metal accumulation rates and toxicity in the aquatic oligochaete Tubifex . Aquatic Toxicology, 157, 109–119. [DOI] [PubMed] [Google Scholar]
- Miranda M, Lopez Alonso M and Benedito J, 2005. Copper, zinc, iron, and manganese accumulation in cattle from Asturias (northern Spain). Biological Trace Elements Research, 109, 135–143. [DOI] [PubMed] [Google Scholar]
- Monteiro SC, Lofts S and Boxall ABA, 2010. Scientific/Technical Report submitted to EFSA. Pre‐assessment of environmental impact of zinc and copper used in animal nutrition, NP/FEEDAP/2008/01, 325 pp. 10.2903/sp.efsa.2010.EN-74 [DOI]
- Natal‐da-Luz Gevaert T, Pereira C, Alves D, Arena M and Sousa JP, 2019. Should oral exposure in Hypoaspis aculeifer tests be considered in order to keep them in Tier I test battery for ecological risk assessment of PPPs? Environmental Pollution, 244, 871–876. 10.1016/j.envpol.2018.10.113 [DOI] [PubMed] [Google Scholar]
- Nys C, Vlaeminck K, Van Sprang P and De Schamphelaere KAC, 2020. A generalized bioavailability model (gBAM) for predicting chronic copper toxicity to freshwater fish. Environmental Toxicology and Chemistry, 39, 2424–2436. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Co‐operation and Development), 2000a. Adsorption–desorption using a batch equilibrium method (No. 106). OECD Guidelines for the Testing of Chemicals, Section 1, Éditions OCDE, Paris. Available online: 10.1787/9789264069602-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2000b. Soil microorganisms: nitrogen transformation test (No. 216). OECD Guidelines for the Testing of Chemicals. OECD, Paris, France.
- OECD (Organization for Economic Co‐operation and Development), 2004a. Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264069947-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2004b. Test No. 218: Sediment‐Water Chironomid Toxicity Using Spiked Sediment, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264070264-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2004c. Test No. 219: Sediment‐Water Chironomid Toxicity Using Spiked Water, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264070288-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2004d. Earthworm reproduction test (Eisenia fetida/Eisenia andrei) (No. 222). OECD Guidelines for the Testing of Chemicals. OECD, Paris, France.
- OECD (Organization for Economic Co‐operation and Development), 2006a. Test No. 221: Lemna sp. Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264016194-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2006b. Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test (No. 208). OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264070066-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2006c. Terrestrial Plant Test: Vegetative Vigour Test (No. 227). OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264067295-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2007. Test No. 225: Sediment‐Water Lumbriculus Toxicity Test Using Spiked Sediment, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264067356-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2008. Predatory mite (Hypoaspis/Geolaelaps aculeifer) reproduction test in soil (No. 226). OECD Guidelines for the Testing of Chemicals. OECD, Paris, France.
- OECD (Organization for Economic Co‐operation and Development), 2009. Collembolan reproduction test in soil (No. 232). OECD Guidelines for the Testing of Chemicals. OECD, Paris, France.
- OECD (Organization for Economic Co‐operation and Development), 2011. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264069923-en. [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2012. Test No. 211: Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264185203-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2013. Test No. 210: Fish, Early‐life Stage Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264203785-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2014. Test No. 238: Sediment‐Free Myriophyllum Spicatum Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264224131-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2016. Guidance on the incorporation of bioavailability concepts for assessing the chemical ecological risk and/or environmental threshold values of metals and inorganic metal compounds. OECD Series on Testing and Assessment, No. 259, OECD Publishing, Paris. 10.1787/9789264274839-en [DOI]
- OECD (Organization for Economic Co‐operation and Development), 2019. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. 10.1787/9789264069961-en [DOI]
- Olesen MH and Jensen PK, 2013. Collection and evaluation of relevant information on crop interception. Report by the Aarhus University, Denmark. 67 pp. Available online: www.efsa.europa.eu/en/supporting/pub/438e.htm [Google Scholar]
- Orgiazzi A, Ballabio C, Panagos P, Jones A and Fernández‐Ugalde O, 2018. LUCAS Soil, the largest expandable soil dataset for Europe: a review. European Journal of Soil Science, 69, 140–153. 10.1111/ejss.12499 [DOI] [Google Scholar]
- Oruc HH, Cengiz M and Beskaya , 2009. A. Chronic copper toxicosis in sheep following the use of copper sulfate as a fungicide on fruit trees. Journal of Veterinary Diagnostic Investigations, 21, 540–543. [DOI] [PubMed] [Google Scholar]
- Panagos P, Ballabio C, Lugato E, Jones A, Borrelli P, Scarpa S, Orgiazzi A and Montanarella L, 2018. Potential sources of anthropogenic copper inputs to European agricultural soils. Sustainability, 2018, 2380. 10.3390/su10072380 [DOI] [Google Scholar]
- Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM and Wu K, 2002. The biotic ligand model: a historical overview. Special issue: the biotic ligand model for metals – current research, future directions, regulatory implications. Comparative Biochemistry and Physiology C, 133, 3–35. [DOI] [PubMed] [Google Scholar]
- Peakall D and Burger J, 2003. Methodologies for assessing exposure to metals: speciation, bioavailability of metals and ecological host factors. Ecotoxicology and Environmental Safety, 56, 110–121. [DOI] [PubMed] [Google Scholar]
- Peijnenburg WJGM and Jager T, 2003. Monitoring approaches to assess bioaccessibility of metals: matrix issues. Ecotoxicology and Environmental Safety, 56, 63–77. [DOI] [PubMed] [Google Scholar]
- Peijnenburg WJGM, Capri E, Kula C, Liess M, Luttik R, Montforts M, Nienstedt K, Ombke O, Sousa JP and Jensen J, 2012. Evaluation of exposure metrics for effect assessment of soil invertebrates. Critical Reviews in Environmental Science and Technology, 42, 1862–1893. 10.1080/10643389.2011.574100 [DOI] [Google Scholar]
- Plumlee KL (ed.), 2004. Clinical Veterinary Toxicology. Mosby, St Louis, USA. 477 pp. [Google Scholar]
- Rader KJ, Richard F, Carbonaro RF, van Hullebusch ED, Baken S and Delbeked K, 2019. The fate of copper added to surface water: field, laboratory, and modeling studies. Environmental Toxicology and Chemistry, 38, 1386–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow PS, 2007. Trace metal bioaccumulation: models, metabolic activity and toxicity. Environment International, 33, 576–582. [DOI] [PubMed] [Google Scholar]
- RAR Copper Compounds , 2017. Draft Renewal Assessment Report prepared according to the Commission Regulation (EC No 1007/2009). RMS France; Co‐RMS Germany. Available online: http://registerofquestions.efsa.europa.eu/roqFrontend/outputLoader?output=ON-5152 [Google Scholar]
- Rautmann D, 2000. New basic drift values in the authorisation procedure for plant protection products. Paper for the FOCUS‐Surface Water Group, 2000. 9 pp.
- Redeker ES, Bervoets L and Blust R, 2004. Dynamic model for the accumulation of cadmium and zinc from water and sediment by the aquatic oligochaete, Tubifex tubifex. Environmental Science and Technology, 38, 6193–6200. [DOI] [PubMed] [Google Scholar]
- Riepert F, Steindl A and Eibach R, 2010. Monitoring of total contents of copper in organically and conventionally managed soils. Part 1: study plan and preliminary sampling of copper and their anthropogenic induced heavy metal contents in vineyard soils. Journal für Kulturpflanzen, 62, 42–50. [Google Scholar]
- Riepert F, Baier B, Felgentreu D and Strumpf Th, 2012. Earthworm cenoses used as indicators of soil fertility applied to sites of viticulture. Julius‐Kühn‐Archiv, 436, 37–43. 10.5073/jka.2012.436.005 [DOI] [Google Scholar]
- Römbke J, Schmelz RM and Knaebe S, 2009. Field studies for the assessment of pesticides with soil mesofauna, in particular enchytraeids, mites and nematodes: design and first results. Soil Organisms, 81, 237–264. [Google Scholar]
- Roulier JL, Tusseau‐Vuillemin MH, Coquery M, Geffard O and Garric J, 2008. Measurement of dynamic mobilization of trace metals in sediments using DGT and comparison with bioaccumulation in Chironomus riparius: first results of an experimental study. Chemosphere, 70, 925–932. [DOI] [PubMed] [Google Scholar]
- Rüdel H, Díaz Muñiz C, Garelick H, Kandile NG, Miller BW, Pantoja Munoz L, Peijnenburg WJGM, Purchase D, Shevah Y, van Sprang P, Vijver M and Vink JPM, 2015. Consideration of the bioavailability of metal/metalloid species in freshwaters: experiences regarding the implementation of biotic ligand model‐based approaches in risk assessment frameworks. Environmental Science and Pollution Research, 22, 7405–7421. 10.1007/s11356-015-4257-5 [DOI] [PubMed] [Google Scholar]
- Ruiz E, Alonso‐Azcárate J and Rodríguez L, 2011. Lumbricus terrestris L. activity increases the availability of metals and their accumulation in maize and barley. Environmental Pollution, 159, 722–728. 10.1016/j.envpol.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Sahlin S and Ågerstrand M, 2018. Copper in sediment, EQS Data Overview, ACES report 28. Department of Environmental Science and Analytical Chemistry (ACES). Stockholm University. [Google Scholar]
- Schaeffer A, van den Brink PJ, Heimbach F, Hoy SP, de Jong FMW, Römbke J, Roß‐Nickoll M and Sousa JP, 2010. Semi‐field methods for the environmental risk assessment of pesticides in soil. CRC Press. 144. [DOI] [PubMed] [Google Scholar]
- Schlekat C, Stubblefield W and Gallagher K, 2020. State of the science on metal bioavailability modeling: introduction to the outcome of a Society of Environmental Toxicology and Chemistry technical workshop. Environmental Toxicology and Chemistry, 39, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizmur T and Hodson ME, 2009. Do earthworms impact metal mobility and availability in soil? – a review. Environmental Pollution, 157, 1981–1989. 10.1016/j.envpol.2009.02.029 [DOI] [PubMed] [Google Scholar]
- Sizmur T, Palumbo‐Roe B and Hodson ME, 2010. Why does earthworm mucus decrease metal mobility? Integrated Environmental Assessment and Management, 6, 1551–3777. 10.1002/ieam.124 [DOI] [PubMed] [Google Scholar]
- Sizmur T, Watts MJ, Brown GD, Palumbo‐Roe B and Hodson ME, 2011. Impact of gut passage and mucus secretion by the earthworm Lumbricus terrestris on mobility and speciation of arsenic in contaminated soil. Journal of Hazardous Materials, 197, 169–175. 10.1016/j.jhazmat.2011.09.071 [DOI] [PubMed] [Google Scholar]
- Slaveykova VI and Wilkinson KJ, 2005. Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environmental Chemistry, 2, 9–24. 10.1071/EN040476 [DOI] [Google Scholar]
- Smolders E, Oorts K, Van Sprang P, Schoeters I, Janssen CR, McGrath SP and Mclaughlin MJ, 2009. Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environmental Toxicology and Chemistry, 28, 1633–1642. 10.1897/08-592.1 [DOI] [PubMed] [Google Scholar]
- Soldo D and Behra R, 2000. Long‐term effects of copper on the structure of freshwater periphyton communities and their tolerance to copper, zinc, nickel and silver. Aquatic Toxicology, 47, 181–189. [Google Scholar]
- Sturzenbaum SR, Winters C, Galay M, Morgan AJ and Kille P, 2001. Metal ion trafficking in earthworms: identification of a cadmium‐specific metallothionein. Journal of Biological Chemistry, 276, 34013–34018. [DOI] [PubMed] [Google Scholar]
- Tercier‐Waeber ML, Stoll S and Slaveykova VI, 2012. Trace metal behavior in surface waters: emphasis on dynamic speciation, sorption processes and bioavailability. Archives des sciences, 65, 119–142. [Google Scholar]
- Thompson LJ, 2007. Copper. In: Guptah RC (ed.). Veterinary Toxicology: Basic and Clinical Principles. Elsevier, Amsterdam. pp. 427–430. [Google Scholar]
- Topping CJ, Craig PS, de Jong F, Klein M, Laskowski R, Manachini B, Pieper S, Smith R, Sousa JP, Streissl F, Swarowsky K, Tiktak A and van der Linden , 2015. Towards a landscape scale management of pesticides: ERA using changes in modelled occupancy and abundance to assess long‐term population impacts of pesticides. Science of the Total Environment, 537, 159–169. 10.1016/j.scitotenv.2015.07.152 [DOI] [PubMed] [Google Scholar]
- Topping CJ, Aldrich A and Berny P, 2020. Overhaul environmental risk assessment for pesticides. Science, 367, 360–363. 10.1126/science.aay1144 [DOI] [PubMed] [Google Scholar]
- Toschki A, Hammers‐Wirtz M, Poßberg C, Roß‐Nickoll M, Schaeffer A, Schmidt B, Scholz Starke B, Römbke J, Scheffzyk A, Klein M and Hommen U, 2015. Evaluation of the risk for soil organisms under real conditions – development of a national position in the context of the new European Plant Protection Regulation (EC 1107/2009). Federal Environment Agency, Umweltbundesamt FKZ 3710 67 410. Final report.
- Tranvik L and Eijsackers H, 1989. On the advantage of Folsomia fimetarioides over Isotomiella minor (Collembola) in a metal polluted soil. Oecologia, 80, 195–200. 10.1007/bf00380150 [DOI] [PubMed] [Google Scholar]
- Udovic M, Plavc Z and Lestan D, 2007. The effect of earthworms on the fractionation, mobility and bioavailability of Pb, Zn and Cd before and after soil leaching with EDTA. Chemosphere, 70, 126–134. 10.1016/j.chemosphere.2007.06.044 [DOI] [PubMed] [Google Scholar]
- VDI (Verein Deutscher Ingenieure), 2014. Monitoring the effects of genetically modified organisms – Effects on soil organisms. In: VDI, Technologies of Life Sciences (eds.). VDI manual GMO Monitoring. 72 pp. [Google Scholar]
- Vijver MG, Jager T, Posthuma L and Peijnenburg W, 2001. Impact of metal pools and soil properties on metal accumulation on metal accumulation in Folsomia candida (Collembola). Environmental Toxicology and Chemistry, 20, 712–720. [DOI] [PubMed] [Google Scholar]
- Vijver MG, Vink JPM, Miermans CJH and van Gestel CAM, 2003. Oral sealing using glue: a new method to distinguish between intestinal and dermal uptake of metals in earthworms. Soil Biology and Biochemistry, 35, 125–132. [Google Scholar]
- Vijver MG, Hunting ER, Nederstigt TA, Tamis WL, van den Brink PJ and van Bodegom PM, 2017. Postregistration monitoring of pesticides is urgently required to protect ecosystems. Environmental Toxicology and Chemistry, 36, 860–865. 10.1002/etc.3721 [DOI] [PubMed] [Google Scholar]
- Vink JPM, 2009. The origin of speciation: trace metal kinetics over natural water/sediment interfaces and the consequences for bioaccumulation. Environmental Pollution, 157, 519–527. [DOI] [PubMed] [Google Scholar]
- VRAR , 2008. Voluntary risk assessment reports – copper and copper compounds. Available online: https://echa.europa.eu/copper-voluntary-risk-assessment-reports
- Wang W, 1987. Factors affecting metal toxicity to (and accumulation by) aquatic organisms – overview. Environment International, 13, 437–457. [Google Scholar]
- Watanabe T, Kiron V and Satoh S, 1997. Trace minerals in fish nutrition. Aquaculture, 151, 185–207. [Google Scholar]
- Weltens R, Goossens S and van Puymbroeck S, 2000. Ecotoxicity of contaminated suspended solids for filter feeders (Daphnia magna). Archives of Environmental Contamination and Toxicology, 39, 315–323. [DOI] [PubMed] [Google Scholar]
- WFD (Water Framework Directive), 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy.
- Wikfors GH and Ukeles R, 1982. Growth and adaptation of estuarine unicellular algae in media with excess copper, cadmium or zinc, and effects of metal‐contaminated algal food on Crassostrea virginica larvae. Marine Ecology Progress Series, 7, 191–206. [Google Scholar]
- Xu H, Demetriades A, Reimann C, Jiménez JJ, Filser J and Zhang C and GEMAS Project Team , 2019. Identification of the co‐existence of low total organic carbon contents and low pH values in agricultural soil in north‐central Europe using hot spot analysis based on GEMAS project data. Science of the Total Environment, 678, 94–104. 10.1016/j.scitotenv.2019.04.382 [DOI] [PubMed] [Google Scholar]
- Zeng S, Li J, Wei D and Ma Y, 2017. A new model integrating short‐ and long‐term ageing of copper added to soils. PLoS ONE, 12, e0182944.18. 10.1371/journal.pone.0182944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yu CG, Zeng GM, Jiang M, Yang ZZ, Cui F, Zhu MY, Shen LG and Hu L, 2014. Effects of sediment geochemical properties on heavy metal bioavailability. Environment International, 73, 270–281. [DOI] [PubMed] [Google Scholar]


