Abstract
Background
Omega-3 polyunsaturated fatty acids (n3-PUFAs) may exert beneficial effects on the immune system of patients with viral infections. This paper aimed to examine the effect of n3-PUFA supplementation on inflammatory and biochemical markers in critically ill patients with COVID-19.
Methods
A double-blind, randomized clinical trial study was conducted on 128 critically ill patients infected with COVID-19 who were randomly assigned to the intervention (fortified formula with n3-PUFA) (n = 42) and control (n = 86) groups. Data on 1 month survival rate, blood glucose, sodium (Na), potassium (K), blood urea nitrogen (BUN), creatinine (Cr), albumin, hematocrit (HCT), calcium (Ca), phosphorus (P), mean arterial pressure (MAP), O2 saturation (O2sat), arterial pH, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3), base excess (Be), white blood cells (WBCs), Glasgow Coma Scale (GCS), hemoglobin (Hb), platelet (Plt), and the partial thromboplastin time (PTT) were collected at baseline and after 14 days of the intervention.
Results
The intervention group had significantly higher 1-month survival rate and higher levels of arterial pH, HCO3, and Be and lower levels of BUN, Cr, and K compared with the control group after intervention (all P < 0.05). There were no significant differences between blood glucose, Na, HCT, Ca, P, MAP, O2sat, PO2, PCO2, WBCs, GCS, Hb, Plt, PTT, and albumin between two groups.
Conclusion
Omega-3 supplementation improved the levels of several parameters of respiratory and renal function in critically ill patients with COVID-19. Further clinical studies are warranted.
Trial registry
Name of the registry: This study was registered in the Iranian Registry of Clinical Trials (IRCT); Trial registration number: IRCT20151226025699N3; Date of registration: 2020.5.20; URL of trial registry record: https://en.irct.ir/trial/48213
Keywords: Omega-3 fatty acids, Coronavirus, Kidney function, Respiratory function
Introduction
In recent years, coronavirus infections have been a significant source of morbidity and mortality around the world [1]. Novel coronavirus 2019 (nCoV-2019), now known as acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is responsible for the pandemic of coronavirus disease COVID-19 [2]. This infection usually causes a defined pattern of metabolic and clinical changes in affected patients [3]. Leukocytopenia, lymphopenia, and elevated C-reactive protein (CRP) can occur in the primary form of COVID-19. With disease progression, increasing levels of leukocytes, creatine kinase, and creatinine may also occur [4].
Host nutritional status has an important role in defense against viral infections [5]. Numerous studies have reported that malnourished humans are more susceptible to a wide variety of infections [6]. Indeed, proper nutrition can preserve the immune system and improve its function [7]. Nutritional deficiencies influence both immune response and viral pathogenic functions [5, 8]. Moreover, they may lead to oxidative stress in the host which can alter the genome of the virus, so that a normally benign or mildly pathogenic virus can convert to a highly virulent pathogen [6].
Omega-3 polyunsaturated fatty acids (n3-PUFAs) are important mediators of inflammation and acquired immune responses and can amplify anti-inflammatory responses [9]. Recent studies have been shown that n3-PUFAs including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and α-linoleic acid (ALA) can increase the stability of the cell membrane, regulate immune function, block hyper inflammatory reactions, and reduce the incidence of systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), and complications of infection [10].
Chen et al. reported a lower mortality rate in septic patients who received total parenteral nutrition containing 100 ml (ml) n3-PUFAs daily for 4 weeks. Also, the treatment group had a higher ratio of T helper to inducer lymphocytes and CD4 to CD8 lymphocytes in comparison with the control group during the first 7 days of follow-up [11]. Pluta et al. found that patients with chronic kidney disease, who daily received one capsule of omega 3 including 1000 mg n3-PUFAs containing 330 mg of EPA, 220 mg of DHA, and 100 mg of other acids such as alpha-linolenic acid (ALA) had lower urinary excretion of monocyte chemoattractant protein-1 (MCP-1) after 6 months of follow-up. However, white blood cell (WBC) count and serum levels of CRP and MCP-1 did not change significantly [12]. Soleimani et al. reported that patients with diabetic nephropathy, who received 1 g omega-3 acids daily had higher circulating nitric oxide (NO) after 12 weeks of follow-up with no change in serum concentrations of high sensitivity (hs)-CRP or total antioxidant capacity (TAC) [13]. Coghill et al. demonstrated that human immunodeficiency virus (HIV) and human herpesvirus-8 (HHV-8) co-infected patients, who received 3 gr of omega-3 fatty acids daily (each pill contained about 230 mg EPA and 150 mg DHA), had lower serum concentrations of the inflammatory cytokine interleukin-6 (IL-6) and higher CD8 + counts at 12 weeks [14]. Omar et al. in a recent study found that end-stage renal disease patients on chronic hemodialysis, who daily received two grams of omega-3 (containing 360 mg EPA and 240 mg DHA) had lower serum levels of IL-6 and CRP at 3 months [15]. On the other hand, Rice et al. conducted a randomized, double-blind, placebo-controlled, multicenter trial and reported that the n-3 supplementation did not improve the primary end point of ventilator-free days or other clinical outcomes in patients with acute lung injury and may be harmful. Patients receiving the n-3 supplement had fewer ventilator-free days, intensive care unit–free days, and nonpulmonary organ failure–free days [16]
Given suggestive evidence of the beneficial effects of n3-PUFAs on the immune system and the contradictory results of recent studies concerning the effects of omega-3 fatty acids in patients with viral infections, we aimed to examine the effect of omega-3 supplementation on inflammatory and biochemical markers in critically ill patients with COVID-19.
Methods
Study design and population
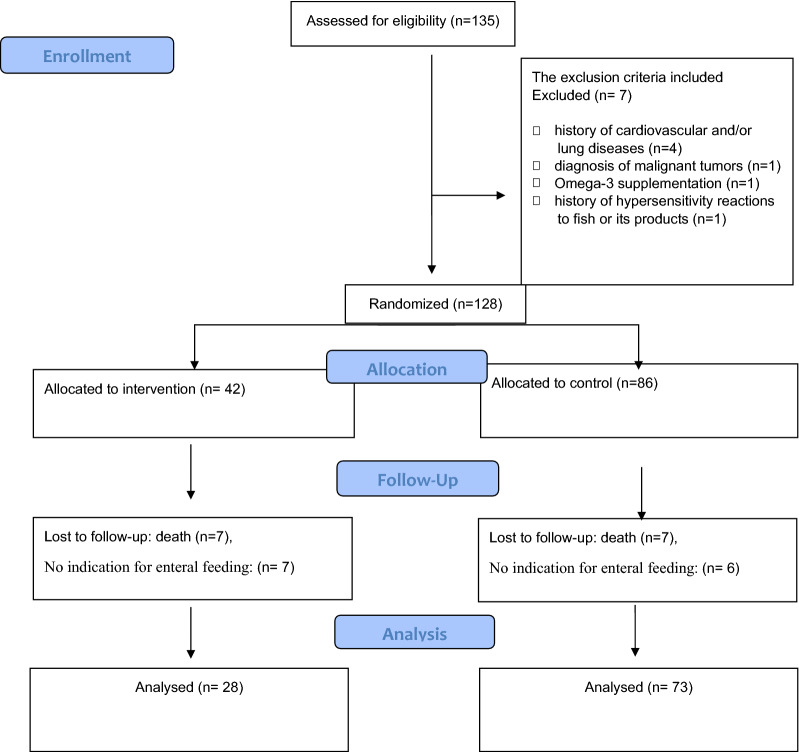
This study was a double-blind, randomized clinical trial study carried out from May to July 2020 in critically ill patients infected with COVID-19 in Razi Hospital, Rasht, Iran. The inclusion criteria were the age between 35 and 85 years, diagnosis of COVID-19 confirmed by a positive RT-PCR nasopharyngeal swab, as well as symptoms such as severe pneumonia, fever, fatigue, dry cough, respiratory distress, and indicated for enteral nutrition. One hundred thirty five patients were assessed for eligibility and 128 patients met the inclusion criteria. Sample size was calculated using α = 0.95, β = 20%, ratio of unexposed to exposed of 2:1, and power of 0.8. An unequal randomization ratio (2:1) was used because of a fixed and limited budget for this research project. Therefore, more participants were randomized to the cheaper arm in order to facilitate greater overall recruitment in the face of a possibly high drop-out rate. The exclusion criteria included history of cardiovascular and/or lung diseases (n = 4), and diagnosis of malignant tumors (n = 1) because of their effects on current medical treatment and diet, consumption of omega-3 fatty acids during the prior 3 months before the study (n = 1), history of hypersensitivity reactions to fish or its products (n = 1), did not complete the study because of death (n = 14) or no indication for enteral feeding any more (n = 13). The final analysis was performed on 101 critically ill patients infected with COVID-19 who were assigned to the intervention (n = 28) and control (n = 73) groups.
Interventions
This study was done in form of a double-blind trial. Though no placebo was used, the patients were not aware of their feeding contents, and the patients and researchers were not aware of the arms of the study. Finally, the results were analyzed by a person outside of the treatment team. The allocation to the groups was done through web-based randomization using https://www.randomizer.org. Sealed non-transparent envelopes with randomized sequences were used to hide the allocation. All participants received high protein formula as 30 kcal/kg/d through enteral feeding. The intervention group received one capsule of 1000 mg omega-3 daily (Vita Pharmed, Switzerland) containing 400 mg EPAs and 200 mg DHAs for 14 days through adding the supplement to their enteral formula. Omega-3 fatty acids fortified formula was administered to the case group by a nurse, who was not a member of the research team, for 2 weeks after the first 24 h of hospitalization in ICU. The omega-3 capsules were pierced with a syringe, then its contents were squeezed out into a prepared enteral formula, with thorough mixing. The control group received nutritional support including the isocaloric-isovolemic formula using the same route, except for the intervention.
Data collection
After collecting the written consent forms, the required information was collected using a pre-determined checklist including the following sub-scales:
Anthropometric and medical history
Data on age, weight, height, previous illnesses, medications, blood pressure, serum lipids, random blood glucose, and respiratory status was collected using medical records.
Dietary intake
Data on nutritional intake including caloric intake, type of formula, and dietary supplements were assessed using documents recorded in ICU sheets and patient logs.
Biochemical and pathological indices
Arterial blood gas (ABG) parameters [i.e., O2 saturation, arterial pH, partial pressure of oxygen (PO2), partial pressure of carbon dioxide (PCO2), bicarbonate (HCO3), and base excess (Be)], kidney function parameters [i.e., blood urea nitrogen (BUN), creatinine (Cr), and urine volume], as well as blood glucose, mean arterial pressure, white cell blood count (including neutrophil, lymphocyte, monocyte), the Acute Physiology and Chronic Health Evaluation II (APACHE II), Glasgow Coma Scale (GCS), hemoglobin (Hb), platelet (Plt), the partial thromboplastin time (PTT), serum electrolytes [i.e. sodium (Na), potassium (K), calcium, phosphorus (P)], albumin, and hematocrit (Hct) were measured at baseline and after 14 days of the intervention. These measurements were routinely performed in the hospital using standard kits and the required data were gathered from the lab test section of the ICU sheets.
Statistical analyses
Quantitative data were presented as the mean ± standard deviation and qualitative data were presented as the percentage and number to describe the status of demographic, social, and anthropometric measurements of the participants. The Kolmogorov–Smirnov test was used to verify the normal distribution. Independent T-test and chi-square test were used to compare results between groups at baseline. The core research question was whether the difference between groups of interest changed from the basal values to the values after treatment. The general linear model repeated measure was used to identify the group-by-time interaction effect of omega-3 supplementation on inflammatory and biochemical markers, comparing the values in intervention and control groups. Confounding factors including age, sex, body mass index (BMI), dietary intake, smoking, presence of background diseases such as diabetes and hypertension, and medication and supplements were adjusted for. Statistical analyses were performed using the SPPS software (version 20). P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 135 eligible patients were included in this study and randomization was done 1:2. Of these, seven patients were excluded based on the exclusion criteria (Fig. 1). In addition, another seven patients in the treatment group and twenty controls were excluded from analysis after randomization, because they died during the intervention period. Finally, 101 patients were included in the analysis, of which 28 were assigned to the treatment group and 73 were assigned to the control group. All baseline data in Table 1 were normally distributed. The groups were not significantly different in demographic or clinical characteristics at baseline, except for the lymphocyte count, which was lower in the intervention group (11.59 vs 13.26, P = 0.01).
Fig. 1.
Study flowchart
Table 1.
Baseline demographic and clinical data of the study groups
| Interventions (n = 28) | Controls (n = 73) | P | |
|---|---|---|---|
| Age y | 66 (14.58) | 64 (14.25) | 0.12 |
| Males n (%) | 15 (53.60) | 45 (61.60) | 0.46 |
| Weight kg | 77.39 (21.12) | 75.09 (7.26) | 0.58 |
| Height meter | 1.67 (7.28) | 1.66 (6.42) | 0.39 |
| BMI kg/m2 | 27.68 (7.54) | 27.39 (3.15) | 0.85 |
| APACHE II | 15.54 (1.73) | 15.42 (1.92) | 0.78 |
| Blood group n (%) | |||
| A | 13 (50) | 24 (34.3) | 0.14 |
| B | 3 (11.5) | 16 (22.9) | |
| AB | 1 (3.8) | 0 | |
| O | 9 (34.6) | 30 (42.9) | |
| Addiction n (%) | |||
| Yes | 1 (3.6) | 6 (8.6) | 0.39 |
| No | 27 (96.4) | 64 (91.4) | |
| Tobacco n (%) | |||
| Yes | 2 (7.1) | 5 (7.1) | 1 |
| No | 26 (92.9) | 65 (92.9) | |
| Underlying disease n (%) | |||
| Yes | 14 (50) | 28 (40) | 0.57 |
| No | 14 (50) | 41 (60.2) | |
| 1 month survival rate | 5 (21) | 2 (3) | 0.005 |
| Blood glucose mg/mL | 148.98 (10.00) | 152.41 (8.71) | 0.15 |
| Na mEq/L | 138.48 (3.47) | 139.32 (1.74) | 0.11 |
| K mEq/L | 4.03 (0.1) | 4.00 (0.1) | 0.51 |
| BUN mg/mL | 37.06 (4.14) | 35.17 (4.10) | 0.96 |
| Cr mg/mL | 1.33 (0.25) | 1.29 (0.24) | 0.76 |
| Alb g/dL | 3.02 (0.14) | 3.35 (0.39) | 0.44 |
| Hct % | 31.88 (0.61) | 29.65 (0.66) | 0.59 |
| Ca mg/dL | 7.96 (0.22) | 7.65 (0.12) | 0.80 |
| P mg/dL | 3.30 (0.21) | 3.33 (0.19) | 0.87 |
| MAP mmHg | 74.23 (1.64) | 74.42 (1.74) | 0.97 |
| O2 sat. % | 84.47 (1.93) | 77.79 (1.80) | 0.16 |
| Arterial pH | 7.27 (0.01) | 7.30 (0.01) | 0.15 |
| PO2 mmHg | 68.93 (3.72) | 67.72 (3.97) | 0.53 |
| PCO2 mmHg | 45.20 (2.41) | 43.53 (2.03) | 0.06 |
| HCO3 mEq/L | 21.11 (1.13) | 22.00 (1.19) | 0.91 |
| Be mEq/L | − 4.97 (1.04) | − 3.59 (1.05) | 0.11 |
| WBC 106/L | 14.26 (1.12) | 20.47 (3.83) | 0.40 |
| Neutrophil 106/L | 86.54 (0.74) | 86.76 (0.94) | 0.22 |
| Lymphocyte 106/L | 11.59 (0.55) | 13.26 (0.81) | 0.01 |
| Monocyte 106/L | 2.12 (0.26) | 1.67 (0.28) | 0.11 |
| GCS | 8.37 (0.22) | 7.90 (0.20) | 0.09 |
| Hb g/dL | 10.32 (0.28) | 9.50 (0.26) | 0.07 |
| Plt | 248.69 (16.27) | 187.91 (16.18) | 0.07 |
| Ptt second | 38.90 (2.20) | 43.07 (2.52) | 0.10 |
| Urine volume mL/day | 1572.60 (201.15) | 1803.44 (180.29) | 0.05 |
| Number of dialysis days | 0.26 (0.66) | 0.19 (0.72) | 0.67 |
| Number of insulin injection days | 0.26 (0.71) | 0.07 (0.50) | 0.23 |
Data are mean (SD) for quantitative variables and number (%) for categorical variables
sBMI body mass index, APACHE II acute physiologic assessment and chronic health evaluation II, Na sodium, K potassium, BUN blood urea nitrogen, Cr creatinine, Alb albumin, HCT hematocrit, Ca calcium, P phosphorus, MAP mean arterial pressure, O2 sat. oxygen saturation, pH potential hydrogen, PO2 partial pressure of oxygen, PCO2 partial pressure of carbon dioxide, HCO3 bicarbonate, Be base excess, WBC white blood cell, GCS glasgow coma score, Hb hemoglobin, Plt platelet, Ptt partial thromboplastin time (test)
Effects of omega-3 supplementation on 1-month survival rate
The intervention group had significantly higher 1-month survival rate compared with the control group (21% vs 3%, P = 0.003). About 21% (n = 6) of the participants in the intervention group and only about 3% (n = 2) of the participants in the control group survived at least for 1 month after the beginning of the study.
Effects of omega-3 supplementation on kidney function
As summarized in Table 2, levels of BUN (35.17 vs 43.19, F = 4.76, P = 0.03) and Cr (1.29 vs 1.68, F = 5.90, P = 0.02) were significantly lower and the amount of urine excreted (2101 vs 1877.02, F = 12.26, p = 0.01) was significantly higher in the intervention group compared with the control group.
Table 2.
Comparison of clinical and biochemical parameters between the intervention group and control group after the intervention
| Factors | Intervention group mean (SE) | Control group mean (SE) | Group timea | |
|---|---|---|---|---|
| After | After | F | P | |
| Blood sugar (mg/mL) | 152.41 (8.71) | 142.27 (5.35) | 0.16 | 0.69 |
| Na (mEq/L) | 139.32 (1.74) | 140.99 (1.08) | 1.25 | 0.27 |
| K (mEq/L) | 4.00 (0.1) | 4.14 (0.06) | 10.15 | 0.01 |
| BUN (mg/mL) | 35.17 (4.10) | 43.19 (2.50) | 4.76 | 0.03 |
| Cr (mg/mL) | 1.29 (0.24) | 1.68 (0.15) | 5.90 | 0.02 |
| Alb (g/dL) | 3.35 (0.39) | 2.88 (0.26) | 0.59 | 0.45 |
| Hct (%) | 29.65 (0.66) | 29.84 (0.43) | 0.08 | 0.77 |
| Ca (mg/dL) | 7.65 (0.12) | 7.88 (0.72) | 0.27 | 0.61 |
| P (mg/dL) | 3.33 (0.19) | 3.12 (0.12) | 0.80 | 0.78 |
| MAP (mmHg) | 74.42 (1.74) | 69.72 (1.06) | 3.25 | 0.08 |
| O2 sat. (%) | 80.73 (1.80) | 75.50 (1.11) | 0.13 | 0.72 |
| Arterial pH | 7.30 (0.01) | 7.26 (0.01) | 19.11 | 0.01 |
| PO2 (mmHg) | 67.72 (3.97) | 61.40 (2.58) | 0.00 | 0.96 |
| PCO2 (mmHg) | 43.53 (2.03) | 44.26 (1.25) | 1.55 | 0.22 |
| HCO3 (mEq/L) | 22.00 (1.19) | 18.17 (0.73) | 10.83 | 0.01 |
| Be (mEq/L) | − 3.59 (1.05) | − 7.09 (0.65) | 23.01 | 0.01 |
| WBC (106/L) | 20.47 (3.83) | 13.57 (2.35) | 0.36 | 0.55 |
| Neutrophil (106/L) | 86.76 (0.94) | 87.29 (0.85) | 2.00 | 0.17 |
| Lymphocyte (106/L) | 11.59 (0.81) | 11.80 (0.73) | 4.08 | 0.05 |
| Monocyte (106/L) | 1.67 (0.28) | 2.16 (0.42) | 0.03 | 0.87 |
| GCS | 7.90 (0.20) | 7.49 (0.12) | 6.07 | 0.05 |
| Hb (g/dL) | 9.50 (0.26) | 9.68 (0.16) | 1.60 | 0.21 |
| Plt | 187.91 (16.18) | 162.49 (9.69) | 1.11 | 0.30 |
| Ptt (second) | 43.07 (2.52) | 44.14 (1.55) | 1.81 | 0.18 |
| Urine volume (mL) | 2101 (884) | 1877.02 (917) | 12.26 | 0.01 |
sBMI body mass index, APACHE II acute physiologic assessment and chronic health evaluation II, Na sodium, K potassium, BUN blood urea nitrogen, Cr creatinine, Alb albumin, HCT hematocrit, Ca calcium, P phosphorus, MAP mean arterial pressure, O2 sat. oxygen saturation, pH potential hydrogen, PO2 partial pressure of oxygen, PCO2 partial pressure of carbon dioxide, HCO3 bicarbonate, Be base excess, WBC white blood cell, GCS glasgow coma score, Hb hemoglobin, Plt platelet, Ptt partial thromboplastin time (test)
aRepeated measure ANOVA
Effects of omega-3 supplementation on arterial blood gas (ABG) parameters
After 14 days, the levels of arterial pH (7.30 vs 7.26, F = 19.11, P = 0.01), HCO3 (22.00 vs 18.17, F = 10.83, P = 0.01), and Be (−4.97 vs −3.59, F = 23.01, P = 0.01) were significantly higher in the intervention group compared with the control group. However, there were no significant differences among PO2 and PCO2 between two groups (Table 2).
Effects of omega-3 supplementation on the Glasgow coma scale (GCS)
On admission to the study, the mean GCS was 8.37 in the intervention group and 7.90 in the control group (P > 0.05). As shown in Table 2, it was significantly lower after 14 days of the study in both groups (7.90 vs 7.49, F = 6.07, P = 0.05). No significant difference was found in APACHE II score between the groups (15.54 ± 1.73 vs 15.42 ± 1.92., P = 0.78).
Effects of omega-3 supplementation on serum electrolytes
As shown in Table 2, the level of K was significantly reduced in the intervention group compared to the control group (4.00 vs 4.14, F = 10.15, P = 0.01) after 14 days. No significant differences were found between the levels of serum electrolytes including Na, Ca, and P in between two groups after intervention.
Effects of omega-3 supplementation on blood clotting function and cell blood count (CBC)
The lymphocyte count increased in the omega-3 group compared to the control group, though this effect was marginally significant (11.59 vs 11.80, F = 4.08, P = 0.05). There were no significant differences in levels of PTT, hematocrit, neutrophil, monocyte, hemoglobin, and Plt between the two groups (Table 2).
Effects of omega-3 supplementation on the other blood factors
As shown in Table 2, no significant differences were observed in the other factors including blood glucose, albumin, MAP, and O2 sat between two groups after intervention.
Discussion
To our knowledge, this is the first randomized clinical trial assessing the effect of omega-3 fatty acid supplementation in ICU patients with COVID-19. We found that administration of omega-3 PUFA significantly improved arterial PH, HCO3, and Be. This trial also supports our hypothesis that omega-3 supplementation can improve the level of indicators of kidney function including BUN, Cr, K, and urine volume. The results have indicated that omega-3 supplementation may increase the lymphocyte count and GCS. However, these increases were not statistically significant. In our study, omega-3 supplementation had a positive effect on 1-month survival rate of the critically ill patients with COVID-19.
Many severely or critically ill COVID-19 patients have been reported to develop severe metabolic and respiratory acidosis [17, 18], indicating possible microcirculation dysfunction [18]. This study identified that omega-3 supplementation improved arterial PH, HCO3, and Be, which may be related to the effect of omega-3 supplementation on microcirculatory function. Several studies [19–21] have reported that omega-3 supplementation improved endothelial function and microcirculation. Vasil'ev et al. reported that therapy with omega-3 PUFAs increased tissue hemoperfusion, capillary blood flow, and tissue blood flow and generally improved microcirculation. Trevor et al. studied the effects of DHA and EPA supplementation on vascular reactivity and microcirculation. They found that DHA significantly enhanced vasodilator mechanisms, attenuated constrictor responses, and improved endothelium reactivity and microcirculation [22]
It has been reported that renal abnormalities occurred in the majority of patients with COVID-19 [18, 23–25], and microemboli in renal vessels as a consequence of the prothrombotic state results in kidney damage. Furthermore, hypertension induced by pro-inflammatory status, increasing IFN-γ, IL-6, and IL-17 expression and CD8 + T cells in the kidney, angiotensin II-induced hypertension, and the endothelial dysfunction caused by the innate immune response seems to be involved in the kidney injury [26]. Renal involvement in COVID-19 was reported to be associated with higher mortality [18, 23–25]. Hence, it is crucial to identify adjuvant and protective therapies that improve kidney function in patients with severe COVID-19. The present study found that the administration of omega-3 PUFAs significantly decreased BUN, Cr, and K and increased urine volume in ICU patients infected with COVID-19. These findings suggest that omega-3 supplementation may be protective against progression to renal impairment, consistent with observations from animal models that showed that omega-3 PUFA supplementation reduces the progression of renal disease [27–31]. Moreover, studies in human subjects suggested that a higher dietary intake of PUFAs may improve creatinine clearance [32] and also may have a role in maintaining healthy kidney function [33]. In contrast, some studies have reported that omega-3 treatment did not protect renal function. Higashihara et al. assessed the effects of omega-3 PUFAs on kidney function in 41 patients with stages two or three chronic kidney disease (CKD); omega-3 supplementation did not alter kidney function, assessed using 24-h urine creatinine clearance. Another study demonstrated that omega-3 PUFA pre-treatment did not protect against renal function deterioration or ischemia-induced renal inflammation; however, tubular transport was improved [30]. Previous studies that have evaluated omega-3 PUFAs in the treatment of kidney disease have yielded contradictory results, which may reflect differences in study duration, dosage of omega-3, route of administration, and type of omega-3 fatty acids.
Omega-3 PUFAs can change the lipid composition and cell function of lymphocytes and affect cellular immune function [10, 34, 35]. There is no consensus regarding the efficacy of omega-3 PUFAs on lymphocyte count. A meta-analysis of randomized control trials found that the value of postoperative lymphocyte count increased in response to omega-3 PUFAs supplementation [10]. Consistent with this finding, the results of the present study identified a marginal increase in lymphocyte count after omega-3 PUFAs supplementation.
The results of the current study identified a significant difference in 1-month survival rate between two groups. Supplementation with omega-3 fatty acids may have immune-modulating [36–38] and organ-protective effects [39, 40]. Previous studies have reported that higher plasma levels of omega-3 PUFAs are associated with survival [41–43]. Moreover, a recent systematic review found that omega-3 supplements can improve survival in critically ill patients [44]. However, another recent review article concluded that there is weak evidence for beneficial effects of omega-3 supplementation on ICU or hospitalized survival in critically ill patients [45]. Heller et al. have reported that the effects and effect sizes of omega-3 on clinical outcome is dependent on the disease [46].
Our study had some limitations. Although the sample size was calculated with an acceptable power for the study, the results need to be confirmed in larger studies. Moreover, only one dosage of omega-3 was used, which makes it difficult to discuss the dose–response efficacy of the supplement. It is possible that we may have seen more benefit with a higher dose. Another limitation of this study was the short duration of the study. Furthermore, prognostic biochemical markers in COVID-19 including the inflammatory cytokines C-reactive protein (CRP), interleukin-6, and interleukin-10, were not measured due to limited resources. Another limitation of this study was that there was no access to an omega-3 fatty acid enriched formula, and therefore we had to add it manually.
Conclusion
In conclusion, this randomized, double-blind, clinical trial has shown that omega-3 supplementation has promising effects on acidosis and renal function and possibly can improve clinical outcomes of patients infected with COVID-19. Further clinical studies with different dosages of n-3 PUFAs, larger sample sizes, and longer duration are warranted.
Acknowledgements
This study was conducted at Guilan University of Medical Sciences, Rasht, Iran. We acknowledge the staff of the mentioned centers for their kind cooperation.
Abbreviations
- n3-PUFAs
Omega-3 polyunsaturated fatty acids
- Na
Sodium
- K
Potassium
- BUN
Blood urea nitrogen
- Cr
Creatinine
- HCT
Hematocrit
- Ca
Calcium
- P
Phosphorus
- MAP
Mean arterial pressure
- O2 sat
O2 saturation
- PCO2
Partial pressure of oxygen
- WBC
White blood cells
Authors’ contributions
SD, SG, SR, MGH, FM, SEB, FS and MEA designed the study, involved in the data collection, analysis, and drafting of the manuscript. MSH, FGH, AMJ, NA, HTM, AH, PJ, AM and MG were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this study was provided by Sabzevar University of Medical Sciences (code: 99068), Sabzevar, Iran.
Availability of data and materials
All data will be made available upon request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Sabzevar University of Medical Sciences and Health Services, Khorasan Razavi, Iran (Ethical code: IR.MEDSAB.REC.1399.054). Furthermore, the necessary details of the project were explained clearly to the patients or their first-degree relatives and written informed consent was obtained from them before they joined the study. All participants were free to decide whether or not to participate or to withdraw at any time and for any reason without further penalty either personal or professional or affecting their future medical care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Al-Osail AM, Al-Wazzah MJ. The history and epidemiology of middle east respiratory syndrome corona virus. Multidiscip Respir Med. 2017;12(1):1–6. doi: 10.1186/s40248-017-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z-W, Yuan S, Yuen K-S, Fung S-Y, Chan C-P, Jin D-Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–97. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurpad AV. The requirements of protein & amino acid during acute & chronic infections. Indian J Med Res. 2006;124(2):129–48. [PubMed] [Google Scholar]
- 4.Han Q, Lin Q, Jin S, You L. Recent insights into 2019-nCoV: a brief but comprehensive review. J Infect. 2020;80(4):373–7. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systemic review. J Med Virol. 2020;92(5):479–90. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133(5):1463S–7S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 7.López Plaza B, Bermejo López LM. Nutrición y trastornos del sistema inmune. Nutr Hosp. 2017;34:68–71. doi: 10.20960/nh.1575. [DOI] [PubMed] [Google Scholar]
- 8.BourBour F, Mirzaei Dahka S, Gholamalizadeh M, Akbari ME, Shadnoush M, Haghighi M, Taghvaye-Masoumi H, Ashoori N, Doaei S. Nutrients in prevention, treatment, and management of viral infections; special focus on coronavirus. Arch Physiol Biochem. 2020;7:1–10. doi: 10.1080/13813455.2020.1791188. [DOI] [PubMed] [Google Scholar]
- 9.Bheliya VK, Pathak AK. clinical research and role of dietary supplement in the treatment of middle east respiratory syndrome current status. J Pharm Pharm Sci. 2020;9(3):823–39. [Google Scholar]
- 10.Zhao Y, Wang C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: a meta-analysis of randomized control trials in China. Medicine. 2018;97(16):e0472. doi: 10.1097/MD.0000000000010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Wang W, Hong Y, Zhang H, Hong C, Liu X. Single-blinded, randomized, and controlled clinical trial evaluating the effects of omega-3 fatty acids among septic patients with intestinal dysfunction: a pilot study. Exp Ther Med. 2017;14(2):1505–11. doi: 10.3892/etm.2017.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pluta A, Stróżecki P, Kęsy J, Lis K, Sulikowska B, Odrowąż-Sypniewska G, Manitius J. Beneficial effects of 6-month supplementation with omega-3 acids on selected inflammatory markers in patients with chronic kidney disease stages 1–3. Biomed Res Int. 2017;2017:1680985. doi: 10.1155/2017/1680985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soleimani A, Taghizadeh M, Bahmani F, Badroj N, Asemi Z. Metabolic response to omega-3 fatty acid supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(1):79–84. doi: 10.1016/j.clnu.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Coghill AE, Schenk JM, Mahkoul Z, Orem J, Phipps W, Casper C. Omega-3 decreases interleukin-6 levels in HIV and HHV-8 co-infected patients: results from a randomized supplementation trial in Uganda. AIDS. 2018;32(4):505–12. doi: 10.1097/QAD.0000000000001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omar ZA, Montser BA, Farahat MAR. Effect of high-dose omega 3 on lipid profile and inflammatory markers in chronic hemodialysis children. Ren Fail. 2010;32(9):1031–5. doi: 10.3109/0886022X.2010.510231. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Wheeler AP, Thompson BT, DeBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–81. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26(5):499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–20. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller A, Koch T. Immunonutrition with omega-3-fatty acids. Are new anti-inflammatory strategies in sight? Zentralbl Chir. 2000;125(2):123–36. [PubMed] [Google Scholar]
- 20.Vasil'ev A, Strel'tsova N, Sekisova M. Effect of omega-3 fatty acids on the serum lipid profile and microcirculation in patients with metabolic syndrome and hypertensive disease. Klin Med. 2009;87(4):37–41. [PubMed] [Google Scholar]
- 21.Weitzel L-RB, Mayles WJ, Sandoval PA, Wischmeyer PE. Effects of pharmaconutrients on cellular dysfunction and the microcirculation in critical illness. Curr Opin Anaesthesiol. 2009;22(2):177–83. doi: 10.1097/ACO.0b013e328328d32f. [DOI] [PubMed] [Google Scholar]
- 22.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(11):1264–9. doi: 10.1161/01.CIR.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 23.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–65. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 26.Robba C, Battaglini D, Pelosi P, Rocco PR. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020 doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simopoulos A, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63(3):119–21. doi: 10.1054/plef.2000.0176. [DOI] [PubMed] [Google Scholar]
- 28.Brown SA, Finco DR, Brown CA. Is there a role for dietary polyunsaturated fatty acid supplementation in canine renal disease? J Nutr. 1998;128:2765S–7S. doi: 10.1093/jn/128.12.2765S. [DOI] [PubMed] [Google Scholar]
- 29.Garman JH, Mulroney S, Manigrasso M, Flynn E, Maric C. Omega-3 fatty acid rich diet prevents diabetic renal disease. Am J Physiol Renal Physiol. 2009;296(2):F306–16. doi: 10.1152/ajprenal.90326.2008. [DOI] [PubMed] [Google Scholar]
- 30.Rund KM, Peng S, Greite R, Claaßen C, Nolte F, Oger C, Galano J-M, Balas L, Durand T, Chen R. Dietary omega-3 PUFA improved tubular function after ischemia induced acute kidney injury in mice but did not attenuate impairment of renal function. Prostaglandins Other Lipid Mediat. 2020;146:106386. doi: 10.1016/j.prostaglandins.2019.106386. [DOI] [PubMed] [Google Scholar]
- 31.Hassan IR, Gronert K. Acute changes in dietary ω-3 and ω-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182(5):3223–32. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 32.Lauretani F, Maggio M, Pizzarelli F, Michelassi S, Ruggiero C, Ceda G, Bandinelli S, Ferrucci L. Omega-3 and renal function in older adults. Curr Pharm Des. 2009;15(36):4149–56. doi: 10.2174/138161209789909719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopinath B, Harris DC, Flood VM, Burlutsky G, Mitchell P. Consumption of long-chain n-3 PUFA, α-linolenic acid and fish is associated with the prevalence of chronic kidney disease. Br J Nutr. 2011;105(9):1361–8. doi: 10.1017/S0007114510005040. [DOI] [PubMed] [Google Scholar]
- 34.Fan Y-Y, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133(6):1913–2035. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–7. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 37.Berger MM, Delodder F, Liaudet L, Tozzi P, Schlaepfer J, Chiolero RL, Tappy L. Three short perioperative infusions of n-3 PUFAs reduce systemic inflammation induced by cardiopulmonary bypass surgery: a randomized controlled trial. Am J Clin Nutr. 2013;97(2):246–54. doi: 10.3945/ajcn.112.046573. [DOI] [PubMed] [Google Scholar]
- 38.Gogos CA, Ginopoulos P, Salsa B, Apostolidou E, Zoumbos NC, Kalfarentzos F. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy: a randomized control trial. Cancer. 1998;82(2):395–402. doi: 10.1002/(SICI)1097-0142(19980115)82:2<403::AID-CNCR21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Hall TC, Bilku DK, Al-Leswas D, Neal CP, Horst C, Cooke J, Metcalfe MS, Dennison AR. A randomized controlled trial investigating the effects of parenteral fish oil on survival outcomes in critically ill patients with sepsis: a pilot study. JPEN J Parenter Enteral Nutr. 2015;39(3):301–12. doi: 10.1177/0148607113518945. [DOI] [PubMed] [Google Scholar]
- 40.Pontes-Arruda A, DeMichele S, Seth A, Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32(6):596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 41.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158(7):515–25. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156(9):824–31. doi: 10.1093/aje/kwf118. [DOI] [PubMed] [Google Scholar]
- 43.Eide IA, Jenssen T, Hartmann A, Diep LM, Dahle DO, Reisæter AV, Bjerve KS, Christensen JH, Schmidt EB, Svensson M. The association between marine n-3 polyunsaturated fatty acid levels and survival after renal transplantation. Clin J Am Soc Nephrol. 2015;10(7):1246–56. doi: 10.2215/CJN.11931214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK. Parenteral fish oil lipid emulsions in the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):20–8. doi: 10.1177/0148607113486006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koekkoek WK, Panteleon V, van Zanten AR. Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: a systematic review and meta-analysis. Nutrition. 2019;59:56–68. doi: 10.1016/j.nut.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Heller AR, Rössler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. J Clin Med. 2016;5(3):34. doi: 10.3390/jcm5030034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be made available upon request.