Abstract
Introduction
A previous study demonstrated that the surface area-normalized standard Kt/V (SAstdKt/V) was better associated with mortality than standard Kt/V (stdKt/V). This study investigates the association of SAstdKt/V and stdKt/V with mortality, anemia, and hypoalbuminemia in a larger patient cohort with a longer follow-up period.
Methods
We included adult patients on thrice weekly hemodialysis in the USRDS database and excluded amputated patients. StdKt/V, and SAstdKt/V were calculated from the available single-pool Kt/V. Patients were categorized into 5 groups according to their stdKt/V and SAstdKt/V: <2.00, 2.00–2.19, 2.20–2.39, 2.40–2.59, and ≥2.60. Hazard ratios (HR) and odds ratios (OR) were calculated using Cox and Logistic regression analysis.
Findings
There were 507,656 patients included in the analysis. The patients had a median age of 65.5 years with a median follow-up period of 2 years. Thirty-four percent died during follow-up. HRs for mortality progressively decreased as SAstdKt/V increased in both unadjusted and adjusted models. Unlike SAstdKt/V, HRs were the lowest in the categories with stdKt/V of 2.40–2.59 and they increased in the higher stdKt/V category. The adjusted HR for SAstdKt/V versus stdKt/V were 0.68 versus 0.62 in the category of 2.40–2.59, and 0.63 versus 0.73 in the category of ≥2.60. The adjusted ORs for anemia progressively decreased as SAstdKt/V increased, whereas ORs decreased to the lowest in stdKt/V category 2.40–2.59 and increased in the ≥2.60 category. The adjusted ORs for hypoalbuminemia progressively decreased as SAstdKt/V and stdKt/V increased which were both 0.45 in 2.40–2.59 category and decreased to 0.29 and 0.42 in the ≥2.60 category.
Discussion
SAstdKt/V better associated with mortality, anemia, and hypoalbuminemia than stdKt/V. SAstdKt/V is a better parameter in defining hemodialysis dosing which can be calculated by an available online tool. Further studies to determine the optimal SAstdKt/V dose required to achieve improved clinical outcomes with better cost effectiveness are needed.
Keywords: Adequacy of dialysis, survival, nutrition, anemia
Introduction
Hemodialysis (HD) adequacy is commonly determined by measuring small-solute clearance using urea Kt/V, where “K” denotes urea clearance, “t” time of dialysis treatment, and “V” urea distribution volume which equates to total body water 1. HD patients with single-pool Kt/V (spKt/V) less than 1.2 have worse clinical outcomes and higher mortality 2,3. A National Institutes of Health-sponsored Hemodialysis study (HEMO) showed that patients who received standard dose versus high dose spKt/V (1.3 versus 1.7) had no difference in mortality rate 4. Data from the HEMO study was reanalyzed and it was found that female patients benefited from the higher dialysis dose. Unsurprisingly, females had a significantly lower “V” than their male counterparts 5. These results raised questions about the effect of normalizing the dialysis dose normalization for “V”.
Since Kt/V is most commonly calculated using a complex mathematical equation which does not allow easy determination of the Kt value alone 1, practical rescaling would involve multiplying Kt/V with the estimated “V” and then dividing it by the desirable variables instead. Among the alternative variables that have been proposed for Kt/V rescaling, body surface area (BSA) has been a promising option with some physiologic justifications 6,7. Firstly, the liver is known to be the major organ responsible for uremic toxin metabolism and its volume is best estimated as a function of BSA 8. Secondly, normalization of glomerular filtration rate (GFR) with BSA has been used for decades 9. The scaling of estimated GFR with either BSA or liver volume has been shown to be not significantly different between genders and body sizes, whereas scaling with “V” resulted in significant differences 10.
The V/BSA ratio was found to be relatively lower in females and in males with a smaller body size 6,11,12. Therefore, the rescaling of Kt/V with BSA or surface area (SA)-based dose in these patients would result in lower Kt/Vs than previously calculated. As a result, a volume (V)-based dose would result in less dialysis given to these patients than is truly required. In the HEMO study, female patients in the conventional dose group had a lower surface-area-normalized standard Kt/V (SAstdKt/V) than male patients in the conventional dose group, although they had similar standard Kt/V (stdKt/V) results. In addition, females and smaller body-sized patients in the higher dose group had lower mortality rates compared to patients in the conventional dose group 12. This result indicates that these patients received inadequate dialysis.
In a prior study of HD patients receiving thrice weekly HD, the hazard ratio (HR) for 1-year mortality decreased progressively as the SAstdKt/V increased in both genders, while the HR actually increased in the higher quintiles of stdKt/V 13. This suggests that SAstdKt/V may perform better than stdKt/V as a measure of dialysis adequacy when evaluating the association between dialysis dose and mortality, especially at extreme values.
Studies determining relationship between SAstdKt/V and mortality are scarce, and none have been performed for other clinical outcomes. Therefore, we set out to study the association between the HD dose, expressed using stdKt/V and SAstdKt/V, and the clinical outcomes of mortality, anemia, and hypoalbuminemia in a larger cohort with a longer follow-up period.
Materials and Methods
Participants and Study Design
Prevalent HD patients from the United States Renal Data System (USRDS) database14 with an age of 18–100, who received HD for ≥90 days and did not receive any other renal replacement therapy (RRT) modality were included in the study.
Patients with no monthly data from Consolidated Renal Operations in a Web-enabled Network (CROWNWeb) clinical data file, had an amputation, or who had not received thrice weekly HD treatment were excluded. The CROWNWeb file contained monthly laboratory and dialysis adequacy results from May 2012 to May 2017. Patients who had at least 1 month of data were included in the analysis. Patients with missing necessary data for SAstdKt/V calculation, such as gender, spKt/V, height, weight, dialysis frequency, and duration were excluded. The demographics, sequence of dialysis treatment, and death were obtained from patients’ medical evidence, treatment history, and death files. Outlier data values were excluded from the analysis (supplementary Table S1). After exclusion of this data, patients who had no spKt/V, pre- and post-HD weights, dialysis treatment times, or who didn’t have all this data at the same time-point for SAstdKt/V calculation were excluded.
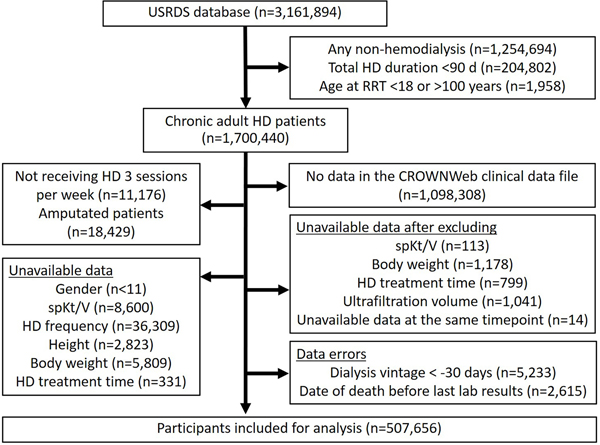
Vintage of dialysis was calculated as the date of RRT initiation minus the first date of data available on CROWNWeb. As CROWNWeb does not report the date of the labs, only the month and year, we defaulted the date to the 1st of the month which may lead to values of up to negative 30 days. Patients with negative dialysis vintage or with date of death occurring before the last reported date from the CROWNWeb clinical data file were excluded (Figure 1).
Figure 1.
The study flow.
HD, hemodialysis; RRT, renal replacement therapy, spKt/V, single-pool Kt/V; USRDS, United States Renal Data System.
The primary outcome was mortality and the secondary outcomes were anemia (mean hemoglobin level <10 g/dL) and hypoalbuminemia (mean serum albumin level <3.5 g/dL). Deaths were censored at 30 days after the last reported date from the CROWNWeb clinical data file.
Formal modeling
The spKt/V results were collected from the CROWNWeb clinical data file. SAstdkt/V was calculated using the following steps:
Step 1: The equilibrated Kt/V (eKt/V) was calculated using the modified Tattersall Equation 1,15,16.
where “t” is the HD treatment time in minutes and 30 is the correction value.
Step 2: The stdKt/V was calculated using the simplified, fixed-volume equation by Leypoldt 1,17.
where “N” = 3, the number of treatments per week.
Step 3: The volume of distribution of urea (V) was calculated using the equation by Watson et al 18:
Volume of distribution of urea (V)
for males = 2.447 – (0.09156 x A) + (0.1074 x H) + (0.03362 x W)
for females = −2.097 + (0.1069 x H) + (0.2466 x W)
where “A” is age in years, “H” is height in centimeters, and “W” is post-HD weight in kilograms.
Step 4: The stdKt/V was adjusted with volume change during dialysis using the equation by Chin et al 19.
where “N” = 3, the number of treatments per week, “UFw” is weekly fluid removal in mL which can be estimated by multiplying volume of ultrafiltration with 3 (number of treatments per week), and “Vu” is volume distribution of urea in mL from urea kinetic model. Since the urine output and the pre- and post-dialysis BUN were not available from the monthly reported results in the CROWNWeb clinical data file, the urea clearance from residual native function was omitted and the “Vu” is estimated by multiplying V with 0.9 19. The value of adjusted stdKt/V with this equation will be typically 6–7% higher than the value obtains from step 2.
Step 5: The body surface area (BSA; m2) was calculated using the equation by DuBois and DuBois 20:
Step 6: The SAstdKt/V was calculated using the equation by Daugirdas et al 1,6:
where 20 is a normalizing factor (population mean V/BSA).
Statistical Analyses
The monthly laboratory and dialysis dose measurements were averaged. The baseline data was presented as a percentage and a median (interquartile range, IQR) for categorical and continuous variables, respectively. Baseline descriptive statistics were compared using stdKt/V <2.0 versus ≥2.0 and SAstdKt/V <2.0 versus ≥2.0 using Fisher’s Exact test for categorical variables and Wilcoxon Rank-Sum test for continuous variables. The mean stdKt/V and SAstdKt/V were categorized into 5 groups: 1) less than 2.00, 2) 2.00–2.19, 3) 2.20–2.39, 4) 2.40–2.59, and 5) 2.60 or more. The Kaplan-Meier survival analysis was performed, and the survival estimates among the 5 categories compared by the Log-Rank test. We assumed that the stdKt/V and SAstdK/V levels were steady throughout the follow-up period and used their averaged monthly levels as the variable of interest in the Cox model. The association between outcomes and calculated stdKt/V and SAstdKt/V were determined by Cox proportional hazard models for primary outcome and logistic regression for secondary outcomes. The 3 Cox regression analysis models were adjusted for 1) age, gender, race and Hispanic ethnicity, 2) model 1 plus comorbidities (list of comorbidities provided in supplementary Table S2), and 3) model 2 plus serum albumin and dialysis vintage. The adjusted odds ratio (OR) for anemia and hypoalbuminemia were adjusted by similar covariates as used for model 3, except not including serum albumin for adjusted hypoalbuminemia. The group with stdkt/V and SAstdKt/V of 2.00–2.19 were used as the reference.
This study was approved by Icahn School of Medicine Program for the Protection of Human Subjects/Institutional Review Board (IRB-18–01255). The analysis was done using Stata/IC 15.1 software (StataCorp, College Station, TX).
Results
Patient and treatment characteristics
There were 3,161,894 patients initially in the study. After applying the exclusion criteria, there were a total of 507,656 patients (Figure 1). The median age was 65.5 (55.8–74.9) years with a median follow-up period of 2.0 (0.9–3.8) years. Fifty-six percent were male, 60% were white, 33% were African American, and 50% of the patients used an AVF or AVG at the time of entry into the analysis. The patients had a median spKt/V of 1.6 (1.4–1.7), eKt/V 1.4 (1.3–1.5), stdKt/V 2.3 (2.2–2.4), and SAstdKt/V 2.4 (2.2–2.5). The SAstdKt/V was greater than the stdKt/V with a mean difference of 0.065. During the follow-up period 174,748 (34%) patients died.
Compared to patients with stdKt/V ≥2.0, those with stdKt/V <2.0 had a greater proportion of male (74 versus 56%), white (63 versus 60%), younger (59.7 versus 65.7 years), and taller (175 versus 168 cm) patients. Other differences included BSA (2.1 versus 1.9 m2), pre-HD weight (97.7 versus 79.5 kg), post-HD weight (95.9 versus 77.3), V (45.9 versus 38.2 L), and V/BSA (22.1 versus 20.4 L/m2) (Table 1).
Table 1.
Characteristics of participants included in the analysis
| stdKt/V | SAstdKt/V | |||||
|---|---|---|---|---|---|---|
| Characteristics | <2.0 | ≥2.0 | P value | <2.0 | ≥2.0 | P value |
| Total, n (%) | 12,353 (2) | 495,303 (98) | - | 10,028 (2) | 497,628 (98) | - |
| Male, n (%) | 9,180 (74) | 276,157 (56) | <0.001 | 3,796 (38) | 281,541 (57) | <0.001 |
| Age, year, median (IQR) | 59.7 (49.6–69.0) | 65.7 (55.9–75.0) | <0.001 | 63.8 (53.3–74.2) | 65.6 (55.8–74.9) | <0.001 |
| Race, n (%) | <0.001 | <0.001 | ||||
| White | 7,813 (63) | 298,502 (60) | 6,231 (62) | 300,084 (60) | ||
| African American | 4,036 (33) | 163,416 (33) | 3,344 (33) | 164,108 (33) | ||
| Asian | 207 (2) | 20,110 (4) | 240 (2) | 20,077 (4) | ||
| Other | 297 (2) | 13,275 (3) | 213 (2) | 13,359 (3) | ||
| Hispanic ethnicity, n (%) | 1,342 (11) | 88,017 (18) | <0.001 | 1,034 (10) | 88,325 (18) | <0.001 |
| Comorbidities at RRT initiation, n (%) | ||||||
| Hypertension | 10,705 (87) | 441,996 (89) | <0.001 | 8,647 (86) | 444,054 (89) | <0.001 |
| Diabetes mellitus | 7,491 (61) | 300,141 (61) | 0.9 | 5,705 (57) | 301,927 (61) | <0.001 |
| Congestive heart failure | 3,871 (31) | 150,901 (30) | 0.04 | 3,013 (30) | 151,759 (31) | 0.3 |
| Atherosclerotic heart disease | 1,721 (14) | 81,275 (16) | <0.001 | 1,447 (14) | 81,549 (16) | <0.001 |
| Peripheral vascular disease | 1,225 (10) | 50,833 (10) | 0.2 | 961 (10) | 51,097 (10) | 0.03 |
| Stroke | 797 (6) | 44,792 (9) | <0.001 | 792 (8) | 44,797 (9) | <0.001 |
| COPD | 1,205 (10) | 42,829 (9) | <0.001 | 947 (9) | 43,087 (9) | 0.006 |
| Smoking | 1,021 (8) | 31,209 (6) | <0.001 | 754 (8) | 31,476 (6) | <0.001 |
| Malignancy | 869 (7) | 30,377 (6) | <0.001 | 805 (8) | 30,441 (6) | <0.001 |
| AVF or AVG at entry, n (%) | 4,753 (39) | 250,867 (51) | <0.001 | 3,953 (40) | 251,657 (51) | <0.001 |
| Duration of follow-up, year, median (IQR) | 0.7 (0.2–2.1) | 2.1 (0.9–3.8) | <0.001 | 0.7 (0.2–2.2) | 2.1 (0.9–3.8) | <0.001 |
| Dialysis vintage, year, median (IQR) | 0 (0–0.2) | 0 (0–1.5) | <0.001 | 0 (0–0.5) | 0 (0–1.5) | <0.001 |
| Height, cm, median (IQR) | 175 (168–182) | 168 (160–175) | <0.001 | 168 (162–175) | 168 (160–175) | <0.001 |
| Body surface area (BSA), m, median (IQR) | 2.1 (1.9–2.3) | 1.9 (1.7–2.1) | <0.001 | 1.9 (1.7–2.1) | 1.9 (1.7–2.1) | <0.001 |
| Hemodialysis parameters, median (IQR) | ||||||
| Pre-HD body weight, kg | 97.7 (81.1–118.2) | 79.5 (66.9–95.6) | <0.001 | 81.5 (67.4–99.0) | 79.8 (67.1–96.2) | <0.001 |
| Post-HD body weight, kg | 95.9 (79.5–116.1) | 77.3 (65.0–93.1) | <0.001 | 80.1 (66.1–97.4) | 77.6 (65.2–93.6) | <0.001 |
| Ultrafiltrate volume, L | 1.8 (1.1–2.6) | 2.2 (1.6–2.8) | <0.001 | 1.4 (0.9–2.0) | 2.2 (1.7–2.8) | <0.001 |
| Total body volume (V), L | 45.9 (39.8–53.5) | 38.2 (32.9–44.2) | <0.001 | 37.6 (32.4–44.4) | 38.4 (33.0–44.4) | <0.001 |
| V/BSA, L/m2 | 22.1 (20.4–23.5) | 20.4 (18.8–21.9) | <0.001 | 19.4 (18.6–21.5) | 20.4 (18.8–22.0) | <0.001 |
| HD treatment time, minutes | 201 (180–224) | 218 (203–240) | <0.001 | 185 (169–209) | 218 (203–240) | <0.001 |
| spKt/V | 1.0 (0.9–1.1) | 1.6 (1.4–1.7) | <0.001 | 1.1 (0.9–1.3) | 1.6 (1.4–1.7) | <0.001 |
| eKt/V | 0.9 (0.8–1.0) | 1.4 (1.3–1.5) | <0.001 | 0.9 (0.7–1.1) | 1.4 (1.3–1.5) | <0.001 |
| stdKt/V | 1.8 (1.6–1.9) | 2.4 (2.3–2.5) | <0.001 | 1.9 (1.6–2.1) | 2.4 (2.3–2.5) | <0.001 |
| SAstdKt/V | 2.0 (1.7–2.2) | 2.5 (2.3–2.6) | <0.001 | 1.9 (1.7–2.0) | 2.5 (2.3–2.6) | <0.001 |
| nPCR, g/kg/day | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | <0.001 | 0.7 (0.6–0.9) | 0.9 (0.8–1.0) | <0.001 |
| Laboratory values, median (IQR) | ||||||
| Hemoglobin, g/dL | 10.3 (9.5–10.8) | 10.6 (10.3–11.0) | <0.001 | 10.2 (9.5–10.8) | 10.6 (10.2–11.0) | <0.001 |
| Serum ferritin, pg/L | 428 (243–714) | 784 (508–1045) | <0.001 | 489 (269–801) | 782 (504–1043) | <0.001 |
| Iron saturation, % | 24 (19–29) | 30 (25–35) | <0.001 | 25 (19–31) | 30 (25–35) | <0.001 |
| Serum albumin, g/dL | 3.6 (3.2–3.9) | 3.7 (3.5–3.9) | <0.001 | 3.5 (3.1–3.8) | 3.7 (3.5–3.9) | <0.001 |
| Serum calcium, mg/dL | 9.2 (8.9–9.5) | 9.2 (8.9–9.5) | <0.001 | 9.3 (8.9–9.6) | 9.2 (8.9–9.5) | <0.001 |
| Serum phosphate, mg/dL | 5.0 (4.3–5.9) | 4.9 (4.4–5.6) | <0.001 | 4.9 (4.2–5.7) | 4.9 (4.4–5.6) | <0.001 |
| Parathyroid hormone, pg/mL | 330 (197–527) | 315 (220–461) | 0.1 | 314 (186–509) | 315 (220–461) | 0.2 |
| Death, n (%) | 4,417 (36) | 170,331 (34) | 0.002 | 3,967 (40) | 170,781 (34) | <0.001 |
| Cardiovascular | 2,068 (17) | 82,483 (17) | 0.8 | 1,762 (18) | 82,789 (17) | 0.01 |
| Myocardial infarction | 143 (1) | 6,765 (1) | 0.049 | 127 (1) | 6,781 (1) | 0.4 |
| Infection | 449 (4) | 14,503 (3) | <0.001 | 385 (4) | 14,567 (3) | <0.001 |
| Malignancy | 144 (1) | 5,435 (1) | 0.5 | 146 (1) | 5,433 (1) | 0.001 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; COPD, chronic obstructive pulmonary disease; eKt/V, equilibrated Kt/V, HD, hemodialysis; IQR, interquartile range; nPCR, normalized protein catabolic rate; RRT, renal replacement therapy; SAstdKt/V, surface area-normalized standard Kt/V; spKt/V, single-pool Kt/V; stdKt/V, standard Kt/V; V/BSA, total body water/body surface area ratio
In contrast, compared to patients with SAstdKt/V ≥2.0, those with SAstdKt/V <2.0 had a lower proportion of male (38 versus 57%), young (63.8 versus 65.6 years) and higher proportion of White patients (62 versus 60). Other differences included pre-HD weight (81.5 versus 79.8 kg), post-HD weight (80.1 versus 77.6), V (37.6 versus 38.4 L), and V/BSA (19.4 versus 20.4 L/m2) (Table 1).
As shown in supplementary Table S7, higher levels of stdKt/V were associated with increased age and lower indices of body size (height, BSA, V, V/BSA, pre- and post-HD weights). There was a trend toward decreasing of male proportion as the stdKt/V increased. Hemoglobin and serum albumin were relatively stable amongst all stdKt/V categories.
However, these trends were different in the SAstdKt/V categorization (supplementary Table S8). Higher levels of SAstdKt/V were associated with increased hemoglobin and serum albumin. There was a trend toward increasing of male proportion as the stdKt/V increased. The trend for age and body size indices were not clear.
Primary outcome
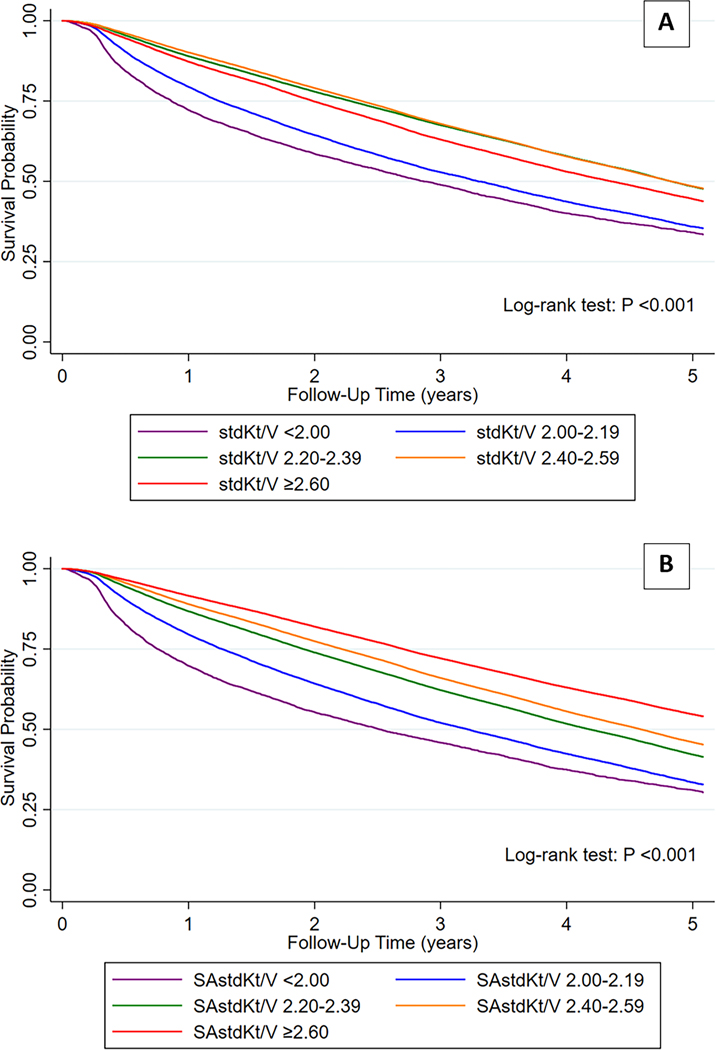
The Kaplan-Meier survival curves for mortality among patients in the 5 categories of stdKt/V (Figure 2A) and SAstdKt/V (Figure 2B) showed significant intra-category differences for both stdKt/V and SAstdKt/V (p value <0.001 by Log-Rank test). The survival outcomes were worst in the lowest categories of stdKt/V and SAstdKt/V. Moreover, survival progressively decreased with lowering of SAstdKt/V. Such a direct, orderly association was not seen with stdKt/V.
Figure 2.
The survival curves for mortality among patients who were in 5 different categories of standard Kt/V (stdKt/V) (Figure 2A) and surface area-normalized standard Kt/V (SAstdKt/V) (Figure 2B). The number of patients at risk were shown in Table S3 and S4 in supplementary.
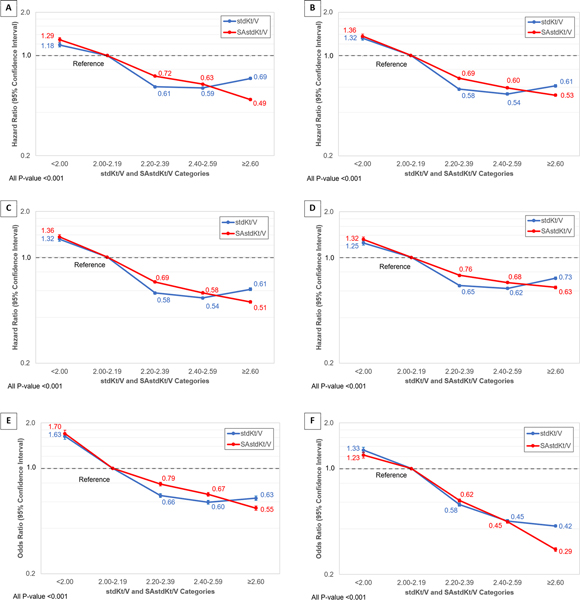
In multivariable models, the HRs for mortality progressively decreased as the SAstdKt/V increased in unadjusted and all 3 adjusted models (Figure 3A-3D and supplementary Table S5). Unlike SAstdKt/V, HRs were the lowest in the categories with stdKt/V of 2.40–2.59, and then HRs increased in the higher stdKt/V categories. In Figure 3D, the adjusted HRs for SAstdKt/V versus stdKt/V were 0.68 versus 0.62 in categories of 2.40–2.59, and 0.63 versus 0.74 in categories of ≥2.60, respectively.
Figure 3.
The hazard ratio (HR) and 95% confidence interval for mortality: (A) unadjusted, adjusted by (B) age, gender, race, and Hispanic ethnicity, (C) age, gender, race, Hispanic ethnicity, and comorbidities, and (D) age, gender, race, Hispanic ethnicity, comorbidities, serum albumin, and dialysis vintage. The adjusted odds ratio (OR) and 95% confidence interval for (E) anemia (defined by hemoglobin level of less than 10 g/dL) by age, gender, race, Hispanic ethnicity, comorbidities, serum albumin, and dialysis vintage, and (F) for hypoalbuminemia (defined by serum albumin level of less than 3.5 g/dL) by age, gender, race, Hispanic ethnicity, comorbidities, and dialysis vintage. The group with stdKt/V and SAstdKt/V of 2.00–2.19 were used as reference group. The unadjusted OR for anemia and hypoalbuminemia were shown in supplementary Figure S1.
Secondary outcomes
As stdKt/V increased, the unadjusted and adjusted ORs for anemia decreased to their lowest in stdKt/V category 2.40–2.59, and then increased in the highest categories. Unlike stdKt/V, the unadjusted and adjusted ORs were continuously decreased as the SAstdKt/V increased. (Figure 3E, supplementary Figure S1 A-B and Table S6).
The unadjusted ORs for hypoalbuminemia progressively decreased to the lowest in 2.40–2.59 category of stdKt/V and increased in the higher category. Whereas, the ORs were continuously decreased as the SAstdKt/V increased. The adjusted ORs for hypoalbuminemia progressively decreased as the SAstdKt/V and stdKt/V increased. The ORs were both 0.45 in the 2.40–2.59 categories. The ORs slightly decreased to 0.42 for stdKt/V and more noticeable decreased to 0.29 for SAstdKt/V (Figure 3F, supplementary Figure S1 C-D and Table S6).
Sensitivity analysis
The Kaplan-Meier survival curves for mortality in both genders showed significantly lower survival probability in patients with stdKt/V and SAstdKt/V <2.0 (p value <0.001) (supplementary Figure S2).
Discussion
Our results suggest that SAstdKt/V had a better association with mortality, anemia and hypoalbuminemia than stdKt/V in a large cohort of chronic HD patients with long-term follow-up. While extremely low and high levels of stdKt/V were found to be associated with worse mortality and anemia, lower levels of SAstdKt/V were associated with worse clinical outcomes and higher levels with increasingly better outcomes. Although the ORs were decreased progressively as SAstdKt/V and stdKt/V increase, the ORs in the highest categories was more noticeably decreased for SAstdKt/V than stdKt/V. These findings are similar to those reported in a previous study by Ramirez et al. which included a smaller cohort and a shorter follow-up period 13. They found that both unadjusted and adjusted HRs for mortality were decreased as stdKt/V increased and became lowest in patients with stdKt/V level of 2.5 for males and 2.6 for females. HRs for mortality were higher if stdKt/V increased beyond these points. However, better clinical outcomes were associated with higher SAstdKt/V regardless of gender. Our results emphasize the effect of stdKt/V rescaling to SAstdKt/V on mortality. We also demonstrated the effects of rescaling the dialysis dose on anemia and hypoalbuminemia. These associations persisted after adjusting for both gender and the associated clinical variables.
Gender and body size have been shown to be important factors in the mortality of HD patients who received different V-based dialysis doses. Previous studies showed that females and smaller body-sized males required higher dialysis doses according to the SA-based dose in the HEMO study dataset as well as other datasets 6,11,21. The results from a large observational study and the HEMO study showed that the benefit of increasing V-based dose beyond standard targeted dose were restricted only to females 5,22. In addition, smaller body-sized HD patients had a higher mortality than larger patients at similar V-normalized dose levels 23. These results suggested that determining dialysis dose with V-normalized dose might be problematic, particularly in females and smaller body-size patients.
Body composition is also affected by gender and body size, and these factors could be contributing to mortality. Smaller body-sized patients have relatively lower fat mass, muscle mass, and V in proportion to their body weight, but had relatively higher high metabolic rate organs (HMRO) than larger body-sized patients 24–26. These differences in body compartments results in higher HMRO per V ratio in smaller body-sized patients. This ratio was also found to be higher in female patients 25. The HMRO mass relates to protein catabolic rate which indirectly reflects protein intake and uremic toxin generation from proteins. Thus, females and small body-sized patients have higher uremic toxin concentration, and this could be the reason why increasing V-based dialysis dose in these patients may be beneficial.
Our results demonstrated that SAstdKt/V is consistently associated with anemia and hypoalbuminemia. As SAstdKt/V values increased, the median serum albumin, nPCR, and hemoglobin levels progressively increased as well. In contrast, these laboratory parameters were quite steady with stdKt/V values of 2.0 or more (supplementary Table S7-S8). These trends persisted in the adjusted models (Figure 3 and supplementary Figure S1). These findings might be explained by rescaling the dialysis dose with BSA leading to a more consistent association between uremic toxin generation and removal (dialysis dose). Smaller body-sized patients and females have relatively higher uremic toxin generation with lower V’s 24–26. Therefore, determining dialysis delivery with V-based dosing could result in inadequately dialyzing these patients.
Prior studies have demonstrated an association with HD dose and several clinical outcomes. Azar et al. compared nutritional status and biochemical outcomes before and after increasing HD dose 27. They found that nPCR, serum albumin and hemoglobin levels were significantly improved after increasing the HD dose. Moreover, previous studies on HD patients have shown the association between dialysis adequacy and hematocrit 28–30, as well as serum albumin level 31,32. Alteration of these factors could affect patient mortality. It has been known that nutritional status, particularly serum albumin, is strongly associated with mortality in HD patients 33,34. Anemia was also associated with all-cause mortality in patients with CKD either with or without dialysis dependence 35. Thus, ensuring adequate dialysis clearance could improve the nutritional status, anemia, and mortality of HD patients.
Although previous studies showed a discrepancy in the outcomes between SA- and V-normalized dosing among different body-sized patients and genders, V-normalized targets have been the standard in determining the delivered HD dose and applied to all HD patients 1. Since SA-based dosing showed better dose-outcome relationships, dialysis dosing should also be determined by SA-based dosing. Our results suggest that using SAstdKt/V (Figure 2-3) has a better association with mortality and other clinical outcomes than using stdKt/V. Although the formulae for calculation of SAstdKt/V seems complicated, the calculation can be simply done by using open-source program, Solute-Solver, which can be found at www.ureakinetics.org 36. This tool aid in implementing the SAstdKt/V into clinical practice without requiring multiple-step complex calculations. However, our findings have to be interpreted with following caveats: Firstly, since these results came from an observational dataset, there might be several factors that could affect the outcomes other than the effect of HD dosing. Secondly, residual renal function (RRF) data was unavailable and was assumed to be zero. This could result in potential underestimation of the stdKt/V and SAstdkt/V. Thirdly, it has been known that RRF is associated with lower mortality rates 37,38. The effects of RRF on mortality were not assessed in this study. Lastly, since we enrolled only the patient with thrice weekly hemodialysis to this study, the association of SAstdKt/V to the outcomes cannot be reassured in other population. We suggest not to apply these findings to the patients who not receive thrice weekly hemodialysis.
In conclusion, we demonstrated that SAstdKt/V showed better association with mortality, anemia, and hypoalbuminemia than stdKt/V. Targeted V-normalized dosing carries the risk of inappropriate dialysis dose modification. Therefore SAstdKt/V is a better parameter to define HD dosing which can be simply calculated by using an available online tool without requiring multiple-step complex formulae. However, the results should be cautiously interpreted with the aforementioned limitations. Further studies to determine the optimal dialysis SA-normalized dose required to achieve improved clinical outcomes with better cost effectiveness are needed.
Supplementary Material
Acknowledgements
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
GNN is supported by a career development award from the National Institutes of Health (NIH) (K23DK107908) and is also supported by R01DK108803, U01HG007278, U01HG009610, and 1U01DK116100. SGC is also supported by the following grants: R01DK106085, R01HL85757, R01DK112258, and U01OH011326.
GNN has received operational funding from Goldfinch Bio in the past three years. SGC has received consulting fees from CHF Solutions, Quark Biopharma, Takeda Pharmaceuticals, Janssen Pharmaceuticals, pulseData, Goldfinch Bio, Relypsa, Bayer and inRegen in the past three years. SGC owns stock options in pulseData. GNN and SGC receives financial compensation as consultants and advisory board members for RenalytixAI, Inc., and own equity and stock options in RenalytixAI. GNN and SGC are scientific co-founders of RenalytixAI. Other authors declare that they have no conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
GNN has received operational funding from Goldfinch Bio in the past three years. SGC has received consulting fees from CHF Solutions, Quark Biopharma, Takeda Pharmaceuticals, Janssen Pharmaceuticals, pulseData, Goldfinch Bio, Relypsa, Bayer and inRegen in the past three years. SGC owns stock options in pulseData. GNN and SGC receives financial compensation as consultants and advisory board members for RenalytixAI, Inc., and own equity and stock options in RenalytixAI. GNN and SGC are scientific co-founders of RenalytixAI.
Disclosure of grants or other funding
Footnotes
Conflict of Interest Statement
Other authors declare that they have no conflict of interest.
References
- 1.Daugirdas JT, Depner TA, Inrig J, et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am J Kidney Dis 2015; 66: 884–930. [DOI] [PubMed] [Google Scholar]
- 2.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 1985; 28: 526–534. [DOI] [PubMed] [Google Scholar]
- 3.Held PJ, Port FK, Wolfe RA, et al. The dose of hemodialysis and patient mortality. Kidney Int 1996; 50: 550–556. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 5.Depner T, Daugirdas J, Greene TOM, et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int 2004; 65: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 6.Daugirdas JT, Depner TA, Greene T, et al. Surface-area-normalized Kt/V: a method of rescaling dialysis dose to body surface area-implications for different-size patients by gender. Semin Dial 2008; 21: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugirdas JT, Levin NW, Kotanko P, et al. Comparison of proposed alternative methods for rescaling dialysis dose: resting energy expenditure, high metabolic rate organ mass, liver size, and body surface area. Semin Dial 2008; 21: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson TN, Tucker GT, Tanner MS, et al. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl 2005; 11: 1481–1493. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh JF, Moller E, Van Slyke DD. Studies of urea excretion. III: The Influence of Body Size on Urea Output. J Clin Invest 1928; 6: 467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugirdas JT, Meyer K, Greene T, et al. Scaling of measured glomerular filtration rate in kidney donor candidates by anthropometric estimates of body surface area, body water, metabolic rate, or liver size. Clin J Am Soc Nephrol 2009; 4: 1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spalding EM, Chandna SM, Davenport A, et al. Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int 2008; 74: 348–355. [DOI] [PubMed] [Google Scholar]
- 12.Daugirdas JT, Greene T, Chertow GM, et al. Can Rescaling Dose of Dialysis to Body Surface Area in the HEMO Study Explain the Different Responses to Dose in Women versus Men? Clin J Am Soc Nephrol 2010; 5: 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez SPB, Kapke A, Port FK, et al. Dialysis Dose Scaled to Body Surface Area and Size-Adjusted, Sex-Specific Patient Mortality. Clin J Am Soc Nephrol 2012; 7: 1977–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Renal Data System. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018. [Google Scholar]
- 15.Tattersall JE, DeTakats D, Chamney P, et al. The post-hemodialysis rebound: Predicting and quantifying its effect on Kt/V. Kidney Int 1996; 50: 2094–2102. [DOI] [PubMed] [Google Scholar]
- 16.Daugirdas JT, Greene T, Depner TA, et al. Factors that affect postdialysis rebound in serum urea concentration, including the rate of dialysis: results from the HEMO Study. J Am Soc Nephrol 2004; 15: 194–203. [DOI] [PubMed] [Google Scholar]
- 17.Leypoldt JK. Urea standard Kt/V(urea) for assessing dialysis treatment adequacy. Hemodial Int 2004; 8: 193–197. [DOI] [PubMed] [Google Scholar]
- 18.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 1980; 33: 27–39. [DOI] [PubMed] [Google Scholar]
- 19.Chin AI, Depner TA, Daugirdas JT. Assessing the Adequacy of Small Solute Clearance for Various Dialysis Modalities, with Inclusion of Residual Native Kidney Function. Semin Dial 2017; 30: 235–240. [DOI] [PubMed] [Google Scholar]
- 20.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311; discussion 312–313. [PubMed] [Google Scholar]
- 21.Sridharan S, Vilar E, Davenport A, et al. Scaling Hemodialysis Target Dose to Reflect Body Surface Area, Metabolic Activity, and Protein Catabolic Rate: A Prospective, Cross-sectional Study. Am J Kidney Dis 2017; 69: 358–366. [DOI] [PubMed] [Google Scholar]
- 22.Port FK, Wolfe RA, Hulbert-Shearon TE, et al. High dialysis dose is associated with lower mortality among women but not among men. Am J Kidney Dis 2004; 43: 1014–1023. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RA, Ashby VB, Daugirdas JT, et al. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis 2000; 35: 80–88. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar SR, Kuhlmann MK, Kotanko P, et al. Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int 2006; 70: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 25.Kotanko P, Levin NW. The impact of visceral mass on survival in chronic hemodialysis patients. Int J Artif Organs 2007; 30: 993–999. [DOI] [PubMed] [Google Scholar]
- 26.Davenport A Differences in prescribed Kt/V and delivered haemodialysis dose—why obesity makes a difference to survival for haemodialysis patients when using a ‘one size fits all’ Kt/V target. Nephrol Dial Transplant 2013; 28: iv219–iv223. [DOI] [PubMed] [Google Scholar]
- 27.Azar AT, Wahba K, Mohamed ASA, et al. Association between dialysis dose improvement and nutritional status among hemodialysis patients. Am J Nephrol 2007; 27: 113–119. [DOI] [PubMed] [Google Scholar]
- 28.Ifudu O, Feldman J, Friedman EA. The intensity of hemodialysis and the response to erythropoietin in patients with end-stage renal disease. N Engl J Med 1996; 334: 420–425. [DOI] [PubMed] [Google Scholar]
- 29.Ayesh Haj Yousef MH, Bataineh A, Elamin E, et al. Adequate hemodialysis improves anemia by enhancing glucose-6-phosphate dehydrogenase activity in patients with end-stage renal disease. BMC Nephrol 2014; 15: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ifudu O, Uribarri J, Rajwani I, et al. Adequacy of dialysis and differences in hematocrit among dialysis facilities. Am J Kidney Dis 2000; 36: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 31.Y C-S, C S-W, C C-H, et al. Effects of increasing dialysis dose on serum albumin and mortality in hemodialysis patients. Am J Kidney Dis 1996; 27: 380–386. [DOI] [PubMed] [Google Scholar]
- 32.Teixeira Nunes F, de Campos G, Xavier de Paula SM, et al. Dialysis adequacy and nutritional status of hemodialysis patients. Hemodial Int 2008; 12: 45–51. [DOI] [PubMed] [Google Scholar]
- 33.Owen WFJ, Lew NL, Liu Y, et al. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993; 329: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 34.Leavey SF, Strawderman RL, Jones CA, et al. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis 1998; 31: 997–1006. [DOI] [PubMed] [Google Scholar]
- 35.Toft G, Heide-Jorgensen U, van Haalen H, et al. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol 2020; 33: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ureakinetics.org, http://www.ureakinetics.org/ (accessed 22 May 2020).
- 37.Termorshuizen F, Dekker FW, van Manen JG, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004; 15: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 38.Obi Y, Rhee CM, Mathew AT, et al. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol 2016; 27: 3758–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.