Abstract
Purpose.
Topical therapy of local disease (e.g. skin) is advantageous over oral therapy since there is less systemic drug distribution (so fewer side-effects), no first-pass effect, etc. However, patient compliance with topical therapy can be poor as it may require many applications a day and can last months. Here we propose a topical controlled release formulation with thermoresponsive gelation at body temperature and improved adhesiveness, making it easier to remain in contact with the body.
Methods.
The formulation contains two excipients, poloxamer 407 (P407) and casein. Casein can modify the properties of the hydrogel through molecular entanglement. In addition, tissue reaction and drug release profile were evaluated.
Results.
Changes in casein concentration affected adhesive strength, viscosity, mechanical properties and drug release, presumably by hydrophobic interactions between casein and P407. Two different concentrations of P407 were tested with two different concentrations of casein. Formulations containing 5% and 10% casein released 80% of model drug in 48h, while formulations without casein released the same fraction in around 24h hours. Formulations with 10% casein had almost twice the adhesive strength of those without casein.
Conclusions.
Addition of casein modified the mechanical properties and drug release rate of the hydrogel. There was no inflammation or injury after brief exposure in vivo.
Keywords: casein, poloxamer 407, drug delivery, mixed micelles, topical treatment
INTRODUCTION
Topical therapy can be advantageous over oral therapy, since it can achieve very high local drug concentrations locally (in absolute terms and in proportion to systemic distribution), there is no first-pass effect, and it usually does not require laboratory monitoring during treatment. Hydrogels have been extensively studied for drug delivery, particularly for topical applications (1, 2). With topical applications, being able to improve the properties of the hydrogel, such as adhesiveness and viscosity, would make it easier for the formulation to remain in contact with the skin, particularly in places that are relatively difficult to access or with high friction (e.g. between toes, intertriginous areas). Improvement of release kinetics could reduce the number of daily applications, enhancing patient compliance. The properties of hydrogels such as drug release and mechanical properties (and therefore duration of release and frequency of administration) can be tuned by changing the composition of the hydrogel (3).
Poloxamer 407 (P407) is an uncharged synthetic amphiphilic triblock copolymer composed of a hydrophobic polypropylene oxide block flanked by two lateral hydrophilic polyethylene oxide blocks. It can be applied as liquid at room temperature and forms a hydrogel by reverse thermal gelation upon contact with the warmer human body (4). With increasing temperature, poloxamer molecules aggregate into micelles due to dehydration of hydrophobic blocks of the poloxamer. Reverse thermal gelation has been useful in a variety of drug delivery applications (5–7). However, P407 presents some deficiencies such as relatively low gel mechanical strength and weak mucoadhesiveness. The mechanical properties of poloxamers, which are important for retention in situ, can be improved by blending with other polymers (3).
Casein, a naturally occurring anionic, amphiphilic protein. It is a diblock copolymer, with hydrophobic and hydrophilic domains at the C- and N-terminal regions, respectively (8, 9). It can be added to a hydrogel to increase its adhesiveness through molecular entanglement (10–12). Casein and poloxamers are miscible and form micelles in solution, forming mixed micelles (13, 14). Casein has been used to improve the mechanical properties of PVA hydrogels (15). Therefore, we hypothesized that casein may improve mechanical properties of P407 hydrogels.
Here we investigate whether the addition of casein to P407 would create a stronger, more adhesive topical hydrogel for extended controlled release.
MATERIALS AND METHODS
Materials
Poloxamer 407 (P407) (BASF, New Jersey, USA), casein (C3400-Sigma-Aldrich, Minnesota, USA) (approximate casein composition of milk is (g/L): α-s1, 12–15; α-s2, 3–4; ß, 9–11; and k, 2–4), bupivacaine hydrochloride (Sigma-Aldrich, Minnesota, USA), sulforhodamine B (230162 -Sigma-Aldrich, Minnesota, USA). Bupivacaine hydrochloride and sulforhodamine B were chosen as model drugs.
Preparation of hydrogels and characterization
Casein solution (50 mg/mL) was prepared by dissolving casein in deionized water and adjusting the solution pH to 7 using a 5 mol/L NaOH solution. The obtained casein solution was stored in a 4°C refrigerator. P407 at 25% (w/v) and 18% (w/v) of was added and the solutions and stirred overnight at 4°C.
ATR-FTIR
The FTIR spectra were recorded in an attenuated total reflectance Fourier transformed infrared (ATR-FTIR) spectrometer (Nicolet iS50, Thermo Fisher Scientific, USA).
Mechanical tests
Rheological experiments (viscosity and G’G”)
Hydrogels samples rheological analysis were carried out using a Discovery HR-2 hybrid rheometer (TA Instruments, Delaware, USA). Measurements were performed at a temperature range from 10 to 45 °C using linear oscillatory shear rheology measurements (100 rads−1, 1% strain, and 1°C/min) and 25mm parallel plate, Peltier plate steel (105100), a sample volume of 350 μL, a gap between the plates of 0.300 mm. The oscillatory measurements were used to determine parameters related to the elastic modulus (G′), the viscous modulus (G″). Gelation temperature was taken as the temperature at which G′ becomes greater than G″.
Gelation temperature and viscosity were quantified using linear oscillatory shear rheology measurements (100 rads−1, 1% strain, and 1°C/min). Gelation temperature was taken as the temperature at which G′ becomes greater than G″. The changes of G′ and G″ over temperatures ranging from 10°C to 45°C were recorded to reflect changes in mechanical properties.
Bioadhesion properties
Bioadhesive properties of the formulations were evaluated using a Stable Micro Systems texture analyzer (Model TA-XT Plus) in the TPA mode (16–18). Portions of porcine tissue (epidermis/dermis) were fixed horizontally to the lower end of the analytical probe (P/10) using double-sided adhesive tape and suture, respectively. Hydrogels were placed into a fixed volume compartment and conditioned in a water bath at 37 °C. In TPA mode, the analytical probe descended on the surface of each formulation at a constant rate of 0.5 mm/s, penetrated 10 mm in the formulation and a downward tensile force of 2.45 N was applied at fixated time of 90s. In sequence, biological substrate returned vertically to the formulation surface at a constant rate of 10.0 mm/s. After each cycle, hydrogels and biological substrate were replaced.
Drug Release
The release of sulforhodamine B, bupivacaine hydrochloride from each formulation was performed using a diffusion system. Using Yang, et al. (7) adapted, transwell membrane inserts (0.4-mm pore size and 0.33-cm2 area; Costar) and 24-well culture plates were used as the donor and acceptor chambers, respectively. Two hundred microliters of each formulation was pipetted directly onto pre-warmed filter inserts to obtain a solid hydrogel. Filter inserts (donor compartments) with formed gels were suspended in wells (acceptor compartments) filled with pre-warmed phosphate-buffered saline (PBS) pH 7.4, and the plates were then incubated in an oven (37°C). At each sampling time (0.5, 1, 2, 6, 12, 24, 48h), 1 mL aliquots of the PBS-receiving media were collected and inserts were sequentially moved into a new well with fresh PBS. Sample aliquots were analyzed by spectrophotometer UV to determine sulforhodamine B (560 nm) and bupivacaine hydrochloride (263nm) concentrations. Experiments were performed in quadruplicate.
Tissue Reaction
Animals were cared for in compliance with protocol approved by the Boston Children’s Hospital Committee, in conformity with NIH guidelines for care and use of laboratory animals (Protocol number: 18-07-3348). In order to prevent animals from rubbing the hydrogel off their skin, these experiments were performed under isoflurane anesthesia. 6h was the maximum time frame allowed at our institutions. Attempts to keep the hydrogel on the animals for longer periods without anesthesia using occulsive dressings and other devices were unsuccessful.
Male Sprague–Dawley rats weighting 400–600 g were anesthetized under 2% isoflurane in oxygen, and their dorsal aspect was shaved and disinfected with 70% ethanol and betadine. 3 groups of hydrogels (P25; P25C10; P18C5) were tested by applying them on to separate sites / flanks of the rat. Each flank had a different formulation and each formulation group had an N=4. Normal rat skin (with no formulation applied) was used as a negative control. The rats were kept under anesthesia for 6 hours to ensure that the formulation stayed in contact with the skin during the entire experiment. After 6 h, the animals were euthanized with an injection of Pentobarbital 100 mg/Kg. A 1.5 cm2 area that included the site of the formulation application was excised. Tissues were processed, embedded in paraffin, cut into 10 μm sections, and stained with hematoxylin and eosin. Histologic evaluation of tissues by light microscopy was performed by a pathologist in a masked fashion.
Statistics
Data are expressed as means ± standard deviations. All statistical comparisons were made with Student’s t-test, unpaired for independent samples. A p value < 0.05 was considered to indicated statistical significance.
RESULTS
Preparation of hydrogels and characterization
We formulated combinations of P407 and casein in varying proportions (Table 1). Formulations are labeled PxCy, where P = P407, C = casein, and x and y are the % (w/v) of each. FT-IR spectroscopy of casein, P407 and P407-casein (Fig. 1), showed the presence of the two molecules in the hydrogels (peaks for casein: 1644.49 cm−1 [carbonyl groups], 1517.33 cm−1 [N-H bending and C-N stretching vibration]; for P407: 1105.36 cm−1 [C-O stretch] (7, 11)). There was no evidence of new bond formation by FT-IR.
Table 1-.
P407-Casein hydrogels formulation. The concentrations are all in (w/v). See text for definition of abbreviations.
| P18 | P18C5 | P18C10 | P25 | P25C5 | P25C10 | |
|---|---|---|---|---|---|---|
| P407 | 18% | 18% | 18% | 25% | 25% | 25% |
| Casein | - | 5% | 10% | - | 5% | 10% |
Fig. 1 -.
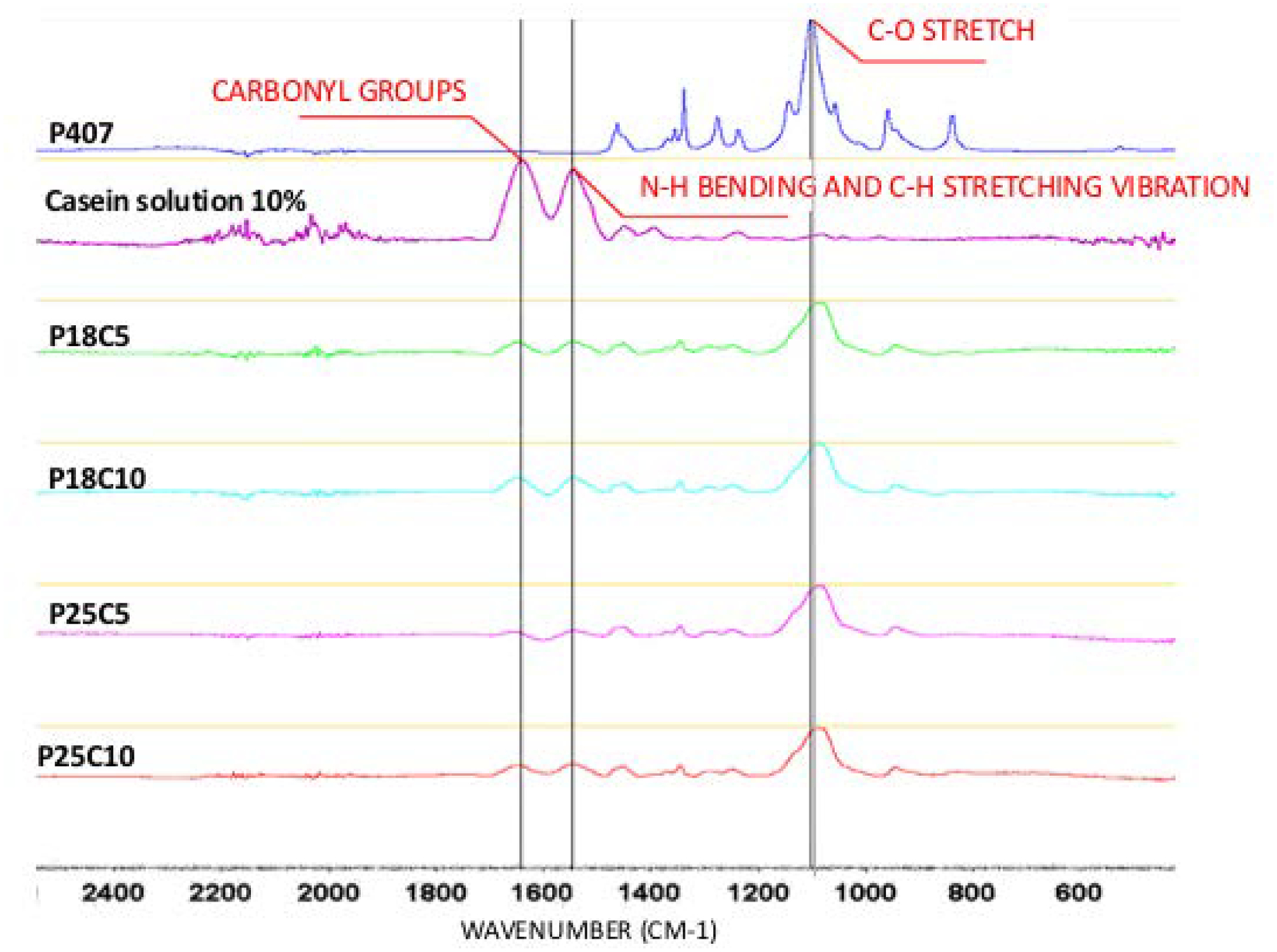
ATR-FTIR spectra of P407, Casein 10%, P407 18%-Casein 5% and 10%, P407 25%-Casein 5% and 10%, respectively.
To assess the mechanical effects of the addition of casein to P407, the rheology of the hydrogels was examined by linear oscillatory shear rheology measurements (100 rads−1, 1% strain, and 1°C/min) over a range of temperatures. Increasing storage modulus (G’) relates to mechanical strength, i.e. solid-like properties. The loss modulus (G”) relates to liquid-like properties. G’ > G” suggests gelation Yang, et al. (7). The addition of casein had little effect on the G’ of P25 at 37 °C, 1.07 fold with addition of 10% casein (p<0.05) and no increase with 5% casein. However, with P18 the addition of 5% and 10% casein increased the peak G’ 1.43 and 3.73 (p<0.005) fold respectively. The gelation temperature of P407, at which G′ became greater than G″, was not affected by the addition of casein at any of the concentrations tested.
Polymer viscosity was studied at 10 °C and 37°C (body temperature). At 10 °C, all formulations had very low viscosity (< 1Pa.s). The enhancement of G’ that casein caused in P25 at 37 °C was also seen with viscosity (Fig. 3). At 37 °C, i.e. after gelation, the viscosity of P25 was unaffected by the addition of casein (Fig. 3), while that of P18 increased with the addition of casein. For example, when 10% casein was added to P18, the viscosity of the mixture was 31.83 and 2.23 times higher than that of P18 and P18C5 respectively.
Fig. 3 -.
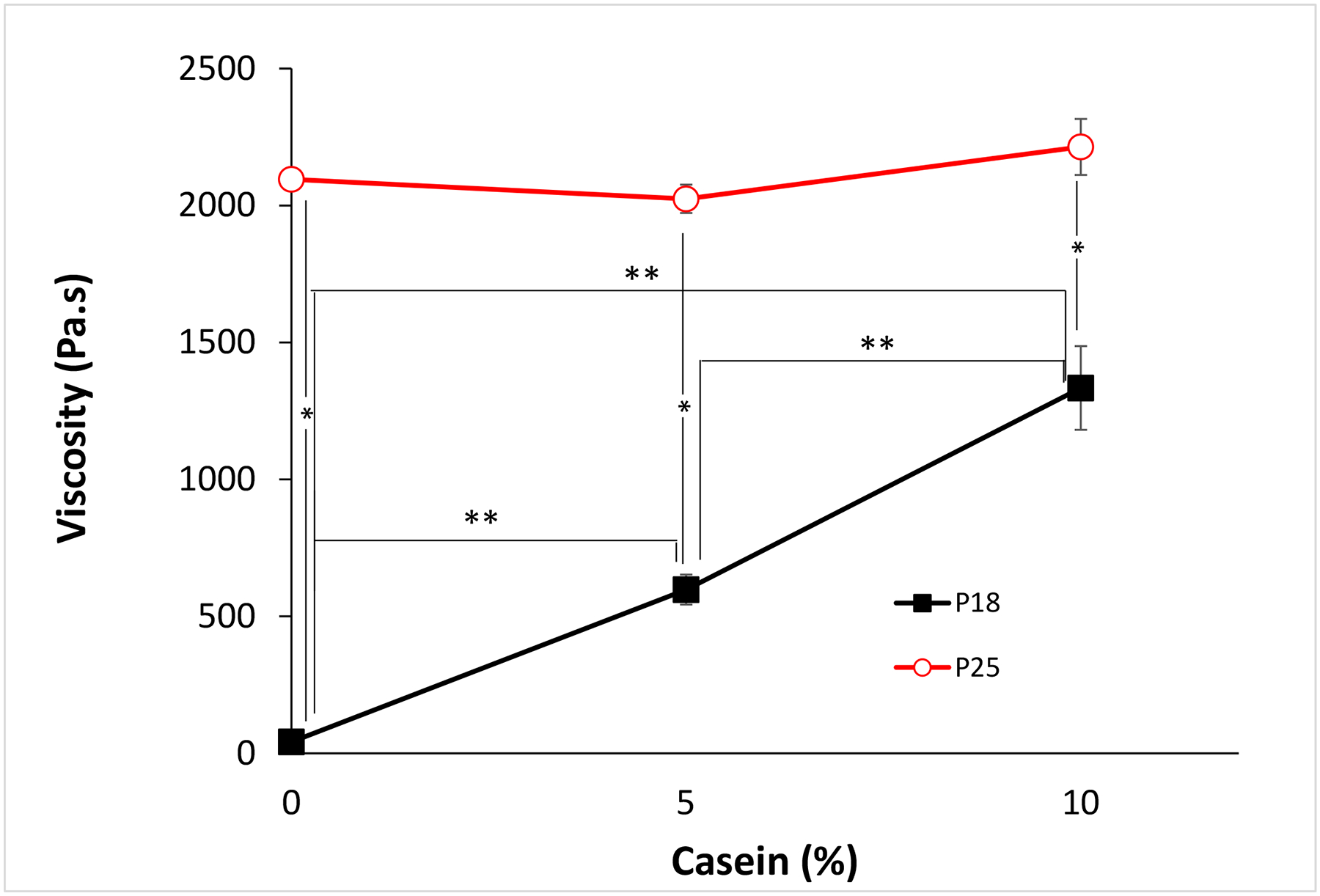
Hydrogel viscosity at 37 °C at different casein concentrations. Data are means ± SD (n=4). *p<0.05 between different P407 concentrations with the same casein concentration. **p< 0.001 between different casein concentrations with the same P407 concentration.
The bioadhesive properties of the P407-casein were assessed by measuring the loading strength required to detach the hydrogel (at 37°C) from porcine skin (Fig. 4). The addition of 5% casein to P18 decreased the adhesive strength of P18 1.36-fold but increased that of P25 by 1.46-fold. 10% casein increased the adhesive strength of P18 1.91-fold and that of P25 1.81-fold.
Fig. 4 -.
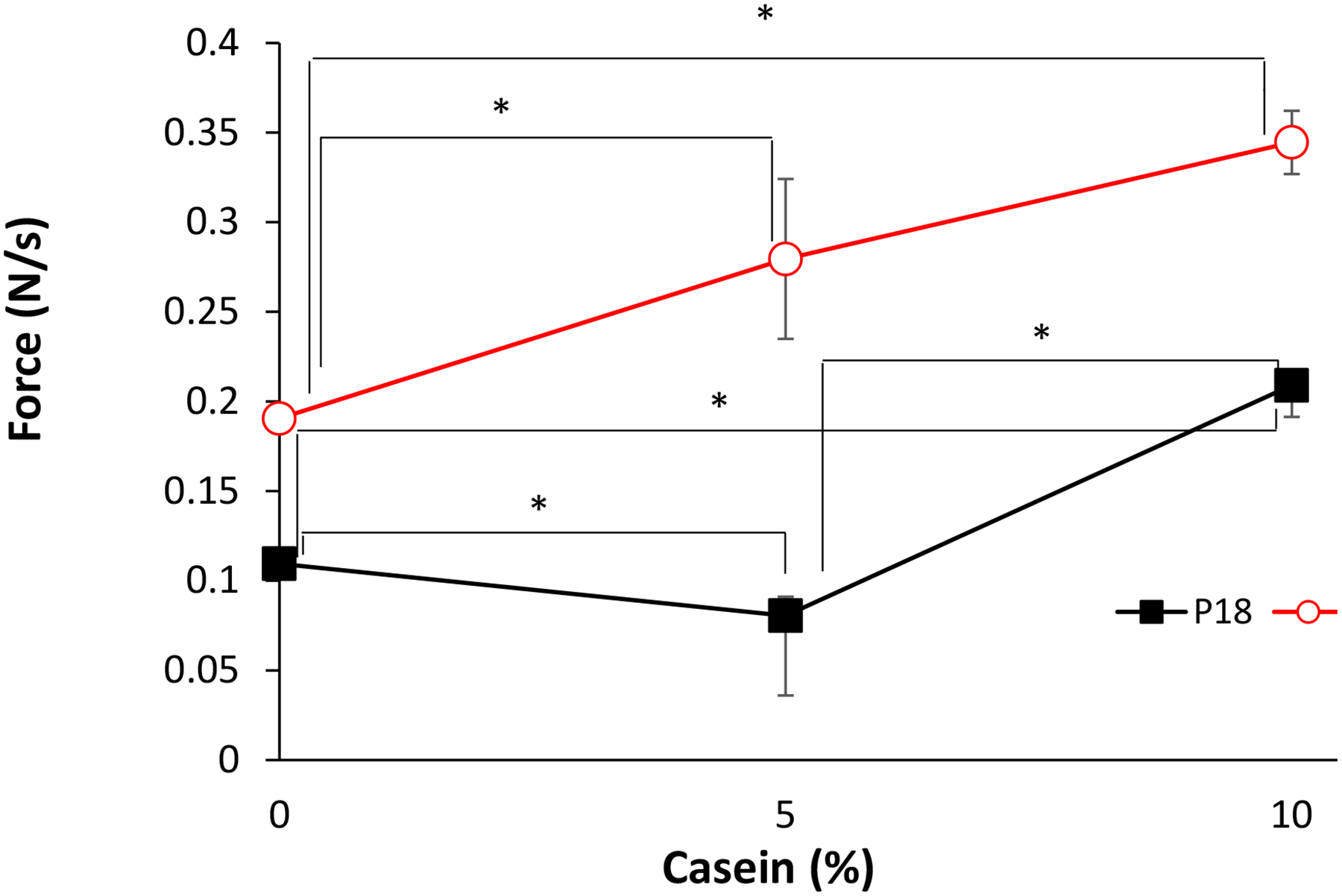
Hydrogel bioadhesion to pig skin at 37 °C. Data are means ± SD (n=3). *p < 0.05.
Drug release
Release kinetics from the hydrogel was assessed with sulforhodamine B (logP = 1.25; ChemAxon Database) and bupivacaine hydrochloride (log P = 3.71 at pH 7; (ChemAxon Database). As described in Methods, formed hydrogels with either sulforhoramine B or bupivacaine hydrochloride, were placed in Transwell ™ inserts then immersed in wells containing pre-warmed PBS pH 7.4, and incubated at 37°C. At predetermined intervals, aliquots of the PBS (the receiving media) were collected and inserts moved into a new well with fresh PBS.
In the absence of casein, there was little difference in the release rates of sulforhodamine B (Fig.5) or bupivacaine (Fig. 6) from P18 or P25. However, release of both drugs decreased with increasing casein concentration at both P407 concentrations. In the absence of casein, the rate of release of bupivacaine was slower than (P18) or equal to (P25) that of sulforhodamine B release (e.g. at 24h) (p<0.05). In the presence of 5% or 10% casein, release of bupivacaine (e.g. at 24 h) was slightly faster than that of sulforhodamine B, by 1.2- to 1.4-fold.
Fig. 5 -.
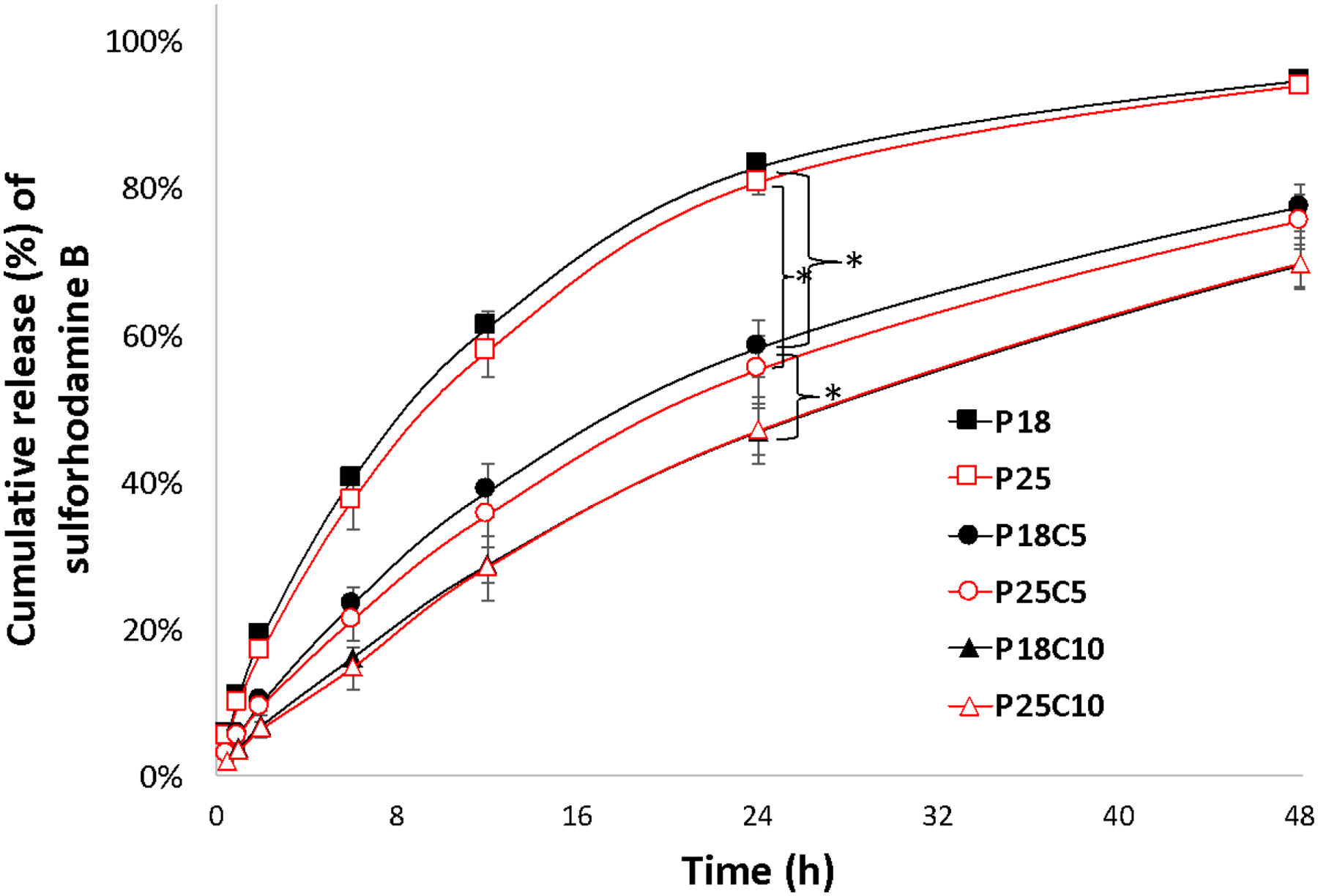
Cumulative release (%) of sulforhodamine B from P407/casein hydrogel formulations at 37°C in PBS buffer pH 7.4. Data are means ± SD (n=4). Some error bars are obscured by the symbols due to their small size. At 24h *p<0.05 when compared between different casein concentrations. Please see table 1 for definition of abbreviations.
Fig. 6 -.
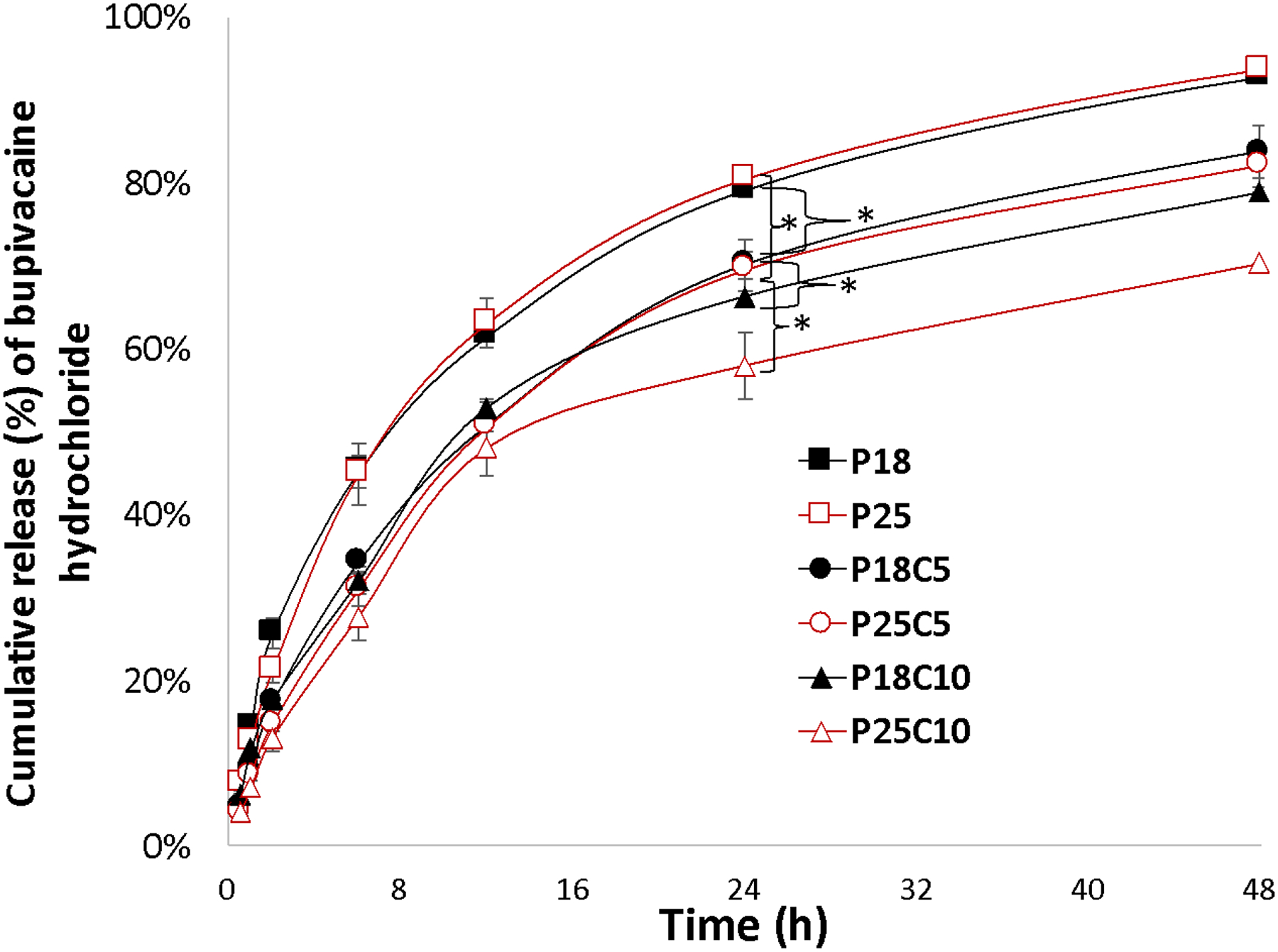
Cumulative release (%) of bupivacaine hydrochloride from P407/casein hydrogel formulations at 37°C in PBS buffer pH 7.4. Data are means ± SD (n=4). Some error bars are obscured by the symbols due to their small size. *p<0.05 when compared between different casein concentrations. Please see table 1 for definition of abbreviations.
Tissue Reaction
To assess tissue reaction in vivo, two areas on the backs of the rats were shaved and 3 hydrogels, including those with the highest and lowest total mass %w/v (P25, P25C10, and P18C5), were applied (Fig. 7a). After 6h, animals were euthanized, and skin samples were collected, and processed into hematoxylin and eosin-stained slides. On histologic evaluation (Fig. 7b), no signs of inflammation or tissue injury were seen in any layers of the skin, subcutaneous tissue, or underlying skeletal muscle layer and fascia.
Fig. 7 -.
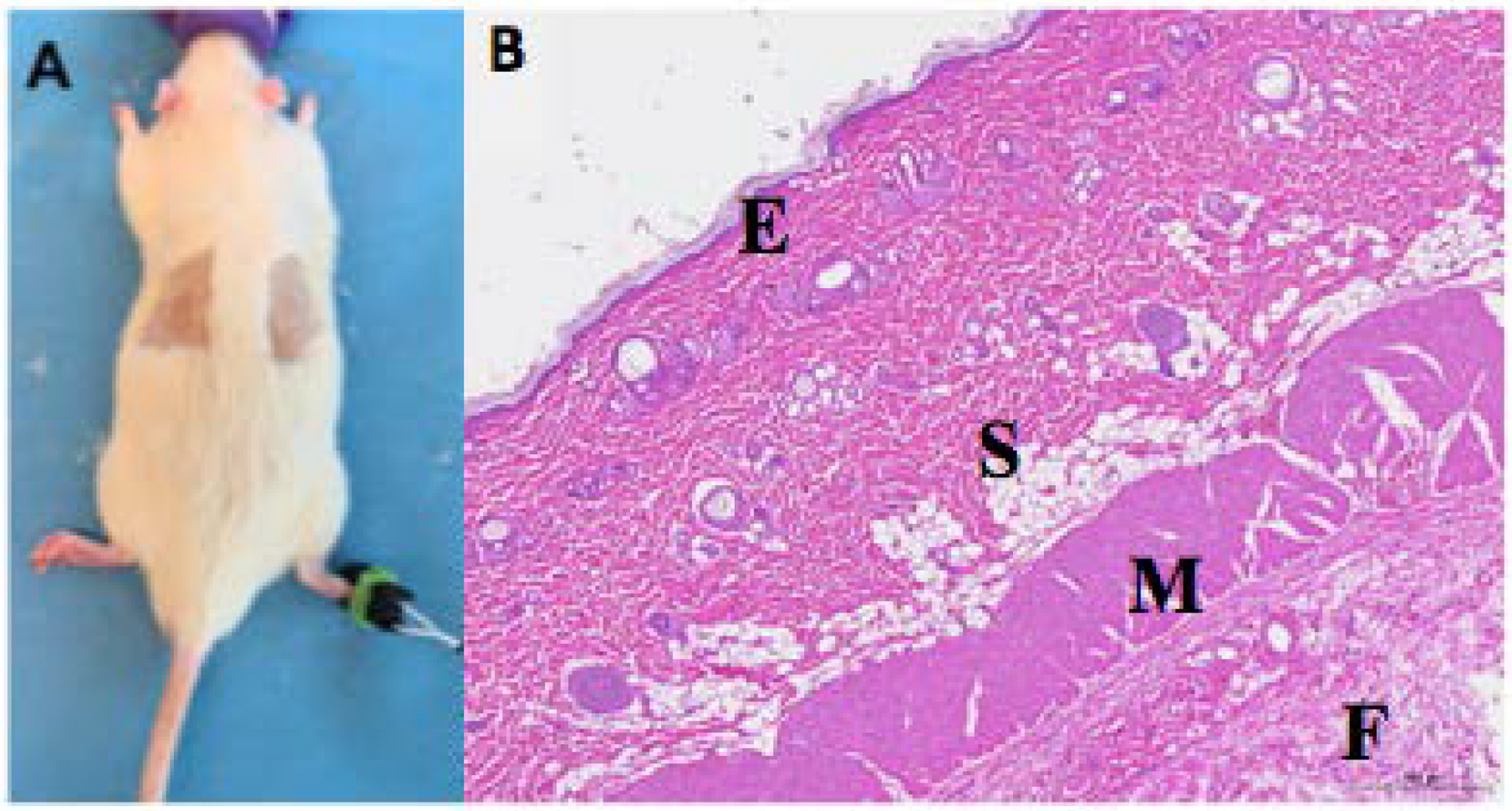
Histologic evaluation after surface application of formulations (A) Placement of hydrogels on the skin. (B) Photomicrograph of hematoxylin-eosin-stained section of skin exposed to the P25C10 formulation, showing epidermis (E), subcutaneous tissue (S), underlying skeletal muscle (M) and fascia (F).
DISCUSSION
The addition of casein to P407 generally enhanced the mechanical properties (G’ and viscosity) of P407, as well as its adhesive properties, and slowed the release of low-molecular weight compounds. The combination of these macromolecules did not result in new bond formation, suggesting that the interactions that caused those effects occurred by entanglement and the formation of mixed micelles (13). The lack of enhancement of G’ with the addition of casein to P25 (which was seen with P18) could be due to that effect having been maximized.
Viscosity can enhance hydrogel retention, helping patients’ compliance and the efficiency of the treatment (16, 19). In general, and here, viscosity is driven in part by polymer concentration. In solutions of two polymers, it can also depend on interaction between those polymers, which can have a maximum effect at a given concentration, when all the possible interactions between the two molecules are made (20). (This could explain why the viscosity of P18 was increased by the addition of casein, while that of P25 was not.) In the system, the physical properties of the gel would have also been affected by micellization of P407, the formation of mixed micelles, etc (13).
Viscosity is also an important determinant of the release of drug from hydrogels, as it is harder for the drug to diffuse at higher viscosity (21). In addition to increasing the hydrogel’s viscosity, the addition of casein could have altered drug release kinetics by altering the micelle properties. Micellization creates distinct hydrophilic and hydrophobic domains within the hydrogel. The latter would provide the opportunity for relatively hydrophobic drugs to partition into them, potentially extending the duration of release. In that context, it is worth noting that, for example, bupivacaine has an aromatic group and a tertiary amine. Protonation of the tertiary amine confers water solubility and interaction with anionic surfaces, while the aromatic group imparts the ability to partition into hydrophobic domains of drug delivery systems or biological systems (e.g. lipid bilayers in liposomes or cells). The hydrophilicity/-phobicity of the compound is determined by pH-dependent protonation of the amine.
In addition to the creation of micelles, the type of micelles that can be formed can affect drug release. In these formulations, the P407 concentration was always greater than the casein concentration (w/v). If micelle formation is determined by the amount of casein available in each system (22), the number of mixed micelles formed should be similar at a given casein concentration, and might not vary between the two concentrations of P18 and P25. Assuming this is true for our system, one possible explanation for the prolongation of drug release is that increasing the casein concentration might encourage the formation of mixed micelles. As they are much bigger than polymeric micelles, this would make it more difficult for molecules to diffuse through and out of the hydrogel, as the spaces between the micelles became smaller, less free space to diffuse freely (22, 23).
As noted above, bupivacaine is amphiphilic. Based on the potential for interaction with the hydrophobic segments of P407 and with the anionic domains of casein, bupivacaine might have been expected to be released more slowly from the hydrogel than was sulforhodamine B. However, interactions with the hydrophobic domains of P407 and casein were likely impeded by the fact that bupivacaine is mostly in its hydrophilic cationic form at pH 7 (24). Moreover, the long PEO segments of poloxamers can mask the surface charge of α-casein micelles (13). (The isoelectric point of casein is 4.7, so it carries a negative charge at neutral or physiological pH.) This masking could impede electrostatic interactions of the protonated tertiary amine on bupivacaine with anionic casein. The slightly faster release of bupivacaine than of sulforhodamine B may be attributable to the fact that it has a lower molecular weight (25).
Bioadhesion is important to ensure retention at the site of application, improving patient compliance (19). As P407 is a hydrogel with no charge, its interaction with skin is likely based on hydrogen bonds (26). The addition of casein increased adhesiveness in 3 of the 4 formulations tested. If such charge masking occurred with the mixed micelles formed by P407 and casein in our system as well, then interaction with skin would likely not be due to electrostatic interactions. Increased adhesiveness was therefore likely mediated by the increase of hydrogen bonds, new hydrophobic interactions due to the addition of casein or both (11, 15). Casein can interact with other molecules by several mechanisms, including hydrophobic interactions, van der Waals attraction and hydrogen bonds (12, 27).
Tissue reaction, over the relatively short period of exposure studied here, was benign. This was not surprising given that P407 (3) and casein (12) have good biocompatibility. However, as noted in Methods, we were unable to maintain contact between the hydrogels and skin for longer than six hours. While this might correspond to the duration of a single application in humans, the time frame over which serial applications would be applied to treat most cutaneous diseases is much longer. While the fact that there was no tissue reaction whatsoever within the 6h is somewhat reassuring, it cannot preclude the possibility of tissue reaction with more extended exposure.
CONCLUSION
The addition of casein to P407 increased its adhesiveness, mechanical strength and viscosity, and slowed the release rates of the model drugs. Choosing casein, a food-grade biopolymer, to modify the properties of the gel presented advantages in safety, cost, commercial availability. The properties of the hydrogel could be tuned by changing the proportions of casein and P407. These improvements could have an impact on patient compliance, particularly with topical therapies that may last months and require many applications a day. Bioadhesive hydrogels driven by the addition of readily available proteins have potential as translatable controlled drug release systems.
Fig. 2 -.
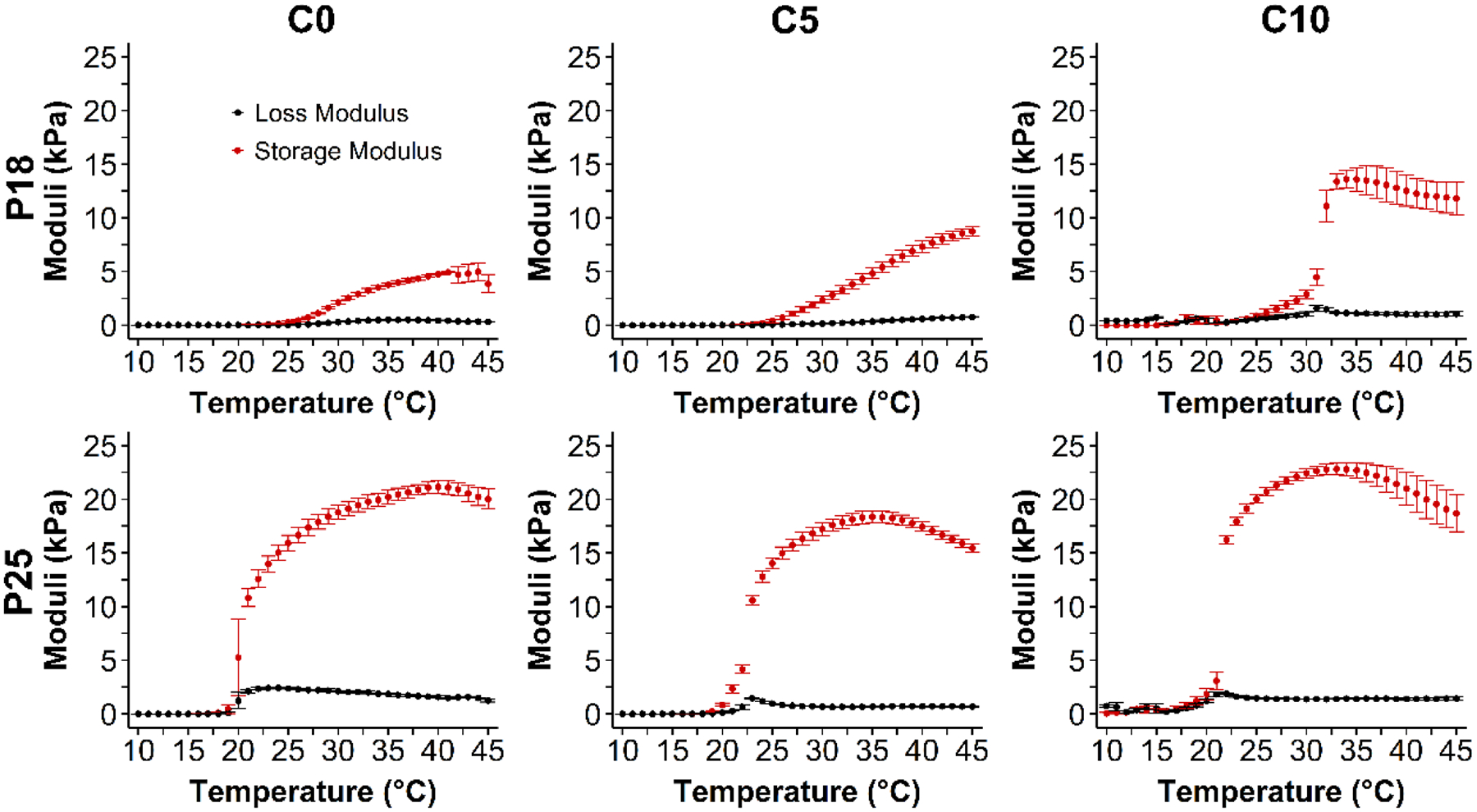
Rheology of polymer combinations as a function of temperature. G’: storage modulus, G”: loss modulus. Data are means ± SD (n=4).
ACKNOWLEDGMENTS
This study was supported in part by grants FAPESP 2017/10728-2 and 2018/05421-8 (to L.L.T.), FAPESP 2018/07707-6 and CNPQ (to P.G.M.), R35 GM131728 (to D.S.K.)
Footnotes
DATA STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFS
- 1.Croisfelt FM, Tundisi LL, Ataide JA, Silveira E, Tambourgi EB, Jozala AF, Souto EMB, Mazzola PG. Modified-release topical hydrogels: a ten-year review. Journal of Materials Science. 2019. [Google Scholar]
- 2.Schmolka IR. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. Journal of Biomedical Materials Research. 1972;6(6):571–582. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Shamat MA, Calvo-Castro J, Stair JL, Cook MT. Modifying the Properties of Thermogelling Poloxamer 407 Solutions through Covalent Modification and the Use of Polymer Additives. Macromolecular Chemistry and Physics. 2019;220(16):1900173. [Google Scholar]
- 4.Tung IC. Rheological behavior of poloxamer 407 aqueous solutions during sol-gel and dehydration processes. International Journal of Pharmaceutics. 1994;107(2):85–90. [Google Scholar]
- 5.Pai-Chie C-C, Frank SG. In vitro release of lidocaine from pluronic F-127 gels. International Journal of Pharmaceutics. 1981;8(2):89–99. [Google Scholar]
- 6.Chen-Chow SGF PC. Pluronic F-127 Gels as a Vehicle for Topical Administration of Anticancer Agents. Chemical & pharmaceutical bulletin. 1984;32(10):4205–4208. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Sabharwal V, Okonkwo OS, Shlykova N, Tong R, Lin LY, Wang W, Guo S, Rosowski JJ, Pelton SI, Kohane DS. Treatment of otitis media by transtympanic delivery of antibiotics. Science Translational Medicine. 2016;8(356):356ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambardzumyan A, Aguié-Béghin V, Daoud M, Douillard R. β-Casein and Symmetrical Triblock Copolymer (PEO−PPO−PEO and PPO−PEO−PPO) Surface Properties at the Air−Water Interface. Langmuir. 2004;20(3):756–763. [DOI] [PubMed] [Google Scholar]
- 9.Portnaya I, Ben-Shoshan E, Cogan U, Khalfin R, Fass D, Ramon O, Danino D. Self-Assembly of Bovine β-Casein below the Isoelectric pH. Journal of agricultural and food chemistry. 2008;56:2192–2198. [DOI] [PubMed] [Google Scholar]
- 10.Shaw DJ. 8 - Colloid stability. In: Shaw DJ, editor. Introduction to Colloid and Surface Chemistry (Fourth Edition). Oxford: Butterworth-Heinemann; 1992. p. 210–243. [Google Scholar]
- 11.Xu J, Gao G, Duan L, Sun G. Protein and Hydrophobic Association-Regulated Hydrogels with Adhesive Adjustability in Different Materials. Advanced Materials Interfaces. 2020;7(1):1901541. [Google Scholar]
- 12.Elzoghby AO, Abo El-Fotoh WS, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. Journal of Controlled Release. 2011;153(3):206–216. [DOI] [PubMed] [Google Scholar]
- 13.Kessler A, Menéndez-Aguirre O, Hinrichs J, Stubenrauch C, Weiss J. αs-Casein—PE6400 mixtures: Surface properties, miscibility and self-assembly. Colloids and Surfaces B: Biointerfaces. 2014;118:49–56. [DOI] [PubMed] [Google Scholar]
- 14.Portnaya I, Khalfin R, Kesselman E, Ramon O, Cogan U, Danino D. Mixed micellization between natural and synthetic block copolymers: β-casein and Lutrol F-127. Physical Chemistry Chemical Physics. 2011;13(8):3153–3160. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Fan Z, Duan L, Gao G. A tough, stretchable, and extensively sticky hydrogel driven by milk protein. Polymer Chemistry. 2018;9(19):2617–2624. [Google Scholar]
- 16.Alves T, Souza J, Rebelo M, Pontes K, Santos C, Lima R, Jozala A, Grotto D, Severino P, Rai M, Chaud M. Formulation and evaluation of thermoresponsive polymeric blend as a vaginal controlled delivery system. Journal of Sol-Gel Science and Technology. 2018;86(3):536–552. [Google Scholar]
- 17.Oshiro Junior JA, Carvalho FC, Soares CP, Chorilli M, Chiavacci LA. Development of Cutaneous Bioadhesive Ureasil-Polyether Hybrid Films. International Journal of Polymer Science. 2015;2015:727324. [Google Scholar]
- 18.Zurdo Schroeder I, Franke P, Schaefer UF, Lehr CM. Development and characterization of film forming polymeric solutions for skin drug delivery. Eur J Pharm Biopharm. 2007;65(1):111–121. [DOI] [PubMed] [Google Scholar]
- 19.Hurler J, Engesland A, Poorahmary Kermany B, Škalko-Basnet N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. Journal of Applied Polymer Science. 2012;125(1):180–188. [Google Scholar]
- 20.Tadros TF. Colloids in Agrochemicals. United Kingdom: Wiley; 2009. [Google Scholar]
- 21.Cheong LWS, Heng PWS, Wong LF. Relationship Between Polymer Viscosity and Drug Release from a Matrix System. Pharmaceutical Research. 1992;9(11):1510–1514. [DOI] [PubMed] [Google Scholar]
- 22.Kessler A, Menéndez-Aguirre O, Hinrichs J, Stubenrauch C, Weiss J. αs-Casein–PE6400 mixtures: a fluorescence study. Faraday Discussions. 2013;166(0):399–416. [DOI] [PubMed] [Google Scholar]
- 23.Maolin Z, Min Y, Jian S, Jinshan W, Hongfei H. Radiation preparation and diffusion behavior of thermally reversible hydrogels. Radiation Physics and Chemistry. 1998;52(1):313–317. [Google Scholar]
- 24.Stoelting RK, Miller R. Local anesthetics, Basics of anesthesia. New York, NY: Churchill Livingstone Inc.; 1994. [Google Scholar]
- 25.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–d1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghobril C, Grinstaff MW. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem Soc Rev. 2015;44(7):1820–1835. [DOI] [PubMed] [Google Scholar]
- 27.Livney YD. Milk proteins as vehicles for bioactives. Current Opinion in Colloid & Interface Science. 2010;15(1):73–83. [Google Scholar]


