Abstract
E-cigarette or vaping product use–associated lung injury (EVALI) is a respiratory illness that has significant overlap with the symptoms of coronavirus disease 2019 (COVID-19). In the current pandemic, diagnosis of EVALI may be delayed because of anchoring bias when patients present with symptoms consistent with COVID-19. We present 3 cases of patients who were hospitalized with a presumed diagnosis of COVID-19 but were later diagnosed with EVALI.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; EVALI, e-cigarette or vaping product use–associated lung injury; IV, intravenous; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
E-cigarette or vaping product use–associated lung injury (EVALI) is an increasingly recognized condition that was declared a public health emergency in 2019 by the Centers for Disease Control and Prevention.1 Patients who use e-cigarettes or vaping products may present with respiratory symptoms including cough, shortness of breath, and chest pain, but constitutional symptoms (subjective fever, chills, fatigue, malaise, weight loss) and gastrointestinal complaints are just as common, a feature that may not be obvious or well known.2,3 Whereas cases reached a peak in late 2019, lately cases have been reduced in large part owing to the removal of vitamin E acetate, which is an additive that was found in many tetrahydrocannabinol-containing vaping products.1 Despite this reduction, the removal of vitamin E acetate has not completely eliminated EVALI.
The current coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is also a predominantly respiratory illness that bears striking resemblance to EVALI. It is currently dominating the health care provider’s mindset, leading to many opportunities for cognitive bias, including anchoring and availability bias. We present 3 cases of patients who were admitted to the hospital for presumed COVID-19 and were treated as such but were later found to have EVALI.
Case 1
A 34-year-old man with depression, anxiety, attention-deficit/hyperactivity disorder, gastroesophageal reflux disease, hypertension, polysubstance abuse, and recent vaping exposure with cannabis oil presented to the hospital with 10 days of worsening shortness of breath, nonproductive cough, pleuritic chest pain, fevers, myalgias, abdominal pain, nausea, and headaches. He had been evaluated 1 week earlier in a local emergency department and was not hypoxic at that time. Pertinent laboratory work showed a leukocytosis with neutrophilia and lymphopenia (Table). A chest radiograph showed asymmetric bilateral patchy basilar opacities. He had 2 negative SARS-CoV-2 nasopharyngeal test results around that time. He was given a dose of ceftriaxone and then completed a 5-day course of amoxicillin/clavulanic acid (Augmentin) and azithromycin for presumed community-acquired pneumonia. His symptoms worsened, however, and he presented 10 days after symptom onset.
Table.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Leukocytes (3.4-9.6 × 109/L) | 26.1 | 10.9 | 14.3 |
| Neutrophils (1.56-6.45 × 109/L) | 23.49 | 9.84 | 13.16 |
| Lymphocytes (0.95-3.07 × 109/L) | 0.85 | 0.72 | 0.73 |
| Hemoglobin (13.2-16.6 g/dL) | 10.3 | 13.5 | 14.5 |
| Platelets (135-317 × 109/L) | 577 | 355 | 308 |
| CRP (≤8.0 mg/L) | 283.4 | 234.1 | 261.2 |
| D-dimer (≤500 ng/mL FEU) | 776 | 389c | 1046 |
| Lactate (0.5-2.2 mmol/L) | 2.1 | 1.4 | 1.1 |
| Creatinine (0.74-1.35 mg/dL) | 0.7 | 0.58 | 0.78 |
| Sodium (135-145 mmol/L) | 132 | 138 | 134 |
| AST (8-48 U/L) | 100 | 33 | 24 |
| ALP (40-129 U/L) | 181 | 68 | 71 |
| Albumin (3.5-5.0 g/dL) | 2.5 | 2.6 | 4.3 |
ALP, alkaline phosphatase; AST, aspartate transaminase; CRP, C-reactive protein; FEU, fibrinogen-equivalent unit.
To convert creatinine levels to μmol/L, multiply by 88.4; ALP and AST values to μkat/L, multiply by 0.0167; albumin levels to g/L, multiply by 10.
D-dimer level for patient 2 was obtained by a different assay with a normal range of 0 to 229 ng/mL D-dimer units.
He was now hypoxic, requiring 2 L of oxygen by nasal cannula. He was febrile to 38.2°C, tachycardic with a heart rate of 110 beats/min, and tachypneic with a respiratory rate of 30 breaths/min. Laboratory work showed a worsening leukocytosis, neutrophilia, lymphopenia, elevated C-reactive protein (CRP) concentration, and elevated D-dimer level (Table). Chest computed tomography (CT) showed diffuse, midlung-predominant, ill-defined ground-glass opacities with interlobular septal thickening (Figure 1). He was admitted to the hospital, given intravenous (IV) fluids, and prescribed levofloxacin. The results of a repeated SARS-CoV-2 test and respiratory pathogen panel (both nasopharyngeal swabs) were negative, as was the serologic test response for SARS-CoV-2. His respiratory status worsened, and he was transferred to the intensive care unit, where high-flow nasal cannula 55% fraction of inspired oxygen at 45 L/min was started. Given the lack of improvement on antibiotics, repeated negative SARS-CoV-2 test result, and history of vaping, he was treated for EVALI with prednisone. He had rapid improvement within the first day of treatment and completed a 4-day course of prednisone 40 to 80 mg daily. He was discharged on room air on hospital day 5.
Figure 1.
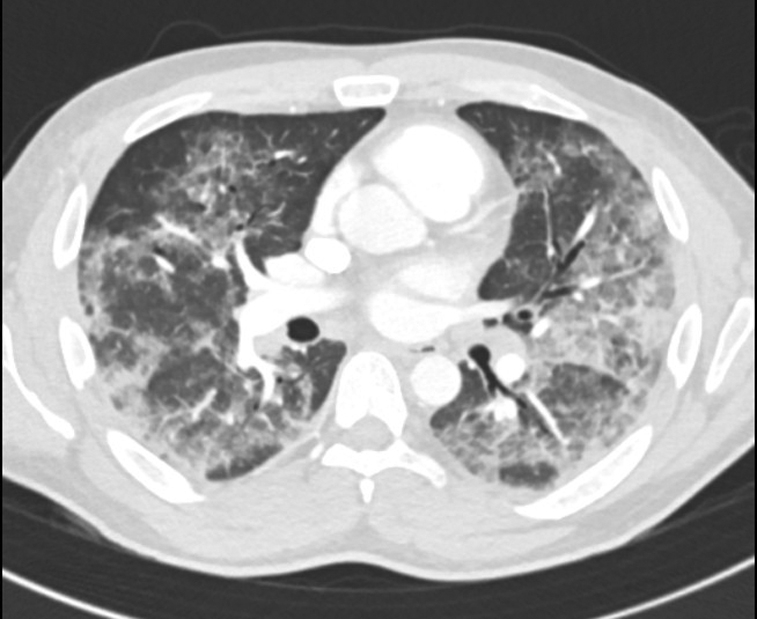
Computed tomography scan from case 1 demonstrating bilateral ill-defined ground-glass opacities with a midlung predominance and interlobular septal thickening.
Case 2
A 47-year-old man with no past medical history presented with 7 days of fevers, nonproductive cough, myalgias, malaise, nausea, and diarrhea. He was febrile to 38.0°C and hypoxic, requiring 3 L of oxygen by nasal cannula. Laboratory work showed leukocytosis with lymphopenia along with elevated CRP concentration, platelets, and D-dimer level (Table). Chest radiography showed bilateral patchy airspace opacities. He was admitted to the hospital, given IV fluids, and treated with ceftriaxone and azithromycin for possible community-acquired bacterial pneumonia. The results of a SARS-CoV-2 polymerase chain reaction (PCR) and a respiratory pathogen panel obtained by nasopharyngeal swab were negative. His respiratory status worsened, and he required 6 L of oxygen to maintain saturations. After further discussion, the patient reported vaping with cannabis oil several times daily in the weeks before admission. Results of repeated SARS-CoV-2 testing by both a nasopharyngeal swab and sputum sample were negative, as was the serologic test response. The CT scan showed extensive bilateral ground-glass opacities in a predominantly central distribution with associated interlobular septal thickening (Figure 2). Given significant hypoxia and diagnostic uncertainty, he underwent bronchoscopy. A thorough infectious and autoimmune panel, including a fourth SARS-CoV-2 PCR, on bronchoalveolar lavage specimens was negative. A cell count showed 47% alveolar macrophages, 36% neutrophils, 12% lymphocytes, and pigment-laden macrophages. At this point, a presumptive diagnosis of EVALI was made. He remained intubated after bronchoscopy but was quickly extubated. He required high-flow nasal cannula but was able to be weaned to room air without steroid treatment. He was discharged to home on hospital day 11.
Figure 2.
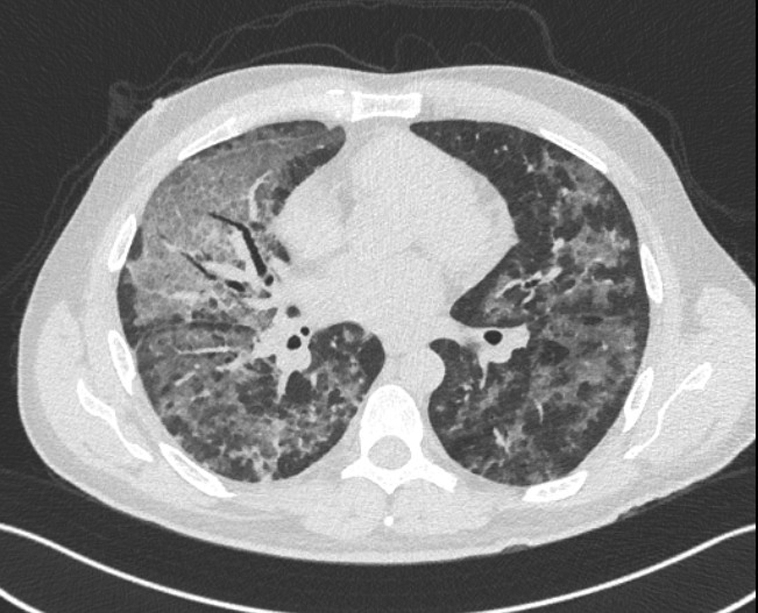
Computed tomography scan from case 2 showing extensive bilateral ground-glass opacities with a predominantly central distribution.
Case 3
A 20-year-old man with a known history of vaping tetrahydrocannabinol products presented with 5 days of shortness of breath, fever, nonproductive cough, and nausea. One day before hospital admission, he had been evaluated as an outpatient, had a negative SARS-CoV-2 test result by nasopharyngeal swab, and was treated with doxycycline for community-acquired pneumonia. Because of continued symptoms, he presented to the emergency department the following day. He was febrile to 38.4°C and tachycardic with a heart rate of 110 beats/min but was normotensive and saturating well on room air. A chest radiograph showed bilateral infrahilar and basilar interstitial infiltrates. A CT scan of the chest showed diffuse bilateral ground-glass opacities with a peripheral predominance (Figure 3). Laboratory work showed a mild leukocytosis with a lymphopenia and an elevated CRP concentration and D-dimer level (Table). He was admitted to the hospital, given IV fluids, and prescribed ceftriaxone and azithromycin for community-acquired pneumonia. He remained persistently febrile with elevated inflammatory markers. He had 2 additional nasopharyngeal swabs that were negative for SARS-CoV-2, as was serology testing. Given his lack of improvement and significant vaping history, he was treated for EVALI with 125 mg of methylprednisolone for 3 days. He had prompt resolution of the fevers, improvement in cough, and reduction of inflammatory markers. He was discharged to home on hospital day 5.
Figure 3.
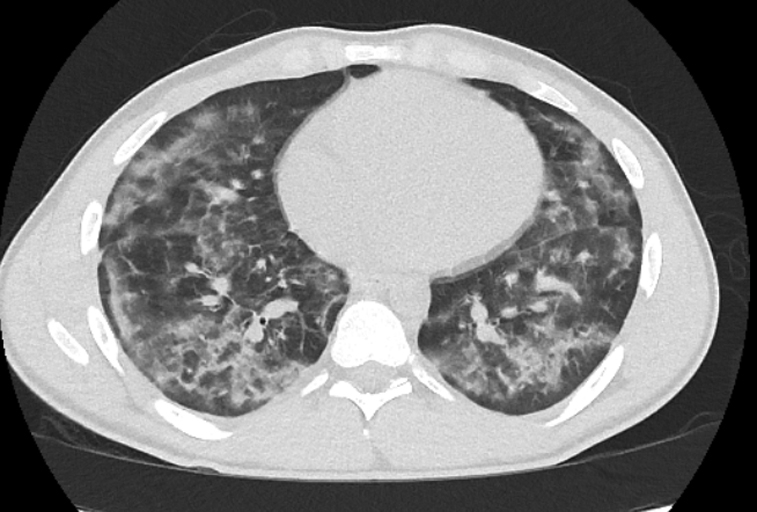
Computed tomography scan from case 3 showing diffuse bilateral ground-glass opacities with subpleural sparing.
Discussion
E-cigarette or vaping product use–associated lung injury (EVALI) is a respiratory illness caused by the inhalation of harmful substances associated with e-cigarettes or vaping products.2 The most common symptoms include cough, chest pain, and shortness of breath, which occur in 95% of patients.4 Constitutional and gastrointestinal symptoms are also common, occurring in approximately 85% to 100% and 77% of patients, respectively.3,4 The association of constitutional and gastrointestinal symptoms with EVALI may be underrecognized. It is an acute to subacute illness that progresses during a 1- to 2-week course; progressive hypoxemic respiratory failure due to acute respiratory distress syndrome develops in approximately 22% of people, necessitating intubation and mechanical ventilation.4 As of February 18, 2020, there have been a reported total of 2807 hospitalizations and 68 deaths in the United States due to EVALI.1 Treatment largely consists of cessation of product use and supportive care, although there has been some success with corticosteroid use.4 Corticosteroids should typically not be initiated without the input of a pulmonologist and adequate exclusion of an infectious cause.4
Also a predominantly respiratory illness caused by the novel SARS-CoV-2 virus, COVID-19 is currently causing a global pandemic with more than 28 million infections in the United States and more than 500,000 deaths.5 The symptoms are wide-ranging and varied. A large study of patients in Wuhan, China, the epicenter of the pandemic, reported fever (99%), fatigue (70%), dry cough (59%), anorexia (40%), myalgias (35%), dyspnea (31%), and sputum production (27%) as the most common clinical manifestations.6 Subsequent studies and clinical experience have shown that gastrointestinal symptoms are somewhat frequent, with reports of diarrhea, nausea, vomiting, and abdominal pain occurring in up to 18% of patients.7 Similar to EVALI, it typically begins as a mild respiratory illness that can progress during the course of 1 to 2 weeks, with acute respiratory distress syndrome developing in 20% to 41% of patients; approximately 12% require intubation in some series.6,8 Treatment is largely supportive as there are currently no approved pharmacologic therapies, although several trials are in progress.
Aside from clinical history, objective measures to help differentiate EVALI and COVID-19 include chest imaging and bronchoscopy. Chest radiography in patients with EVALI can reveal diffuse hazy opacities that are most pronounced centrally9, 10, 11; CT imaging can show diffuse ground-glass opacities with a central predominance, although all lobes can be affected.9, 10, 11 Additional characteristic findings include subpleural sparing, some areas of lobular sparing, and centrilobular nodules.9, 10, 11 Consolidation, if present, is often mild.9, 10, 11 The classic finding on bronchoscopy is the presence of lipid-laden macrophages with negative test results for infectious organisms.4 Chest imaging findings in COVID-19 are characterized by ground-glass opacities with a peripheral (as opposed to the central distribution of EVALI) and lower lobe predominance (Figure 4).10,12 Multiple areas of bilateral lobular and subsegmental consolidation can also be seen, particularly in patients with severe infection requiring intensive care unit admission.10,12 Pleural effusion and lymphadenopathy are uncommon.10,12 In addition, a central distribution of infiltrate (although common in EVALI) is less common in COVID-19, and its finding should prompt consideration of an alternative diagnosis.10,12 If it is needed for diagnostic purposes, reverse transcriptase–PCR detection of SARS-CoV-2 can be performed on bronchoalveolar lavage fluid; some studies suggest that this as the highest yield specimen.13 Bronchoscopy is not needed for a definitive diagnosis of COVID-19 as a diagnosis can be made by nucleic acid amplification testing of nasopharyngeal swabs or expectorated sputum.
Figure 4.
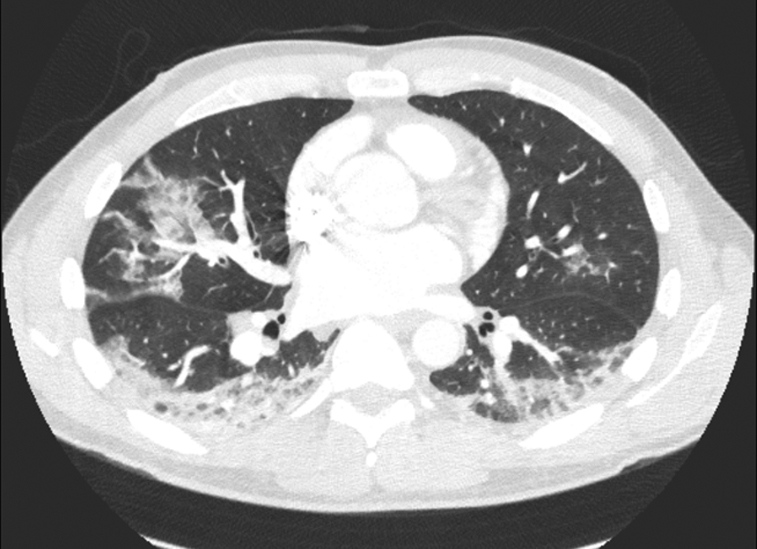
Computed tomography scan from a patient with COVID-19 demonstrating multifocal peripheral ground-glass opacities with central sparing.
Conclusion
We presented 3 cases of a febrile respiratory illness initially thought to be due to COVID-19. The symptoms of COVID-19 are nonspecific and can be seen in a variety of other infectious and noninfectious conditions. There is substantial overlap in the presenting signs and symptoms of EVALI and COVID-19, and constitutional symptoms are as common a feature of EVALI as they are of COVID-19.3,4,6, 7, 8 This highlights the importance of a thorough history. All patients presenting with these symptoms should be asked about substance use. Whereas EVALI is a diagnosis of exclusion, identification of vaping or e-cigarette use in the history alters the differential diagnosis and subsequent evaluation in treatment. Aside from history, use of chest CT is also important to differentiate these 2 entities. However, whereas CT imaging can be useful, it is not recommended as a first-line diagnostic test.14 In addition to an accurate history, for COVID-19, the “gold standard” of diagnosis remains the nucleic acid amplification test, the sensitivity and specificity of which are high in ideal conditions.15,16 However, the test performance is dependent on both specimen quality and duration of illness at time of testing, with false-negative rates ranging from less than 5% to 40%, depending on the specimen source.15,16 Even when diagnostic uncertainty exists, the use of CT should not be pursued without first weighing the potential risks of exposure to other medical personnel with potential benefits of the CT scan itself. This report highlights the importance of an accurate history as well as the risk of diagnostic error and bias in the setting of a global pandemic. Given the similar signs and symptoms, most clinicians will consider COVID-19 first, rather than another cause. This is appropriate during a pandemic, but it is also imperative to consider alternative diagnoses if the result of repeated testing is negative. Clinicians must be cognizant of the potential for cognitive bias, including anchoring and availability bias. Anchoring occurs when clinicians put significant emphasis on features of a patient’s initial presentation that may lead to dismissal of information learned later in the clinical course.17 Availability bias refers to a clinician’s judgment of how likely a diagnosis is on the basis of how easily an example of that diagnosis is mentally generated.17 Recent or emotional events are often deemed more probable to recur again because of the clarity with which they are recalled.17 This is especially true during the COVID-19 pandemic. Whereas COVID-19 is a condition that should be at the top of a differential diagnosis of a febrile respiratory illness, a thorough history is imperative to identify risk factors and features for other diagnoses so prompt diagnostic and therapeutic interventions can be performed and anchoring and availability biases avoided.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Centers for Disease Control and Prevention Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html Published February 25, 2020.
- 2.Salzman G.A., Alqawasma M., Asad H. Vaping associated lung injury (EVALI): an explosive United States epidemic. Mo Med. 2019;116(6):492–496. [PMC free article] [PubMed] [Google Scholar]
- 3.Layden J.E., Ghinai I., Pray I. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 4.Siegel D.A., Jatlaoui T.C., Koumans E.H. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—-United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;98(41):919–927. doi: 10.15585/mmwr.mm6841e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map
- 6.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [erratum appears in JAMA. 2021;325(11):1113] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung K.S., Hung I.F., Chan P.P. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [erratum appears in JAMA Intern Med. 2020;180(7):1031] JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kligerman S., Raptis C., Larsen B. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use-associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294(3):491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 10.Foust A.M., Winant A.J., Chu W.C. Pediatric SARS, H1N1, MERS, EVALI, and now coronavirus disease (COVID-19) pneumonia: what radiologists need to know. AJR Am J Roentgenol. 2020;215(3):736–744. doi: 10.2214/AJR.20.23267. [DOI] [PubMed] [Google Scholar]
- 11.Henry T.S., Kanne J.P., Kligerman S.J. Imaging of vaping-associated lung disease. N Engl J Med. 2019;381(15):1486–1487. doi: 10.1056/NEJMc1911995. [DOI] [PubMed] [Google Scholar]
- 12.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update—Radiology Scientific Expert Panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 15.Cheng M.P., Papenburg J., Desjardins M. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172(11):726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissleder R., Lee H., Ko J., Pittet M. COVID-19 diagnostics in context. Sci Transl Med. 2020;12(546):eabc1931. doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- 17.Tversky A., Kahneman D. Judgement under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]


