Abstract
The ongoing Coronavirus Disease 2019 (COVID-19) pandemic has affected more than 100 million people and clinics are being established for diagnosing and treating lingering symptoms, so called long-COVID. A key concern are neurological and long-term cognitive complications. At the same time, the prevalence and nature of the cognitive sequalae of COVID-19 are unclear. The present study aimed to investigate the frequency, pattern and severity of cognitive impairments 3–4 months after COVID-19 hospital discharge, their relation to subjective cognitive complaints, quality of life and illness variables. We recruited patients at their follow-up visit at the respiratory outpatient clinic, Copenhagen University Hospital, Bispebjerg, approximately four months after hospitalisation with COVID-19. Patients underwent pulmonary, functional and cognitive assessments. Twenty-nine patients were included. The percentage of patients with clinically significant cognitive impairment ranged from 59% to 65% depending on the applied cut-off for clinical relevance of cognitive impairment, with verbal learning and executive functions being most affected. Objective cognitive impairment scaled with subjective cognitive complaints, lower work function and poorer quality of life. Cognitive impairments were associated with d-dimer levels during acute illness and residual pulmonary dysfunction. In conclusion, these findings provide new evidence for frequent cognitive sequelae of COVID-19 and indicate an association with the severity of the lung affection and potentially restricted cerebral oxygen delivery. Further, the associations with quality of life and functioning call for systematic cognitive screening of patients after recovery from severe COVID-19 illness and implementation of targeted treatments for patients with persistent cognitive impairments.
Keywords: COVID-19, Cognitive impairment, Quality of life, Pulmonary dysfunction
1. Introduction
The ongoing Coronavirus Disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus has so far affected more than 100 million people and led to more than two million deaths worldwide. While a key focus in the acute treatment of hospitalized patients is to limit fatality, it has become clear that there may also be significant detrimental long-term effects of a COVID-19 infections. Hence, healthcare systems have now also begun to open clinics dedicated to diagnosing and treating lingering symptoms following COVID-19, so called long-COVID. Long-lasting symptoms are frequent; a new large-scale study found that 75% of people hospitalized with COVID-19 experience symptoms for at least six months after recovery, including fatigue, muscle weakness, depression and sleep difficulties (Huang et al., 2021). While the severity of persistent symptoms is often related to the severity of symptoms during the acute illness phase, studies found that long-lasting symptoms can also occur after mild illness and across all ages (Townsend et al., 2020).
A key concern is the neurological complications of COVID-19, which are most common in severely affected patients (Avula et al., 2020; Desforges et al., 2019; Dube et al., 2018; Gu and Korteweg, 2007; Helms et al., 2020; Li et al., 2020; Mao et al., 2020; Moriguchi et al., 2020; Nath, 2020; Oxley et al., 2020; Toscano et al., 2020; Troyer et al., 2020; Wu et al., 2020). These include mild symptoms like headaches, loss of smell and taste, tingling sensations, dizziness, nausea and severe fatigue, as well as more severe outcomes such as aphasia, strokes and seizures. It is still unclear whether the effects of SARS-CoV-2 on the brain are indirect (mediated by oxygen starvation of the brain and/or the body's extreme inflammatory response in severely affected patients) or direct (mediated by virus invasion in the brain), or both (Lucchese and Floel, 2020; Natoli et al., 2020; Zhou et al., 2021). A large proportion of COVID-19 patients exhibit extremely low (around 70%) blood oxygen saturation but, remarkably, experience disproportionately few symptoms of cerebral hypoxia, a phenomenon coined ‘happy hypoxia’ (Couzin-Frankel, 2020). Underrecognised oxygen starvation of the brain could therefore be partially responsible for neurological damage – particularly in brain regions highly susceptible to hypoxia, such as the hippocampus. There is also evidence for a role of inflammation, leakiness of the blood brain barrier and multiple brain abnormalities – including intracerebral haemorrhagic lesions, white matter microhemorrhages and hyperintensities – in COVID patients with neurological symptoms (Achar and Ghosh, 2020; Kremer et al., 2020; Lersy et al., 2021).
Regardless of the mechanisms, the neuronal damage caused by the SARS-CoV-2 can have long-term negative impact on cognitive functions, daily functioning and quality of life. Accordingly, emerging reports indicate that a large proportion of patients experience persistent cognitive problems with memory difficulties and impaired ability to concentrate for several months after their recovery from COVID-19. A large US survey study with data from >1500 respondents with previous COVID-19 found that difficulty concentrating and focusing was experienced by more than 50% of patients and was the fourth most reported long-term symptom after COVID-19 (Lambert, 2020). In keeping with this, a study of 29 middle-aged patients recovered from COVID-19 found sustained attention impairments (Zhou et al., 2020) while executive dysfunction was found in a study of 58 patients 2–3 months after hospital discharge (Raman et al., 2021). At larger scale, internet-based testing of cognitive functions in >84,000 people with suspected or biologically confirmed COVID-19 revealed even broader cognitive impairments across memory, attention and executive functions after recovery from COVID-19 illness when controlling for age, sex, education levels and pre-existing comorbidity (Hampshire et al., 2020). Cognitive impairments were most pronounced in people who had been hospitalized but, importantly, were also observed in non-hospitalised patients with no reported breathing difficulties (Hampshire et al., 2020).
Aside from the above few studies, little is known about the pattern and severity of cognitive impairments after COVID-19 and their association with illness variables. Specifically, it is unclear how many patients continue to show clinically relevant cognitive impairments after their recovery. Another challenge is that subjective self-reported cognitive problems do not necessarily scale with objective performance deficits on neuropsychological tests (Jensen et al., 2015; Miskowiak et al., 2016; Ott et al., 2015). It is also unclear whether persistent objective cognitive impairments after COVID-19 illness are related to patients’ quality of life and work functions. Investigation of the relation between subjective and objective cognitive sequalae of COVID-19 – and their association with quality of life and functioning – would thus be important to guide cognition assessments in long-COVID clinics. Finally, investigation of the association between the severity of long-term cognitive impairments and biological measures of illness activity will provide key insights into the pathogenic mechanisms. Importantly, understanding the specific pathogenic drivers of cognitive impairments – such as cerebral hypoxia or systemic inflammation – is pertinent to guiding the best management strategies for acute COVID-19. These knowledge gaps are addressed in the present report, which is based a cohort study of residual symptoms of COVID-19 after recovery in hospitalised patients (IMPACT-COVID). Specifically, we here investigate: (I) the frequency, pattern and severity of cognitive impairments 3–4 months after COVID-19 applying different cut-offs for ‘clinically relevant’ impairments, (II) the associations between objectively measured cognitive impairments, subjectively reported cognitive difficulties, quality of life and work function, and (III) the association between objective cognitive impairments, severity of the acute COVID-19 illness and measures of lung function and oxygen uptake post-COVID.
2. Methods
The IMPACT-COVID study is a prospective study examining all patients admitted acutely for COVID-19 to Bispebjerg Hospital from the start of the epidemic in Denmark in March 2020 until the end of the first wave of cases in June 2020. All admitted patients were referred from a post discharge assessment and offered participation, with a visit 3–4 months and 12 months after discharge.
Here, we present data from patients who underwent cognitive testing from June to November 2020 as part of our cohort study 3–4 months post COVID-19 at the respiratory outpatient clinic at Bispebjerg Hospital, Capital region of Denmark (IMPACT-COVID). Diagnosis of COVID-19 was made by a positive polymerase chain reaction (PCR) test for SARS-CoV-2 from the upper respiratory tract or a positive IgG titre for COVID-19. The study was approved by the regional ethics committee in the Capital Region of Denmark (protocol no. H-20035553) and all patients gave written informed consent.
2.1. Procedures
During hospitalisation for COVID, blood tests were taken for evaluation of immune response including d-dimer, C-Reactive Protein (CRP) and ferritin levels, and High Flow Nasal Canula (HFNC), and total oxygen requirements were recorded. As part of the follow-up evaluation, patients were assessed with physical examination, blood tests, chest high resolution computed tomography scanning (HRCT), high resolution 12-lead electrocardiogram (ECG), and questionnaires including the Chronic Obstructive Pulmonary Disease (COPD Assessment Test; CAT; Celli et al., 2004), Medical Research Council (MRC) dyspnoea assessment (Stenton, 2008), asthma control questionnaire (ASQ; Juniper et al., 1999), Work Productivity and Activity Impairment Questionnaire (WPAI; Reilly et al., 1993) and EQ-5D-5L quality of life questionnaire (EQ5D; Lloyd and Pickard, 2019) and were assessed for objective and subjective cognitive functions.
2.2. Cognition assessments
Objective cognitive functions were assessed with a brief (<20 min) performance-based cognition test battery consisting of the Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D (Jensen et al., 2015; Purdon, 2005) and the Trail Making Test-Part B (TMT-B; Army Battery, 1944). These tests measure verbal learning and memory, working memory, verbal fluency, processing speed, and executive function. In addition, subjective cognitive functions were assessed with the Cognitive Failures Questionnaire (CFQ; Broadbent et al., 1982).
2.3. Statistical analyses
Statistical analyses were carried out using IBM SPSS statistics 25 for windows (IBM Corporation, Armonk, New York). Statistical significance for all analyses was set to an alpha-level of p<0.05 (two-tailed).
Research question (I) regarding the frequency, pattern and severity of cognitive impairments 3–4 months after COVID-19 was investigated with two complementary approaches: (A) by comparing patients’ cognitive performance to their expected performance calculated with the regression based formulas based on their age, sex and years of education (Supplementary Table 1), and (B) by comparing patients’ cognitive performance to that of an age-, and education-matched sample of n = 100 healthy control (HC) participants from our pre-established normative data set (Ott et al., 2021).
Regarding (A), we used regression-based formulas for prediction of each patient's expected SCIP test scores based on their age, sex and years of education. The use of regression-based formulas enable calculation of demographically corrected normative scores that are applicable at an individual level (Duff, 2012). For each patient, this provides an easy and precise estimation of whether their cognition score is similar to or deviates from what is expected of a person with similar demographic characteristics. Specifically, reliable change indexes (RCI) provide standardized scores for the deviation of the observed scores from the predicted scores. The RCIs are calculated as (observed score – predicted score)/SEE, where SEE is the standard error of the estimate for the regression equation (Attix et al., 2009). The implemented regression-based models to calculate RCI are displayed in Supplementary Table 1. Given the lack of consensus on what constitutes clinically relevant cognitive impairments (see Miskowiak et al., 2018; Ott et al., 2021), we applied two different cut-offs for clinical relevance of global cognitive impairments: (a) the previously recommended cut-off defined as performance ≥0.5 standard deviations (SD) under the expected SCIP total score based on patients’ age, sex and education (Ott et al., 2021) and (b) a more conservative cut-off defined as performance ≥1 SD under the expected SCIP total score based on patients’ age, sex and education. Further, selective cognitive impairments were defined as performance ≥1SD under the expected scores on ≥2 individual tests based on their demographic characteristics based on previous recommendations (Miskowiak et al., 2018; Ott et al., 2021).
Regarding (B), we estimated the frequency of clinically relevant cognitively impairments based on the matched HC sample; cut-off for global cognitive impairments was defined as performance ≥1SD under the normative HC mean and – for selective impairments – as ≥1SD under the mean of HC on ≥2 individual tests. Second, the pattern and severity of cognitive impairments was investigated through group comparisons across the individual cognition test scores between patients and the HC group with independent samples t-tests or Mann–Whitney U tests for normally and non-normally distributed data, respectively. Effect sizes for significant differences were estimated with Cohen's d.
Research questions (II) and (III), regarding the relation between objective cognitive impairments and subjective cognitive complaints, quality of life and work function, and between objective cognition and illness variables were analysed with Pearson's correlations or Spearman's rho for normally and non-normally distributed data, respectively. Consistent with our previous approach (Ott et al., 2021), the RCI for patients’ SCIP Total scores was used as a measure of ‘global cognitive impairment’ in the analyses of associations between cognitive impairment and illness variables.
3. Results
3.1. Participant characteristics
Fig. 1 illustrates the recruitment process. Following hospitalisation for COVID-19 at Bispebjerg Hospital, 70% of hospitalised patients (n = 83) were offered a 3–4 months follow-up visit of whom n = 71 (86%) accepted. Of these, n = 12 patients were excluded due to substantial language barriers or disabilities. Of the 59 eligible patients, n = 34 (67%) chose to take part in the study. The remaining patients stated that they had insufficient time and energy to take part. Of the patients who agreed to take part, n = 5 patients were excluded from the cognition assessments because of insufficient fluency in Danish or pre-existing neurological comorbidity. The final sample or the cognition assessments thus included n = 29 patients.
Fig. 1.
Flow-chart for recruitment of patients in post-COVID cognition assessments.
Table 1 displays demographic and clinical characteristics of patients and demographic characteristics of the pre-existing matched healthy control (HC) group (n = 100). Patients were generally healthy prior to their COVID-19 infection with the main comorbidity being asthma (34%). The HC control group drawn from our pre-existing normative database was matched to patients on age, sex and education levels (Table 1).
Table 1.
Demographics and clinical characteristics, quality of life and work function at the four months follow-up assessment after hospitalisation with COVID 19.
| Patients (n = 29) | Healthy controls (n = 100) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 56.2 (10.6) | 56.0 (6.9) | 0.92 |
| Sex, no. females (%) | 12 (41) | 59 (59) | 0.09 |
| Years of education, mean (SD) | 14.3 (3.9) | 14.3 (3.0) | 0.90 |
| BMI (kg/m2) | 29. (5.7) | – | – |
| Work status, no. employed (%) | 21 (72) | – | – |
| Clinical characteristics | |||
| Charlson comorbidity score | 2.9 (2.4) | – | – |
| Asthma, no (%) | 10 (34.5) | – | – |
| MRC score, mean (SD) | 2.2 (0.8) | ||
| CAT score, mean (SD) | 12.9 (6.7) | ||
| ACQ score, mean (SD) | 1.3 (0.8) | ||
| EQ-5D-5L Quality of Life Questionnaire | |||
| Movement | 1.7 (0.9) | – | – |
| Personal care | 1.2 (0.5) | – | – |
| Usual activity | 2.0 (1.2) | – | – |
| Pain | 2.3 (1.1) | – | – |
| Anxiety/depression | 1.7 (1.0) | – | – |
| Work productivity and activity impairment | |||
| Percent work time missed due to* health (absenteeism) | 0.0 [0.0, 41.0] | – | – |
| Percent impairment while working due to health (presenteeism) | 10.0 [0.0, 80.0] | – | – |
| Percent overall work impairment due to health* | 10.0 [0.0, 82.0] | – | – |
| Percent activity impairment due to health* | 20.0 [0.01, 100.0] | – | – |
Data is presented as mean (SD) or number (percentage) for demographics, clinical characteristics and quality of life data. Work Productivity and Activity Impairment data is reported as median [minimum, maximum]. Abbreviations: no, number, BMI, body mass index, SD, standard deviation; EQ5D, EQ-5D-5L quality of life questionnaire. *Data for these variables only available for 13–15 of the 21 employed patients.
3.2. (I) What is the frequency, pattern and severity of patients’ cognitive impairments?
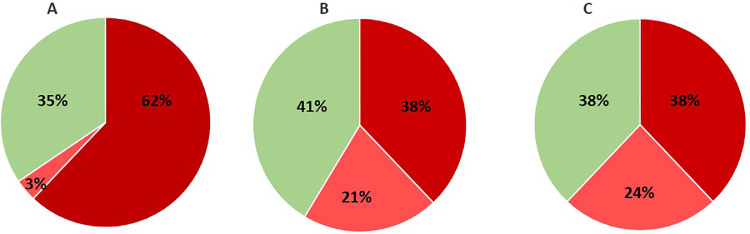
Fig. 2 illustrates the proportion of patients with clinically relevant global and selective cognitive impairments, respectively, based on two applied cut-offs for clinically relevant impairments in comparison with the expected performance based on their age, sex and education levels (A and B) and in comparison with the age-, sex- and education-matched HC group (C). Using the recommended criterion for global impairment defined as SCIP total scores ≥ 0.5 SD below the demographically adjusted predicted scores, 18 patients (62%) were classified as globally cognitively impaired with one additional patient (3%) showing selective cognitive impairment (A); hence, yielding a total of n = 19 (65%) of patients being classified as cognitively impaired. Using the more conservative cut-off for global cognitive impairment, defined as SCIP total scores being ≥1 SD below demographically adjusted norms, yielded n = 11 patients (38%) as globally impaired and another six (21%) with selective impairments; hence, in total n = 17 (59%) of the patient sample being cognitively impaired (B). Finally, the frequency of clinically relevant impairments was estimated based on comparisons with the matched HC sample. Using the cut-off of SCIP total scores ≥1SD below the HC group mean (i.e., cut-off ≤66), n = 11 patients (38%) were identified as globally impaired, and n = 7 patients (24%) as selectively impaired (scores ≥1SD below HC group mean on ≥2 tests); in total, n = 18 patients (62%) showing cognitive impairments; i.e., the same as in (B). Subjective cognitive difficulties in daily life were generally high, with 83% of patients experiencing severe cognitive difficulties (CFQ scores≥43; Table 2 ).
Fig. 2.
Proportion of patients with clinically relevant global or selective cognitive impairments using different approaches for determining the clinically relevance of impairments. (A) Using the recommended cut-off for global impairment defined as scores ≥0.5 below the expected SCIP Total scores and – for selective impairments – scores ≥1 SD below the expected scores on ≥2 individual tests based on patients’ age, sex and education years yielded n = 18 patients 62%) with global impairments (dark red) and n = 1 patient (3%) with selective impairments (light red); i.e., 65% patients being cognitively impaired. (B) With a more conservative cut-off for global impairment defined as SCIP Total scores ≥1 below demographically adjusted norms and – for selective impairments – performance ≥1 SD below the demographically adjusted norms on ≥2 individual tests yielded n = 11 patients (38%) with global impairments (dark red) and n = 6 patients (21%) with selective impairments (light red); i.e., 59% patients being cognitively impaired. (C) Finally, comparisons with age- and education matched healthy controls (n = 100) and use of the cut-off for global and selective impairments as in (B), indicated that n = 11 patients (38%) had global impairments (dark red) and n = 7 (24%) had selective impairments (light red); i.e., 62% showed clinically relevant cognitive impairments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Objective and subjective cognition data from patients and a matched control group as well as the expected scores based on patients age, sex and education.
| Patients (n = 29) | Expected scores based on age, sex, and education | Healthy controls (n = 100) | P-value all patients actual vs. expected | P-value all patient vs. healthy controls | |
|---|---|---|---|---|---|
| SCIP total score, mean (SD) | 67.4 (13.9) | 75.2 (4.6) | 75.0 (9.1) | 0.001 | 0.01 |
| VLT-L, mean (SD) | 19.9 (4.2) | 22.1 (1.2) | 22.1 (3.0) | 0.004 | 0.003 |
| WMT, mean (SD) | 18.2 (4.2) | 19.9 (0.7) | 1.9 (2.5) | 0.002 | 0.04 |
| VFT, mean (SD) | 14.3 (4.7) | 16.1 (1.4) | 16.0 (4.5) | 0.03 | 0.17 |
| VLT-D, mean (SD) | 6.3 (2.8) | 7.0 (0.6) | 7.0 (1.9) | 0.16 | 0.08 |
| PMT, mean (SD) | 9.0 (3.2) | 10.1 (1.2) | 10.1 (2.3) | 0.04 | 0.09 |
| TMT-B, mean (SD) | 116.2 (65.0) | 80.6 (18.7) | – | 0.002 | – |
| CFQ total | 61 (15) | – | – | – | – |
| Proportion with severe complaints, scores ≥43, number (%) | 19 (83) |
Data is presented as mean (SD) or number (percentage). CFQ data was only available for 23 of the 29 patients; SCIP, Screen for Cognitive Impairment in Psychiatry; SD, standard deviation; VLT-L, verbal learning test-learning; WMT, working memory test; VFT, verbal fluency test; VLT-D, verbal learning test-delayed recall; PMT, psychomotor speed test; TMT-B, Trail Making Test B; CFQ, Cognitive Failures Questionnaire.
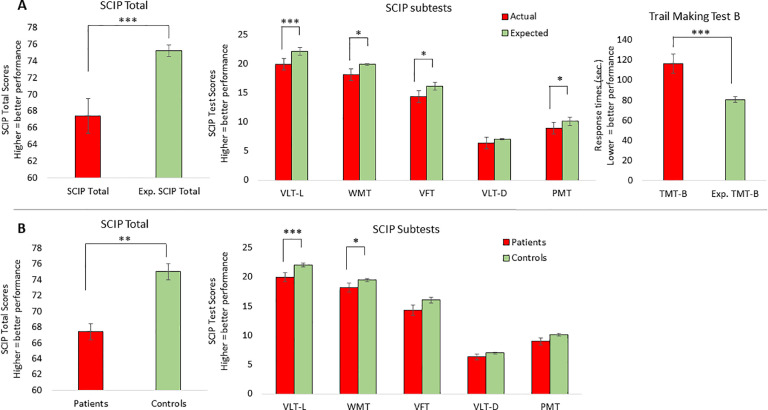
Fig. 3 illustrates the patients’ actual SCIP and TMT-B scores in comparison with (A) their expected demographically adjusted normative scores on the SCIP Total and five subtests based on the regression-based formula (Supplementary Table 1) and the Danish age- and education adjusted TMT-B norms (Joergensen, 2012) and (B) the performance of the matched HC sample on the SCIP Total and the five SCIP subtests (TMT-B scores had not been collected in our HC sample). Comparison between patients actual and expected scores revealed that on average, patients displayed pronounced global cognitive impairment on the SCIP with a large effect size (SCIP Total: t=−3.60, df=28, p = 0.001, Cohen's d=−0.85) and verbal learning and executive function impairments with large effect sizes for (VLT-l: t=−3.12, df=28, p = 0.004, Cohen's d=−0.81; TMT-B: t=−3.46, df=28, p = 0.002, Cohen's d=−0.81). Moderate impairments were observed within working memory, verbal fluency and psychomotor speed (WMT: t=−2.33, df=28, p = 0.03, Cohen's d=−0.70; VFT: t=−2.32, df=28, p = 0.03, Cohen's d=−0.59; PMT: t=−2.15, df=28, p = 0.04, Cohen's d=−0.51). In contrast, patients were not significantly impaired on the test of delayed verbal memory (VLT-D: p = 0.16).
Fig. 3.
Pattern of cognitive impairments in patients (n = 29) four months after COVID-19 in comparison (A) with normative scores adjusted for age, sex and education estimated with regression models and (B) with an age-, sex- and education-matched healthy control group (n = 100). Most pronounced impairments were seen in verbal learning (VLT-L) and executive function (TMT-B). Graphs represent the mean and error bars the standard error of the mean. * p<0.05; ** p<0.01; *** p<0.001.
Similar findings were obtained in analyses comparing patients with the matched HC group; Patients displayed global cognitive impairments with a moderate to large effect size (SCIP total: t=−2.78, df=35.3, p = 0.01; Cohen's d=−0.70), and moderate impairments in verbal learning and working memory (VLT-l: t=−3.06, df=127, p = 0.003, Cohen's d=−0.62; WMT: t=−2.11, df=34.0, p = 0.04, Cohen's d=−0.44). Again, patients’ delayed memory performance was unimpaired (VLT-D: p = 0.17), whereas there was only a non-significant trend toward verbal fluency and psychomotor speed impairments in patients compared with HC (VFT: p = 0.08; PMT: p = 0.09).
3.3. (II) Are objective cognitive impairments associated with subjective cognitive complaints, quality of life and work function?
Subjective cognitive complaints (CFQ Total scores) correlated significantly with objectively measured global cognitive impairments (Pearson's correlation; SCIP Total deviation from expected scores; r=−0.62, p = 0.002) and executive dysfunction (Pearson's correlation; TMT-B deviation from expected scores; r=−0.45, p = 0.03).
Regarding quality of life measurements, more global cognitive impairment and executive dysfunction both correlated with greater difficulty within EQ5D ‘usual activity’ and ‘anxiety and depression’ (Pearson's correlations between SCIP and: usual activity: r=−0.47, p = 0.03; anxiety and depression: r=−0.64, p = 0.001, and between TMT-B and: usual activity: r = 0.49, p = 0.02; anxiety and depression: r = 0.51, p = 0.01). More executive dysfunction also correlated with greater difficulties in the domain of ‘personal care’ (Pearson's correlation; r = 0.46, 0.03) and more absenteeism (Spearman's rho: r=−0.62, p = 0.01).
3.4. (III) Is the severity of cognitive impairments associated with illness severity variables?
Investigation of the relation between cognitive status and severity of acute COVID-19 illness revealed no associations between global cognitive impairments and length of hospitalisation, total oxygen requirements during hospitalisation, time since discharge or the following acute illness severity markers: lymphocytes, CRP, ferritin, or need for high flow nasal oxygen (HFNC) (p-levels>0.56). There was also no significant correlation between maximum d-dimer levels and global cognitive impairment (p-levels≥0.10). However, higher d-dimer levels did correlate moderately with poorer delayed verbal recall and psychomotor speed, respectively (Pearson's correlations: VLT-D: r=−0.50, p = 0.03; PMT: r=−0.64, p = 0.004).
With respect to pre-existing comorbidity and other potential sources of cognitive impairments, cognitive impairments were not significantly associated with co-existing asthma, number of pre-existing comorbidities according to the Charlson comorbidity index or high body mass index (BMI) (p-values≥0.09). Only one patient had required mechanical ventilation, rendering the effects of mechanical ventilation on cognition impossible to assess in this study.
Regarding associations between cognitive and pulmonary dysfunction, more global cognitive impairment and executive dysfunction both correlated with severity of respiratory symptoms according to the ACQ (Spearman's rho: SCIP Total deviation: r=−0.56, p = 0.009; TMT-B deviation: r = 0.44, p = 0.02) and CAT (Spearman's rho: SCIP total deviation, r=−0.39, p = 0.050; TMT-B: r = 0.64, p<0.001). More global cognitive impairment also correlated with poorer pulmonary function, as reflected by lower forced expiratory volume in one second (FEV1; Spearman's rho, r = 0.37, p = 0.049). In contrast, there was no correlation between global cognitive impairments and MRC dyspnoea, CT or DLCO scores (p-levels≥0.18).
4. Discussion
In this study at the outpatient clinic at the Department of Respiratory Medicine, Copenhagen University Hospital, we examined objective performance-based and subjectively rated cognitive functions in 29 COVID-19 patients 3–4 months after their hospital discharge. The percentage of patients with clinically significant objective cognitive impairment ranged from 59% to 65%, depending on the cut-off scores applied for determining clinically relevance of the impairment, with verbal learning and executive functions being most affected. More than 80% of patients reported experiencing severe cognitive difficulties in daily life. Greater objective cognitive impairments were associated with more subjective cognitive difficulties, absenteeism and poorer quality of life. Poorer pulmonary function and more respiratory symptoms after recovery were associated with more cognitive impairments. Among acute illness severity markers, higher maximum d-dimer levels correlated with poorer verbal recall and psychomotor speed. In contrast, cognitive performance was not associated with length of hospitalisation, oxygen requirements, CT scores, comorbidity or inflammation.
Given the lack of consensus what constitutes clinically relevant cognitive impairment, we applied two distinct cut-offs –one that was previously recommended for patients with psychiatric disorders (see Miskowiak et al., 2018; Ott et al., 2021), and a more conservative cut-off consistent with other studies (Gualtieri and Morgan, 2008; Jensen et al., 2015; Ott et al., 2015). We also compared patients’ cognitive performance to that of a healthy control group matched for age, sex and education years to examine the validity of our newly developed regression-based formulas for estimating patients’ expected cognitive performance adjusted for their age, sex and educational levels (Ott et al., 2021). These different cut-offs and complementary approaches revealed highly consistent results, categorising 59–65% of patients with clinically relevant impairments with large effect sizes for verbal learning and executive functions (Figs. 2 and 3). Our observation of broad cognitive impairments across verbal learning, executive function and working memory is consistent with the findings of a largescale internet-based study of >84,000 people that showed substantial cognitive impairments across several cognitive domains in both hospitalised and non-hospitalised patients (Hampshire et al., 2020). Impaired executive functions were also observed in 33% of COVID-19 patients after hospital discharge (Helms et al., 2020) and in patients with other acute respiratory distress syndrome diseases before the COVID-19 pandemic (Sasannejad et al., 2019). In contrast, only selective sustained attention difficulties were observed in a study in 29 patients who had recovered from COVID-19 without hospitalisation (Zhou et al., 2020), while only selective executive dysfunction was found in 58 patients 2–3 months after hospital discharge (Raman et al., 2021) despite structural brain abnormalities. This discrepancy may be explained by suboptimal cognitive test batteries in the two latter studies that either tapped into only sustained attention and executive function (Zhou et al., 2020) or were designed for dementia evaluation and thus likely associated with ceiling effects in younger, non-demented populations (Raman et al., 2021).
While it is unclear how long the cognitive impairments after COVID-19 prevail, hospitalised patients with other respiratory diseases have been found to display impairments for several years (Hopkins et al., 2005; Sasannejad et al., 2019). We found no association between the length of time since hospital discharge and the severity of cognitive impairments in this cross-sectional report but our planned follow-up assessments again 12 months after hospital discharge will provide better insight into whether impairments resolve with time. Nevertheless, the observed cognitive impairment in 59–65% of our patient cohort four months after hospital discharge and its association with poorer quality of life (including more depression and anxiety) and impaired work functioning point to a pressing need for systematic cognition screening and targeted treatments for patients with persistent cognitive impairments after COVID-19. Based on these observations, we now offer patients who have been hospitalised with COVID-19 at Bispebjerg Hospital, Copenhagen, cognition screening as part of their four months follow-up assessment at the hospital's multi-disciplinary long-COVID outpatient clinic. It is likely that the observed association between cognitive impairments and anxiety and depression was bi-directional: more cognitive impairments may create more anxiety and depression due to difficulties with overcoming cognitive challenges in daily life; conversely, more anxiety and depression symptoms could also impair cognitive test performance. Attention should therefore be given to both cognitive and mood symptoms after severe COVID-19. Specifically, treatments that improve cognitive functions may also aid patients’ stress resilience and, thereby, their mental health outcomes after COVID-19 hospitalisation (Vinkers et al., 2020). One of the most promising treatments is with the multifunctional hormone erythropoietin (EPO), which plays a key role in neuroprotection and neuroplasticity and has been found by our and other groups to improve cognitive functions across a range of neuropsychiatric diseases (Ehrenreich et al., 2020). Future studies are thus warranted to investigate the potential of EPO to reverse cognitive impairments after COVID-19.
In our patient cohort, persistent pulmonary dysfunction was observed in most patients (Johnsen et al., in review). The relation between more cognitive impairments and poorer pulmonary function suggests that reduced oxygen delivery to the brain may play a role in patients’ cognitive impairments. In keeping with this interpretation, poorer verbal memory and lower psychomotor speed correlated with higher d-dimer levels – a marker of thrombosis or pulmonary coagulation disorder, which may suggest vascular consequences cerebrally, perhaps with hypoxia of the brain or direct damage from micro-embolisms. This would explain the pronounced impairments in verbal learning and memory, which depend on the integrity of the hippocampus, a brain region highly susceptible to hypoxia (Hota et al., 2007). Importantly, these putative pathogenic processes may not be specific to COVID-19; hypoxia has been highlighted as a common cause of cognitive decline in acute respiratory distress syndrome that is associated with cerebral atrophy (Hopkins et al., 2006) and degree of verbal memory and executive function impairments (Hopkins et al., 2005). In contrast, the lack of associations between cognitive impairments and inflammation markers indicate that immune response and associated cerebral microglia activation may not play a strong role in the cognitive sequelae of COVID-19.
A key question is that of the generalisability of our findings given potential biased sampling. As shown in Fig. 1, one-third of patients declined to take part in our study at their follow-up assessment. It is possible that these patients declined because they experienced no lingering symptoms consistent with evidence that 25% of hospitalised patients do not experience long-COVID (1). Consequently, our findings may primarily be generalised to more severely affected patients who experience long-COVID and should be interpreted in the context of potentially confounding factors such as hospitalization, isolation and medications. If, we hypothetically take account of these 25% and assuming that these patients had no subjective or objective cognitive impairments, the percentage of patients with severe subjective cognitive difficulties would fall to about 60%, while the percentage with clinically significant objective cognitive impairments would fall to 35–40%; numbers that would still be clinically significant. Further, it is notable that our patient sample had little pre-existing somatic comorbid conditions and that cognitive impairments were not associated with comorbidity. Consistent with this, a largescale internet-based cognition study found that the cognitive impairments after COVID-19 prevailed in analyses adjusted for pre-existing comorbid conditions (Hampshire et al., 2020).
A strength of the study was its multi-disciplinary approach, which enabled comprehensive investigation of physical and cognitive consequences of illness and their relation. A limitation, however, was the modest sample size which may have masked any additional significant findings (type II error). Another limitation was the absence of a control group of patients who had been hospitalised with another respiratory disease, which precluded inferences regarding the specificity of patients’ cognitive impairments to COVID-19 illness. Further, the cross-sectional design was a limitation since it is unclear whether the cognitive impairments existed prior to COVID-19 or were a consequence of illness. However, our comparison between patients’ performance and (i) individually calculated expected performance based on their age, sex and education and (ii) a matched HC sample provided highly consistent estimations of the proportion with clinically relevant impairments as well as the profile of these impairments. Finally, the SCIP is a brief cognition screener, originally developed and validated for patients with psychiatric disorders (Ott et al., 2021) and cannot replace a comprehensive neuropsychological examination. In the interest of brevity, the SCIP also does not assess executive functions. Executive function tests (like the TMT-B) must thus be co-administered for insights into this cognitive domain.
In conclusion, we demonstrate in our sample of 29 patients that 59–65% suffer from clinically relevant cognitive impairments 3–4 months after hospitalisation with COVID-19, which have large effect sizes for global cognition, verbal learning and executive functions. Cognitive impairments were associated with the degree of long-term pulmonary dysfunction and respiratory symptoms and with d-dimer levels during acute illness, suggesting a potential link to restricted oxygen delivery to the brain. Our findings are consistent with emerging evidence for high prevalence of cognitive consequences of COVID-19 and associated functional impairments. Based on these findings, it is imperative that future studies investigate the long-term consequences of COVID-19 for cognition, vocational functioning and quality of life. There is also a pressing need for investigation of potential pro-cognitive treatments, such as EPO, for the substantial proportion of patients who suffer from persistent cognitive and functional impairments after COVID-19 illness.
Role of the funding source
Department of Pulmonology Medicine and Respiratory Research Unit, Bispebjerg University Hospital, provided financial support for the study. The funder had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Contributors
KWM and CMP defined the aim and hypotheses of this report. SJ, SS, SN, KK and TL were involved in conducting the study and assessing the patients under supervision of CMP. KWM conducted the statistical analyses and wrote the first draft. All authors contributed to and approved the final manuscript.
Declaration of Competing Interest
The authors report no conflicts of interest in relation to the current manuscript. Outside of the present work, KWM reports having received consultancy fees from Janssen-Cilag and Lundbeck; JR reports having received consultancy fees from Novo Nordisk, Boehringer-Ingelheim and Astra-Zeneca; CP reports having received consultancy honararia and unrestricted grants from Astra Zeneca, Novartis, Sanofi, GSK, TEVA, ALK, Chiesi and Pharmaxis in the past three years; TL, SJ, SLN, KK and SMS report no conflicts of interest outside of the present work.
Acknowledgements
The authors thank the Department of Pulmonology Medicine and Respiratory Research Unit, Bispebjerg University Hospital, for the financial support for the study. KWM would like to thank the Lundbeck Foundation for her five-year Lundbeck Foundation Fellowship (grant no. R215-2015-4121). We thank miss Beata Trawińska for her work with creating Figure 1.
References
- Achar A., Ghosh C. COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells. 2020;9(11):2360. doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attix D.K., Story T.J., Chelune G.J., Ball J.D., Stutts M.L., Hart R.P., Barth J.T. The prediction of change: normative neuropsychological trajectories. Clin. Neuropsychol. 2009;23:21–38. doi: 10.1080/13854040801945078. [DOI] [PubMed] [Google Scholar]
- Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., Glaser A., Elsayegh D. COVID-19 presenting as stroke. Brain Behav. Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army Individual Test Battery . War Department, Adjutant General's Office; Washington, DC: 1944. Manual of Directions and Scoring. [Google Scholar]
- Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Celli B.R., MacNee W., Force A.E.T. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur. Respir. J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. The mystery of the pandemic's 'happy hypoxia. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dube M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92 doi: 10.1128/JVI.00404-18. e00404-00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch. Clin. Neuropsychol. 2012;27:248–261. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H.E., Weissenborn K., Begemann M., Busch M., Vieta E., Miskowiak K. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol. Med. 2020;16(1):58. doi: 10.1186/s10020-020-00186-y. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri C.T., Morgan D.W. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J. Clin. Psychiatry. 2008;69:1122–1130. doi: 10.4088/jcp.v69n0712. [DOI] [PubMed] [Google Scholar]
- Hampshire, A., Trender, W., Chamberlain, S.R., Jolly, A., Grant, J.E., Patrick, F., Miazibuko, N., Williams, C., Barnby, J.M., Hellyer, P., Mehta, M.A., 2020. Cognitive Deficits in People Who Have Recovered From COVID-19 Relative to Controls: An N=84,285 Online Study. Preprints.org. [DOI] [PMC free article] [PubMed]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.O., Gale S.D., Weaver L.K. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20:263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- Hopkins R.O., Weaver L.K., Collingridge D., Parkinson R.B., Chan K.J., Orme J.F., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- Hota S.K., Barhwal K., Singh S.B., Ilavazhagan G. Differential temporal response of hippocampus, cortex and cerebellum to hypobaric hypoxia: a biochemical approach. Neurochem. Int. 2007;51:384–390. doi: 10.1016/j.neuint.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Støttrup M.M., Nayberg E., Knorr U., Ullum H., Purdon S.E., Kessing L.V., Miskowiak K.W. Optimising screening for cognitive dysfunction in bipolar disorder: validation and evaluation of objective and subjective tools. J. Affect. Disord. 2015;187:10–19. doi: 10.1016/j.jad.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Joergensen K. first ed. Dansk Psykologisk Forlag; Viborg, Denmark: 2012. Danske Normer Til Neuropsykologiske Tests. [Google Scholar]
- Juniper E.F., O'Byrne P.M., Guyatt G.H., Ferrie P.J., King D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- Kremer S., Lersy F., de Seze J., Ferre J.C., Maamar A., Carsin-Nicol B., Collange O., Bonneville F., Adam G., Martin-Blondel G., Rafiq M., Geeraerts T., Delamarre L., Grand S., Krainik A., Caillard S., Constans J.M., Metanbou S., Heintz A., Helms J., Schenck M., Lefebvre N., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Lacalm A., Oesterle H., Bolognini F., Messie J., Hmeydia G., Benzakoun J., Oppenheim C., Bapst B., Megdiche I., Henry Feugeas M.C., Khalil A., Gaudemer A., Jager L., Nesser P., Talla Mba Y., Hemmert C., Feuerstein P., Sebag N., Carre S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Comby P.O., Ricolfi F., Thouant P., Desal H., Boulouis G., Berge J., Kazemi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Zorn P.E., Matthieu M., Baloglu S., Ardellier F.D., Willaume T., Brisset J.C., Boulay C., Mutschler V., Hansmann Y., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., David J.S., Meyer N., Anheim M., Cotton F. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, N.J.S.C., 2020. COVID_19 "Long Hauler" Symptoms Survey Report.
- Lersy F., Benotmane I., Helms J., Collange O., Schenck M., Brisset J.C., Chammas A., Willaume T., Lefebvre N., Solis M., Hansmann Y., Fabacher T., Caillard S., Mertes P.M., Pottecher J., Schneider F., Meziani F., Fafi-Kremer S., Kremer S. Cerebrospinal fluid features in COVID-19 patients with neurologic manifestations: correlation with brain MRI findings in 58 patients. J. Infect. Dis. 2021;223(4):600–609. doi: 10.1093/infdis/jiaa745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A., Pickard A.S. The EQ-5D and the EuroQol group. Value Health. 2019;22:21–22. doi: 10.1016/j.jval.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Lucchese G., Floel A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;2020(6):683–690. doi: 10.1001/jamaneurol.2020.1127. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Burdick K.E., Martinez-Aran A., Bonnin C.M., Bowie C.R., Carvalho A.F., Gallagher P., Lafer B., Lopez-Jaramillo C., Sumiyoshi T., McIntyre R.S., Schaffer A., Porter R.J., Purdon S., Torres I.J., Yatham L.N., Young A.H., Kessing L.V., Vieta E. Assessing and addressing cognitive impairment in bipolar disorder: the International Society for Bipolar Disorders Targeting Cognition Task Force recommendations for clinicians. Bipolar Disord. 2018;20:184–194. doi: 10.1111/bdi.12595. [DOI] [PubMed] [Google Scholar]
- Miskowiak K.W., Petersen J.Z., Ott C.V., Knorr U., Kessing L.V., Gallagher P., Robinson L. Predictors of the discrepancy between objective and subjective cognition in bipolar disorder: a novel methodology. Acta Psychiatr. Scand. 2016;134:511–521. doi: 10.1111/acps.12649. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Neurologic complications of coronavirus infections. Neurology. 2020;94(19):809–810. doi: 10.1212/WNL.0000000000009455. [DOI] [PubMed] [Google Scholar]
- Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020;27(9):1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C.V., Bjertrup A.J., Jensen J.H., Ullum H., Sjaelland R., Purdon S.E., Vieta E., Kessing L.V., Miskowiak K.W. Screening for cognitive dysfunction in unipolar depression: validation and evaluation of objective and subjective tools. J. Affect. Disord. 2015;190:607–615. doi: 10.1016/j.jad.2015.10.059. [DOI] [PubMed] [Google Scholar]
- Ott C.V., Knorr U., Jespersen A., Obenhausen K., Roen I., Purdon S.E., Kessing L.V., Miskowiak K.W. Norms for the screen for cognitive impairment in psychiatry and cognitive trajectories in bipolar disorder. J. Affect. Disord. 2021;281:33–40. doi: 10.1016/j.jad.2020.11.119. [DOI] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon S. Edmonton; Alberta, Canada: 2005. The Screen for Cognitive Impairment in Psychiatry: Administration and Psychometric Properties. [Google Scholar]
- Raman R., Rajalakshmi R., Surya J., Ramakrishnan R., Sivaprasad S., Conroy D., Thethi J.P., Mohan V., Netuveli G. Impact on health and provision of healthcare services during the COVID-19 lockdown in India: a multicentre cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-043590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M.C., Zbrozek A.S., Dukes E.M. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- Sasannejad C., Ely E.W., Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit. Care. 2019;23:352. doi: 10.1186/s13054-019-2626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenton C. Occupational Medicine; Oxford, England: 2008. The MRC Breathlessness Scale. [DOI] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-Barre syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds J., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A.K., J. N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers C.H., van Amelsvoort T., Bisson J.I., Branchi I., Cryan J.F., Domschke K., Howes O.D., Manchia M., Pinto L., de Quervain D., Schmidt M.V., van der Wee N.J.A. Stress resilience during the coronavirus pandemic. Eur. Neuropsychopharmacol. 2020;35:12–16. doi: 10.1016/j.euroneuro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2021;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]