Abstract
As controversy exists about the efficacy of substance P (SP) in treating ulcerative colitis (UC) with no previous study highlighting the impact of SP on mitochondrial dysfunction in this diseased condition, it became logical to perform the present study. C57BL/6 J mice were administered with DSS @ 3.5%/gm body weight for 3 cycles of 5 days each followed by i.v. dose of SP @ 5nmole per kg for consecutive 7 days. Histopathological features were noticed in the affected colon along with colonic mitochondrial dysfunction, alterations in mitochondrial stress variables and enhanced colonic cell death. Interestingly, SP failed to reverse colitic features and proved ineffective in inhibiting mitochondrial dysfunction. Unexpectedly SP alone seemed to impart detrimental effects on some of the mitochondrial functions, enhanced lipid peroxidation and increased staining intensities for caspases 3 and 9 in the normal colon. To substantiate in vivo findings and to assess free radical scavenging property of SP, Caco-2 cells were exposed to DSS with or without SP in the presence and absence of specific free radical scavengers and antioxidants. Interestingly, in vitro treatment with SP failed to restore mitochondrial functions and its efficacy proved below par compared to SOD and DMSO indicating involvement of O2•− and •OH in the progression of UC. Besides, catalase, L-NAME and MEG proved ineffective indicating non-involvement of H2O2, NO and ONOO− in UC. Thus, SP may not be a potent anti-colitogenic agent targeting colonic mitochondrial dysfunction for maintenance of colon epithelial tract as it lacks free radical scavenging property.
Keywords: Apoptosis, Colon epithelial cell, Free radical scavenger, Oxidative stress, Intestinal neuropeptide
Introduction
In the last couple of decades, ulcerative colitis (UC) has become one of the most incident global diseases triggered by multifactorial contributing factors involving the complex interactions between environment, genetic predisposition, microbial dysbiosis and immune deregulation (Love et al. 1992; Sands 2000; Podolsky 2002; Zhang and Li 2014). Considering the limitations of the conventional treatment procedures, there is an ongoing search for safer and effective therapeutics for treating patients suffering from UC. This search for better therapeutics has led to the exploration of the intestinal neuropeptides as an effective alternative for their unique physiological roles in the human gastrointestinal tract. One such neuropeptide is substance P (SP), belonging to the tachykinin family comprised of 11 amino acids, has gained prominence for its role in treating UC, though controversy exists regarding its therapeutic efficacy with reports existing in favour and against the use of this intestinal neuropeptide. On one hand, there are reports that claim administration of SP can ameliorate intestinal damage in UC by preserving tissue barrier function and epithelial integrity and promoting tissue proliferation (Hwang et al. 2017; Koon et al. 2007). Conversely, few reports that are available have shown incorporation of exogenous SP receptor antagonists especially NK-1R antagonists have attenuated oxidative stress and inflammation in colonic tissue and in UC reducing the disease activity index and colonic tissue damage score (Engel et al. 2011; Jonsan et al. 2005; Stucchi et al. 2000).
In this context, colonic mitochondrial study in this diseased condition has become all the more important as mitochondria in the intestinal epithelial cells serve as targets as well as facilitators of pathways that are involved in inflammation as similarly observed in UC (Jackson and Theiss 2019). Majority of the cellular processes involving tissue barrier function and mucosal healing through cell proliferation are energy-dependent processes and this energy dependency makes the role of mitochondrial function in colonocytes under this UC condition even more important (Bar et al. 2013; Santhanam et al. 2007, 2012; Sifroni et al. 2010). Presently, it is an established fact that there occurs colonic mitochondrial stress and variations in mitochondrial functions in UC patients (Santhanam et al. 2007, 2012; Sifroni et al. 2010). However, there is no detailed report on the effect of SP on functionality and integrity of mitochondria in colonocytes in this diseased condition and this makes our current study even more logical.
Thus, the current work was designed to clarify the direct impact of exogenous SP on mitochondrial function in colonic tissue in an in vivo setting wherein mice were administered with dextran sulphate sodium (DSS) to induce colitis that mimics human UC. To further validate our in vivo findings and to evaluate the possible mode of action of SP, we performed in vitro mitochondrial functional assays involving Caco-2 cells as these cells are known to express NK-1 receptors and thus can justify the action of SP.
Materials and methods
Chemicals
Substance P (SP), and Sephadex G-25 were purchased from Sigma Aldrich, India. DTNB (Ellman’s Reagent), MTT [3-(4, 5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] and thiobarbituric acid (TBA) were procured from Hi-media and other chemicals including dextran sulfate sodium (DSS, molecular weight 30–50 kDa), cytochrome c, nicotinamide adenine dinucleotide hydrogen (NADH), D-mannitol, sodium succinate, bovine serum albumin (BSA), pyridine, potassium ferricyanide, EGTA [Ethylene glycol-o-bis- (2-amino ethyl) N,N,N,N-tetra acetic acid], n-butanol and HEPES buffer were procured from Sisco Research Laboratories (SRL), India.
Animal
C57BL/6 wild-type mice in the age bracket of 8–10 weeks were supplied by Adita Biosys Pvt. Ltd., Tumkur, India. Mice were kept in well ventilated pathogen-free condition with 12-h light and dark illumination. All animals were provided laboratory chow and water and were made to adapt to the environment for 7–8 days prior to the start of actual experiments. The experimental protocols were approved as per international norms by the institutional animal ethical care committee for animal use of Dayananda Sagar University, Bengaluru, Karnataka (Registration No. 606/02/C/CPCSEA).
Administration of DSS
Before the onset of the experiment, all animals were weighed and grouped. Each animal group comprised of six mice (3 male + 3 female). Group I was considered as Control, group II as SP alone (+ SP), group III as DSS alone (+ DSS) and group IV as + DSS + SP. Induction of chronic colitis in DSS alone and in + DSS + SP groups was carried out by the administration of 3.5% of DSS (molecular weight 30–50 kDa) per gram body weight in drinking water for 3 cycles of 5 days each (Yoshihara et al. 2006). To be precise each cycle comprised of 5 days of continuous DSS treatment with a break of 5 days of drinking water in between each DSS cycle. Animals in the control group received drinking water throughout the experimental duration. The animal body weights were measured on a daily basis.
Administration of SP
Following DSS exposure, mice in groups II and IV were injected intravenously (i.v.) with SP @ 5 nM per kg dissolved in 0.9% saline once a day for 7 consecutive days (Dae et al. 2017).
Tissue collection
Animals were sedated using isoflurane and euthanized by means of cervical dislocation. The whole colon from the cecum to the rectum was collected which was subjected to washing using ice-cold phosphate-buffered saline (PBS) to remove faecal contents and other dirt. The last 2 cm of the distal colon initiating from the anal verge was collected. About 1 cm of the colon was placed in 10% formalin for histological analysis and the rest 1 cm was collected in chilled phosphate buffer (50 mM, pH 7.4) for mitochondrial isolation.
Histological analysis
Formalin (10%) fixed colon tissues were embedded in paraffin and were sectioned to 4–5 µm. These sectioned tissues were subjected to staining procedure using haematoxylin and eosin (H&E) and scored for colitis assessment.
Evaluation of colitis score
Colitis assessment was carried out in H&E stained tissue sectioned slides by scoring the following parameters within a range of 0–3 (0–3 signifies; 0 = normal morphology and 3 = maximum damage): damaged tissue, altered crypt length and crypt architecture, depletion of goblet cell and increase in lamina propria counts.
Cell culture
Caco-2 cells (NCCS, Pune, India) were cultured in Dulbecco’s MEM medium containing 1.5 mM L-glutamine, supplemented with 1 mM sodium pyruvate, 1.5 g/L of sodium bicarbonate, 1% penicillin–streptomycin solution and 20% fetal bovine serum (FBS). The medium was replenished every alternate day. To perform cell line experiments, a humidified environment with 5% CO2 was maintained wherein cells from passages were used. For further experimentation including the evaluation of the effect of DSS on Caco-2 cells, 24-well plates (Costar, Corning, NY, USA) were used. These 24-well plates were seeded at a density of 1 × 104 viable cells/cm2 and to achieve 90–100% confluence, monolayers of Caco-2 cells were differentiated for further 14 days.
In vitro SP dosage selection via cell viability assays
Initial rounds of experiments were carried out for SP dosage selection by performing trypan blue and lactate dehydrogenase (LDH) assays. To standardise the optimum dosage of SP, initially different concentrations of SP (1,10, 20, 50 and 100 nM) were used for 24 h in DMEM lacking fetal bovine serum (FBS) and control was maintained in DMEM containing 10% FBS. To evaluate the effect of DSS alone, a separate group was included wherein cells were exposed to 5% concentration of DSS devoid of SP treatment. This particular dosage of DSS was found to be safe and effective as observed in our previous round of experiment. Further, to assess the combined influence of DSS and SP on cell viability, another separate group was also included wherein the cells were exposed to 24 h of 5% DSS and 10 nM of SP treatment at 37 °C. Following the treatment periods, freshly prepared phosphate-buffered saline (PBS) was utilized to prepare cell suspensions for cell viability assays.
Trypan blue activity: Trypan blue solution (0.4% PBS) was mixed equally with an aliquot of cell suspension in PBS. The viability of cells was indicated after counting the viable cells in a Neubauer chamber and expressed as the total percentage of positive cells for trypan blue within unstained and stained cells. (Yermolaieva et al. 2004).
LDH assay: In this NADH-based spectrophotometric assay, the total LDH released into the medium was measured, i.e., intracellular and extracellular LDH levels. (Kitazawa et al. 2004).
Treatment paradigm for in vitro cell line
Each monolayer of Caco-2 cells has been incubated with 5% DSS for 24 h at 37 °C in different combinations. One such combination involved SP (10 nM) and the other combinations were with O2•− scavenger SOD (Khan et al. 2005) or •OH scavenger DMSO (Khan et al. 2005) or H2O2 scavenger catalase (Khan et al. 2005) or •NO scavenger L-NAME (Rehman et al. 1997) or nitric oxide synthase (NOS) inhibitor and ONOO − scavenger MEG (Cuzzocrea et al. 1998; Szabo et al. 1997) at concentrations of 20 mM, 20 mM, 50 μg/ml, 100 μM and 0.5 mM respectively. The above-mentioned concentrations of free radical scavengers and antioxidants have been primarily selected based on previous work (Hokari et al. 2005; Kitazawa et al. 2004; Koon et al. 2007; Maiti et al. 2017; Maiti et al. 2018; Nielsen et al. 2016; Yermolaieva et al. 2004). Mitochondria were isolated from control and treated cells after incubation for evaluation of mitochondrial functional activities.
Preparation of mitochondrial isolates
Murine colon: Mitochondria from the colon epithelial cells were isolated using the method adopted by Maiti et al. (2015). Post isolation, mitochondria have been collected in 50 mM potassium phosphate buffer, pH 7.4 for evaluation of mitochondrial complex I and IV activities, and for measurement of MTT assay, the isolated mitochondria have been dissolved in isotonic buffer (145 mM KCl, 50 mM sucrose, 1 mM EGTA, 1 mM magnesium chloride, 10 mM phosphate buffer, pH 7.4). For assessing the oxidative stress parameters, isolated mitochondria were resuspended in 50 mM phosphate buffer, pH 7.4. Mitochondrial samples have been stored in several aliquots, kept frozen at – 20 ℃ and used within a couple of days.
Caco-2 cells: Mitochondrial isolates were prepared from cultured cells with minor modifications as outlined in Cui et al. (1997). Control and treated groups of cells were initially washed in PBS containing 2.7 mM KCl, 4 mM Na2HPO4, 1.5 mM KH2PO4 and 137 mM NaCl. The cell pellet obtained was resuspended in ice-cold cell-lysis buffer composed of 10 mM Tris, 1.5 mM MgCl2, 10 mM NaCl and 0.1 mM PMSF (phenyl methyl sulfonyl fluoride) which was stored on ice for 10 min and further homogenization was performed through the addition of one-third quantity of mitochondrial stabilization buffer comprising of 35 mM EDTA, 1 M sucrose and 50 mM Tris. This was followed by centrifugation of homogenate mixture at 2000 × g for 5 min at 4 °C, the supernatant was accumulated and to obtain the mitochondrial isolate, further centrifugation at 12,000 × g for 10 min at 4 °C was performed. Finally, mitochondrial isolate was resuspended in 50 mM phosphate buffer, pH 7.4 after washing in isotonic buffer B (5 mM NaCl, 1 mM EGTA, 145 mM KCl, 1 mM MgCl2, 50 mM sucrose, 10 mM phosphate, pH 7.4).
Citrate synthase assay to evaluate mitochondrial integrity
The free thiol groups of coenzyme A from acetyl CoA reacted with DTNB in this assay. Latency values of citrate synthase of 10 and above were considered as good indicators of intact mitochondrial membrane integrity.
Mitochondrial respiratory complex assays
The mitochondria isolated from murine colon and Caco-2 cells were utilized for evaluation of respiratory enzyme complexes of mitochondria as detailed below.
Evaluation of NADH-ferricyanide reductase activity (complex I) was performed through the adaption from Hatefi (1978). In the ratio of 1:1:100, Triton X-100 (0.1% v/v), potassium ferricyanide (0.6 mM, acting as electron acceptor) and 50 mM phosphate buffer, pH 7.4, were added. To this assay mixture, NADH 0.17 mM (electron donor was added and blank readings were recorded and then mitochondrial sample (10–30 µg) was added to initiate the reaction. The fall in absorbance at 340 nm for two min at every 20 s interval was noted to evaluate the oxidation rate of NADH. The complex I activity was expressed in nmoles of NADH oxidized/mg protein/min.
Evaluation of succinate: ubiquinone oxidoreductase activity (complexes II–III) was performed by evaluating MTT reduction as mentioned in Berridge and Tan (1993). Difference in absorbance between 550 and 620 nm was observed and analyzed for the final demonstration of complex II–III activity.
Assessment of cytochrome oxidase activity (complex IV) was initiated by the preparation of cytochrome c (reduced) using commercially available ferricytochrome c (oxidized, SRL, India). Reduced cytochrome c (50 µM) and potassium phosphate buffer (10 mM), pH 7.4 were added in blank and sample cuvettes. In the blank cuvette, ferrocytochrome c (reduced) was oxidized by the addition of 10 µl of potassium ferricyanide (1 mM). Upon addition of 10 µl of mitochondrial sample (10–30 µg), the reaction was initiated at room temperature in the sample cuvette. The absorbance was measured at 550 nm for 1 min at 15 s interval and the results have been reported as nmoles of cytochrome c oxidised/min/mg protein (Wharton and Tzagaloff 1967).
Estimation of mitochondrial transmembrane potential
Murine colon: Isotonic buffer A and 50 µM JC-1 (1 mM ADP, 10 mM succinate, and 10 mM pyruvate) have been added to mitochondria which were freshly isolated and incubated for 30 min at 37 °C. Subsequent to incubation, the mixture was centrifuged at 1000 × g to precipitate the dyed mitochondrial pellet. Once dyed mitochondrial pellet was collected, to cast off the excess dye, it was subjected to washing with isotonic buffer A and thereafter dissolved with the same buffer. The emission intensities (530 nm and 590 nm) of fluorescence have been measured with excitation at 490 nm. As green fluorescence (λex 490 nm, λem 530 nm) is emitted by JC-1 monomers and red fluorescence (λ ex 490 nm, λ em 590 nm) emits J-aggregates, it was easy to measure the mitochondrial transmembrane potential with the aid of the ratio of fluorescence intensities at 590 nm to 530 nm.
Caco-2 cells: Mitochondrial transmembrane potential was evaluated with the aid of a fluorescence dye TMRE (tetramethylrhodamine ethyl ester) in both control and treated cells as mentioned in the mitochondrial membrane potential assay kit (Abcam, ab113852). Cells with concentration of 2 × 105 cells/well in a 96-well plate were used for the measurement of fluorescence in a microplate reader. Once the incubation was completed, TMRE has been added to the replaced cultured media with a final concentration of 50 nM which was then subjected to incubation at 5% CO2 for 20 min at 37 °C. Positive control cells along with 20 μM FCCP were introduced, 10 min prior to addition of TMRE. After incubation, cells were subjected to trypsinization followed by a round of centrifugation wherein the cell pellets were collected and observed for TMRE fluorescence (excitation/emission = 549/575 nm). The fluorescence readings were taken by suspending cell pellet in 0.4 ml of DPBS with 0.2% BSA.
Assessment of mitochondrial ATP generation
For evaluating ATP generation, glycerol was phosphorylated generating a measurable product which was measured at 570 nm by colorimetry. The entire protocol was performed using commercial ATP Assay Kit (Abcam, ab83355) by following the manufacturer’s instructions.
Assessment of oxidative stress in in vivo murine model
Assessment of oxidative stress variables in murine colon mitochondria was performed using well-established protocols as summarized here. Malondialdehyde (MDA), a marker for lipid peroxidation was estimated using thiobarbituric acid (TBA) reaction of lipid peroxides by adapting the method of Ohkawa et al. 1979 which is the most accepted methodology to measure MDA content. To measure the effect of oxidative stress on mitochondrial proteins, protein thiol content was assessed by Ellman’s reagent adapting the method of Habeeb 1972. In addition, protein carbonyl estimation was also carried out to measure mitochondrial protein stress by adapting the procedure of Levine et al. 1990.
Immunohistochemistry of murine colon
Colon murine samples were used to perform immunohistochemistry following the methodology as described in Maiti et al. 2015. To detect respiratory enzyme complex I and caspases 3 and 9, rabbit polyclonal primary antibodies have been used as stated here with their dilutions in antibody diluent (Dako, S0809): complex I (anti-NADH dehydrogenase subunit 6, MTND6, orb6548, Biorbyt) at 1:2000, caspase-3 (activated form, ab4051,Abcam) at 1:500 and caspase-9 (ab52298, Abcam) at 1:500 respectively. Caspases 3 and 9 were incubated at 4 °C for 18 h with primary antibodies and complex I was incubated at 25 °C for 4 h. After incubation, PBS containing Tween 20 (0.05%) was used to wash sections for four times. Subsequently, the tissues have been incubated with horseradish peroxidase (HRP) labelled anti-rabbit polymer (Dianova GmbH, PT03-L) for 15 min. Next for visualization of the bound polymer 0.05% 3, 3′-diaminobenzidine hydrochloride (DAB) chromogen (CD-12, Dako) was applied for 10 min and then the tissues were counterstained with haematoxylin. The staining intensity (scale of 0–5) was scored by blinded independent observers.
Protein estimation
Lowry’s method of protein estimation was performed to estimate the protein content in the mitochondrial samples, in which 1% SDS was used to solubilize the mitochondrial proteins (Lowry et al. 1951).
Biostatistical analysis
The experimental results obtained were statistically analyzed by Graph Pad Prism (version 5.03). The data containing more than two experimental groups have been analysed using one-way ANOVA followed by post-Student–Newman–Keuls multiple comparison test. The obtained values have been stated as mean ± S.E.M and variations have been considered significant at p < 0.05.The number of repetitions (n) for each assay is mentioned in respective figure legends.
Results
SP failed to revert colitis induced by DSS
To evaluate the impact of SP on colitis induced by DSS, C57BL/6 J WT mice were induced with chronic colitis by feeding 3.5% of DSS in 3 cycles for 30 days orally as outlined in the experimental flowchart in Fig. 1a. Post-DSS exposure, from 31st day, mice were subjected to SP treatment @ 5nmole/kg intravenously for 7 consecutive days. All animals have been sacrificed following SP treatment after 37th day. In mice groups III and IV treated with DSS, a trend was observed in body weight reduction during and after DSS cycles leading to a considerable decrease in body weight by the end of the DSS exposure (Fig. 1b). Importantly, mice belonging to group IV lost body weight and was unable to recover the lost bodyweight even after 7 days of continuous SP treatment ( – 22.60% to – 21.73% vs. control on 37th day, p > 0.05, Fig. 1b) similar to group III mice devoid of SP treatment. But there was no loss of body weight in mice belonging to groups I and II by the end of the experimental duration. Regarding the length of the colon, as predicted there was shortening of the colon in ulcerated mice; treatment with SP failed to restore the decreased colon length ( – 31.25% to – 29.70% vs. control on 37th day, p > 0.05) and treatment of SP alone did not affect the same (Fig. 1c).
Fig. 1.
Experimental flowchart, dynamics of body weight alteration and changes in colon length in in vivo C57BL/6 WT murine model of colitis. a Flowchart of the experimental setup. b Dynamics of alteration in body weight in control, SP treated alone, DSS treated alone and DSS + SP groups of mice throughout the experimental period. c Final colon length in control, SP treated alone, DSS treated alone and DSS + SP-treated mice. Values are mean ± S.E.M (n = 6). Statistics: For (b), ANOVA with Student–Newman–Keuls multiple comparison post hoc test on the 37th day: *P < 0.05 vs. control. For (c), ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05
Histological analysis of chronic DSS administered mice showed severe histopathological injury; upon SP treatment, significant recovery was not observed relative to the control values as represented in Fig. 2. DSS-fed mice suffered from complete colitis as represented by alteration in crypt architecture (Fig. 2c, p < 0.001), tissue damage (Fig. 2d, p < 0.001), elongation of crypt length (Fig. 2e, p < 0.05), loss of goblet cells (Fig. 2f, p < 0.01) and enhanced presence of lamina propria neutrophils (Fig. 2g, p < 0.01) as compared to control values. Interestingly, upon SP treatment, there seemed no recovery in colitis scores, rather detrimental impact of SP alone was observed in most of the colitis parameters with the exception of lamina propria neutrophils.
Fig. 2.
Histological analysis of colon tissue sections for colitis. a H&E-stained colon sections of control, SP alone, DSS alone and DSS + SP (scale bar- 50 μm, magnification × 200); quantification of clinical scores, b total colitis, c crypt architecture, d tissue damage, e crypt length, f goblet cell depletion and g lamina propria neutrophil. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001
SP failed to up-regulate mitochondrial respiratory complex activities during experimentally induced colitis
To explore the effect of SP on DSS-induced colitis in terms of its mitochondrial function, mitochondrial respiratory complex activities were measured as outlined in the methodology section. DSS-induced colitis mice showed decreased mitochondrial activities for complexes I–IV, SP treatment proved ineffective in reverting the same as represented in Fig. 3. As shown in Fig. 3a, the complex I activity was inhibited up to 60.00% in DSS-induced mice when compared to control (p < 0.01). However, SP treatment failed to revert complex I inhibition with inhibition close to 57.69% (p < 0.01). Remarkably, SP alone seemed to affect complex I activity with an inhibition of 34.45% as compared to control (p < 0.05). Unlike complex I, SP alone did not impart any individual inhibitory impact on complex II–III and complex IV activities. However, SP treatment in DSS-induced mice seemed ineffective in combating the inhibition of complex II–III ( – 50.19% to – 35.00% vs. control, p > 0.05, Fig. 3b) and complex IV activities ( – 67.74% to – 54.37% vs. control, p > 0.05, Fig. 3c).
Fig. 3.
The impact of SP on mitochondrial dysfunction in DSS-induced murine colitis. a Complex I activity. b Complex II–III activity. c Complex IV activity. d Mitochondrial membrane potential. e Mitochondrial ATP generation. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01
SP treatment failed to ameliorate lost mitochondrial membrane potential and mitochondrial ATP generation in murine colitis in vivo
As shown in Fig. 3d, SP treatment failed to restore mitochondrial membrane potential in DSS-induced colitis ( – 39.01 to – 24.88%, p > 0.05) when compared to control. Loss of mitochondrial ATP production in colonic epithelial cells was not restored significantly by exogenous SP treatment ( – 30.45 to – 27.98%, p > 0.05) when compared to control as represented in Fig. 3e. Importantly, SP treatment alone seemed to have a detrimental impact on membrane potential and ATP generation of mitochondria with loss of -34.97% (p < 0.01) and – 17.69% (p < 0.05), respectively, as compared to control values (Fig. 3d–e).
In vivo, SP administration in murine colitis unable to recover lost mitochondrial complex I immunohistochemical staining intensity
As SP seemed to impart a direct individual inhibitory effect only on complex I activity as compared to complexes II–III and complex IV as shown earlier in Fig. 3, the IHC staining was carried out only for complex I in all the treated groups. Upon DSS exposure, there was extensive loss of staining intensity in complex I; SP treatment in DSS exposed mice seemed to improve the loss of staining intensity though this impact was found to be statistically insignificant ( – 46.01% to – 31.61% vs. control, p > 0.05, Fig. 4). Surprisingly, SP alone seemed to cause extensive loss of complex I staining intensity as observed in SP-alone-treated mice group and the impact was found to be statistically significant ( – 31.10% vs. control, p < 0.05) as represented in Fig. 4.
Fig. 4.
Tissue localization and semi-quantification of mitochondrial respiratory enzyme complex I in C57BL/6 WT murine model of colitis in the presence and absence of SP treatment. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01. Scale bar 50 μm, magnification × 200
In vivo, exogenous administration of SP failed in lowering DSS promoted colonic cell death
Significant increase in colonic cell death was observed in DSS-alone- and DSS-induced SP-treated mice as demonstrated by caspases 3 and 9 immunostaining (Fig. 5a, b). SP treatment proved to be ineffective in attenuating cell death in colitic diseased condition. While there was a minor decrease in occurrence of cell death with SP treatment but it was found to be statistically insignificant as evidenced in caspase-3 (5.13-fold to 4.25-fold, p > 0.05, Fig. 5a) and caspase-9 (6.41-fold to 5.64-fold, p > 0.05, Fig. 5b), respectively, relative to control. Surprisingly, SP-alone-treated mice showed an increase in cell death of nearly 3.75-fold (p < 0.01, Fig. 5a) and 2.45-fold (p < 0.05, Fig. 5b) in case of caspases 3 and 9, respectively, when compared to control.
Fig. 5.
Caspase-3 and caspase-9 staining in sections of colon in in vivo C57BL/6 WT murine model of colitis with or without SP treatment. a Caspase-3 staining intensity measured following immunohistochemistry and images of different murine groups represented in this panel. b Caspase-9 staining intensity measured following immunohistochemistry in similar groups with their representative images. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar 50 μm, magnification × 200
SP failed to inhibit oxidative damage caused by DSS-induced colitis
Isolated colon epithelial mitochondria from all four treated mice groups were utilized for measuring oxidative stress variables. In case of MDA measurement, DSS exposure incurred near about 3.26-fold increase whereas SP-treated DSS-exposed mice showed similar increase of 3.42-fold indicating ineffectiveness of SP in lowering lipid peroxidation (p > 0.05, Fig. 6a). Importantly, SP alone contributed an increase of 2.13 fold in MDA production (p < 0.05, Fig. 6a). Pertaining to protein thiol loss and protein carbonyl generation; however, SP alone seems to have no effect on colonic mitochondria (p > 0.05, Fig. 6b, c). As shown in Fig. 6b, SP treatment led to minimal recovery of protein thiol loss in DSS-treated mice that was found to be statistically insignificant ( – 59.93% to – 43.23%, p > 0.05) as compared to control. Similarly, in case of protein carbonylation, treatment of DSS exposed mice with SP led to aggravation of protein carbonylation (+ 63.15% to + 71.05%, p > 0.05, Fig. 6c) as compared to control showcasing ineffectiveness of SP in countering oxidative stress caused by experimental colitis.
Fig. 6.
Measurement of mitochondrial oxidative stress indices in the murine colon after DSS-induced colitis with or without SP treatment. a Malondialdehyde (MDA). b Protein thiol. c Protein carbonyl. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.005, **P < 0.01, ***P < 0.001
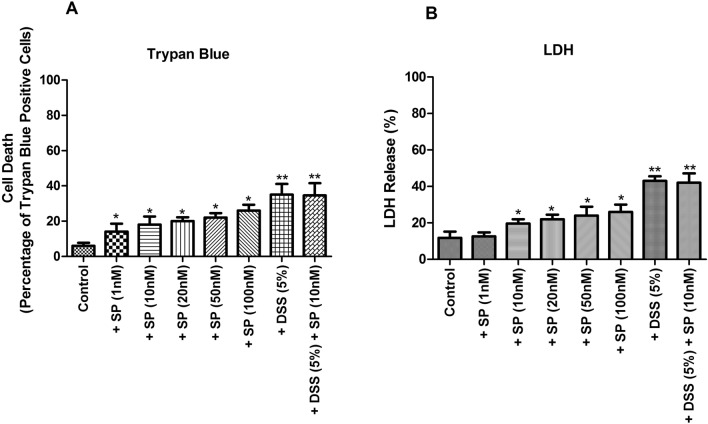
In vitro, dose-dependent impact of SP on Caco-2 cells for combinational treatment with DSS
To figure out the ideal concentration of SP for in vitro cell line experiments, Caco-2 cells have been treated with varying concentrations of SP (1, 10, 20, 50, and 100 nM) for 24 h. The final dosage of SP suitable for in vitro experiments was found out to be 10 nM. Importantly, the combination of 5% DSS and 10 nM SP exposure did not lead to significant lowering of trypan blue-stain-positive cells when compared to 5% DSS exposure alone (5.83 to 5.76 fold, p > 0.05, Fig. 7a). In this connection, it must be mentioned that 5% DSS concentration was found to be suitable concentration by previous in vitro experiments (data not provided). LDH activity assay was also used to evaluate cell death in intestinal epithelial cells wherein total free LDH released into the medium was estimated. In case of 5% DSS-treated cells, there was an increase in the percentage of LDH released; upon further addition of SP (10 nM) in the presence of 5% DSS, no significant alteration in total LDH release was observed (3.64 to 3.55 fold, p > 0.05, Fig. 7b).
Fig. 7.
In vitro dose-dependent impact of SP on Caco-2 cells in cell viability assays in the presence and absence of DSS. Varying concentrations of SP (1 nM–100 nM) were used in the presence or absence of DSS (5%), and the cell viability was measured by (a) trypan blue assay and (b) LDH activity assay as described in the materials and methods section. Values are mean ± S.E.M (n = 6). Statistics: ANOVA with Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01
Lack of free radical scavenging property of SP failed to revert mitochondrial dysfunction in Caco-2 cell experiments
To substantiate the in vivo mitochondrial findings and to assess the free radical scavenging property of SP, Caco-2 cell line experiments pertaining to mitochondrial dysfunction were performed utilizing an array of free radical scavengers and antioxidants. As observed in in vivo studies, similar latent values of citrate synthase were observed in in vitro mitochondrial preparations (data not shown). Interestingly, SP treatment failed to revert the different functional variables of mitochondrial dysfunction as represented in Fig. 8. As shown in Fig. 8a, mitochondrial complex I activity was comprehensively inhibited with 5% DSS exposure in Caco-2 cells (100 to – 45.19%, p < 0.01). Treatment with exogenous SP did not prove efficacious in reverting complex I inhibition ( – 45.19 to – 44.71%, p > 0.05) unlike SOD ( – 45.19 to – 18.06%, p < 0.05) and DMSO ( – 45.19 to – 13.73%, p < 0.05) as compared to control. Like SP, CAT ( – 45.19 to – 34.47%, p > 0.05), L-NAME ( – 45.19 to – 32.69%, p > 0.05) and MEG ( – 45.19 to – 39.14%, p > 0.05) did not prove effective in providing protection as compared to control (Fig. 8a). In case of complexes II–III, 5% DSS-induced inhibition was not improved with SP treatment ( – 55.83 to – 49.16% vs. control, p > 0.05) compared to SOD ( – 55.83 to – 23.3.6%, p < 0.05) and DMSO ( – 55.83 to – 18.13%, p < 0.05). Like SP similar trend of ineffectiveness was noticed in case of CAT ( – 55.83 to – 41.6%, p > 0.05), L-NAME ( – 55.83 to – 40.83%, p > 0.05) and MEG ( – 55.83 to – 45.83%, p > 0.05) regarding complex II–III activity as compared to control as shown in Fig. 8b. Similarly, in complex IV inhibition, treatment with SP failed to provide protection against DSS-induced colitis ( – 47.31 to – 41.04%, p > 0.05) as compared to SOD ( – 47.31 to – 14.23%, p < 0.05) and DMSO ( – 47.31 to – 19.07%, p < 0.05) whereas CAT ( – 47.31 to – 41.43%, p > 0.05), L-NAME ( – 47.31 to – 42.24%, p > 0.05) and MEG ( – 47.31 to – 34.49%, p > 0.05 Fig. 8c) proved ineffective in reducing inhibition of complex IV. In case of loss of mitochondrial membrane potential, SOD ( – 58.42 to – 24.28%, p < 0.05) and DMSO ( – 58.42 to – 30.57%, p < 0.05) delivered better protection as compared to SP ( – 58.42 to – 41%, p > 0.05), MEG ( – 58.42 to – 42.14%, p > 0.05), CAT ( – 58.42 to – – 42.85%, p > 0.05) and L-NAME ( – 58.42 to – 50%, p > 0.05) as depicted in Fig. 8d. In the assessment of mitochondrial ATP generation, SP proved ineffective in reinstating the loss of ATP generation ( – 57.62 to – 48.21%, p > 0.05) as compared to SOD ( – 57.62 to – 21.28%, p < 0.05) and DMSO ( – 57.62 to – 20.15%, p < 0.05). Treatment with CAT ( – 57.62 to – 47.83%, p > 0.05), L-NAME ( – 57.62 to – 47.08%, p > 0.05) and MEG ( – 57.62 to – 40.11%, p > 0.05) showed similar trend of ineffectiveness as SP when compared to control (Fig. 8e).
Fig. 8.
Effects of free radical scavengers and antioxidants on mitochondrial functions in in vitro Caco-2 cells exposed to DSS in the presence or absence of SP. a Complex I activity. b Complex II–III activity. c Complex IV activity. d Mitochondrial membrane potential. e Mitochondrial ATP generation. Values are mean ± S.E.M (n = 6). Statistics: ANOVA Student–Newman–Keuls multiple comparison post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
The current study was carried out with the intention to ascertain the impact of exogenous SP on colonic mitochondrial function in a diseased model mimicking human UC. As there exists controversy about the usage of SP as an anti-colitogenic agent, it was thought logical to carry out the present study as cellular processes either involved in promoting mucosal healing or aggravating mucosal damage in this diseased condition are energy dependent and for this reason, the status of mitochondrial function needs to be properly investigated. Interestingly, some of the data obtained from this study as detailed later in this section seemed unexpected that creates concern about the efficacy of SP in treating UC. In our DSS-induced murine model, as expected colitic histopathological symptoms were observed involving aberration of colonic crypt architecture and crypt length, infiltration of lamina propria neutrophils to the inflamed site and depletion of goblet cells around the mucosa. Interestingly, treatment with SP failed to alleviate these histopathological changes and this finding was not in agreement with some of the earlier reports where administration of SP proved effective in treating colitis and improved disease index (Hwang et al. 2017; Koon et al. 2007). In this background, the fact that deserves even more attention is that SP alone caused considerable damage in colon epithelial lining as observed in some of the individual parameters concerned with colitis scoring.
The failure of SP in treating colitis may have a direct connection with the impact of SP alone or in combination with DSS on mitochondrial function in colon epithelial cells. It is a known fact that mitochondrial stress and variations in mitochondrial functional status occur in colonocytes of UC patients (Santhanam et al. 2007, 2012; Sifroni et al. 2010). Similarly, in our murine colitis model, alteration of colonic mitochondrial function was observed in the form of inhibition of respiratory complexes I–IV, loss of mitochondria membrane potential and ATP generation of mitochondria. But the most important observation in this connection is the ineffectiveness of SP in alleviating mitochondrial dysfunction. Not only SP failed to inhibit mitochondrial dysfunction in DSS-induced mice, SP alone seemed to impart a detrimental effect on some of the mitochondrial functions in normal colonocytes by directly inhibiting complex I activity, lowering mitochondrial membrane potential and depleting mitochondrial ATP production. These observations about the individual impact of SP on mitochondrial function raise serious concern about its anti-colitogenic efficacy in treating UC. Considering the fact that SP seemed to inhibit complex I activity alone as compared to other mitochondrial respiratory complexes, we carried out in situ quantification in the form of immunohistochemistry for complex I. This particular technique helped to authenticate our earlier findings related to mitochondrial complex I as this technique eliminated any influence of stress caused during the mitochondrial isolation as outlined in our methodology section. The results obtained from this immunohistochemical study evidently indicated aberration in protein level expression of complex I after treatment with SP alone or in combination with DSS.
The concern about the ineffectiveness of SP in treating UC becomes even more aggravated by analysing the immunostaining data for caspases 3 and 9. Caspases are well-known markers for cell death involving mitochondrion-dependent pathways. Occurrence of cell death was evident in DSS-induced colon with substantial increase in staining intensity for both caspases. But in DSS-exposed colon treated with SP, there seemed to be some lowering of staining intensity for caspases; however, these results were found to be statistically insignificant indicating failure of SP in negating cell death even though SP might have showcased anti-apoptotic tendency. This anti-apoptotic tendency of SP as observed in DSS-induced mice treated with SP gets opposed by the results obtained in SP alone treated mice devoid of DSS exposure wherein an increase in staining intensity for both caspases were observed indicating SP to promote apoptotic impact in colonocytes. Thus, the trend as observed in DSS + SP vs. SP alone seems to contradict each other, thus aggravating further controversy about the anti-colitogenic role of SP. In this context, an interesting fact that one has to consider is that in our murine colitic model SP-induced programmed cell death by means of apoptosis that is dependent on activation of caspase. This particular finding stands against an earlier report that highlighted cell death only through non-apoptotic form induced by SP though in non-colitic conditions (Castro-Obregon et al. 2002). However, the role of proinflammatory cytokines induced by SP has not been considered in our study as proinflammatory cytokines may also promote cell death as observed in non-colitic diseases (Bang et al. 2004).
In an effort to delineate the influence of oxidative stress in the progression of UC and to elucidate the involvement of mitochondrial stress in this diseased state, assessment of lipid peroxidation, protein thiol and protein carbonylation was carried out with isolated colon mitochondria. As expected, the oxidative variables got altered in colitis-affected mitochondria indicating the role of stress experienced by mitochondria in the progression of UC. However, treatment with SP failed to alleviate mitochondrial stress in DSS exposed colon signifying the ineffectiveness of SP as a free-radical-scavenging agent. Besides, SP alone affected normal colon mitochondria showing higher MDA levels signifying the inefficacy of SP as an anti-colitogenic agent. This oxidative stress-inducing property of SP was in agreement with earlier reports where SP-induced oxidative stress; however, in non-colitic conditions (Block et al. 2006; Lordal et al. 1997; Liu et al. 2013).
For better understanding the mode of action that SP is most likely to follow in in vivo model, it became essential to replicate similar mitochondrial functional experiments in an artificial setting involving Caco-2 cells. One of the prime reasons for preferring Caco-2 cells was the presence of NK-1 receptors in these cells, the NK-1R being the key receptor type responsible for the action of SP (Bockmann 2002; Kang et al. 2009). 10 nM concentration of SP turned out to be the optimum dosage after conducting initial cell viability tests. In our in vitro experimental setup, cells were initially exposed to 5% DSS for 24 h at 37 °C with and without SP in the presence or absence of different free radical scavengers or antioxidants that included •OH scavenger DMSO (20 mM), H2O2 scavenger CAT (catalase, 50 µg/ml), O2•− scavenger SOD (20 mM), NO scavenger L-NAME (100 µM) and nitric oxide synthase inhibitor cum ONOO- scavenger MEG (0.5 mM). Importantly, SP in combination with DSS failed to provide at par or improved protection as compared to DMSO and SOD in connection to mitochondrial respiratory activities, membrane potential and ATP generation indicating the involvement of O2•− and •OH in the progression of UC. However, other free radical scavengers and antioxidants like catalase, L-NAME and MEG failed to counter the mitochondrial dysfunction as compared to SOD and DMSO indicating the non-involvement of H2O2, NO and ONOO− in the process. Similar to in vivo findings, SP alone inhibited complex I activity, induced mitochondrial membrane potential loss and depleted mitochondrial ATP production in Caco-2 cells, thus substantiating the in vivo findings. Analyzing the entire in vitro mitochondrial data, two basic findings can be concluded—first, elevated levels of O2•− and •OH are involved in the progression of UC and second, SP lacks free radical scavenging properties that demerits SP as a potential anti-colitogenic agent.
In conclusion, the data obtained from the current study seem to suggest the absence of anti-colitogenic property for SP as SP treatment failed to counter the detrimental impact of DSS-induced colitis mediated by mitochondrial dysfunction. This stands as the first report to evaluate the effect of SP on mitochondrial function in normal and diseased colonocytes. To better comprehend the mode of action of SP, further research needs to be carried out especially focusing on the role of pro-inflammatory cytokines that might get elevated in this UC condition (Aleksandra et al. 2005; Fujino et al. 2003; Komatsu et al. 2001; Noguchi et al. 1998). As pro-inflammatory cytokines are known to mediate mitochondrial functions (Hahn et al. 2014; Kim et al. 2010) and as SP might elevate certain pro-inflammatory cytokines under different diseased conditions as reported earlier (O'Connor et al. 2004; Yamaguchi et al. 2017), it will be worthwhile to investigate the impact of pro-inflammatory cytokines on mitochondrial function in this diseased state and examine whether SP is able to alleviate such impact. Though focus on the role of pro-inflammatory cytokines in UC was beyond scope in the present scenario, the present data will certainly lay the foundation to address concerns about the controversy related to anti-colitogenic properties of SP in treating UC.
Significance of the work
The present work being novel involving intestinal neuropeptide in the treatment of UC targeting mitochondrial dysfunction holds significance in the current status, as India is considered as the UC capital of South Asia. The treatment methods available being partially effective has resulted in increased colectomy, thus the search for good anti-colitogenic agents has become essential. The current study was carried out with the intention to ascertain the impact of exogenous SP on colonic mitochondrial function in a diseased model mimicking human UC. As there exists controversy about the usage of SP as an anti-colitogenic agent, it became essential to carry out the present study as cellular processes either involved in promoting mucosal healing or aggravating mucosal damage in this diseased condition are energy dependent and for this reason the status of mitochondrial function needs to be properly investigated. In pretext of the present scenario this work holds merit considering its implication in the field of UC that will lead to opening up of new avenues for UC treatment by using intestinal neuropeptides as anti-colitogenic agents by attenuating mitochondrial dysfunction in affected colon epithelial cells for the maintenance of colon epithelial tract.
Acknowledgements
The authors are thankful to the Department of Science and Technology (DST), New Delhi, India, for funding this research (Sanctioned No. EMR/SERB/2016/001981).
Author contributions
Spoorthi B. C. (S.B.C); Shashwati Ghosh (S.G); Ishita Saha (I.S); Sunil S More (S.S.M); Gautham S.A (G.S.A); Arpan K Maiti (A.K.M). S.B.C, S.G, I.S, S.S.M and A.K.M were involved in bench work and performed the experiments. S.B.C, G.S.A, S.S.M and A.K.M designed the work and involved in conceptualization. All authors supported technically. A.K.M provided financial assistance. S.B.C, S.S.M and A.K.M interpreted data and wrote the manuscript, and all authors reviewed it.
Funding
Department of Science and Technology (DST), New Delhi, India (Grant No. EMR/SERB/2016/001981).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical issue
All experimental protocols were permitted by the ethical committee of animal care and use of Dayananda Sagar University, Bengaluru, Karnataka, India (Registration No. 606/02/C/CPCSEA).
Contributor Information
Spoorthi B. Chandraiah, Email: spoorthi26bc@gmail.com
Shashwati Ghosh, Email: shashwatighosh592@gmail.com.
Ishita Saha, Email: hymntohope@protonmail.com.
Sunil S. More, Email: sunilacr@yahoo.co.in, Email: drsunil@dsu.edu.in
Gautham S. Annappa, Email: gautham.annappa@gmail.com, Email: gautham@dsu.edu.in
Arpan K. Maiti, Email: arpankumar.maiti@gmail.com, Email: arpankrmaiti@nbu.ac.in
References
- Aleksandra NA, Nederby NJ, Schmedes A, et al. Saliva Interleukin-6 in patients with inflammatory bowel disease. Scand J Gastroenterol. 2005;40:1444–1448. doi: 10.1080/00365520510023774. [DOI] [PubMed] [Google Scholar]
- Bang R, Biburger M, Neuhuber WL, et al. Neurokininreceptor antagonists protect mice from CD95- and TNF {alpha}-mediated apoptotic liver damage. J Pharmacol Exp Ther. 2004;308:926–934. doi: 10.1124/jpet.103.059329. [DOI] [PubMed] [Google Scholar]
- Bar F, Bochmann W, Widok A, et al. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterol. 2013;145:1055–1063. doi: 10.1053/j.gastro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- Block ML, Li G, Qin L, et al. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J. 2006;20:251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- Bockmann S. Substance P (NK(1)) receptor expression by human colonic epithelial cell line Caco-2. Peptides. 2002;23:1783–1791. doi: 10.1016/s0196-9781(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Castro-Obregon S, Del RG, Chen SF, Swanson RA, Frankowski H, Rao RV, Stoka V, Vesce S, Nicholls DG, Bredesen DE (2002) A ligand-receptor pair that triggers a non-apoptotic form of programmed cell death. Cell Death Differ 9:807–817 [DOI] [PubMed]
- Cui L, Locatelli L, Xie MY. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC12 cells. J Pharmacol Exp Therapeut. 1997;280:1228–12234. [PubMed] [Google Scholar]
- Cuzzocrea S, Zingarelli B, Hake P, et al. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med. 1998;24:450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- Dae YH, Suna K, Hyun SH (2017) Substance-P ameliorates dextran sulfate[1]induced intestinal damage by preserving tissue barrier function. Tissue Eng Regen Med 15(1):63–73 [DOI] [PMC free article] [PubMed]
- Engel MA, Leffler A, Niedermirtl F, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterol. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb AF. Reaction of protein sulfhydryl groups with Ellman's reagent. Methods Enzymol. 1972;25:457–464. doi: 10.1016/S0076-6879(72)25041-8. [DOI] [PubMed] [Google Scholar]
- Hahn WS, Kuzmicic J, Burrill JS, et al. Proinflammatory cytokines differentially regulate adipocyte mitochondrial metabolism, oxidative stress, and dynamics. Am J physiol Endocrinol Metab. 2014;306:E1033–E1045. doi: 10.1152/ajpendo.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. Preparation and properties of NADH: Ubiquinone oxidoreductase (complex I), EC 1.6.5.3. Methods Enzymol. 1978;53:11–14. doi: 10.1016/S0076-6879(78)53006-1. [DOI] [PubMed] [Google Scholar]
- Hokari R, Lee H, Crawley SC, et al. Vasoactive intestinal peptide upregulates MUC2 intestinal mucin via CREB/ATF1. Am J Physiol Gastrointest Liver Physiol. 2005;289:G949–G959. doi: 10.1152/ajpgi.00142.2005. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Kim S, Hong HS. Substance-P Ameliorates Dextran Sodium Sulfate-Induced Intestinal Damage by Preserving Tissue Barrier Function. Tissue Eng Regen Med. 2017;15:63–73. doi: 10.1007/s13770-017-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DN, Theiss AL. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut microbes. 2019;11:285–304. doi: 10.1080/19490976.2019.1592421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, Taghert PH (2005) A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J Exp Biol 208:1239–1246 [DOI] [PubMed]
- Kang MH, Kim DY, Yi JY, et al. Substance P accelerates intestinal tissue regeneration after gamma-irradiation-induced damage. Wound Repair Regen. 2009;17:216–223. doi: 10.1111/j.1524-475X.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- Khan FH, Sen T, Maiti AK, et al. Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: Implications in Parkinson’s disease. Biochim Biophys Acta. 2005;1741:65–74. doi: 10.1016/j.bbadis.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Kim J, Xu M, Xo R, et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr cartel. 2010;18:424–432. doi: 10.1016/j.joca.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy A, et al. Dieldrin promotes proteolytic cleavage of poly (ADPribose) polymerase and apoptosis in dopaminergic cells: protective effect of mitochondrial anti-apoptotic protein Bcl-2. Neurotoxicol. 2004;25:589–598. doi: 10.1016/j.neuro.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kobayashi D, Saito K, et al. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297–1301. doi: 10.1093/clinchem/47.7.1297. [DOI] [PubMed] [Google Scholar]
- Koon HW, Zhao D, Zhan Y, et al. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci U S A. 2007;104:2013–2018. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Liu N, Wang LH, Guo LL, et al. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS ONE. 2013;8:e61574. doi: 10.1371/journal.pone.0061574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordal M, Soder O, Hellstrom PM. Tachykinins stimulate lipid peroxidation mediated by free radicals in gastrointestinal tract of rat. Dig Dis Sci. 1997;42:1524–1529. doi: 10.1023/a:1018835314834. [DOI] [PubMed] [Google Scholar]
- Love JR, Irvine EJ, Fedorak RN. Quality of life in inflammatory bowel disease. J Clin Gastroenterol. 1992;14:15–19. doi: 10.1097/00004836-199201000-00005. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Far AL, et al. Protein measurement with Folin-Phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Maiti AK, Sharba S, Navabi N, et al. Colonic levels of Vasoactive Intestinal Peptide decrease during infection and exogenous VIP protects epithelial mitochondria against the detrimental effects of TNFα and IFNγ induced during Citrobacter rodentium infection. PLoS ONE. 2015;13:e0204567. doi: 10.1371/journal.pone.0204567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti AK, Spoorthi BC, Saha NC, et al. Mitigating peroxynitrite mediated mitochondrial dysfunction in aged rat brain by mitochondria-targeted antioxidant MitoQ. Biogerontol. 2018;19:271–286. doi: 10.1007/s10522-018-9756-6. [DOI] [PubMed] [Google Scholar]
- Maiti AK, Saha NC, More SS, Panigrahi AK, Paul G (2017) Neuroprotective efficacy of mitochondrial antioxidant MitoQ in suppressing peroxynitrite mediated mitochondrial dysfunction inflicted by lead toxicity in rat brain. Neurotox Res 31:358–372 [DOI] [PubMed]
- Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N (2016) Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev 5 [DOI] [PubMed]
- Noguchi M, Hiwatashi N, Liu Z, et al. Secretion imbalance between tumor necrosis factor and its inhibitor in inflammatory bowel disease. Gut. 1998;43:203–209. doi: 10.1136/gut.43.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TM, O'Connell J, O'Brien DI, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Rehman A, Whiteman M, Halliwell B. Scavenging of hydroxyl radicals but not of peroxynitrite by inhibitors and substrates of nitric oxide synthases. Br J Pharmacol. 1997;122:1702–1706. doi: 10.1038/sj.bjp.0701556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands BE. Therapy of inflammatory bowel disease Gastroenterol. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- Santhanam S, Venkatraman A, Ramakrishna BS. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut. 2007;56:1543–1549. doi: 10.1136/gut.2006.108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam S, Rajamanickam S, Motamarry A, et al. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2158–2168. doi: 10.1002/ibd.22926. [DOI] [PubMed] [Google Scholar]
- Sifroni KG, Damiani CR, Stoffel C, et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol Cell Biochem. 2010;342:111–115. doi: 10.1007/s11010-010-0474-x. [DOI] [PubMed] [Google Scholar]
- Stucchi AF, Shofer S, Leeman S, et al. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- Szabo C, Ferrer-Sueta G, Zingarelli B, et al. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J Biol Chem. 1997;272:9030–9036. doi: 10.1074/jbc.272.14.9030. [DOI] [PubMed] [Google Scholar]
- Wharton DC, Tzagaloff TA. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967;10:245–250. doi: 10.1016/0076-6879(67)10048-7. [DOI] [Google Scholar]
- Yamaguchi K, Kumakura S, Murakami T, et al. Ketamine suppresses the substance P-induced production of IL-6 and IL-8 by human U373MG glioblastoma/astrocytoma cells. Int J Mol Med. 2017;39:687–692. doi: 10.3892/ijmm.2017.2875. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Xu R, Schinstock C, et al. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci U S A. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K, Yajima T, Kubo C, et al. Role of interleukin 15 in colitis induced by dextran sulphate sodium in mice. Gut. 2006;55:334–341. doi: 10.1136/gut.2005.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]