Abstract
Alcoholic liver disease (ALD) is associated with gut dysbiosis and hepatic inflammasome activation. While it is known that antimicrobial peptides (AMPs) play a critical role in the regulation of bacterial homeostasis in ALD, the functional role of AMPs in the alcohol-induced inflammasome activation is unclear. The aim of this study was to determine the effects of cathelicidin-related antimicrobial peptide (CRAMP) on inflammasome activation in ALD. CRAMP knockout (Camp−/−) and wild-type (WT) mice were subjected to binge-on-chronic alcohol feeding and synthetic CRAMP peptide was administered. Serum/plasma and hepatic tissue samples from human subjects with alcohol use disorder and/or alcoholic hepatitis were analyzed. CRAMP deficiency exacerbated ALD with enhanced inflammasome activation as shown by elevated serum interleukin (IL)-1β levels. Although Camp−/− mice had comparable serum endotoxin levels compared to WT mice after alcohol feeding, hepatic lipopolysaccharide (LPS) binding protein (LBP) and cluster of differentiation (CD) 14 were increased. Serum levels of uric acid (UA), a Signal 2 molecule in inflammasome activation, were positively correlated with serum levels of IL-1β in alcohol use disorder patients with ALD and were increased in Camp−/− mice fed alcohol. In vitro studies showed that CRAMP peptide inhibited LPS binding to macrophages and inflammasome activation stimulated by a combination of LPS and UA. Synthetic CRAMP peptide administration decreased serum UA and IL-1β concentrations and rescued the liver from alcohol-induced damage in both WT and Camp−/− mice. In summary, CRAMP exhibited a protective role against binge-on-chronic alcohol-induced liver damage via regulation of inflammasome activation by decreasing LPS binding and UA production. CRAMP administration may represent a novel strategy for treating ALD.
Keywords: CRAMP, ALD, LPS, uric acid, IL-1β
Introduction
Patients with alcoholic liver disease (ALD) have high morbidity and mortality in its severe forms [1,2]. Altered gut microbiota have been identified as one of the major features in different forms of ALD [3]. Alcohol consumption causes dysbiosis and ‘leaky gut’, which allows the translocation of bacteria and its products through the portal vein to the liver, resulting in damage to the liver cells and the development of hepatic steatosis and liver injury through the toll-like receptor 4 (TLR4)-mediated pathway [4,5].
One of the most important mechanisms maintaining gut microbiota homeostasis is antimicrobial peptide (AMP)-mediated regulation. AMPs are produced in Paneth cells, enterocytes, intestinal epithelial cells, and macrophages, and form a central part of the innate immune system to counter microbial infections [6] and inhibit the overgrowth of pathogenic bacteria in gut lumen [7,8]. Previous studies have demonstrated the critical role of AMPs in the pathogenesis of ALD [9–11]. Lack of intestinal epithelial cell-derived regenerating islet-derived (REG)-3 lectins, Reg3β or Reg3γ, exacerbated alcohol-induced dysbiosis and ALD, while overexpression of Reg3g attenuated ALD [9]. Recent work further showed that alcohol-induced α-defensin dysfunction exaggerated endotoxin translocation and liver damage [12]. In addition to Reg3 lectins and defensins, cathelicidins are one of the major AMP families. LL-37 is the sole member of the human cathelicidin family, and has a murine ortholog, cathelicidin-related antimicrobial peptide (CRAMP). LL-37/CRAMP has piqued great research interest for its dual role in modulating microbiota and immune response in several infectious diseases and inflammatory bowel disease (IBD) [13–15]. Wild-type (WT) mice co-housed with Camp−/− (CRAMP knockout) mice had markedly increased sensitivity to dextran sulfate sodium-induced colitis, with a skewed composition of fecal microbiota [16]. CRAMP deficiency exacerbated type 1 diabetes through the dysregulation of β-cell immunity [17]. Notably, the production of CRAMP in the pancreas is controlled by gut-derived butyric acid, implying the importance of gut microbiota in the CRAMP regulation [17].
Inflammasome activation is an important component of the liver pathophysiology in ALD [18,19]. The NLRP3 inflammasome [an intracellular protein complex consisting of NLRP3, apoptosis-associated speck protein with caspase activation and recruitment domain (ASC), and caspase-1] is one of the most studied inflammasomes. The NLRP3 inflammasome requires both Signal 1 (pathogen-associated molecular patterns, PAMPs) and Signal 2 (danger-associated molecular patterns, DAMPs) for its full activation. The activation of caspase-1 enables the cleavage of pro-interleukin (IL)-1β to form active IL-1β, which can then be secreted out and bind to the IL-1 receptor to induce inflammation [20]. LL-37 prevented the TLR-4 mediated pro-inflammatory pathway by inhibiting the binding of LPS to the CD14/TLR-4 complex and by promoting the LPS degradation by lysosomes [21,22]. However, whether LL-37/CRAMP is involved in the regulation of inflammasome activation in ALD remains unclear.
Our previous studies showed a decreased expression of intestinal CRAMP in mouse models of ALD, and that treatment with probiotics improved gut microbiota homeostasis and restored intestinal CRAMP expression in mice [23,24]. In the current study, we aimed to test the hypothesis that CRAMP protects the liver from alcohol-induced injury through the regulation of inflammasome activation. We found that CRAMP deficiency exacerbates, while exogenous CRAMP peptide alleviates, alcohol-induced liver injury through inhibition of inflammasome activation by decreasing LPS function and uric acid (UA) production.
Materials and methods
Animals
All mice were treated according to the protocols reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville. Camp−/− male mice (B6.129X1, JAX stock #017799; Jackson Laboratory, Bar Harbor, ME, USA) at the age of 8–10 weeks were used with their age-matched WT (C57BL/6J) mice. Littermates were used. Mice were maintained at 22 °C with a 12 h light/dark cycle and had free access to a normal chow diet and tap water. Mice were fed the Lieber DeCarli Diet containing 5% alcohol (w/v) (alcohol-fed, AF) or isocaloric maltose dextrin (pair-fed, PF). For the AF groups, mice were initially fed the control Lieber-DeCarli liquid diet (Bio-Serve, Flemingtown, NJ, USA) for 5 days to acclimate to the liquid diet. The content of alcohol in the liquid diet was gradually increased from 1.6% (w/v) to 5% (w/v) over the next 6 days and remained at 5% for the subsequent 24 days. Mice in the PF group were fed isocaloric maltose dextrin in substitution for alcohol in the liquid diet. On day 24, a bolus of EtOH (5 g/kg body weight) was given to AF mice by gavage 6 h before harvesting, while mice in PF groups received a gavage of maltose dextrin (24D+1B model). The CRAMP peptide (H-ISR-LAGLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE-OH) was synthesized (Peptides International, Louisville, KY, USA) and 100 μg of CRAMP peptide in 100 μl of saline was injected intraperitoneally (i.p.) once daily for 3 days before the conclusion of the feeding period. Interventions were done during the light cycle.
Human subjects
All studies were IRB-approved by the appropriate IRB, and all patients consented to having their tissues/samples used for further research. Human serum/plasma samples and hepatic tissues were used in this study. Serum/plasma samples were from an Alcohol Use Disorder study at NIAAA and from an Acute Alcoholic Hepatitis study at the University of Louisville. Hepatic tissues from patients with severe alcoholic hepatitis were obtained from the Resources Center of Johns Hopkins University. Details of characterizations and eligibility of human subjects have been described previously [25–27] and also in Supplementary materials and methods.
Statistics
Statistical analyses were performed using the statistical computer package GraphPad Prism version 6 (GraphPad Software Inc, San Diego, CA, USA), MS Excel 2016 (Microsoft Corp, Redmond, WA, USA) or SPSS 26.0 (IBM, Chicago, IL, USA). Results are expressed as mean standard error of the mean (SEM). Statistical comparisons were made using two-way analysis of variance (ANOVA) with Tukey’s post hoc test or Student’s t-test, where appropriate. Differences were considered to be significant if p < 0.05. Columns with different letters denote significance, or significance may be noted as *p < 0.05, **p < 0.01, ***p < 0.001 between groups.
Details for Cell culture, Hepatocyte and siRNA transfection, ALT and AST measurements, Histological analyses, RNA isolation and RT-qPCR, Immunoblotting, Plasma LL-37, Serum CRAMP and IL-1β assays, Endotoxin assay, LPS-binding assay, Serum ATP, uric acid and xanthine oxidase assays, and Gut microbiota analysis and bacterial culturing are presented in Supplementary materials and methods and supplementary material, Table S1.
Results
Cathelicidin is dysregulated in mice fed alcohol and in patients with ALD
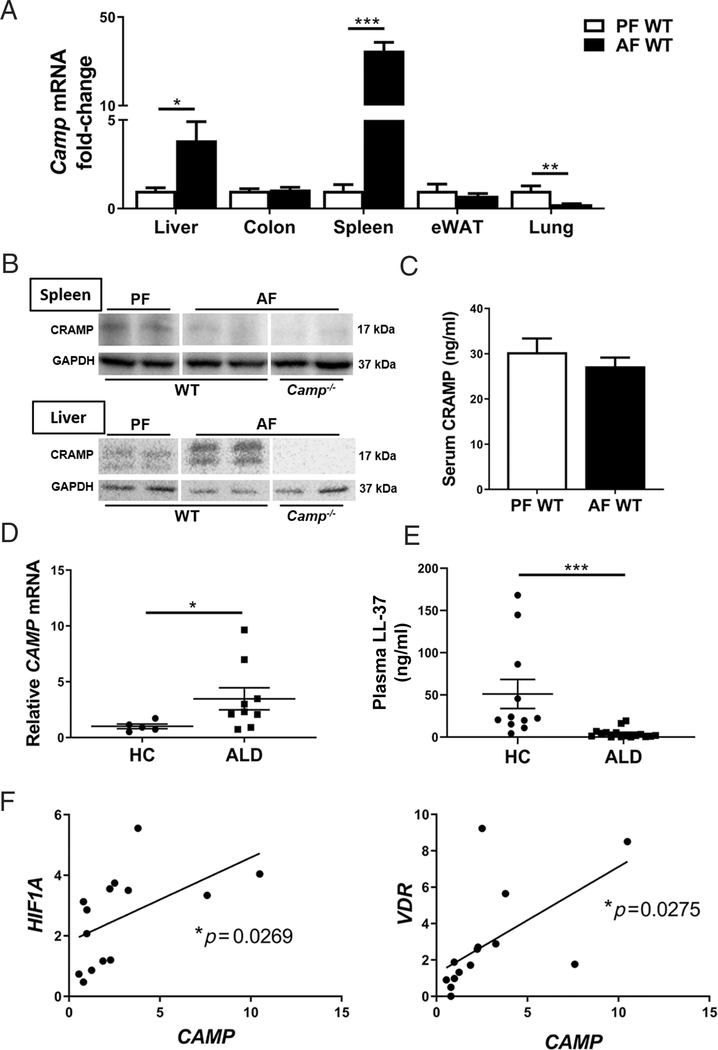
Our previous studies showed that ileal CRAMP mRNA expression was decreased in mice treated with binge alcohol or on chronic alcohol feeding for 4 weeks [23,24]. To further evaluate CRAMP tissue expression in response to alcohol feeding, we analyzed the mRNA levels of Camp (gene name for mouse CRAMP) in various tissues of the mice fed alcohol. Mice followed the 24D+1B binge-on-chronic alcohol-feeding model. Alcohol feeding significantly increased Camp mRNA expression in the liver and spleen, and decreased it in the lung tissue, while no change was found in the epididymal white adipose tissue (eWAT) (Figure 1A). Interestingly, spleen CRAMP protein was decreased by alcohol, while liver levels of CRAMP were increased (Figure 1B). There was no change in serum CRAMP levels in mice fed alcohol (Figure 1C). Similarly, in patients with ALD, the hepatic mRNA levels of LL-37 (gene name CAMP) were elevated compared with healthy controls (Figure 1D). There was no significant difference between active drinkers and abstinent subjects in patients with ALD (data not shown). However, plasma LL-37 protein levels were decreased in ALD patients (Figure 1E). The levels of plasma LL-37 between moderate acute alcoholic hepatitis (mAAH) and severe acute alcoholic hepatitis (sAAH) were comparable in these ALD patients. LL-37 is transcriptionally regulated by hypoxia-inducible factor 1α (HIF-1α) [23,28] and vitamin D receptor (VDR) [29]. We found that hepatic CAMP mRNA was positively correlated with the mRNA levels of HIF1A and VDR (Figure 1F), indicating likely upregulated hepatic HIF1A and VDR signaling in ALD patients.
Figure 1.
Cathelicidin dysregulation in alcohol-fed animals and ALD patients. (A) Alcohol-induced changes in Camp expression in different organs. PF, pair-fed; AF, alcohol-fed. (B) CRAMP protein immunoblotting of liver and spleen tissues. The bands are composite images of selected bands, and the corresponding GAPDH controls are shown. (C) Serum CRAMP protein levels. Data are expressed as mean SEM (n = 5–9). (D) Hepatic CAMP mRNA expression and (E) plasma LL-37 levels in ALD patients and healthy controls (HC). Data are expressed as mean SEM (n = 5–16). (F) Hepatic CAMP expression correlates with HIF1A and VDR expression in ALD patients.
CRAMP deficiency exacerbated alcohol-induced liver steatosis, injury, and pro-inflammatory response
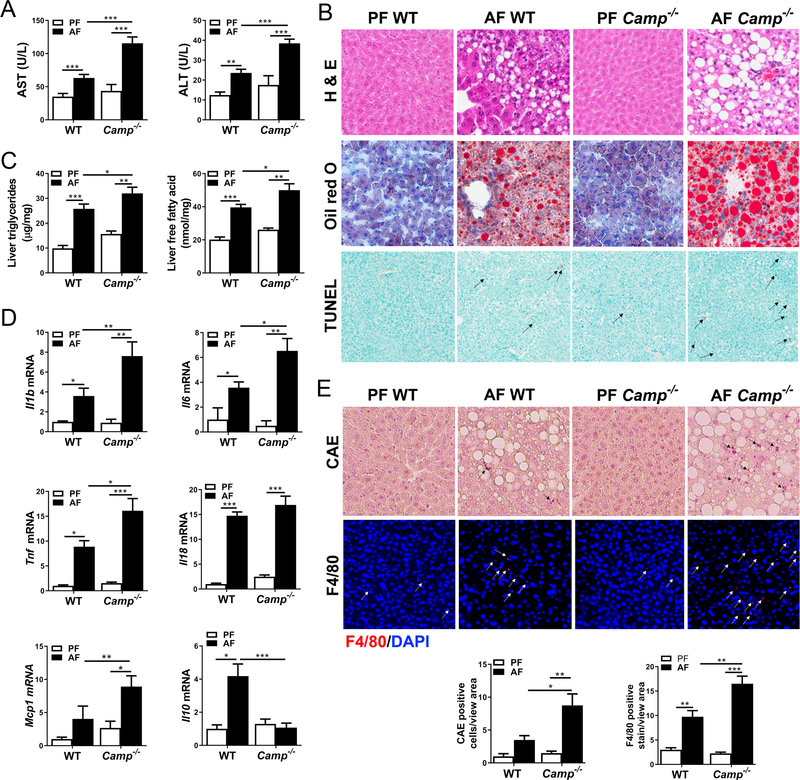
To determine the role of CRAMP in ALD, Camp−/− and WT mice were fed alcohol in a binge-on-chronic model (24D+1B). Alcohol consumption resulted in increased levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in both WT and Camp−/− mice, with more pronounced elevations being found in Camp−/− mice (Figure 2A). Alcohol feeding increased hepatic fat accumulation in both WT and Camp−/− mice, but much more severe macrosteatosis was found in Camp−/− mice (Figure 2B), which was then confirmed by Oil Red O staining of frozen liver sections (Figure 2B). Importantly, the distribution of lipid droplets in the livers of Camp−/− and WT mice was zonally distinct from each other (supplementary material, Figure S1). Enhanced hepatic fat in Camp−/− mice was further confirmed by the measurement of hepatic triglyceride and free fatty acid contents (Figure 2C). Moreover, alcohol exposure induced more hepatic apoptosis in Camp−/− mice, shown as an increased number of terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL)-positive cells (Figure 2B). These results suggest that alcohol feeding exacerbates liver steatosis, injury, and hepatic cell death when CRAMP is deficient. Inflammation is a hallmark of ALD in animal models and human subjects [30,31]. Camp−/− mice had significantly increased mRNA levels for the pro-inflammatory cytokines Il1b, Il-6, Tnf, and Il18, and the chemokine Mcp1 (Figure 2D). Interestingly, Il10, encoding an anti-inflammatory cytokine [32], was substantially lower in the Camp−/− mice than in the WT mice fed alcohol (Figure 2D). Histological analysis revealed increased levels of neutrophil infiltration and macrophage activation in the livers of Camp−/− mice (Figure 2E). These results suggest that lacking CRAMP increased the pro-inflammatory response to alcohol in the liver and was associated with exacerbated neutrophil infiltration and increased macrophage activation.
Figure 2.
Effects of CRAMP deficiency on ethanol-induced liver injury, steatosis, and pro-inflammatory response. (A) Serum levels of AST and ALT. (B) Representative photomicrographs of liver sections with hematoxylin and eosin (H&E) staining (original magnification 200×) (upper panel), Oil Red O staining (original magnification 200×) (middle panel), and TUNEL-positive cell staining (original magnification 100×) (lower panel). Black arrows: TUNEL-positive cells. (C) Hepatic levels of triglycerides and free fatty acids. (D) Hepatic cytokine and chemokine mRNA expression. (E) Representative photomicrographs of paraffin-embedded liver section stained for chloroacetate esterase (CAE) (original magnification 200×) (upper panel) and frozen liver tissue stained using immunofluorescence (IF) for F4/80 (original magnification 100×) (lower panel). Black arrows: infiltrated neutrophils; white arrows: F4/80 positive stained macrophages. Blue: DAPI counterstained nuclei; red: F4/80. Data are expressed as mean SEM (n = 8–10).
Camp−/− mice had dysbiosis in response to alcohol feeding
Gut bacterial dysbiosis has been well documented in mice with experimental ALD [10,33,34] and in ALD patients [35,36]. 16S rRNA sequence analysis showed distinct bacterial populations in the feces between WT and Camp−/− mice fed alcohol. At the phylum level (supplementary material, Figure S2A), Bacteroidetes decreased, while Firmicutes increased in Camp−/− mice fed alcohol, which agrees with previous studies [33,37]. It is notable that there was a great expansion of Saccharibacteria lineage bacteria (formerly known as TM7) in alcohol-fed Camp−/− mice, which was then confirmed by RT-qPCR (supplementary material, Figure S2B). TM7 is one of the colitogenic microbiota phyla that have been shown to correlate with non-alcoholic fatty liver disease (NAFLD) [38]. Total bacterial loads in fecal samples of WT and Camp−/− mice were comparable (supplementary material, Figure S2C). Of note, Akkermansia, a genus in the phylum Verrucomicrobia that is important in ALD [12,23], was increased in WT mice fed alcohol, while it was markedly decreased in the Camp−/− mice (supplementary material, Figure S2D). Analysis of genera (supplementary material, Figure S2E) showed that Bacteroides, which belongs to the Bacteroidetes phylum, completely disappeared in Camp−/− mice fed alcohol. Thermophagus, another genus of Bacteroidetes, increased significantly in Camp−/− mice by eight-fold. Oscillibacter and Papillibacter in Ruminococcaceae families were decreased in Camp−/− mice, and have been reported to be decreased in cirrhotic patients [39]. These results suggest that CRAMP may regulate microbiota homeostasis in ALD. We further showed that CRAMP peptide indeed selectively inhibits bacterial growth. As depicted in supplementary material, Figure S3, CRAMP peptide inhibited the growth of the pathogenic bacteria Listeria (supplementary material, Figure S3A) and Escherichia coli (supplementary material, Figure S3B); however, notably, there was no effect on Bacillus subtilis 22 CP-1 (supplementary material, Figure S3C) or probiotic LGG (supplementary material, Figure S3D).
CRAMP deficiency exacerbated alcohol-induced IL-1β production through the enhancement of LPS signaling and endogenous danger signals’ production
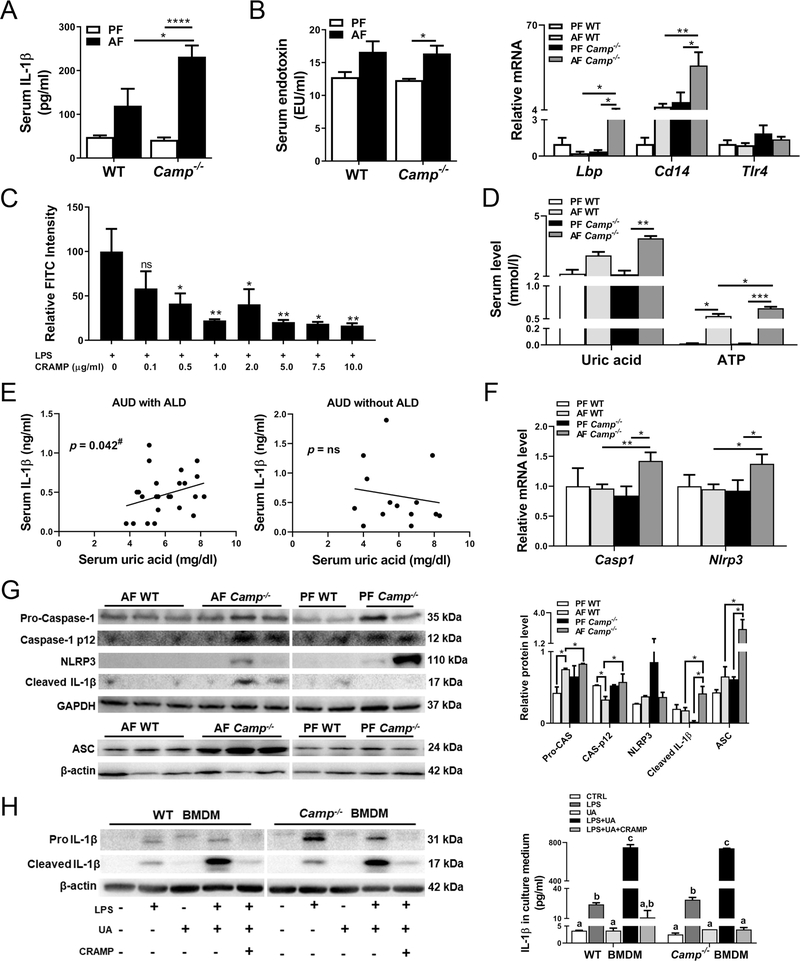
Serum levels of IL-1β were slightly increased by alcohol feeding in the WT mice but significantly increased in alcohol-fed Camp−/− mice, indicating an inflammasome activation (Figure 3A), which requires two signals for its full activation [40]. Serum endotoxin levels were increased by alcohol feeding in both WT and Camp−/− mice. However, alcohol-induced LPS increases were comparable between WT and Camp−/− mice (Figure 3B, left panel). We therefore examined if CRAMP was involved in LPS delivery and activation. There were no significant changes of LPS binding protein (LBP), CD14, or TLR4 expression between pair-fed WT and Camp−/− mice. After alcohol feeding, Camp−/− mice had significantly increased hepatic mRNA expression of Lbp and Cd14, and a slight trend of increase in Tlr4 (Figure 3B, right panel), indicating an enhanced LPS activation. To further examine the role of CRAMP in LPS binding in macrophages, we treated RAW 264.7 cells with a synthetic CRAMP peptide in the presence of fluorescein isothiocyanate (FITC)-labeled LPS. As expected, CRAMP peptide inhibited LPS binding to macrophages in a dose-dependent manner (Figure 3C). Whether CRAMP is involved in the Signal 2 regulation in the NLRP3 inflammasome is not clear. ATP and UA have been identified as endogenous danger signals for NLRP3 inflammasome activation in ALD [41]. Alcohol feeding increased serum levels of UA and ATP in WT mice, which were significantly increased in mice lacking CRAMP (Figure 3D). Importantly, analysis of banked serum samples from alcohol use disorder (AUD) patients revealed a significant positive correlation between serum IL-1β and UA concentrations in AUD patients with ALD, but an insignificant correlation in AUD patients without ALD (Figure 3E). Hepatic Nlrp3 and Casp1 mRNA expression was comparable between pair-fed WT and Camp−/− mice but increased in alcohol-fed Camp−/− mice compared with their WT controls (Figure 3F). Camp−/− mice had similar levels of pro-caspase-1 protein and the active form caspase-1 p12, compared with WT mice under conditions of pair feeding, which were significantly increased by alcohol feeding (Figure 3G). In contrast, NLRP3 and ASC were statistically insignificantly increased, while cleaved IL-1β was decreased in Camp−/− mice compared with WT mice by pair feeding. Importantly, alcohol feeding significantly increased cleaved IL-1β and ASC protein expression in Camp−/− mice compared with WT mice (Figure 3G).
Figure 3.
CRAMP is involved in alcohol-induced IL-1β production and inflammasome activation. (A) Serum concentrations of IL-1β. (B) Serum endotoxin levels (left panel) and hepatic mRNA levels of Lbp, Cd14, and Tlr4 (right panel). (C) LPS binding activity shown as FITC intensity of FITC-conjugated LPS-treated RAW264.7 cells. LPS-FITC: 1 μg/ml. Asterisks indicate the significant difference comparing LPS alone and treated groups. (D) Serum uric acid (UA) and ATP levels. Data are expressed as mean SEM (n = 8–10). (E) Correlation of serum levels of UA and IL-1β in AUD patients. #Covaried with LBP. Univariate and multivariate regression model was used (n = 14–26). (F) Hepatic mRNA levels of Casp1 and Nlrp3. (G) Representative bands of immunoblotting quantification of molecules in inflammasome activation in liver lysates. Data are expressed as mean SEM (n = 8–10). (H) Left panel: Representative bands of immunoblotting quantification of pro-IL-1β and cleaved IL-1β protein in BMDM lysates. The bands are composite images of selected bands, and the corresponding β-actin controls are shown. Right panel: IL-1β protein levels in culture media of BMDMs. Data are expressed as mean SEM (n = 4–6).
We examined whether treatment with CRAMP peptide inhibits inflammasome activation. Bone marrow-derived macrophages (BMDMs) were isolated from both WT and Camp−/− mice and treated with synthetic CRAMP peptide in the presence of LPS. The mRNA expression of Il1b and Il6 was significantly increased, while Il10 was marginally elevated by LPS in Camp−/− BMDMs compared with WT BMDMs (supplementary material, Figure S4A). CRAMP peptide treatment inhibited LPS-induced expression of Il1b, Il18, Il6, Tnf, and Il10. The inhibitory effects of CRAMP peptide at the concentrations of 1 and 10 μg/ml on LPS-induced cytokine expression were comparable, indicating a sensitization of Camp−/− BMDMs to CRAMP treatment in response to LPS stimulation (supplementary material, Figure S4A). Additionally, CRAMP peptide inhibited LPS-induced IL-1β in the cultural media in both WT and Camp−/− BMDMs (supplementary material, Figure S4B). To further confirm our observations in BMDMs, we extended these ex vivo experiments to in vitro using RAW264.7 macrophages. CRAMP peptide significantly reduced the protein level of NLRP3 in a dose-dependent manner (supplementary material, Figure S4C). Pro-caspase-1 protein levels were moderately reduced by CRAMP peptide. Remarkably, CRAMP peptide completely inhibited the pro-IL-1β protein expression by LPS at both high and low doses (supplementary material, Figure S4C). Similar to our observations in LPS-treated Camp−/− BMDMs, CRAMP peptide treatment downregulated LPS-induced Il1b, Il6, and Tnf mRNA expression in a dose-dependent manner (supplementary material, Figure S4D). Importantly, the protein level of IL-1β in the LPS-treated RAW264.7 cell culture medium was decreased by CRAMP peptide treatment (supplementary material, Figure S4E).
We next sought to test whether CRAMP peptide can suppress UA-exacerbated IL-1β production in LPS-primed BMDMs. To exclude the effects of CRAMP peptide on LPS binding, cells were washed with PBS after LPS treatment and immediately exposed to UA and CRAMP peptide in fetal bovine serum-free media. The addition of UA to LPS-primed BMDMs dramatically increased cellular cleaved IL-1β expression, which was significantly reduced by CRAMP peptide (Figure 3H, left panel). IL-1β in the medium was increased by LPS and further upregulated by UA, which was completely inhibited by CRAMP peptide (Figure 3H, right panel). Similar inhibitory effects of CRAMP peptide on LPS- and UA-induced IL-1β production were also found in RAW264.7 macrophages (supplementary material, Figure S5A,B). In addition, we also showed that CRAMP peptide decreased ATP-induced IL-1β release from LPS-primed RAW264.7 cells (supplementary material, Figure S5C). These results suggest that CRAMP deficiency exacerbated inflammasome activation by enhancing the LPS delivery to macrophages and by causing UA dysregulation in alcohol-fed mice that leads to elevated IL-1β production (which was inhibited by CRAMP peptide treatment).
CRAMP deficiency exacerbated alcohol-induced oxidative stress
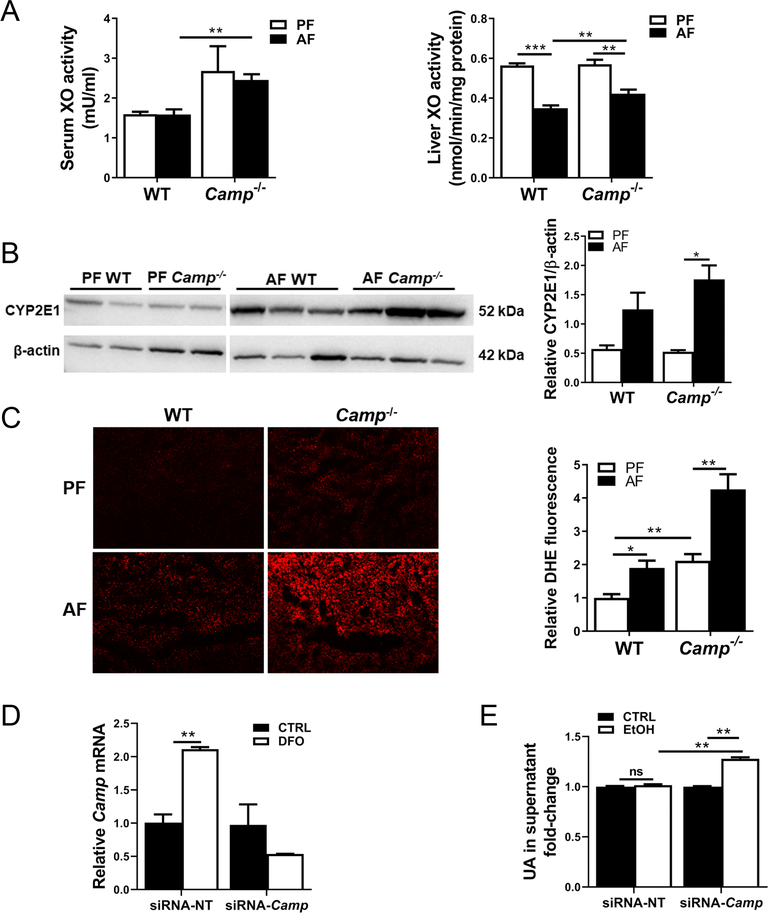
Alcohol metabolism-generated reactive oxygen species is a hallmark in ALD development and progression [42]. UA metabolism is regulated by xanthine oxidase (XO), an important enzyme essential for the endogenous production of UA as a final product of purine metabolism. No changes were observed in serum XO activity by alcohol feeding of either WT or Camp−/− mice when compared with their respective pair-feeding controls, but it was significantly increased in alcohol-fed Camp−/− mice compared with their WT controls (Figure 4A, left panel). Alcohol feeding decreased liver XO activity in both WT and Camp−/− mice. However, Camp−/− mice had significantly increased liver XO activity by alcohol feeding compared with the WT mice (Figure 4A, right panel). Alcohol feeding statistically insignificantly increased hepatic cytochrome P450 2E1 (CYP2E1) protein levels in WT mice, whereas they significantly increased in Camp−/− mice (Figure 4B). Camp−/− mice showed increased hepatic dihydroethidium (DHE, a superoxide marker) staining, which was further increased by alcohol feeding (Figure 4C). These results indicated increased oxidative stress in Camp−/− mice. We then confirmed these observations in an in vitro study using a mouse hepatocyte cell line, Hepa1–6. Desferrioxamine (DFO), a HIF-1α activator, significantly increased CRAMP expression in control siRNA-transfected cells, but not in the Camp-siRNA-transfected cells (Figure 4D). Importantly, knockdown of CRAMP resulted in an increase in UA concentration in the culture supernatant of Hepa1–6 cells treated with ethanol (Figure 4E). Taken together, these results suggest that CRAMP deficiency causes exacerbated oxidative stress that may partially contribute to increased levels of hepatic UA.
Figure 4.
CRAMP KO mice had increased hepatic oxidative stress by alcohol. (A) Xanthine oxidase (XO) activity in the serum and liver lysates of alcohol-fed mice. (B) Immunoblotting and quantification of CYP2E1 protein. The bands are composite images of selected bands, and the corresponding β-actin controls are shown. (C) Liver ROS production and quantification by DHE staining. Original magnification: 100×. Data are expressed as mean SEM (n = 8–10). (D) Validation of knockdown efficiency by siRNA-Camp transfection in desferrioxamine (DFO)-treated mouse hepatocyte Hepa1–6 cells. (E) Ethanol-induced UA production in Hepa1–6 culture supernatant. siRNA-NT: non-targeting siRNA control; siRNA-Camp: Camp-targeting siRNA; EtOH: ethanol (400 mM for 4 h). Data are expressed as mean SEM (n = 4–6).
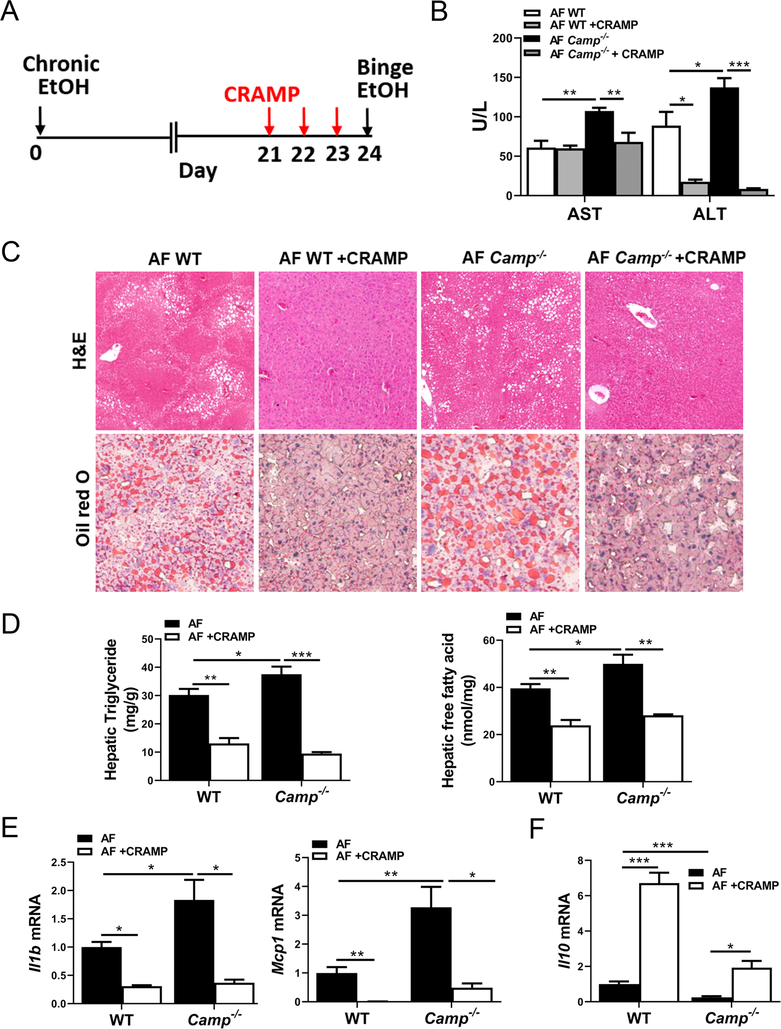
CRAMP peptide administration reversed alcohol-induced liver steatosis and injury
We examined whether administration of CRAMP peptide to WT and Camp−/− mice could attenuate alcohol-induced liver damage. Administration of synthetic CRAMP peptide for 3 days (Figure 5A) marginally suppressed the alcohol feeding-increased Saccharibacteria abundance and restored the Akkermansia abundance in Camp−/− mice, while no change was found in Proteobacteria (data not shown). The total bacteria load in fecal samples was not changed (supplementary material, Figure S2). CRAMP peptide treatment did not alter serum AST levels but significantly reduced ALT levels in WT mice fed alcohol (Figure 5B). In Camp−/− mice, both AST and ALT levels were markedly decreased by CRAMP peptide (Figure 5B). CRAMP peptide administration robustly reduced alcohol-induced hepatic fat accumulation in both WT and Camp−/− mice (Figure 5C), and this was confirmed by the measurements of hepatic triglyceride and free fatty acid contents (Figure 5D). CRAMP peptide treatment significantly reduced hepatic mRNA expression of Il1b and Mcp1 in WT and Camp−/− mice (Figure 5E). In contrast, anti-inflammatory cytokine Il10 mRNA expression was upregulated by CRAMP peptide in both WT and Camp−/− mice (Figure 5F).
Figure 5.
CRAMP peptide administration rescues alcohol-induced liver steatosis and injury. Mice were treated as described in the Materials and methods section. (A) Illustration of feeding and treatment timeline. (B) Serum levels of AST and ALT. (C) Representative photomicrographs of liver sections of H&E (original magnification 100×) and ORO staining (original magnification 200×). (D) Hepatic levels of triglycerides and free fatty acids. (E) Hepatic mRNA expression of Il1b and Mcp1. (F) Hepatic mRNA expression of Il10. Data are expressed as mean SEM (n = 4–8).
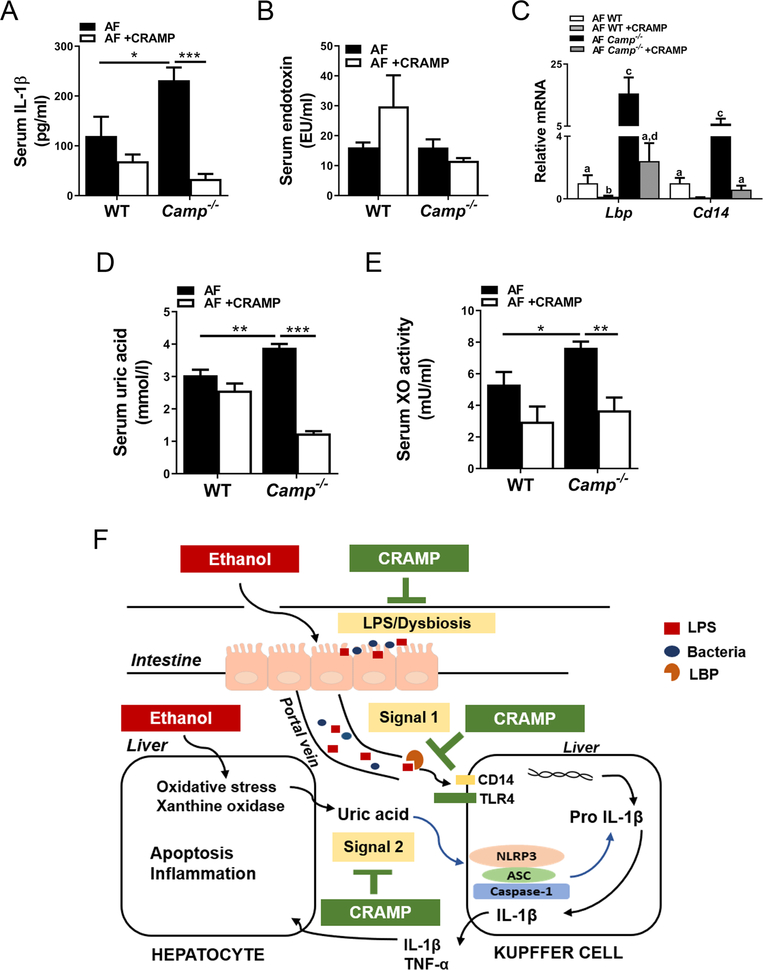
CRAMP peptide administration attenuated alcohol-induced IL-1β production via inhibiting inflammasome activation
Serum IL-1β concentration was marginally decreased in the WT mice but robustly decreased in the Camp−/− mice by CRAMP peptide treatment (Figure 6A), indicating reduced inflammasome activation. Serum LPS levels were not significantly altered by CRAMP peptide (Figure 6B), but Lbp and Cd14 mRNA expression was significantly reduced in both WT and Camp−/− mice (Figure 6C). In addition, CRAMP peptide dramatically decreased serum UA levels and XO activity in Camp−/− mice but only marginally in the WT mice (Figure 6D,E).
Figure 6.
CRAMP peptide administration attenuates alcohol-induced IL-1β production via inhibiting inflammasome activation signaling. (A) Serum IL-1β levels. (B) Serum LPS levels. (C) Hepatic mRNA expression of Lbp and Cd14. (D) Serum UA levels. (E) Serum XO activity. (F) Proposed scheme for the effects of CRAMP on ALD. Data are expressed as mean SEM (n = 4–8).
Discussion
Hepatic inflammation is a hallmark of ALD development. Alcohol exposure reduces antimicrobial peptide expression and causes intestinal bacterial dysbiosis, immune dysregulation, and gut barrier dysfunction, which, in turn, leads to increased endotoxin release and hepatic bacterial translocation that activate TLR-4 on Kupffer cells and causes liver inflammation. In addition to LPS-induced TLR activation, recent studies have demonstrated that endogenous danger molecules are critical in hepatic inflammasome activation in ALD development [41]. Here we showed that CRAMP plays a role in both LPS-mediated initiation and UA-mediated maturation of inflammasome activation in mice fed alcohol.
Alcohol exposure induces gut dysbiosis, which is largely attributed to the reduced expression of antimicrobial factors. Reg3 lectins represent an important family of gut-derived AMPs, and depleting Reg3b and Reg3g exacerbated, while increasing Reg3g expression attenuated, gut dysbiosis and alcohol-induced liver injury [9]. A recent study further showed that alcohol feeding caused α-defensin dysfunction, which was critically involved in the pathogenesis of alcoholic hepatitis in mice [12]. LL-37 (CRAMP in mouse) is the only member in the human cathelicidin AMP family, which is transcriptionally regulated by vitamin D receptor and hypoxia-inducible factor 1α (HIF-1α). Our recent study demonstrated that intestinal HIF-1α plays an important role in maintaining gut microbiota homeostasis and gut barrier function by activating barrier-protecting factors, such as CRAMP, β-defensins, cluster of differentiation 73 (CD73), and claudin-1. Depleting HIF-1α in the intestine exacerbated ALD in mice accompanied by a reduction of CRAMP, while activation of HIF-1α increased CRAMP and attenuated ALD [23]. We hypothesized that CRAMP may represent another important AMP in alcohol-induced gut dysbiosis in ALD. Indeed, alcohol exposure induced a significant decrease of Bacteroidetes and an increase of Firmicutes in Camp−/− mice compared with WT mice, as has been demonstrated in previous studies [33,37]. Ferrere et al showed that the decreased fecal Bacteroidetes/Firmicutes ratio occurred only in alcohol-sensitive mice but not in resistant mice [37]. It is thus possible that CRAMP deficiency sensitizes the mice to alcohol exposure, which contributes to the gut dysbiosis and ALD. At the genus level, we found that Akkermansia was decreased in Camp−/− mice. Although the role of Akkermansia in ALD remains elusive, it has been shown that Akkermansia abundance declined in both ALD patients and mice fed chronic alcohol, and supplementation with Akkermansia showed a protective effect in ALD [43]. Our data suggest a critical involvement of CRAMP in maintaining the homeostasis of gut microbiota by selectively inhibiting bacterial growth in ALD. Fecal microbiota transplantation between Camp−/− and WT mice could be performed to further elucidate the role of CRAMP-mediated microbiota changes in ALD.
Reg3γ and Reg3β are well-studied antimicrobial peptides. Our results showed that Reg3g and Reg3b mRNA expression was markedly decreased when CRAMP was deleted (supplementary material, Figure S6), indicating that there is no compensatory increase but an inhibitory effect on Reg3 expression when CRAMP is deficient. Unlike Reg3, which is expressed mainly in the intestine, CRAMP is expressed in multiple organs, including the intestine, lung, liver, spleen, and adipose tissue. Alcohol treatment increased hepatic but decreased intestinal CRAMP expression in mice. In contrast to intestinal HIF-1α [23], the role of hepatic HIF-1α in lipid metabolism in ALD is the subject of conflicting reports [44–46]. It is known that alcohol exposure induces hepatic hypoxia [47], which then increases HIF-1α expression and upregulates a variety of genes, including CRAMP, that have either deleterious or protective roles. The induction of hepatic Camp in ALD mice is likely a protective mechanism against alcohol-induced injury, since CRAMP deficiency exacerbated ALD. Agreeing with the study in mice, we showed that patients with ALD had higher hepatic CAMP mRNA expression.
We found that depleting CRAMP in mice fed alcohol led to an exacerbated ALD response accompanied by an increased hepatic inflammasome activation, which requires two signals. LPS activation of TLR4 initiates Signal 1, and endogenous, sterile molecules are required to activate caspase-1 to produce mature IL-1β, which kills hepatocytes. Interestingly, although we found dysbiosis in Camp−/− mice exposed to alcohol, serum LPS levels were not altered, indicating that CRAMP deficiency-exacerbated ALD is likely independent of gut barrier function. Instead, hepatic mRNA expression of LBP and CD14 was significantly increased in Camp−/− mice. LBP is an acute phase protein that presents LPS to surface pattern recognition receptors, such as CD14 and TLR4, to elicit immune response [48]. Increased LBP and CD14 in Camp−/− mice suggests a functional increase of LPS by increasing LPS binding to its activation complex. Indeed, CRAMP peptide inhibited LPS binding to macrophages in a dose-dependent manner. These results demonstrate that increased CRAMP in the liver in response to alcohol might serve as an inhibitory mechanism for LPS activation.
Furthermore, our data showed significantly elevated serum IL-1β protein and hepatic Nlrp3 and Caspase-1 mRNA expression in Camp−/− mice, indicating an activation of Signal 2 in alcohol-induced inflammasome activation. Previous research demonstrated that UA or ATP as Signal 2 molecules plays a causal role in the liver inflammation caused by acetaminophen, ischemia–reperfusion injury, and alcohol consumption [41]. Agreeing with these studies, we showed that serum IL-1β levels are positively correlated with levels of UA in patients with ALD, and UA combined with LPS induced a robust elevation of mature IL-1β levels, further demonstrating the requirement of two signals in IL-1β production. Most importantly, mice lacking CRAMP that were fed alcohol had an elevation of serum UA levels, and CRAMP peptide treatment lowered the UA concentration and blocked the IL-1β elevation. UA metabolism is regulated by multiple mechanisms. Our data suggest that the elevation of UA in Camp−/− mice fed alcohol is likely attributed to the increased XO activity resulting from alcohol-induced oxidative stress. Taken together, our data suggest that CRAMP inhibits inflammasome activation by decreasing UA production in mice with experimental ALD. There are intrinsic mechanisms that can control alcohol-induced liver inflammation. We showed that alcohol-increased hepatic CRAMP expression serves as an adaptive response to inhibit both LPS activation and UA-mediated Signal 2 activation in alcohol-induced inflammasome activation.
Our data demonstrate that exogenous CRAMP administration reduces alcohol-induced hepatic steatosis, which is in line with previous research showing that lentivirus-mediated CRAMP overexpression reduced hepatic fat accumulation in obese subjects [49]. We further demonstrated that CRAMP peptide reduces alcohol-induced LPS and inflammasome activation by decreasing hepatic Lbp and Cd14 expression and UA production. These findings suggest that CRAMP may represent a novel drug target for treating fatty liver and liver diseases related to hepatic inflammation.
In summary, our results identify the protective role of LL-37/CRAMP in alcohol-induced liver inflammation, steatosis, and injury by maintaining gut microbiota homeostasis and by attenuation of inflammasome activation via inhibiting LPS activation and UA production (Figure 6F). The knowledge gained from the present study advances our understanding of the role of CRAMP in alcohol-induced liver injury and helps in developing potential therapeutic strategies against ALD.
Supplementary Material
Table S1. Primers used in real-time RT-PCR
Figure S1. Zonal distribution of alcohol-induced hepatic fat accumulation in Camp−/− and WT mice
Figure S2. Fecal microbiota changes in CRAMP KO mice in response to alcohol feeding
Figure S3. Selective inhibition of CRAMP peptide on bacterial growth
Figure S4. Synthetic CRAMP peptide attenuates inflammasome activation in macrophages
Figure S5. Synthetic CRAMP peptide inhibits uric acid and ATP-induced IL-1β production in RAW264.7 macrophages
Figure S6. CRAMP KO mice had lower ileal Reg3g and Reg3b mRNA expression compared with WT mice
Acknowledgements
We thank Marion McClain for proofreading the manuscript. This study was supported by NIH grants R01AA023190 (WF), U01AA021901, U01AA0218 93-01, U01AA022489-01A1, R01AA023681, P20GM 113226, and P50AA024337 (CJM), R01DK115406 (Z-BD), R01AA021434 (S-YC), K23AA021179 (PP) and R24AA025017-01. Support was also provided by the VA (1I01BX002996, CJM).
Footnotes
No conflicts of interest were declared.
SUPPLEMENTARY MATERIAL ONLINE
Supplementary materials and methods
Reference 50 is cited only in the supplementary material.
References
- 1.Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009; 373: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011; 141: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo G Gut–liver axis in alcoholic liver disease. Gastroenterology 2015; 148: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurman RG, Bradford BU, Iimuro Y, et al. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol 1998; 13(suppl): S39–S50. [PubMed] [Google Scholar]
- 5.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep 2011; 13: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta 1999; 1462: 71–87. [DOI] [PubMed] [Google Scholar]
- 7.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathi A, Debelius J, Brenner DA, et al. The gut–liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 2018; 15: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Fouts DE, Starkel P, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016; 19: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011; 53: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrikx T, Duan Y, Wang Y, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019; 68: 1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong W, Wei X, Hao L, et al. Paneth cell dysfunction mediates alcoholic steatohepatitis through promoting bacterial translocation in mice: role of zinc deficiency. Hepatology 2020; 71: 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos JC, Silva-Gomes S, Silva JP, et al. Endogenous cathelicidin production limits inflammation and protective immunity to Mycobacterium avium in mice. Immun Inflamm Dis 2014; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Does AM, Bergman P, Agerberth B, et al. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol 2012; 92: 735–742. [DOI] [PubMed] [Google Scholar]
- 15.Tran DH, Wang J, Ha C, et al. Circulating cathelicidin levels correlate with mucosal disease activity in ulcerative colitis, risk of intestinal stricture in Crohn’s disease, and clinical prognosis in inflammatory bowel disease. BMC Gastroenterol 2017; 17: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura T, McLean MH, Dzutsev AK, et al. The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunol 2018; 200: 2174–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Furio L, Mecheri R, et al. Pancreatic beta-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015; 43: 304–317. [DOI] [PubMed] [Google Scholar]
- 18.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015; 12: 387–400. [DOI] [PubMed] [Google Scholar]
- 19.Szabo G, Iracheta-Vellve A. Inflammasome activation in the liver: focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol 2015; 39(suppl 1): S18–S23. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep 2010; 10: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Z, Murakami T, Suzuki K, et al. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS One 2014; 9: e85765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki K, Murakami T, Hu Z, et al. Human host defense cathelicidin peptide LL-37 enhances the lipopolysaccharide uptake by liver sinusoidal endothelial cells without cell activation. J Immunol 2016; 196: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 23.Shao T, Zhao C, Li F, et al. Intestinal HIF-1α deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J Hepatol 2018; 69: 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Liu Y, Sidhu A, et al. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 2012; 303: G32–G41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Vatsalya V, Gobejishvili L, et al. Porphyromonas gingivalis as a possible risk factor in the development/severity of acute alcoholic hepatitis. Hepatol Commun 2018; 3: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez WE, Wahlang B, Wang YL, et al. Phosphodiesterase 4 inhibition as a therapeutic target for alcoholic liver disease: from bedside to bench. Hepatology 2019; 70: 1958–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vatsalya V, Cave MC, Kong M, et al. Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clin Gastroenterol Hepatol 2020; 18: 2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan D, Coughlin LA, Neubauer MM, et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med 2015; 21: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005; 19: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 30.Wang HJ, Gao B, Zakhari S, et al. Inflammation in alcoholic liver disease. Annu Rev Nutr 2012; 32: 343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol 2012; 2012: 853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011; 29: 71–109. [DOI] [PubMed] [Google Scholar]
- 33.Bull-Otterson L, Feng WK, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013; 8: e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Torralba M, Tan J, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015; 148: 203–214.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bode C, Kolepke R, Schäfer K, et al. Breath hydrogen excretion in patients with alcoholic liver-disease – evidence of small intestinal bacterial overgrowth. Z Gastroenterol 1993; 31: 3–7. [PubMed] [Google Scholar]
- 36.Bajaj JS, Hylemon P, Heuman DM, et al. The cirrhosis dysbiosis ratio provides insight into gut microbiome changes across the spectrum of cirrhosis: a prospective study of 250 patients. Hepatology 2013; 58: 274a–274a. [Google Scholar]
- 37.Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 2017; 66: 806–815. [DOI] [PubMed] [Google Scholar]
- 38.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011; 145: 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2012; 302: G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullen SP, Kearney CJ, Clancy DM, et al. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep 2015; 11: 1535–1548. [DOI] [PubMed] [Google Scholar]
- 41.Iracheta-Vellve A, Petrasek J, Satishchandran A, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 2015; 63: 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 2009; 83: 519–548. [DOI] [PubMed] [Google Scholar]
- 43.Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018; 67: 891–901. [DOI] [PubMed] [Google Scholar]
- 44.Ouyang X, Mehal WZ. The multi-dimensional role of intestinal HIFs in liver pathobiology. J Hepatol 2018; 69: 772–773. [DOI] [PubMed] [Google Scholar]
- 45.Nishiyama Y, Goda N, Kanai M, et al. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol 2012; 56: 441–447. [DOI] [PubMed] [Google Scholar]
- 46.Nath B, Levin I, Csak T, et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 2011; 53: 1526–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arteel GE, Iimuro Y, Yin M, et al. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology 1997; 25: 920–926. [DOI] [PubMed] [Google Scholar]
- 48.Su GL, Rahemtulla A, Thomas P, et al. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol 1998; 152: 841–849. [PMC free article] [PubMed] [Google Scholar]
- 49.Hoang-Yen Tran D, Hoang-Ngoc Tran D, Mattai SA, et al. Cathelicidin suppresses lipid accumulation and hepatic steatosis by inhibition of the CD36 receptor. Int J Obes (Lond) 2016; 40: 1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ying W, Cheruku PS, Bazer FW, et al. Investigation of macrophage polarization using bone marrow derived macrophages. J Vis Exp 2013; (76): 50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in real-time RT-PCR
Figure S1. Zonal distribution of alcohol-induced hepatic fat accumulation in Camp−/− and WT mice
Figure S2. Fecal microbiota changes in CRAMP KO mice in response to alcohol feeding
Figure S3. Selective inhibition of CRAMP peptide on bacterial growth
Figure S4. Synthetic CRAMP peptide attenuates inflammasome activation in macrophages
Figure S5. Synthetic CRAMP peptide inhibits uric acid and ATP-induced IL-1β production in RAW264.7 macrophages
Figure S6. CRAMP KO mice had lower ileal Reg3g and Reg3b mRNA expression compared with WT mice