ABSTRACT
Introduction: SARS-CoV-2, the new coronavirus that originated in 2019, continues to impact every aspect of society in a profound manner. Testing will remain an important tool to mitigate the effects of this pandemic as early and accurate diagnosis can lead to appropriate countermeasures to reduce mortality and morbidity. However, testing isn’t a simple yes/no answer as the target and host are complex, the virus is a moving target, there is a plethora of tests that identify different parts of the virus and have their own limits and range of detection, and when prevalence is low, false positives and negatives can be very high.
Areas covered: This article covers all the major questions related to COVID-19 diagnostics, the why, when, where, who, what and how of testing, the different types of tests, interpretation of results and the ideal ASSURED-SQVM diagnostic. A comprehensive literature review using all the publicly available databases and government websites and reports was performed.
Expert opinion: Diagnostics that meet the ‘ASSURED-SQVM’ (Affordable, Selective and Sensitive, User-friendly, Rapid and Robust, Equipment-free, Deliverable to end-users and additionally, allows for Self-testing, Quantifiable, detects if pathogens are Viable and can detect Multiple pathogens) would make a major impact in our fight against the current pandemic. While a significant majority of researchers focus on developing novel diagnostics that are highly selective and sensitive, it is the opinion of these authors that other aspects of the ASSURED-SQVM principles also be considered early in the development process for widespread use.
KEYWORDS: Covid-19, pandemic, diagnostics, influenza, SARS-CoV-2, rapid diagnostics, PCR, ASSURED-SQVM
1. Introduction
COVID-19, the disease caused by the new coronavirus, SARS-CoV-2, has caused significant human and economic burden. As of 1 March 2021, the number of confirmed cases worldwide is over 110 million and 2.5 million deaths [1,2]. Approximately 7.2 billion human beings live on this planet, indicating that a very small percentage, 1.0%, are infected with the virus. Albeit this low percentage, the high transmissibility, complex patchwork of quarantine measures in different communities, unanswered questions related to long-term immunity and potential antibody enhancement mechanisms of infection indicate that it is very likely that SARS-CoV-2 will continue to create havoc on our lives for several years. The ferocity of this virus cannot be overstated as it has been demonstrated to infect multiple organs with significant damage to the lungs early in the infection process [3,4]. Of the three major methods to mitigate the effect of the virus, diagnostics have become the focal point because vaccines and therapeutics, despite the intense effort of scientists worldwide, will take months to develop, test for safety and efficacy, scale up and distribute to millions [5,6]. Testing has taken center stage as a growing chorus of economists and policy-makers have emphasized that shutdowns are not a long-term solution as ~33 million or ~23% of the working population filed for unemployment in the United States within 5 weeks of the outbreak [7,8]. ‘Immunity passports’ based on current diagnostics are being suggested as one of the potential ways to reopen the economy [6,9]. However, diagnostics for COVID-19 cannot be classified as a simple yes/no answer due to the complex nature of this disease. In addition, the general population (including some healthcare professionals) are not fully aware of the intricacies of testing, differences between analytical and clinical specificity and sensitivity, limitations and differences in tests, and how tests assist in the clinical decision-making process by a professional. This perspective aims to clarify some of the issues related to COVID-19 diagnostics and highlights some of the challenges key stakeholders including policymakers, businesses, managers, scientists and individuals face during this evolving pandemic.
1.1. Why test?
While this is a fundamental question for most people, a significant percentage of the populace are prone to asking these questions (i) Why test when vaccines or therapies to treat the disease are unavailable? (ii) The tests are not 100% accurate, so why take the risk and be quarantined for 14 days when I have very limited or no financial resources? (iii) The cost of an excellent test is prohibitively expensive, especially if testing is required throughout the quarantine period, how can I afford it? and (iv) Why can’t we just wait for herd immunity?
1.2. Health is wealth
The primary reason for testing in an outbreak is to alleviate the health and economic burden for the individual and the entire community [10,11]. The society as a whole, and economic activity will invariably suffer if a significant population is sick and the healthcare system is overburdened. Good health precedes economic activity and growth. Economically, it is cost-effective to quarantine a positive SARS-CoV-2 patient and conduct an aggressive contact tracing program [12,13]. Unfortunately, the silent and rapid transmission of this strain limited this first and best option. The next obvious step was to initiate shutdowns and entire countries were asked to shelter in place. Initially, these shutdowns were mandated to decrease the healthcare burden because most communities did not have sufficient protective equipment, ventilators and resources to fight against the virus [14]. Emerging countries still do not have sufficient hospital beds for their communities. In wealthier nations, governments have increased their stockpiles of essential medical supplies and equipment, leading to the question of why test now when there are sufficient ICUs, beds and other equipment for a projected spike in number of cases? Unfortunately, even in advanced countries with significant resources, skilled personnel need to work around the clock to monitor patients with ARDS (Acute Respiratory Distress Syndrome). There is a shortage of healthcare professionals with specialized training [15]. The argument of a low death rate of 1–2% fades when it is estimated that ~10–20% of positive SARS-CoV-2 patients will suffer from ARDS and –require hospitalization and 2–5% requires major intervention and ICU care [4,16]. The intervention requires experts from different specialties; for example, if a patient is admitted to a medical centre with respiratory distress and a stroke caused by complications related to COVID-19, the individual will require a team of professionals. The team includes an emergency care physician, respiratory therapist, pulmonologist, primary care physician, a neurologist, a cardiologist, a rehabilitation physician, case manager, social worker, nurses and technicians. The economic burden for the patient can mount upto thousands of dollars, depending on the case severity. From a societal perspective, insurance companies and hospitals may have to close [17,18]. As an example, if a community of 100,000 inhabitants are exposed to the virus, ~10,000–20,000 will require hospitalization and 1000–2000 will require ICU admission. It is almost impossible for any healthcare system to manage such a high volume of patients for days or weeks, even if hospitalizations occur over months [19]. In addition to patients, the high concentration of the virus in patients increases exposure to healthcare professionals. When a patient is suffering from ARDS and requires intubation, close patient contact is unavoidable [17,20]. The virus concentration in the ICU, where intubation and other invasive procedures are being performed, is extremely high, leading to a high rate of healthcare professional infections. The economic and health burden increases exponentially when healthcare professionals are infected. Healthcare professionals may have to be hospitalized or quarantined and the hospital will have fewer staff members to care for patients [21].
2. When to test
SARS-CoV-2 enters the host through the ACE-2 receptors on epithelial cells present in the respiratory tract, resulting in nonspecific symptoms, such as a mild cold, headache and general malaise [22,23]. In most people, particularly in children and young adults under the age of 19, these mild symptoms fade with time. A significant number of adults are asymptomatic. However, ~20% of infected individuals develop moderate to severe symptoms which include a fever that doesn’t subside easily, headache, diarrhea and loss of smell and taste in some individuals [23]. Interestingly, the severity increases suddenly in some individuals after 5–10 days. This presents a unique testing challenge for several reasons. (1) If an individual is asymptomatic or has mild symptoms resembling that of a common cold, he/she may not seek medical care until the infection has progressed. During this time, the individual will unwittingly spread the virus to others. (2) A significant percentage of the population is unaware of underlying conditions such as hypertension or diabetes. These patients are more likely to require medical attention or become hospitalized, but only after the virus has established a foothold in their bodies [24,25]. (3) At the present time, the median time to clear the virus is not established and is most likely dependent on the health of the individual, with the caveat that healthy individuals are not refractory to contracting severe disease [26]. Given these features of the virus, testing must be performed early, regularly and randomly in communities with limited number of known cases and even in communities where the number of new cases has apparently decreased significantly. In communities with a large number of cases, a combination of shelter in place coupled with aggressive large-scale and random testing for the presence of the virus and for antibodies produced against the virus must be performed.
The three major classes of diagnostics, i.e. tests for viral nucleic acid, antibody and inflammatory biomarkers, are available for different times and provide uniquely distinct information [27,28]. (Figure 1) For example, the timeline for antibody testing doesn’t follow the timeline for virus testing as it takes 5–10 days after onset of infection for the body to produce sufficient antibody concentration [29]. Additionally, test sensitivity must also be taken into consideration; if the limit of detection of the test is higher than the viral concentration at the time of sample collection, a false-negative result will be recorded. In contrast, if a test has demonstrated to have an extremely low limit of detection, there is an extremely high likelihood that cross-reactivity increases, leading to false-positive results. Therefore, appropriate tests must be used at different times with tests that have excellent limit of detection with minimal or no cross-reactivity.
Figure 1.
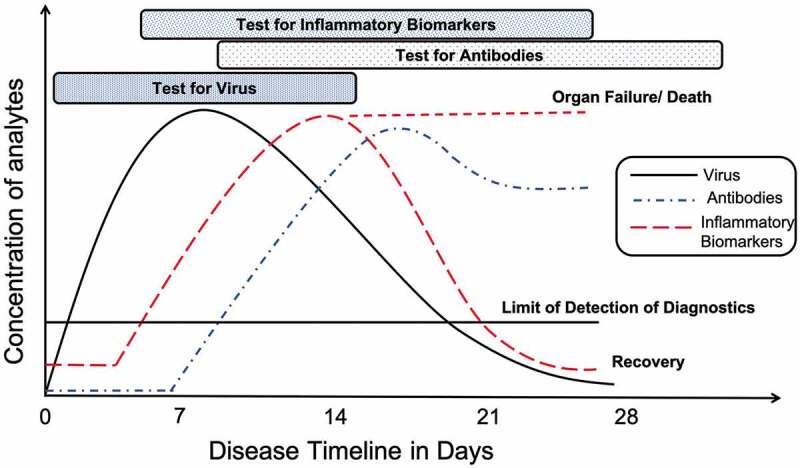
Timeline and concentration of analytes for diagnostic assays after onset of SARS-CoV-2 infection at day zero
3. Where to test
While an ideal approach may be feasible for smaller communities, it is unrealistic to produce and distribute millions of tests for larger communities and countries. A stratification strategy is most pertinent. Testing must be conducted in localities that have been demonstrated to have a high incidence of virus spread and places that cannot strictly adhere to social distancing. These locations include hospitals, emergency rooms, nursing homes and assisted living facilities, retirement communities, food processing industries, grocery chains, prisons, cruise ships, refugee camps, schools, and universities. The common denominator of all these places is high human traffic and interactions in close quarters. Nursing homes and assisted living facilities present unique challenges because close contact between caregivers and residents cannot be avoided [21,30]. Once the virus establishes a foothold in a nursing home, it spreads rapidly, has a very high death rate and is proving to be very difficult to eliminate. Unfortunately, caregivers and residents are falling sick at very high rates. These places require stringent testing.
4. Who to test
It is unrealistic to test millions of people daily. A stratification approach must be undertaken with testing prioritized for high-risk individuals and individuals who are constantly being exposed to the virus [31]. The hospital setting ranks among the top. In a hospital setting, all essential personnel who are working in the hospital must be tested for three reasons: (i) Protecting the health and well-being of the healthcare professional is of the highest importance because they are on the frontlines of this war. Virus concentration is very high in a hospital setting as a significant percentage of patients are spreading the virus. While healthcare professionals are taking all precautions and using protective equipment, accidental exposure can occur in emergency situations where a high volume of patients need assistance [21]. (ii) If a healthcare professional is tested positive, he/she will have to be quarantined, which means one less person to support an already strained healthcare system. (iii) Since a large proportion have no or mild symptoms, healthcare professionals might be passing infections to other patients and their families. The first category includes individuals in very important positions who keep society stable and functioning; these include high-ranking government officials who set and execute policies, researchers and manufacturers developing vaccines, therapeutics, protective equipment and supplies, and people who provide security and maintain food supply chains. However, not everyone in these positions require testing, and daily testing may not be essential as these individuals do not come in regular contact like the hospital professionals. Second, healthcare professionals working in a high-risk community setting, such as nursing homes, assisted living facility, etc., because the virus spreads rapidly. Caregivers are generally in very close contact with the individual performing tasks (such as providing medication, cleaning, showering, etc.) that require very close human contact and therefore transmission is at its peak [30]. Third, professionals at retail settings, specifically all individuals working in areas where customer interactions are high, should be tested daily [31]. The reason is to limit spread: if a server in a restaurant is sick, more spread will occur. Industries where personnel interactions cannot be avoided, but can only be minimized, fall into the same category. The fourth priority for testing should be citizens with co-morbidities since it has been proven that the virus exacts a significant toll on those with underlying health conditions [24,25]. Within this group, age is also a factor that must be considered, because hospitalizations and death rates increase with age. If a new infection is detected, the individual must be quarantined and tested daily to determine if virus concentration is increasing or decreasing. The family and friends of the individual must also be tested and, if tested positive for SARS-CoV-2, they must be quarantined and tested regularly to limit spread. We note that the stratification strategies listed here are designed from the perspective of limiting spread, protecting vulnerable individuals and key personnel essential to the normal functioning of society.
5. What to test
Samples to test can be broken down into human and environmental samples. Testing in the environment is particularly useful in areas where space and resources are limited, such as refugee camps, prisons, assisted living facilities and high-density population centres, to ensure that specific areas are decontaminated [32,33]. For testing in humans, noninvasive samples are, by far, the best sample source as these samples do not require invasive techniques, assuming the tests are of equal precision. SARS-CoV-2 infects the upper and lower respiratory system and therefore, nasopharyngeal and throat swabs are optimal for direct detection of the virus [34]. Sputum is also a good sample source, although it may be difficult to collect samples from very sick patients. Coughing is very difficult for patients with respiratory distress; a similar issue has been well documented for TB patients [35]. However, sputum samples can be collected from individuals with mild or no symptoms. Other noninvasive samples are stool samples. Unfortunately, the virus is not seen in urine samples. Invasive samples include bronchoalveolar lavage fluid specimens, fibrobronchoscope brush biopsy and blood (with the caveat that the concentration of the virus in blood is low) [36]. Direct testing for virus presence in these invasive samples is not preferred. CT lung scans as noninvasive testing have shown significant promise as the lungs of infected patients show significant opaque lesions, but have mainly been used in limited hospital settings, due to low throughput and upfront instrument costs [37].
To determine if an individual has been exposed to the virus regardless of their symptoms and/or for surveillance purposes, antibody testing holds the key. Capillary blood is the current sample source, although the ideal test would be for the presence of IgA in saliva or sputum samples as it is noninvasive [36,38,39]. However, the concentration of IgA in saliva or sputum may be lower than blood samples. Testing for antibodies can be classified broadly into two types: binding and neutralizing antibodies. The latter becomes more relevant if it is used to determine if the individual has immunity upon reinfection by the same or similar strains [9,38,40].
To test if a SARS-CoV-2 positive individual requires intensive intervention, testing for serum inflammatory biomarkers is the ideal candidate as the cytokines, chemokines and enzymes are mainly released in the blood [41]. Urine and saliva are being considered as alternate sample sources to test for inflammatory biomarkers, but research on those sample sources is still evolving [42]. A personalized testing approach using multiple biomarkers and quantification is important since some of the biomarkers may already be at an elevated in patients with chronic conditions, several of which are related to dysregulated immune systems.
6. How to test
To test for the presence of a viral RNA, nasal and/or throat swabs are collected and sent to a central laboratory for processing. The samples are subjected to rRT-PCR analysis. Briefly, viral RNA present in the sample is amplified exponentially using a specific set of primers and detection using fluorescence dyes is performed [43]. There are several variations and improvements to the basic amplification process using LAMP, SAMBA, etc., to reduce time, number of steps and user-friendliness, but the basic principle relies on amplification followed by detection [44,45]. Automation and integration have resulted in systems that have improved user-friendliness and trained personnel are not required. These systems have been developed for a variety of diseases and are being adapted for SARS-CoV-2 [46] (Figure 2).
Figure 2.
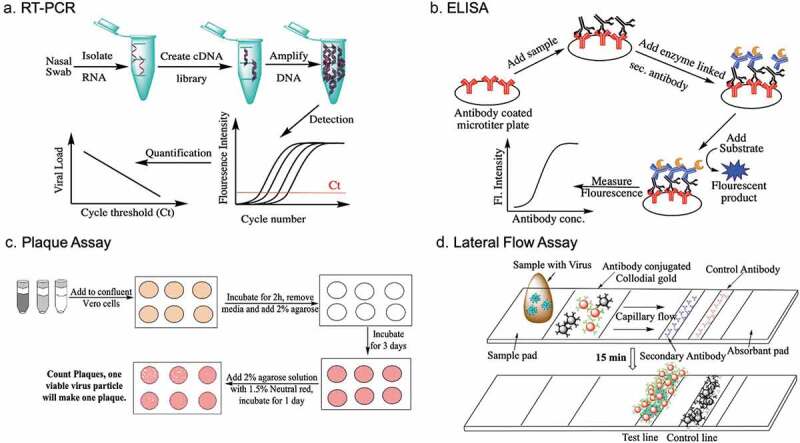
Schematic of some of the tests used to detect SARS-CoV-2. Please note that plaque assays are meant ONLY for research purposes in specialized biosafety laboratories and not for POC diagnostics
To test for the presence of antibodies generated by the body against SARS-CoV-2, whole blood or serum is collected. An ELISA (Enzyme Linked Immunosorbent Assay) is used to determine the presence of antibodies. Briefly, viral antigens are attached to the surface of microwells of a microtiter plate. The sample is added and after a set time, washed to remove nonspecific binding proteins. Enzyme-linked Anti-IgG or anti-IgM antibodies or a combination of enzyme-linked antibodies are added, followed by a washing step and a final addition of the substrate, which will be converted to a colorimetric or fluorescent product [39]. If anti-SARS-CoV-2 antibodies are present, the color change can be measured and correlated to the concentration of the antibodies. Antibodies raised against anti-SARS-CoV-2 antibodies can also be used as a capturing molecule. Once ELISAs are developed and validated, the next obvious step is to convert the assay to a disposable lateral flow device similar to a home pregnancy kit, which can be mass produced at a very low cost and distributed worldwide. ELISAs can also be developed to determine if the antibodies are binding or neutralizing antibodies, but the process if more complicated because the body makes antibodies against a number of pathogens. High-affinity molecules specific to the neutralizing antibodies need to be developed and validated. Alternatively, a concentration-based method to differentiate between high- and low-affinity antibodies can be developed, but these methods are primarily restricted to a laboratory setting and commercial point-of-care (POC) diagnostics to detect neutralizing antibodies are still under development.
The quantification of inflammatory biomarkers is typically performed using venous blood. Inflammatory biomarkers like TNF-α, IL-6, etc. can be quantified using a blood test; using multiple biomarkers provides an overall picture of the ‘cytokine storm’ that is not readily visualized by symptoms alone. There are some devices that measure these biomarkers, but there aren’t a plethora of inexpensive POC diagnostics to detect a possible cytokine storm [47,48]. Clinicians use standard blood tests to monitor these inflammatory biomarkers.
7. Strengths and limitations of diagnostics
7.1. Direct detection of the virus
7.1.1. Viral culture
Pros: Viral culture ranks as the best diagnostic test because it provides information about the viral concentration and viability [49–51]. No other test can inform if the virus is infectious or noninfectious. Subsequent analysis of the genomic analysis of the virus can provide detailed information about the origin, mutations, level of transmission and other intricate details of the virus. Cons: Viral culture requires additional biosafety level precautions, specialized equipment and trained personnel. The time-to-result are generally in days, although efforts are being made to decrease the times. We note that the WHO recommends viral culture ONLY for research purposes and not to be used as a potential POC diagnostic. It is dangerous to culture viruses without adequate precautions as the virus is deadly and highly transmissible.
7.1.2. RT-PCR tests
Pros: The specificity and sensitivity of these tests are unparalleled. The tests can be quantitative, but quantification requires specialized clinical laboratories with longer time-to-results [51,52]. POC qualitative tests that use integrated sample handling, amplification and detection, providing a quick yes/no answer within minutes or hours are being used at some clinics [46,53]. The user-friendliness of the devices and time-to-result are valuable features, especially in high-volume settings. Since specific gene sequences are targeted, multiple pathogens can be detected using one instrument and multiple disposable cassettes. Alternatively, using a cassette that can test for multiple pathogens could also be used. At present, many platforms such as Abbott Diagnostics ID NOW and Qiagen QIAstat-Dx Respiratory SARS-CoV-2 Panel have been authorized by US FDA for emergency use of SARS-CoV-2 detection [54] Cons: The primary weakness is related to the upfront cost of the instrument, which is generally in thousands of dollars depending on the features of the instrument. When the cost of maintenance, disposable cassettes, and multiplexing capabilities are added, this diagnostic is unaffordable, although it could be argued that the economic cost of not testing is higher [55,56]. If an individual needs to be tested daily over a standard 14-day quarantine period, testing becomes impractical with these tests. Second, POC tests are not quantitative and therefore, it is not possible to ascertain if the viral concentration is increasing or decreasing. Third and most importantly, nucleic acid tests cannot differentiate between live and dead virus as the technology amplifies specific gene sequences, regardless of viability. A quantitative test performed daily is required to ascertain if the viral load is increasing over time to determine if the patient has viral remnants or is truly virus free [56].
7.1.3. Detection of viral antigens using lateral flow disposable tests
Pros: These user-friendly, inexpensive tests can be mass produced rapidly and can be used to provide an on-site yes/no answer within minutes [45,57]. These tests can also be made to withstand high temperatures and humidity and therefore, can be deployed rapidly. Cons: The two major concerns are related to sensitivity and selectivity [58]. If antibodies raised against the new coronavirus are used, it is imperative that the antibodies are tested for cross-reactivity against similar strains and validated, or else the selectivity decreases. The tests detect viral antigens and therefore, cannot distinguish between viable and dead pathogens and quantification is generally not feasible with this technology.
7.2. Determination of prior infections
Upon exposure, the body will generate antibodies against the pathogen. These antibodies can be detected using lateral flow devices, where monoclonal antibodies against these antibodies can be generated [59]. Alternately, viral antigens can be used to capture these antibodies in a lateral flow format [57]. In the previous section, antibodies were used to capture viral antigens; here the format is reversed. The pros and cons of these tests are similar to the lateral flow tests described previously for direct detection of the pathogen. Moreover, antibody testing is not recommended for identification of current SARS-CoV-2 infection as antibody response is usually elicited at the late phase of infection [54] (Figure 1).
8. Interpretations of results
Assuming that (i) the sample was collected, handled and transported according to WHO and CDC guidelines, (ii) the test has a high clinical selectivity and sensitivity, (iii) technicians performing the test were well-trained, there are two possibilities for viral tests.
8.1. Positive results
If an individual is tested positive for SARS-CoV-2, the next step would be to consult the physician and follow the physician’s recommendations; these may include hospitalization or quarantine at home. Unfortunately, at home tests are unavailable at the present time, and therefore, symptoms have to be monitored. From a public perspective, it is important that the individual stay under quarantine until symptoms have subsided and the individual is not shedding virus. It is quite possible that the result is a false positive, for example, if the result is positive 3–4 weeks after the onset of infection, it is most likely that the test is detecting dead virus or fragments of the virus.
8.2. Negative results
At face value, a negative viral result means the person doesn’t have the virus. However, the reality is more complicated because of the unique nature of the infection process. Testing early or late in the infection process can result in false negatives (Figure 3). A negative result does not necessarily mean that the individual is SARS-CoV-2 negative; it means that the individual didn’t have the virus at the time of testing [60]. It could also mean that the individual had the virus previously, but the symptoms were mild or not noticeable and the immune system has cleared the pathogen. If a patient is presenting moderate symptoms and the result is negative, it could mean that the immune system has managed to decrease the virus concentration at the time when the sample was collected. Testing more frequently would confirm the diagnosis. A similar logic follows for tests that detect antibodies produced by the body in response to the infection. It is important to note that even if the individual has tested positive for antibodies against the virus, the concentration and neutralizing capability is not known, unless more involved testing in a clinical laboratory setting is performed. Most importantly, it is not clear at the present time if the presence of antibodies prevents re-infection and/or provides long-term immunity [9]. It is also unclear if the individual who has antibodies can act as a carrier and if these antibodies protect people from mutated strains. Taken together, the concept of ‘immunity passports’ shouldn’t be used for major decisions in isolation without considering other factors. Unlike viral or antibody tests, there are no positive or negative results when testing for inflammation. Testing relies on a panel of inflammatory biomarkers [61]. These tests must be quantitative because individuals have baseline levels of these biomarkers, which increase upon injury. Baseline levels are highly dependent on underlying conditions of the patient. In addition, some individuals may be taking immunosuppressive drugs and therefore, the concentration of the biomarkers may be lower [62]. A personalized testing approach is required for inflammatory biomarker testing.
Figure 3.
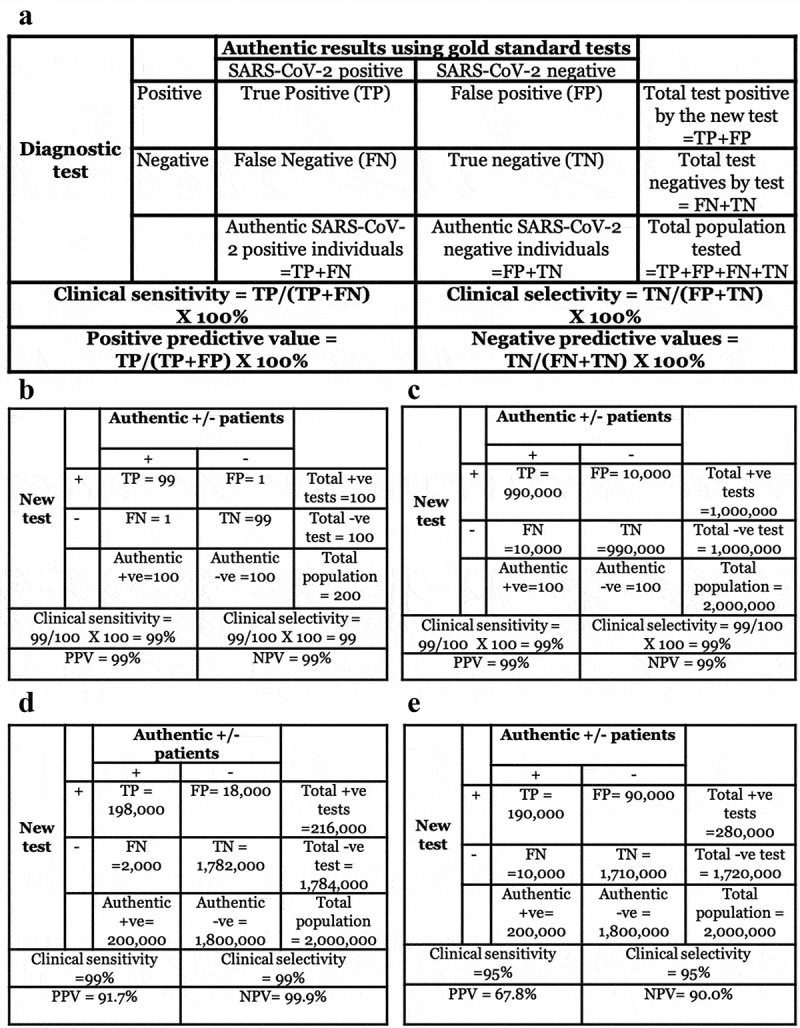
(a) Some of the terms and their derivations used in clinical diagnostics. (b) Situation 1: If 200 tests with excellent clinical selectivity and sensitivity of 99% and 50% disease prevalence are performed, only 2 individuals will be misdiagnosed as false positive or false negative. (c) Situation 2: If 2 million tests with similar parameters as in situation 1 are performed, 20,000 individuals will be misdiagnosed as false positives or false negatives. (d) Situation 3: If the disease prevalence decreases to a more realistic situation observed in the current COVID-19 pandemic, 2,000 individuals will receive a false-negative result and 18,000 individuals will receive a false-positive result. (e) Situation 4: If the test has 95% clinical selectivity and sensitivity with 10% disease prevalence, 10,000 individuals will receive a false-negative result and 90,000 individuals will receive a false-positive result. The last situation is more indicative of most diagnostics in the current COVID-19 pandemic even if a manufacturer claims 99% clinical selectivity and sensitivity, because human errors (e.g. Incorrect sample collection, variability in nasal/throat swabs, etc.) increase when tests are performed in the field
8.2.1. It is very important to note that results from any diagnostic for COVID-19 should be interpreted by a trained professional
For example, even after major technological advances and decades since the first home pregnancy test was introduced in 1976, home pregnancy tests are still not 100% accurate [63]. When diagnostics are being produced within days during an emergency pandemic, it is very likely that tests do not have the selectivity and sensitivity claimed by manufacturers, especially when these tests are being performed in the field. Clinicians consider other symptoms, factors such as underlying conditions, age, community outbreak data, etc. to confirm the diagnosis. These decisions are easier for the 10–20% of the individuals who present typical COVID-19 symptoms and are positive for virus, but the confirmation becomes more difficult when people with mild or no symptoms test negative.
9. The ‘ideal’ diagnostic
Diagnostic requirements for different stakeholders vary significantly. A diagnostic for COVID-19 for clinicians and healthcare professionals require different features than a diagnostic required for a produce manager in a grocery store. A physician may want to know if a set of inflammatory biomarkers are increasing as a function of time in a patient, whereas the produce manager will want to know only if his/her employees have SARS-CoV-2 to avoid spread. The former will allow the clinician to determine if the patient requires more intervention; the latter will use the results to determine if the store needs to be closed. Epidemiologists use diagnostics for surveillance purposes and build models for policymakers to make informed decisions that affect the entire community and not necessarily for a specific individual; in contrast, individuals with underlying health conditions or individuals with family, friends and colleagues with hypertension, diabetes and other morbidities that make then susceptible to severe disease manifestations will find at home diagnostics indispensable.
Some of the questions and decision-making process are given in Figure 4. The questions are not exhaustive, but provide a sense of the different needs of different stakeholders. More detailed guidelines have been released by the multiple government agencies [64].
Figure 4.
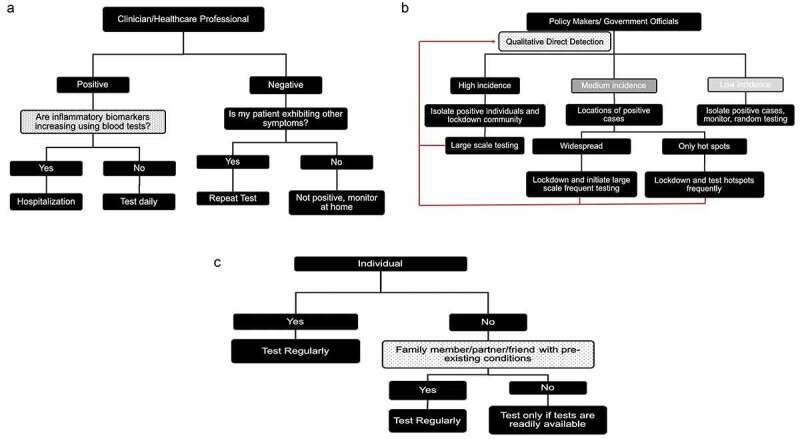
Examples of decision trees based on excellent diagnostics for stakeholders. A. Clinician/Healthcare professional. B. Policymakers. C. Individual. These decision trees are not exhaustive and doesn’t take other factors specific to a particular situation into consideration
9.1. Clinicians, healthcare professionals
The primary aim of a clinician is treating the patient. The diagnostic is expected to assist the clinicians in their decision-making process regarding treatment. Some of the most pressing questions are: (i) Does my patient have SARS-CoV-2 or is it a different pathogen? The treatment options differ depending on the answer. (ii) If my patient is SARS-CoV-2 positive, can the diagnostic quantify the viral load? If it is a very high concentration, patients need to be quarantined and secondly, the patient needs to be monitored for adverse reactions. (iii) Can the diagnostic tell me if the patient’s inflammatory biomarkers are increasing as a function of time? Since every individual is unique, a personalized diagnostic approach is required, where close monitoring of the inflammatory biomarkers as a function of time will ascertain if the patient needs more (or less) intervention is required. Remote monitoring would be ideal because the clinician or healthcare professional would not have to come in close contact with the patient. (iv) If the patient is quarantined in the hospital or at home, can the diagnostic determine the viral load daily to help me determine when to release him/her from quarantine?
9.2. Policymakers and government officials
The primary aim of these stakeholders is to use diagnostics as one of the tools to make informed decisions about society and their communities. Diagnostics that determine number of prior and current rate of infection and hotspots are of utmost importance for them to implement appropriate countermeasures. Epidemiologists use information obtained from these diagnostics to develop models that will assist them in predicting future outbreaks. These diagnostics can be primarily qualitative; quantification and diagnostics for inflammatory biomarkers will generally not affect their decision-making process. The questions that policymakers require from diagnostics are: (i) Will the diagnostics tell us what percentage of the population are positive? (ii) Can the diagnostics identify hotspots and identify local and imported infections? (iii) Can the diagnostics be used to model the infection spread? Often, they have to balance multiple priorities from experts from different sectors to look at cost/benefit analysis. For example, it may seem prudent from an epidemiological standpoint to have a complete shutdown of a community for months; it is unreasonable from an economic perspective as loss of economic activity will lead to significant mortality and morbidity.
9.3. Officials at high density living conditions (e.g. nursing homes, assisted living facilities, cruise lines, prisons, refugee camps)
The aims of the managers in these facilities are multifold, including, but not limited to the health of their residents and their personnel. These facilities are generally no/low resource as they have limited medical equipment and supplies. Therefore, diagnostics must be designed accordingly. Some questions related to the testing aspects are: (i) Can the diagnostic determine if healthcare professionals, technicians, caregivers and visitors are virus free? (ii) If one of the residents is tested positive, can a diagnostic ascertain if the resident needs to be hospitalized or released from quarantine? (iii) Can the diagnostic detect viruses in the environment for decontamination?
9.4. Overseers of areas with high traffic or human interactions (e.g. schools, universities, entertainment complexes, theme parks, beaches, sports venues)
The primary aim of the administrators of these organizations is related to containment and isolation while performing the essential functions of the institutions. For example, school principals and university presidents do not want a SARS-CoV-2 student or employee on their premises for fear of spreading the virus. The number of students in large open campuses is an additional challenge. The requirements from a diagnostic are: (i) Can the diagnostic be administered to students entering campus with minimal interference and privacy? (ii) Can the result be obtained in minutes? (iii) Can the diagnostic detect viruses in the classroom and common areas for decontamination?
9.5. Individuals
The primary aim of the vast majority of citizens is to protect themselves and their loved ones. The requirements for diagnostics are (i) Is it precise and accurate? (ii) Is it complicated? (iii) Is it inexpensive? (iv) Are results rapid? (v) Can it be stored in medicine cabinets for a long time and portable?
10. Challenges for the diagnostic community
10.1. ASSURED-SQVM principles
The ideal diagnostic should follow the ASSURED (Affordable, Selective, Sensitive, User-friendly, Rapid and Robust, Equipment-free Deliverable to end-users) principles of point of care diagnostics developed by the WHO for infectious diseases [65] (Table 1). These ASSURED principles become increasingly important in the current coronavirus pandemic, particularly because of the significant health and economic impact and disparity at a global level. For example, some countries have an efficient healthcare system that can afford expensive diagnostics unlike others, where healthcare systems are limited in scope and severely strained even before this outbreak. Communities with advanced healthcare systems, lower economic disparities and a cohesive evidence-based strategy is able to cope with the economic fallout than resource-poor communities. In addition to these ASSURED requirements, the nature and timeline of the infection requires additional features, that have been coined here as SQVM (Self-testing, Quantification, Viability and Multiplexing). Briefly, Self-testing is highly desirable to limit personal interactions and maintain social distancing in the privacy of homes [66]. Self-testing can be combined with telemedicine for patients to communicate the results to their physicians [67]. Quantification is important for individuals to determine if there is an increase or decrease in the viral load, antibody or inflammatory biomarkers during the 14-day quarantine period [68,69]. Tests that can determine Viability of SARS-CoV-2 in an environmental or human sample could provide additional information to first responders and healthcare professionals to perform adequate countermeasures [32]. Finally, Multiplexing will be of highest priority during the influenza season, because symptoms of the new coronavirus and influenza overlap [70,71]. Rapid detection of the presence and quantification of bacterial respiratory pathogens such as Streptococcus pneumoniae take on increasing urgency during the influenza season, because bacterial pneumonia is often the more severe outcome of the initial influenza infection [72]. There are numerous exciting emerging technologies that address one or more of these ASSURED-SQVM principles; however, none of the existing technologies can satisfy all of these principles. The technological demands are challenging; however, human ingenuity can be applied to develop excellent diagnostics. For example, if a diagnostic lacks user-friendliness, trained personnel can consult with individuals at homes to self-test using video conferencing. Additionally, as described in the previous section, different stakeholders require different information. It is imperative that analytical chemists and assay developers collaborate with stakeholders early in the development process. It doesn’t make sense to develop a test that can detect 10 viral particles for a clinical setting, if patients are presenting 1,000 particles at an urgent clinic or emergency room. Similarly, it doesn’t make sense to develop a multi-step process for reagents, when industries prefer less complicated steps for scale up and distribution. Regular communication and developing realistic plans with go/no-go decisions and alternate strategies with stakeholders and the team is critical.
Table 1.
ASSURED-SQVM principles as applied to current diagnostics for SARS-CoV-2 and other infectious diseases
| Criteria | Detection methods | ||||
| Direct detection of virus | Detection of antibodies |
Detection of inflammatory biomarkers |
|||
| |
Culture |
Nucleic Acid |
Antigen capture |
||
| Affordable | No, requires laboratory setting, trained personnel | No. Instrument, consumables are expensive | Yes | Yes | Requires a clinical laboratory to perform the tests for multiple biomarkers |
| Sensitive | Very high | High | Depends on the QC of the antibodies and manufacturer | Depends on the QC of the antibodies and manufacturer | Depends on the QC of the antibodies and manufacturer |
| Selective | Very high | Very high, but requires the right primers | Depends on the QC of the antibodies and manufacturer | Depends on the QC of the antibodies and manufacturer | Depends on the QC of the antibodies and manufacturer |
| Rapid | No, requires days. | Tabletop instruments provide results in ≤ 15 min | Lateral flow tests will provide results in ≤ 15 min. | Lateral flow tests will provide results in ≤ 15 min. | Blood tests take 24–28 hours |
| Robust | No, reagents require refrigeration. | Yes/No, some reagents may require refrigeration | No, depends on antibody stability. May require refrigeration | No, depends on antibody stability. May require refrigeration | No, reagents require refrigeration |
| User-friendly | No, requires trained personnel | Only the new integrated systems are user friendly. | Yes | Yes | No, requires trained personnel |
| Equipment free | No | No | Yes | Yes | No |
| Deliverable to end users | No, requires laboratory | No, requires space, electricity | Yes, small footprint, portable | Yes, small footprint, portable | No, requires laboratory |
| Self-testing | No | No | Yes | Yes | No |
| Quantitative | Yes, but takes days | Tabletop systems provide yes/no answer; | No | No | Yes, but takes hours/days |
| Viability | Yes | No | No | N/A | N/A |
| Multiplexing | Yes, but requires equipment | Capability exists, but is expensive | Yes, but becomes more expensive | Yes, but becomes more expensive | Yes, but requires equipment |
10.2. Influenza and SARS-CoV-2
The CDC estimates that influenza infections ranged between 39 and 56 million infections in 2019–2020 [73]. In contrast to SARS-COV-2 which has mostly spared children and young adults, influenza infects seniors, children and individuals with weak or compromised immune systems [74]. It is unclear how society will fare if both pathogens attack at the same time. Although influenza vaccines provide substantial health benefits and decrease the number of hospitalizations and deaths, vaccine effectiveness is only ~50% for most seasons [73]. The possibility of a new influenza strain capable of causing a pandemic, similar to the 2009 ‘swine flu strain’ cannot be discounted. The symptoms of the two pathogens are overlapping, which makes it extremely difficult to diagnose. Unfortunately, ASSURED-SQVM diagnostics that can detect influenza, SARS-CoV-2, co-infections and/or bacterial infections are unavailable at the present time. This is a major challenge for diagnostic developers and the larger scientific community [71].
10.3. Communication challenges
In addition to the technological challenges, one of the often overlooked, major challenges for the scientific community is to communicate the nuances of testing, outcomes and the decision-making process to the broader nonscientific community. Diagnosis is especially difficult because some individuals are asymptomatic. This is in contrast to other diseases where symptoms are usually the first indicator before using tests. If a child was not sick, parents would not take the child to the physician. In addition, there is a plethora of information on the internet and in various social media formats that is overwhelming. An internet search for ‘Diagnostics for coronavirus’ yields over 166,000,000 results in less than 1 s. A more focused search for articles with the same terms results in over 42,000 scientific articles and patents; a similar search on the preprint server medrxiv results in over 5,000 non-peer reviewed publications. Unfortunately, with a deep desire of the masses for a rapid return to normalcy, coupled with the aspiration of scientists to be the first to provide solutions, several methods claiming to be the best before validation are published. The research findings are publicized in multiple news outlets and amplified by social media. Every news cycle has reports of new innovations that claim the development of ‘high selectivity and sensitivity’ without testing clinical samples. Claims of ‘rapid’ and ‘within 15 minutes’ without taking sample preparation time into consideration are becoming commonplace. Nonscientific individuals are unfamiliar with limit of detection, differences between analytical and clinical selectivity and sensitivity, positive and negative predictive values, time to test, etc. (Table 2) and hence add to the complexity of the chaos. For example, an individual may test negative on day 1 of the onset of infection if the concentration of the virus on the nasal swab is lower than the limit of detection and positive on day 3, when the viral load is higher than the limit of detection. It is also quite possible that the diagnostic had a low accuracy leading to conflicting results on both days. Temperature sensors are being used at airports and transportation hubs, but may not be particularly useful since individuals generally develop fever after a week of onset of infection. Beyond these issues, the nuances of clinical diagnostics need to be communicated better to the general public. For example, a test has been demonstrated to have 95% clinical sensitivity and selectivity in a well-controlled clinical study, which can lead to 90,000 false-positive cases and 10,000 false-negative cases if the prevalence of the disease is ~ 10% and two million samples have been tested. (Figure 4) The 10,000 false-negative individuals would potentially spread it. Use of multiple biomarkers, frequent testing and quantitative results could solve this problem. Additionally, with hundreds of millions of dollars at stake to develop rapid diagnostics quickly, exaggeration of the research and results becomes commonplace. It is imperative for the diagnostic community to address these issues, or else it will lead to a lack of credibility for testing, which defeats the purpose of testing.
Table 2.
Some of the common terms used in diagnostics
| Term |
Definition and explanation |
| Limit of detection (LOD) or Analytical Sensitivity | LOD is defined as the lowest concentration of virus that can be detected using the diagnostic. The LOD is typically given by the manufacturer of the test and that may vary according to the test characteristics. For example, a nucleic acid based test may be able to detect 10 viral particles, whereas the LOD of an antigen test may be 100 particles. Manufacturers also use LOQ, or the limit of quantification, which is the lowest concentration detected with a high degree of accuracy. LOD is also referred to as Analytical Sensitivity, which is the lowest number of viral particles the test can detect. Researchers generate a series of vials with different concentrations of the virus and test is a positive result is obtained with the different vials. The vial with the lowest concentration is considered the LOD. |
| Analytical Selectivity | Analytical selectivity is the ability of the test to only detect SARS-CoV-2 in the presence of other pathogens, including closely related strains such as SARS-CoV-2, MERS and other respiratory pathogens such as rhinovirus, influenza, Streptococcus pneumonia, etc. Different concentrations of these pathogens are used to determine if the test yields a positive result. Next, low concentrations of SARS-CoV-2 are mixed with high concentrations of one or more pathogens to determine the specificity. |
| Clinical or diagnostic sensitivity | Clinical or diagnostic sensitivity is very different from analytical sensitivity. The latter is associated with the test specifications, the former is the ability of the test to accurately determine SARS-CoV-2 positive patients. Clinical sensitivity has to be benchmarked against gold standard diagnostics. |
| Clinical or diagnostic selectivity | It is the ability of the test to accurately determine SARS-CoV-2 negative patients. Clinical selectivity has to be benchmarked against gold standard diagnostics. |
| Positive predictive value | Positive predictive value is the probability that an individual will definitely have the disease based on a positive result. |
| Negative predictive value | Negative predictive value is the probability that an individual may not have the virus based on a negative result |
| True positive | When a test result is positive, and the individual has SARS-CoV-2 based on additional advanced confirmatory validated tests such as cell culture and or central laboratory based RT-PCR. |
| False positive | When a test result is positive, but the individual does not have SARS-CoV-2. The implication is that the individual will have to be quarantined. Here, the individual will suffer on a more personal level from the wrong result. |
| True negative | When a test result is negative and the individual does not have SARS-CoV-2 based on additional advanced confirmatory validated tests such as cell culture and or central laboratory based RT-PCR. |
| False negative | When a test result is negative, but the individual has SARS-CoV-2. The implication is that the individual will spread the virus. Here, the community will suffer as more people will be infected. |
11. Methodology
A comprehensive review of the literature was performed. This included publicly available databases including Medline, Pubmed, etc. and government websites including and not limited to CDC and WHO.
12. Conclusion
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 continues to be a global concern. Besides, several variants of SARS-CoV-2 have been identified with a potential epidemiologic and pathogenic variation that may influence antibody treatment and vaccine efficiency. Testing will remain an important tool to mitigate the effects of this pandemic as early and accurate diagnosis can lead to appropriate countermeasures to reduce mortality and morbidity. Many diagnostic methods have been introduced to meet the growing test demands. This article summarizes the knowledge about various diagnostics with an emphasis on the development of ASSURED-SQVM diagnostics.
13. Expert opinion
This is a war against an almost ‘perfect’ enemy. Like most successful opponents, the virus has focused on our blind spots and turned our strengths into weaknesses. (i) It infiltrates silently. Humans, by nature and by logic, do not test unless we exhibit symptoms. The virus exploited this seemingly sound logic. Individuals spread virus in the early stages when they are asymptomatic or have mild symptoms. Its early incursions were missed because it transmitted rapidly and silently across the globe within weeks, entering 188 countries and overwhelming healthcare systems. (ii) It stays longer: The median time from onset to clearance is approximately 14 days [23]. This strategy is highly effective to destroy economic activity, which, in the long term, is expected to significantly increase morbidity and mortality. With limited mobility and behavioral changes, economic activity has cratered, allowing the virus to cause more suffering and death due to increased poverty and other social ills. (iii) It kills selectively. It doesn’t kill all vulnerable sections of society, but is highly selective. It kills select individuals with underlying health conditions (particularly in nursing homes and assisted living facilities) but seems to leave the majority of infants and children with mild or minimal symptoms, although rare cases of inflammation in children are being reported [75,76]. At the present time, we don’t understand its selection process. (iv) It uses our best defenses against ourselves: It achieves this objective in two ways; inside and outside the bodies in a seemingly (because we don’t understand it) random manner. (a) Internal: The immune system is our best defense against all pathogens. However, the response must be tempered. If overactivated, it will lead to a ‘cytokine storm’ and subsequent organ failure [41]. Select healthy individuals without co-morbidities, produce a violent immune reaction and have to be hospitalized, whereas the vast majority produce no such reaction. Interestingly, the immune overreaction happens suddenly without warning after 5–10 days from onset of infection [23,69]. This speaks to the craftiness of the virus; some patients have been sent home for quarantine because they have mild symptoms. Some patients have come back when the symptoms increase, unfortunately in other patients, strokes and other complications have been fatal. (b) External: Two of our strengths as a diverse society is to think freely and develop creative solutions toward solving seemingly intractable problems. This strength has allowed us to eradicate several pathogens, but only when a singular public policy is followed. Unfortunately, multiple options, none of which are perfect, has led to different public policies and a patchwork of containment strategies. This is clearly seen in the United States, where different states have pursued different policies and it is widely expected that a resurgence of infections will occur. It has also forced us to forego socialization, one of the fundamental aspects of being human, as a defense mechanism, which has led to significant economic damage. While the forced quarantine and closure of public parks, gymnasiums, events, etc. has been beneficial in reducing the number of infections, there have been unintended consequences, such as anxiety, depression and decreased physical activity. Indeed, several excellent articles have emphasized the importance of maintaining physical activity and maintaining social distancing during this pandemic to counter the effects of quarantines [77–79].
Diagnostics are excellent weapons and can be tailored to blunt the sinister attacks of the virus. Diagnostics can (i) Limit the silent spread, (ii) Determine viral loads in virus positive individuals to release them from quarantine, (iii) Be deployed in hotspots to limit death and (iv) Quantify inflammatory biomarkers in individuals. However, like all weapons, we have to use diagnostics correctly and for the common good. As an example, if precise, but expensive diagnostics are developed, resource-rich communities would benefit in the short term in contrast to resource poor communities. However, the virus knows no boundaries and therefore, a more powerful strain can subsequently attack all communities and cause long-lasting damage. No diagnostic can satisfy all the requirements of all stakeholders and therefore, interpretation, using terms such as positive predictive value, negative predictive value, etc. instead of a simple yes/no result becomes more important. This perspective, hopefully, provides a clear picture of the different questions, nuances and challenges of this important weapon. A focus on the needs of the stakeholders; a collaborative approach toward diagnostics for all; clear and compelling messages for the general public; a lower emphasis on self-promotion; objective analysis of ‘novel’ diagnostics and a deeper sense of humility knowing that this virus and its cousins do not readily allow us to ‘see’ them, will help us develop quality ASSURED-QSVM diagnostics to mitigate the effects of this devastating pathogen.
Acknowledgments
The authors also thank several colleagues whose insightful comments have improved this manuscript significantly.
Funding Statement
The authors thank National Institute of Allergy and Infectious Diseases (1R61AI140475-01A1) for their support for research on point of care diagnostics.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.World Health Organization . Coronavirus Disease. 2020; Available from: https://covid19.who.int/.
- 2.Li H, Liu S-M, Yu X-H, et al. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55(5):105951. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395(10223): 507–513. 2020. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article was one of the first articles that described the epidemiological, demographic, clinical, and radiological features and laboratory data of patients infected with the novel coronavirus.
- 4.Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from coronavirus disease 2019 (COVID-19) infection. Ann Acad Med Singapore. 2020;49(3):108–118. . [PubMed] [Google Scholar]
- 5.Thanh Le T, Andreadakis Z, Kumar A, et al., The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 19(5): 305–306. 2020. . [DOI] [PubMed] [Google Scholar]; •• This article covers the major vaccine efforts to combat the virus.
- 6.Binnicker MJ. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020;66(5):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S . Bureau of labor statistics. labor force statistics from the current population survey. 2020; https://data.bls.gov/timeseries/LNS11000000.
- 8.Blustein DL, et al. Unemployment in the time of COVID-19: a research agenda. Journal of Vocational Behavior. 2020;119:103436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020;395(10236):1527–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binnicker M. Emergence of a novel coronavirus disease (covid-19) and the importance of diagnostic testing: why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020;66(5):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salathé M, et al. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150(11–12):w20225. [DOI] [PubMed] [Google Scholar]
- 12.Crystal Watson AC, Blumenstock J, Fraser M. A national plan to enable comprehensive COVID-19 case finding and contact tracing in the US. Johns Hopikins Bloomberg School of Public Health; 2020. [Google Scholar]
- 13.Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8(4):e488–e496. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranney ML, Griffeth V, Jha AK. Critical supply shortages — the need for ventilators and personal protective equipment during the Covid-19 pandemic. 2020;382(18):e41. [DOI] [PubMed] [Google Scholar]
- 15.Farrell TW, Ferrante LE, Brown T, et al. AGS position statement: resource allocation strategies and age-related considerations in the COVID −19 era and beyond. J Am Geriatr Soc. 2020. DOI: 10.1111/jgs.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh D. Occupational risks for COVID-19 infection. Occup Med (Lond). 2020;70(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020;132(6):1317–1332. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belingheri M, Paladino ME, Riva MA. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect. 2020;105(2):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan J, Ge J, Yu J, et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 581(7807): 215–220. 2020. . [DOI] [PubMed] [Google Scholar]; •• This article describes the molecular level interactions between the spike protein and the ACE-2 receptor.
- 23.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moccia F, et al. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian society of cardiovascular researches. Geroscience. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietz W, Santos-Burgoa C. Obesity and its Implications for COVID-19 mortality. Obesity (Silver Spring). 2020;28(6):1005. [DOI] [PubMed] [Google Scholar]
- 26.Lai CC, Shih T-P, Ko W-C, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics (Basel). 2020;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article describes new and rapid developments of diagnostics for detecting SARS-CoV-2
- 29.Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 2020;71(8):1930–1934. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adalja AA, Toner E, Inglesby TV. Priorities for the US health community responding to COVID-19. JAMA. 2020;323(14):1343. [DOI] [PubMed] [Google Scholar]
- 31.Belingheri M, Paladino ME, Riva MA. COVID-19: health prevention and control in non-healthcare settings. Occup Med (Lond). 2020;70(2):82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietz L, Horve PF, Coil DA, et al. 2019 novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. 2020;5(2). DOI: 10.1128/mSystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosites E, Parker EM, Clarke KEN, et al. Assessment of SARS-CoV-2 infection prevalence in homeless shelters - Four U.S. Cities, march 27-april 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(17):521–522. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udugama B, Kadhiresan P, Kozlowski HN, et al., Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 14(4): 3822–3835. 2020. . [DOI] [PubMed] [Google Scholar]; •• This article describes the development of point of care diagnostics for SARS-CoV-2.
- 35.Datta S, Shah L, Gilman RH, et al. Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. Lancet Glob Health. 2017;5(8):e760–e771. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer M, Jayaramayya K, Subramaniam MD, et al. COVID-19: an update on diagnostic and therapeutic approaches. BMB Rep. 2020;53(4):191–205. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Li X, Wang Y, et al. Diagnostic value and key features of computed tomography in coronavirus disease 2019. Emerg Microbes Infect. 2020;9(1):787–793. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacofsky D, Jacofsky EM, Jacofsky M. Understanding antibody testing for COVID-19. J Arthroplasty. 2020;35(7):S74-S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaaskelainen AJ, Kekäläinen E, Kallio-Kokko H, et al. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill. 2020;25(18). DOI: 10.2807/1560-7917.ES.2020.25.18.2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7):102554. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Kasteren PB, Van Der Veer B, Van Den Brink S, et al. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128:104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan C, Cui J, Huang L, et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26(6):773–779. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baek YH, et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):998–1007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang T, et al. Point-of-care RNA-based diagnostic device for COVID-19. Diagnostics (Basel). 2020;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer SK, Williams K, Wang L, et al. Development of an IL-6 point-of-care assay: utility for real-time monitoring and management of cytokine release syndrome and sepsis. Bioanalysis. 2019;11(19):1777–1785. . [DOI] [PubMed] [Google Scholar]
- 48.Min J, Nothing M, Coble B, et al. Integrated biosensor for rapid and point-of-care sepsis diagnosis. ACS Nano. 2018;12(4):3378–3384. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leland DS, Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007;20(1):49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson JB, et al. Rapid and sensitive viral culture method for human immunodeficiency virus type 1. 1988;26(7):1416–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falsey AR, Formica MA, Treanor JJ, et al. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. Journal of Clinical Microbiology. 2003;41(9):4160–4165. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CJ, Osborn AM. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol. 2009;67(1):6–20. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell SL, George KS. Evaluation of the COVID19 ID NOW EUA assay. J Clin Virol. 2020;128:104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu SY, Yau HS, Yu MY, et al., The diagnostic methods in the COVID-19 pandemic, today and in the future. Expert Rev Mol Diagn. 20(9): 985–993. 2020. . [DOI] [PubMed] [Google Scholar]; •• This article describes recent development in testing for SARS-CoV-2.
- 55.Diel R, Nienhaus A. Cost-benefit analysis of real-time influenza testing for patients in German emergency rooms. Int J Environ Res Public Health. 2019;16(13):2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang YW, et al. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. 2020. DOI: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan C, et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26(6):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z, et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal Chem. 2020;92(10):7226–7231. . [DOI] [PubMed] [Google Scholar]
- 60.West CP, Montori VM, Sampathkumar P. COVID-19 testing: the threat of false-negative results. Mayo Clin Proc. 2020;95(6):1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng MP, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnaldez FI, et al. The society for immunotherapy of cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J Immunother Cancer. 2020;8(1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gnoth C, Johnson S. Strips of hope: accuracy of home pregnancy tests and new developments. Geburtshilfe Frauenheilkd. 2014;74(7):661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuchat A, Team CC-R. Public health response to the initiation and spread of pandemic COVID-19 in the United States, February 24-April 21, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(18):551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95(9):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tidy EJ, Shine B, Oke J, et al. Home self-testing kits: helpful or harmful? Br J Gen Pract. 2018;68(673):360–361. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–1681. . [DOI] [PubMed] [Google Scholar]; •• This article describes the importance and advancement of telemedicine in diagnosing coronavirus and other diseases
- 68.Alteri C, Scutari R, Stingone C, et al. Quantification of HIV-DNA and residual viremia in patients starting ART by droplet digital PCR: their dynamic decay and correlations with immunological parameters and virological success. J Clin Virol. 2019;117:61–67. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham H, Chandler DJ, Dunbar SA. The genesis and evolution of bead-based multiplexing. Methods. 2019;158:2–11. [DOI] [PubMed] [Google Scholar]
- 71.Faust JS, Del Rio C. Assessment of deaths from COVID-19 and from seasonal influenza. JAMA Intern Med. 2020;180(8):1045. [DOI] [PubMed] [Google Scholar]
- 72.Shirey KA, Perkins DJ, Lai W, et al. Influenza “trains” the host for enhanced susceptibility to secondary bacterial infection. mBio. 2019;10(3). DOI: 10.1128/mBio.00810-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mameli C, Cocchi I, Fumagalli M, et al. Influenza vaccination: effectiveness, indications, and limits in the pediatric population. Front Pediatr. 2019;7:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and Control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68(3):1–21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riphagen S, Gomez X, Gonzalez-Martinez C, et al., Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 395(10237): 1607–1608. 2020. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article was one of the first articles that described the inflammatory efects of the coronavirus in some children.
- 76.Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ. 2020;369:m1710. [DOI] [PubMed] [Google Scholar]
- 77.Lesser IA, Nienhuis CP. The Impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health. 2020;17(11):3899. . [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article describes the importance of physical activity to counteract the ffect of forces quarantine.
- 78.Ravalli S, Musumeci G. Coronavirus outbreak in Italy: physiological benefits of home-based exercise during pandemic. J Funct Morphol Kinesiol. 2020;5(2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maugeri G, Castrogiovanni P, Battaglia G, et al. The impact of physical activity on psychological health during Covid-19 pandemic in Italy. Heliyon. 2020;6(6):e04315. . [DOI] [PMC free article] [PubMed] [Google Scholar]


