Abstract
Although experimental transmission of bovine spongiform encephalopathy (BSE) to pigs and transgenic mice expressing pig cellular prion protein (PrPC) (porcine PrP [PoPrP]–Tg001) has been described, no natural cases of prion diseases in pig were reported. This study analyzed pig-PrPC susceptibility to different prion strains using PoPrP-Tg001 mice either as animal bioassay or as substrate for protein misfolding cyclic amplification (PMCA). A panel of isolates representatives of different prion strains was selected, including classic and atypical/Nor98 scrapie, atypical-BSE, rodent scrapie, human Creutzfeldt-Jakob-disease and classic BSE from different species. Bioassay proved that PoPrP-Tg001-mice were susceptible only to the classic BSE agent, and PMCA results indicate that only classic BSE can convert pig-PrPC into scrapie-type PrP (PrPSc), independently of the species origin. Therefore, conformational flexibility constraints associated with pig-PrP would limit the number of permissible PrPSc conformations compatible with pig-PrPC, thus suggesting that pig-PrPC may constitute a paradigm of low conformational flexibility that could confer high resistance to the diversity of prion strains.
Keywords: atypical/Nor98 scrapie, BSE, classic scrapie, pig, prion conversion, prion strains, PrP, swine
Experimental transmission in pig cellular prion protein (PrPC) transgenic mice and in vitro protein misfolding cyclic amplification propagation assay results showed that pig-PrPC is resistant to all tested prion strains except classic bovine spongiform encephalopathy, and it may constitute a paradigm of low susceptibility to prion strain diversity.
Transmissible spongiform encephalopathies (TSEs) or prion diseases are fatal neurodegenerative disorders caused by prion accumulation in the brain and lymphoreticular system [1]. Several TSEs naturally affecting animals are known [2], such as bovine spongiform encephalopathy (BSE) in cattle and scrapie in sheep and goats. Creutzfeldt-Jakob disease (CJD) is the most common human TSE. In the last century, a variant CJD associated to the dietary exposure to BSE-infected cattle was described [3]. Atypical cases of BSE are known to occur, mainly in older animals [2], classified as H-type or L-type according to their biochemical properties. Their low prevalence worldwide is consistent with a sporadic origin. The experimental transmission of atypical BSE to cattle, macaques, and mice evidenced their potentially infectious nature [4–6].
In the case of scrapie, the description of a wide variety of scrapie disease phenotypes suggests that a diversity of scrapie strains is circulating in sheep and goats [7, 8]. Moreover, in 1998, an unusual type of scrapie was discovered in Norway (Nor98) [9]. Atypical/Nor98 scrapie has been reported in Europe, the United States, Canada, the Falkland Islands, Japan, Australia, and New Zealand and has been proposed to have a sporadic origin, because it is uniformly spread and often occurs in older animals as single cases in a flock. Transmission studies in transgenic mice and sheep demonstrated the transmissibility of atypical/Nor98 scrapie [10, 11]. Chronic wasting disease (CWD) in cervids affects both captive and wild animals [12] in North America. Cases of CWD have been detected in wild animals in Scandinavia [13], leading to rising concerns about the spreading of the disease in Europe. These animal prion diseases have become potential threats to public health and the economy.
Naturally occurring TSEs in pigs have never been reported [14, 15]. Experimental data showed that pigs can be infected after parenteral inoculation of BSE but not after oral challenge [16]. Previous studies using transgenic mice overexpressing pig protein (porcine prion protein [PoPrP]–Tg001) [17] suggested that pigs can be susceptible to BSE agent after passage in sheep or to an atypical/Nor98 scrapie isolate but not to 7 European classic scrapie isolates [18]. The susceptibility of pigs to BSE agent after experimental passage in sheep has also been demonstrated [19]. Pigs can be susceptible to US ovine scrapie isolate [20], but only a few intracranial inoculated pigs were scored positive for the infection. In a 2017 report [21], only a few pigs inoculated with an experimental inoculum of CWD were found to be TSE positive, showing low attack rates and long survival times. Moreover, adaptation of both scrapie and CWD prions to pigs was apparently incomplete, because further subpassage in transgenic mice expressing pig protein showed very limited attack rates. The incomplete adaptation of both scrapie and CWD to pigs could be the result of a nonadaptive prion amplification process [22].
In the present study, we use the PoPrP-Tg001 mouse model expressing pig cellular PrP (PrPC) to systematically evaluate the transmission barrier of pigs to a panel of TSE isolates from several species (cattle, sheep, goats, mice, hamsters, and humans). Additional studies have been performed using protein misfolding cyclic amplification (PMCA), an in vitro technique highly sensitive in the detection of prion propagation [23]. Brains from PoPrP-Tg001 mice were used as substrate for the PMCA reactions to evaluate the in vitro misfolding ability of pig-PrPC, using a representative collection of the isolates inoculated in PoPrP-Tg001 mice.
METHODS
Ethic Statements
Animal experiments were carried out in strict accordance with the recommendations in the guidelines of the Code for Methods and Welfare Considerations in Behavioural Research with Animals (directives 86/609EC and 2010/63/EU). Experiments were approved by the Committee on the Ethics of Animal Experiments of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (Madrid, Spain; permits CEEA 2009/004 and 2012/002).
Transmission Studies
The studies used a PoPrP-Tg001 mouse line expressing porcine PrPC (4-fold the level of expression in pig brain) in a background knock-out for the prion protein [17]. All inocula were prepared from brain tissues as 10% (wt/vol) homogenates in 5% glucose in distilled water. The isolates used as inocula are described in Supplementary Table 1. Individually identified 6–10-week-old mice were anesthetized with isofluorane and inoculated with 2 mg of brain homogenate in the right parietal lobe, using 25-gauge disposable hypodermic needles. Mice were observed daily, and their neurological status was assessed weekly.
When progression of a TSE disease was evident or at the end of their lifespan, animals were euthanized. Necropsy was then performed, and the brain was collected. A part of the brain was fixed by immersion in 10% formalin for histopathology and immunohistochemistry studies, and the other part was frozen at −20ºC for porcine protease-resistant PrP (PrPres) detection by means of Western blot (WB) analysis. In some cases, brain homogenates were used for second passages, either from PrPres-positive animals or for blind second passage from PrPres-negative animals. The ability of the inocula used to transmit prion disease was demonstrated using transgenic mice expressing bovine [24], ovine [10, 25], murine [26], and human-PrPC [27] or Syrian golden hamsters (Mesocricetus auratus).
WB Analysis
A mass of 175 mg ± 20 mg of frozen brain tissue was homogenized to a concentration of 10% (wt/vol) in 5% glucose in distilled water in grinding tubes (Bio-Rad), using a TeSeE Precess 48 homogenizer (Bio-Rad). The presence of PrPres in transgenic mice brains was determined by means of WB analysis, as described elsewhere [5, 27]. Ten to 100 μL of a 10% (wt/vol) brain homogenate was digested with Proteinase K, loaded on 12% Bis-Tris Gel (Criterion XT; Bio-Rad), and detected with Sha31 monoclonal antibody [28].
PMCA Procedure
PMCA was done as described elsewhere [23]. Briefly, PoPrP-Tg001 mice were perfused by cardiac puncture with 5 mmol/L ethylenediaminetetraacetic acid (Sigma-Aldrich) prepared in 1× phosphate-buffered saline without calcium and magnesium (Thermo Fisher Scientific). Brains were removed and homogenized using a Potter homogenator (Thermo Fisher Scientific) at 10% in 150 mmol/L sodium chloride (Merck) and 1% (vol/vol) Triton X-100 (Sigma-Aldrich) prepared in 1× phosphate-buffered saline without calcium and magnesium supplemented with protease inhibitors (Roche). Aliquots (500 µL) were stored at −80ºC until use.
The different isolates were diluted 1:100 or 1:10 or undiluted into PoPrP-Tg001 substrate. Then, 7 µL of inocula was mixed with 63 µL of PoPrP-Tg001 brain substrate into 0.2-mL polymerase chain reaction tubes (Thermo Fisher Scientific), and 4 zirconia balls (Biospec) were added on each tube. Next, 20 µL of the mixture was taken and immediately frozen as a nonamplification control. At least 2 tubes for each inoculum-substrate combination were included in the experiment, and 3 independent PMCA experiments were performed. Unseeded substrate was also included in the experiment as negative amplification control. Tubes were placed into the water-filled sonicator horn (QSonica; Q700) at 37ºC. Sonication-incubation cycles of 24 hours were applied to the samples. Each cycle included 20 seconds of sonication plus 30 minutes of incubation (amplitude, 40%).
RESULTS
Isolates with differential properties representative of distinct TSE strains from several species have been used to systematically assess the potential susceptibility of pigs to different prions, using a PoPrP-Tg001 mouse model. The isolates used in this work are compiled in Supplementary Table 1. Prion infectivity of these inocula was previously tested in a homologous PrP animal model (without species barrier), as showed in Tables 1–4.
Table 1.
Transmission of Bovine Inocula to PoPrP-Tg001 and BoPrP-Tg110 Mice
| Inocula | Survival Time, Mean (SD), d [Diseased, PrPres-Positive/Inoculated Mice, No.]a | ||
|---|---|---|---|
| PoPrP-Tg001 | BoPrP-Tg110 (1st Passage) | ||
| 1st Passage | 2nd Passage | ||
| Ca-BSE 2 | 498 (9) [2/12]b | 198 (6) [15/15]b | 308 (5) [5/5]c |
| Ca-BSE 2/Tg110 Bo6OR | 372 [1/6]d | 208 (12) [6/6]d | 265 (35) [6/6]d |
| Ca-BSE 2/Tg008 Bo5OR | 461 (100) [3/18] | ND | 331 (73) [7/7] |
| Ca-BSE-H 02.2695 | >650 [0/6] | >650 [0/6] | 328 (15) [12/12]e |
| Ca-BSE-H 02.2695/Tg110 H | >650 [0/6] | ND | 292 (12) [6/6]e |
| Ca-BSE-H 07-644 | >650 [0/6] | ND | 274 (3) [6/6]e |
| Ca-BSE-H 07-644/Tg110 H | >650 [0/6] | >650 [0/6] | 298 (10) [7/7]e |
| Ca-BSE-L 02.2528 | >650 [0/6] | >650 [0/6] | 207 (7) [6/6] |
| Ca-BSE-L 02.2528/Tg110 L | >650 [0/6] | >650 [0/6] | 198 (1) [6/6] |
| Ca-BSE-L 43 | >650 [0/6] | ND | 188 (1) [6/6] |
| Sheep-Sc Langlade/Tg110 | >650 [0/5] | ND | 321 (8) [5/5] |
| Sheep-Sc PS48/Tg110 | >650 [0/6] | ND | 197 (1) [5/5] |
| Sheep-Sc198-9/Tg110 | >650 [0/5] | ND | 165 (7) [7/7] |
| Sheep-ScPS21/Tg110 | >650 [0/6] | ND | 187 (2) [5/5] |
| Sheep-Sc pool pre-75 cattle P75-7 | >650 [0/6]f | >650 [0/6]f | 203 (5) [6/6]f |
| Sheep-Sc pool post-90 cattle P90-1 | >650 [0/6]f | >650 [0/6]f | 173 (3) [6/6]f |
Abbreviations: BoPrP, bovine prion protein (PrP).BSE, bovine spongiform encephalopathy; ND, not done.; PoPrP, porcine PrP; PrPres, protease-resistant PrP.
aThe mean survival time is indicated for all mice scored positive for PrPres.
bPublished elsewhere [18].
cPublished elsewhere [17].
dPublished elsewhere [29].
ePublished elsewhere [5].
fPublished elsewhere [30].
Table 2.
Transmission of Sheep and Goat Inocula to PoPrP-Tg001 and Ovine Transgenic Mice
| Inocula | Survival Time, Mean (SD), d [Diseased, PrPres-Positive/Inoculated Mice, No.]a | ||
|---|---|---|---|
| PoPrP-Tg001 | Ovine PrP-Tg Miceb (1st Passage) | ||
| 1st Passage | 2nd Passage | ||
| Sheep-Sc pool pre-75 | >650 [0/6] | >650 [0/6] | 69 (1) [6/6]c (Tg338) |
| Sheep-Sc pool post-90 | >650 [0/6] | >650 [0/6] | 480 (19) [6/6]c (Tg338) |
| Sheep-Sc PS09 | >650 [0/6] | >650 [0/5] | 431 (18) [6/6] (Tg338) |
| Sheep-Sc PS21 | >650 [0/6] | >650 [0/6] | 182 (17) [6/6] (Tg338) |
| Sheep-Sc PS42 | >650 [0/7] | >650 [0/5] | 67 (7) [6/6] (Tg338) |
| Sheep-Sc198-9 | >650 [0/5] | ND | 629 (27) [5/5] |
| Sheep-ScO100 | >650 [0/6] | >650 [0/5] | 364 (61) [12/12]d (TgOvPrP4) |
| Sheep-ScO104 | >650 [0/5] | >650 [0/5] | 248 (50) [10/10]d (TgOvPrP4) |
| Goat-Sc S2 | >650 [0/6] | >650 [0/5] | 449 (62) [9/9]e |
| Goat-F10 | >650 [0/5] | ND | 465 (17) [7/7]e |
| Goat-Sc Goujon | >650 [0/6] | >650 [0/5] | 253 (7) [5/5] |
| Goat-AtSc I15 | >650 [0/6] | ND | 552 (78) [6/6]f |
| Sheep-AtSc Engavagen | >650 [0/10] | >650 [0/5] | 227 (15) [11/11] (Tg338) |
| Sheep-AtSc Leknes | >650 [0/9] | ND | ND |
| Sheep-AtSc Kjerringøy | >650 [0/5] | >650 [0/5] | 285 (43) [6/6] (Tg338) |
| Sheep-AtSc Suldalsosen | >650 [0/6] | >650 [0/5] | ND |
| Sheep-AtSc Tennevoll | >650 [0/5] | ND | 245 (15) (Tg338) |
| Sheep-AtSc Vinje | >650 [0/5] | ND | ND |
| Sheep-AtSc 152 | 300–600 [2/12]g | 162 (13) [9/9]g | 418 (6) [6/6] |
| Sheep-AtSc 152 | >650 [0/9] | ND | ND |
| Sheep-AtSc 152/Tg338 | >650 [0/6] | >650 [0/5] | ND |
| BSE in sheep ARQ/ARQ | 458 (11) [15/15]g | 162 (4) [13/13]g | 485 (62) [7/7]e |
| BSE in sheep ARR/ARR | 471 (58) [9/9] | 185 (5) [5/5] | 358 (20) [6/6] |
| BSE in TgOV ARQ | 389 (37) [5/5] | ND | ND |
| BSE in TgOV ARR | 417 (18) [5/5] | 183 (14) [6/6] | ND |
| Experimental BSE in goat | 459 (27) [5/5] | 176 (5) [5/5] | 497 (31) [5/5]e |
| BSE in goat CH636 | 505 (60) [7/7] | 182 (9) [7/7] | ND |
Abbreviations: BSE, bovine spongiform encephalopathy; ND, not done; PoPrP, porcine prion protein; PrPres, protease-resistant prion protein.
aThe mean survival time is indicated for all mice scored positive for PrPres.
bData from ovine PrP-Tg501 mice [25] except for those indicated as either Tg338 [10] or TgOvPrP4 [31].
cPublished elsewhere [30].
dPublished elsewhere [31].
ePublished elsewhere [25].
fPublished elsewhere [32].
gPublished elsewhere [18].
Table 3.
Transmission of Mouse and Hamster Inocula in PoPrP-Tg001, Tga20 transgenic Mice or Hamsters
| Inocula | Survival Time, Mean (SD), d [Diseased, PrPres-Positive/Inoculated Mice, No.]a | |||
|---|---|---|---|---|
| PoPrP-Tg001 | Tga20 (1st Passage) | Hamsters (1st Passage) | ||
| 1st Passage | 2nd Passage | |||
| BSE in Tga20 | 506 [1/6]b | ND | 154 (21) [5/5] | ND |
| BSE in wt mouse | 650 [1/6]b | 201 (12b) [6/6] | 158 (39) [5/5] | ND |
| 22L | >650 [0/6] | >650 [0/6] | 112 (13) [4/4] | ND |
| RML | >650 [0/6]b | >650 [0/6]b | 75 (7) [5/5] | ND |
| 263K | >650 [0/7] | >650 [0/5] | ND | 87 (3) [5/5] |
Abbreviations: BSE, bovine spongiform encephalopathy; ND, not done; PoPrP, porcine prion protein; PrPres, protease-resistant prion protein; wt, wild-type.
aThe mean survival time is indicated for all mice scored positive for PrPres.
bPublished elsewhere [29].
Table 4.
Transmission of Human Inocula in PoPrP-Tg001 and HuPrP-Tg340 Transgenic Mice
| Inocula | Survival Time, Mean (SD), d [Diseased, PrPres-Positive/Inoculated Mice, No.]a | ||
|---|---|---|---|
| PoPrP-Tg001 | HuPrP-Tg340 (1st Passage) | ||
| 1st Passage | 2nd Passage | ||
| sCJD 129 M/M T1 NHBX0/0001 | >650 [0/6]b | >650 [0/6]b | 214 (6) [5/5]b |
| sCJD 129 M/M T1 0.08.02523_001 | >650 [0/6] | ND | 187 (11) [6/6] |
| sCJD 129 V/V T2 0.08.02497_001 | >650 [0/5] | ND | 522 (36) [6/6] |
| vCJD 129 M/M NHBY0/0014 | 556 (81) [6/6]b | 212 (6) [6/6]b | 626 (29) [6/6]b |
| vCJD 129 M/M BC1458 | 530 (48) [6/6] | 197 (9) [5/5] | 545 (146) [5/5]c |
| BSE in HuPrP-Tg340 | 486 (31) [5/6] | ND | 614 (87) [6/6]c |
Abbreviations: BSE, bovine spongiform encephalopathy; HuPrP, human prion protein (PrP); ND, not done; PoPrP, porcine PrP; PrPres, protease-resistant PrP; sCJD, sporadic Creutzfeldt-Jakob disease; vCJD, variant Creutzfeldt-Jakob disease.
aThe mean survival time is indicated for all mice scored positive for PrPres.
bPublished elsewhere [29].
cPublished elsewhere [33].
Susceptibility of PoPrP-Tg001 Mice to TSE Isolates
As described elsewhere [17, 18], PoPrP-Tg001 mice can be infected with classic BSE prions but show a high transmission barrier (Table 1). A similar high transmission barrier was also observed in PoPrP-Tg001 mice inoculated with classic BSE passaged in bovine-PrPC transgenic mice with either 5 or 6 octarepeats (Table 1). Considering that porcine-PrPC harbors 5 octarepeats, this result suggests that the identity in the number of octarepeats does not affect the bovine-porcine transmission barrier for classic BSE.
When atypical BSE-L or atypical BSE-H isolates were inoculated, no transmission was detected in PoPrP-Tg001 mice even after 2 iterative passages. Overall, classic BSE was the only bovine prion able to infect mice expressing pig-PrPC. This observation can be extended to classic BSE after passage in other species. The outcome of the inoculations with a wide panel of prions from other species (sheep, goats, mice, hamsters, and humans) indicates that only those isolates derived from classic BSE strain were able to infect PoPrP-Tg001 mice (Tables 2–4) showing similar survival times after second passage, as reported elsewhere [29]. Furthermore, brains from PoPrP-Tg001 mice exhibited similar neuropathological features for all the classic BSE–derived prions (data not shown), regardless of the species of origin of the inoculum, as reported elsewhere [29].
When other prion isolates representative of different strains, such as classic scrapie, atypical/Nor98 scrapie, or sporadic CJD (sCJD), were used as inocula, no transmission to PoPrP-Tg001 mice was detected (Tables 2–4), neither by PrPres detection with WB analysis nor by histopathological analysis. This occurs independently of the PrP expressed in the donor used as inoculum (cattle, sheep, goats, mice, hamsters, or humans), suggesting that differences in amino acid sequences between pig-PrPC and donor scrapie-type PrP (PrPSc) are not mainly responsible for this transmission barrier.
Strikingly, none of the 9 atypical/Nor98 scrapie isolates from either sheep or goat cases were able to infect PoPrP-Tg001 mice (Table 2). These results contrast with those previously published by our group [18], in which sheep-AtSc 152 isolate was able to infect 2 of 12 inoculated mice. In the present work, sheep-AtSc 152 isolated from the same sheep brain was inoculated in another 9 mice but there was no evidence of transmission to any of them. We also attempted, without success, to infect PoPrP-Tg001 mice with the inoculum previously amplified in VRQ-ovine-PrP-Tg338 mice (sheep-AtSc 152/Tg338 in Table 2).
In Vitro Conversion of Pig-PrP by TSE Isolates
Some isolates tested on the PoPrP-Tg001 mice were selected for testing of their ability to propagate in vitro, using PMCA with PoPrP-Tg001 brain as substrate (Table 5). These isolates were subjected to 15 rounds of PMCA and further analyzed to detect positive propagation by means of PrPres detection with WB analysis (Supplementary Figure 1 shows a representative WB). The results presented here (Table 1) were obtained using 1:100 dilutions of the isolates as inocula. When a higher prion seed concentration was used (1:10 or undiluted), isolates negative at 1:100 dilution remained negative at lower dilutions, whereas for positive isolates, in some cases, additional amplification rounds were needed for positive detection. This may be owing to the presence of PMCA inhibitors in the inocula. Alternatively, the dilution of the inocula may result in a concentration-dependent dissociation of the aggregates, thereby releasing and increasing the concentration of available seeds for PMCA, as suggested elsewhere [34].
Table 5.
Protein Misfolding Cyclic Amplification of Selected Inocula Using PoPrP-Tg001 as Substrate
| Seed | Amplification by Serial PMCA Round, %a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Ca-BSE/Pigb | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ca-BSE 2 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Ca-BSE-H 07-644 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ca-BSE-L 43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc pool pre-75 cattle P75-7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc pool post-90 cattle P90-1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc PS48c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc PS13c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc PS21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc PS42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sheep-Sc198-9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Goat-Sc F10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BSE in sheep ARQ/ARQ | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| BSE in TgOV ARQ | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Experimental BSE in goat | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 22L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RML | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 263K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sCJD 129 M/M T1 0.08.02523_001 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sCJD 129 V/V T2 0.08.02497_001 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| vCJD129 M/M BC1458 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| BSE in HuPrP-Tg340 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Unseeded | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: BSE, bovine spongiform encephalopathy; HuPrP, human prion protein (PrP); PMCA, protein misfolding cyclic amplification; PoPrP, porcine PrP; sCJD, sporadic Creutzfeldt-Jakob disease; vCJD, variant Creutzfeldt-Jakob disease.
aPercentage of positive tubes (showing PrPres) of the total number of tubes sonicated (n = 6).
bDescribed elsewhere [29].
cDescribed elsewhere [18].
PMCA results were comparable to those obtained in animal bioassays: classic BSE was the only strain able to amplify in PoPrP-Tg001 substrate independently of the species-PrP in the isolate (Table 5). In the absence of species barrier, as the case of classic BSE from pigs, the amplification was detected in the first round, whereas several PMCA rounds were required to detect positive amplification when a species barrier existed (ie, classic BSE from cattle, sheep, goats, or humans). Atypical BSE, classic scrapie, and sCJD prion strains were unable to propagate in PoPrP-Tg001 substrate, as also found with the animal bioassay (Table 5).
Biochemical Characterization of Pig-PrPres
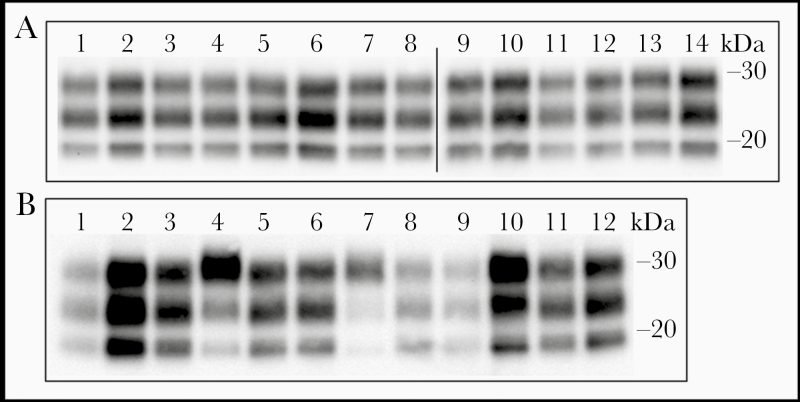
Comparison of the brain PrPres collected from PoPrP-Tg001 mice inoculated with classic BSE–derived prions revealed the same profile in WB analysis, irrespective of the species-PrP in the inoculum (Figure 1A). In all cases, a PrPres glycosylation pattern with a predominant monoglyglcosylated band was observed. This profile was similar to that reported elsewhere in pigs inoculated with classic BSE [18, 21, 29, 35]. Comparison of PrPres obtained from both inoculated mice and PMCA with classic BSE–derived isolates demonstrated that the biochemical strain properties (glycoform proportion and molecular weight of the unglycosylated band) of classic BSE were maintained (Figure 1B). This suggests that the classic BSE prion’s conformation is reliably transmitted to the pig-PrP in vitro, as described elsewhere for PrP from other species [36].
Figure 1.
Immunoblot of porcine protease-resistant prion protein (PrPres). A, Brain PrPres Western blot profile in porcine PrP (PoPrP)–Tg001 mice inoculated with the different classic bovine spongiform encephalopathy (BSE)–derived inocula: Ca-BSE 2 (lane 1), Ca-BSE 2/Tg110 Bo6OR (lane 2), Ca-BSE 2/Tg008 Bo5OR (lane 3), BSE in sheep ARQ/ARQ (lane 4), BSE in sheep ARR/ARR (lane 5), BSE in TgOV ARQ (lane 6), BSE in TgOV ARR (lane 7), experimental BSE in goat (lane 8), BSE in goat CH636 (lane 9), BSE in Tga20 (lane 10), BSE in wild-type mouse (lane 11), variant Creutzfeldt-Jakob disease (vCJD) 129 M/M NHBY0/0014 (lane 12), vCJD 129 M/M BC1458 (lane 13), and BSE in HuPrP-Tg340 (lane 14). B, Brain PrPres Western blot profile in the different inocula and in the PoPrP-Tg001 brain homogenates after in vivo or in vitro experiments. Original inocula used included Ca-BSE/Pig (lane 1), Ca-BSE 2 (lane 4), BSE in Sheep ARQ/ARQ (lane 7), and vCJD 129 M/M BC1458 (lane 10). PoPrP-Tg001 mouse infected with: Ca-BSE/Pig (lane 2), Ca-BSE 2 (lane 5), BSE in Sheep ARQ/ARQ (lane 8) and vCJD 129 M/M BC1458 (lane 11). Brain homogenates from PoPrP-Tg001 were used in protein misfolding cyclic amplification seeded with Ca-BSE/Pig (lane 3), Ca-BSE 2 (lane 6), BSE in sheep ARQ/ARQ (lane 9), and vCJD 129 M/M BC1458 (lane 12). PrPres was detected using Sha31 monoclonal antibody. (Molecular weight in kilodaltons is shown at the right of each panel.)
DISCUSSION
In this work, the transmissibility of a panel of TSE isolates representing diverse prion strains from cattle, sheep, goats, mice, hamsters, and humans was assayed both in vivo using mice overexpressing pig-PrPC and in vitro using the PMCA technique. Although different combinations of prion strains and PrPC expressing donors have been used, only the classic BSE strain was able to propagate in mice expressing pig-PrPC independently of the donor PrP amino acid sequence. It is interesting that classic BSE after passage in other species, such as sheep, goats, or humans, propagate in mice expressing pig-PrPC with better transmission efficiency than cattle BSE, resembling previous observations made in human and bovine PrP transgenic mice [27, 37]. Moreover, all classic BSE–derived prions, regardless of the originating species, exhibited similar strain features, such as survival time and a PrPres glycosylation pattern characterized by a predominant monoglycosylated band, matching that reported elsewhere in pigs infected with BSE [18, 21, 29, 35].
The strain-dependent transmission barrier observed in pig-PrP mice is in accordance with previous observations evidencing that prion strain properties, probably associated with different PrPSc conformers, have a determinant impact on the ability of prions to cross the species barrier [29, 38, 39]. The PMCA results reinforce those obtained in the animal bioassay, confirming that only the classic BSE strain seems able to propagate in a pig-PrP context. In addition, the results indicates that for the isolates analyzed in this study, PMCA is a valuable tool as a complementary method to animal bioassays to assess more quickly the susceptibility or resistance to TSEs in PoPrP-Tg001, because the results obtained using both techniques were equal in terms of isolate propagation and PrPres WB profile.
None of the several atypical/Nor98 scrapie isolates used in this work, including a new inoculation of sheep-AtSc 152 isolate, was transmitted, supporting the contention that porcine species is highly resistant to atypical/Nor98 scrapie prions. This is in contrast with previous results obtained with sheep-AtSc 152 [18], where material from the same infected sheep brain was able to be transmitted, although with a very low attack rate. The ability of sheep-AtSc 152 to infect mice may be due to particular properties distinguishing this isolate from other atypical/Nor98 scrapie isolates. Other possibilities cannot be excluded, such as the coexistence of BSE agent as a minor component present in the donor sheep brain. Moreover, contamination with classic BSE agent in any of the different steps (sample harvesting, homogenization, or inoculation) related with the preparation of the inoculum used in the experiment reported elsewhere [18] cannot be excluded. In any case, the transmission barrier for atypical/Nor98 scrapie infection in pigs can be considered very high as assayed in the mouse model expressing pig-PrPC.
Because positive transmission was not detected in animals inoculated with any of the prion strains used in this work, other than classic BSE, our results indicate a high resistance of the mouse model expressing pig-PrPC to all of them. PMCA is an extremely sensitive technique used to detect prion propagation [23]. Thus, the absence of positive amplification after 15 rounds of PMCA for strains different from classic BSE strongly supports the low susceptibility of PoPrP-Tg001 to prions other than classic BSE. In a recent work, pigs were inoculated with a pool of brains of white tailed deer intracranially inoculated with CWD-affected elk, white-tailed deer, and mule deer [21]. Although RT-QuIC (real-time quaking-induced conversion) enabled detection of PrPSc in both orally and intracranially CWD-inoculated pigs as early as 6 months after inoculation, brain PrPres was detectable with WB analysis a long time (45 months) after inoculation in only a few animals, and in pigs inoculated intracranially but not those inoculated orally. However, 1 orally inoculated pig was positive at immunohistochemistry and enzyme-linked immunosorbent assay 45 months after inoculation. Furthermore, second passages in a pig-PrPC transgenic mouse model showed reduced attacks rates, suggesting that pig-PrPC can support low-level propagation of CWD prions, though with a high species barrier.
Although pig-PrPC could sustain replication of some prion strains, the transmission barrier of pig-PrPC for the analyzed strains is, at least, higher than the already known strong transmission barrier for classic BSE, a prion difficult to transmit in both transgenic mice expressing pig-PrPC and pigs [16, 17]. Interestingly, the ability of pig-PrP to sustain replication of the classic BSE strain regardless of the donor PrPSc amino acid sequence used as inoculum suggests that only a very restricted number of PrPSc conformers, such as classic BSE, present molecular compatibility with pig-PrP for prion propagation. Thus, strain-specific PrPSc conformers seem to play a determinant role in prion strain transmission barrier, even more decisively than amino acid sequence differences (species barrier) [18, 39]. The control of prion host range is thus dictated by selective constraints imposed by the PrPSc rather than the PrPC encoded by the host.
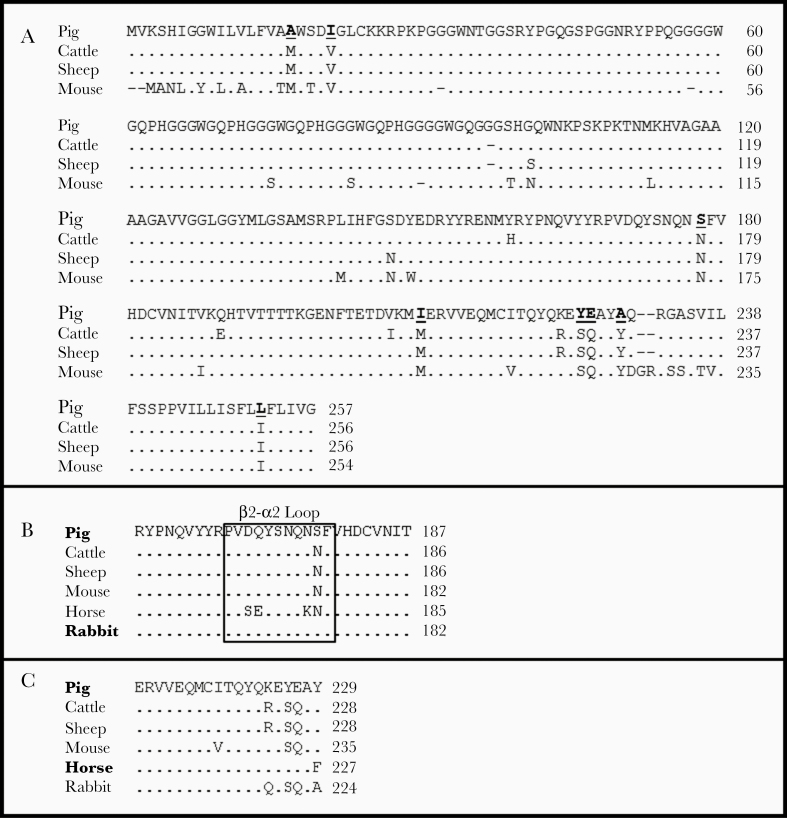
On the other hand, the high resistance of pig-PrPC to replicating a broad diversity of prion strains is in contrast to the prion susceptibility observed in other related species showing minor differences at the PrP amino acid sequence. The pig-PRNP gene is considered very homogeneous, because no relevant polymorphisms have been described [40]. The amino acid sequence of pig-PrP shows ≈94% identity with either cattle or sheep-PrP amino acid sequences (see Figure 2A). This supports the notion that the pig-PrP amino acid sequence has a limited proficiency for recognizing and/or adopting the different PrPSc conformations associated with the diversity of prion strains. This can be due to limitations in the conformational flexibility of the pig-PrP amino acid sequence.
Figure 2.
Amino acid sequence alignment. The amino acid sequences of different species’ prion protein (PrP) were compared with pig-PrP (top rows). Points indicate identical residues; dashes, deletions. Amino acid numbering is indicated on the right, and species on the left. A, Amino acid alignment of pig, cattle, sheep, and mouse prion proteins. Amino acid changes in the pig-PrP amino acid sequence conserved in cattle, sheep, and mice are in bold and underlined. B, Pig-, cattle-, sheep-, mouse-, rabbit-, and horse-PrP alignment of the β2-α2 loop region and the surrounding amino acids. C, Pig-, cattle-, sheep-, mouse-, rabbit-, and horse-PrP alignment of the region around 226YE227 of pig-PrP.
Because only 1 amino acid substitution may drastically alter prion resistance or susceptibility, it is difficult to determine the particular effect of any of the amino acid changes with the high resistance to different prion strains revealed by pig-PrPC. However, we can speculate about the potential effect of some of the amino acid changes observed in the pig-PrP sequence when compared with either bovine or sheep PrP, because both bovine and sheep PrP can adopt the PrPSc conformations associated with the different prion strains used in this work. It is known that minor changes in the β2-α2 loop of PrPC protein (residues 169–179 of porcine sequence) may considerably affect the transmission barrier [41–43]. As a paradigm, the Q171R polymorphism present in the β2-α2 loop of sheep-PrPC is strongly linked to resistance to classic scrapie, but not to BSE [44]. In this sense, the N-to-S amino acid change in the 178 position of the pig-PrPC is present only in species with low susceptibility to prion infection, such as rabbits (Figure 2B).
The amino acid change found in the β2-α2 loop in pig-PrPC would alter the flexibility of the β2-α2 loop, strengthening the transmission barrier for diverse prion strains other than classic BSE. Other amino acid changes in the pig-PrPC would also participate in the limited capacity of this protein to sustain replication of different prion strains and hence to adopt the PrPSc conformations associated with those strains. From these changes, 226YE227 amino acids present in the pig-PrP primary sequence are absent in PrP from other species susceptible to prions showing SQ amino acids at the equivalent position (see Figure 2C).
These 226YE227 amino acid changes are present in PrPC from other species alleged to be reluctant to conformational conversion to PrPSc, such as horses [22]. E227 amino acid in pig-PrP is the equivalent of the Q226E polymorphism observed in cervids, which is E226 in Rocky Mountain elk and Q226 in other CWD-susceptible cervids. CWD prion strain propagation is stable in transgenic mice expressing E226 cervid-PrPC, whereas mice expressing Q226 cervid-PrPC unstably generate CWD mixed strains [45]. Furthermore, these 226YE227 amino acid changes are close to the equivalent position of the Q222K polymorphic variant described in goat populations, considered to confer resistance to classic scrapie prions and reduce susceptibility to the classic BSE strain [25], and the human E219K polymorphism that has been linked to protecting humans against sCJD in epidemiological studies in Asiatic populations [46]. Together, these data suggest that 226YE227 amino acids can be relevant in the restricted ability of pig-PrP to sustain prion replication.
Overall, our results demonstrate that pig-PrPC can be converted in pig-PrPSc only by the classic BSE prion strain, irrespective of the donor species, but not by any other strain used in this work, though other strains not used in this work, such as CWD, may also be able to convert pig-PrPC into pig-PrPSc. Therefore, conformational flexibility constraints associated with pig-PrP would limit the number of permissible PrPSc conformations compatible with pig-PrPC, thus suggesting that pig-PrPC amino acid sequence may constitute a paradigm of low conformational flexibility that could confer high resistance to a wide diversity of prion strains. Amino acid changes in pig-PrPC would be responsible for its limited conformational flexibility compared with other, more susceptible species. This strengthens the transmission barrier for prion strains other than classic BSE, which may represent a thermodynamically favored PrPSc conformation that is readily imprinted on PrP from a range of different species, accounting for the high promiscuity of the BSE strain in mammals.
Finally, the susceptibility of pigs to the classic BSE prion agent and their potential susceptibility to other prion strains not tested here, such as CWD, should not be neglected and underlines the importance of continued monitoring of classic BSE cases and the prohibition of meat and bone meals to reduce the risk of prion transmission to pigs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the staff of the biosafety level 3 animal facility and the Biosafety Office at the Centro de Investigación en Sanidad Animal (Valdeolmos-Madrid) for their excellent animal care and work. We thank all the suppliers listed in Supplementary Table 1 for providing the different inocula used in this work.
Financial support. This work was supported by the Spanish Ministerio de Economía y Competitividad (grants AGL2012-37988-C04-04 and AGL2016-78054-R [Agencia Estatal de Investigación/Fondo Europeo de Desarrollo Regional, Unión Europea] to J. C. E. and J. M. T., fellowship BES-2010–040922 to P. A. C., and fellowship INIA-FPI-SGIT-2015-02 to A. M. M.), the UK Food Standards Agency (project FS231051 [“Permeability of the Human Species Barriers to TSE Circulating Agents”]), and the Fonds Europeens de Developpement Regional Programme Operationnel de Cooperation Territoriale Espagne France Andorre REDPRION (project EFA148/16 [REDPRION] to O. A.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998; 95:13363–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar-Calvo P, García C, Espinosa JC, Andreoletti O, Torres JM. Prion and prion-like diseases in animals. Virus Res 2015; 207:82–93. [DOI] [PubMed] [Google Scholar]
- 3. Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 1996; 383:685–90. [DOI] [PubMed] [Google Scholar]
- 4. Comoy EE, Casalone C, Lescoutra-Etchegaray N, et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One 2008; 3:e3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torres JM, Andréoletti O, Lacroux C, et al. Classical bovine spongiform encephalopathy by transmission of H-type prion in homologous prion protein context. Emerg Infect Dis 2011; 17:1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okada H, Iwamaru Y, Imamura M, et al. Oral transmission of L-type bovine spongiform encephalopathy agent among cattle. Emerg Infect Dis 2017; 23:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pattison IH, Millson GC. Scrapie produced experimentally in goats with special reference to the clinical syndrome. J Comp Pathol 1961; 71:101–9. [DOI] [PubMed] [Google Scholar]
- 8. Bruce ME, Fraser H. Scrapie strain variation and its implications. Curr Top Microbiol Immunol 1991; 172:125–38. [DOI] [PubMed] [Google Scholar]
- 9. Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec 2003; 153:202–8. [DOI] [PubMed] [Google Scholar]
- 10. Le Dur A, Béringue V, Andréoletti O, et al. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A 2005; 102:16031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simmons MM, Konold T, Simmons HA, et al. Experimental transmission of atypical scrapie to sheep. BMC Vet Res 2007; 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haley NJ, Hoover EA. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu Rev Anim Biosci 2015; 3:305–25. [DOI] [PubMed] [Google Scholar]
- 13. Vikøren T, Våge J, Madslien KI, et al. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J Wildl Dis 2019; 55:970–2. [PubMed] [Google Scholar]
- 14. Jahns H, Callanan JJ, Sammin DJ, McElroy MC, Bassett HF. Survey for transmissible spongiform encephalopathies in Irish pigs fed meat and bone meal. Vet Rec 2006; 159:137–42. [DOI] [PubMed] [Google Scholar]
- 15. Konold T, Spiropoulos J, Chaplin MJ, et al. Transmissibility studies of vacuolar changes in the rostral colliculus of pigs. BMC Vet Res 2009; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells GA, Hawkins SA, Austin AR, et al. Studies of the transmissibility of the agent of bovine spongiform encephalopathy to pigs. J Gen Virol 2003; 84:1021–31. [DOI] [PubMed] [Google Scholar]
- 17. Castilla J, Gutiérrez-Adán A, Brun A, et al. Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein. J Neurosci 2004; 24:5063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Espinosa JC, Herva ME, Andréoletti O, et al. Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emerg Infect Dis 2009; 15:1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedman C, Bolea R, Marín B, et al. Transmission of sheep-bovine spongiform encephalopathy to pigs. Vet Res 2016; 47:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greenlee JJ, Kunkle RA, Smith JD, Greenlee MHW. Scrapie in swine: a diagnostic challenge. Food Safety 2016; 4:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore SJ, West Greenlee MH, Kondru N, et al. Experimental transmission of the chronic wasting disease agent to swine after oral or intracranial inoculation. J Virol 2017; 91:e00926-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bian J, Khaychuk V, Angers RC, et al. Prion replication without host adaptation during interspecies transmissions. Proc Natl Acad Sci U S A 2017; 114:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morales R, Duran-Aniotz C, Diaz-Espinoza R, Camacho MV, Soto C. Protein misfolding cyclic amplification of infectious prions. Nat Protoc 2012; 7:1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castilla J, Gutiérrez Adán A, Brun A, et al. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol 2003; 148:677–91. [DOI] [PubMed] [Google Scholar]
- 25. Aguilar-Calvo P, Espinosa JC, Pintado B, et al. Role of the goat K222-PrPC polymorphic variant in prion infection resistance. J Virol 2014; 88:2670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fischer M, Rülicke T, Raeber A, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 1996; 15:1255–64. [PMC free article] [PubMed] [Google Scholar]
- 27. Padilla D, Béringue V, Espinosa JC, et al. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog 2011; 7:e1001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Féraudet C, Morel N, Simon S, et al. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 2005; 280:11247–58. [DOI] [PubMed] [Google Scholar]
- 29. Torres JM, Espinosa JC, Aguilar-Calvo P, et al. Elements modulating the prion species barrier and its passage consequences. PLoS One 2014; 9:e89722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konold T, Nonno R, Spiropoulos J, et al. Further characterisation of transmissible spongiform encephalopathy phenotypes after inoculation of cattle with two temporally separated sources of sheep scrapie from Great Britain. BMC Res Notes 2015; 8:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baron T, Biacabe AG. Molecular behaviors of “CH1641-like” sheep scrapie isolates in ovine transgenic mice (TgOvPrP4). J Virol 2007; 81:7230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aguilar-Calvo P, Espinosa JC, Andréoletti O, et al. Goat K222-PrPC polymorphic variant does not provide resistance to atypical scrapie in transgenic mice. Vet Res 2016; 47:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernández-Borges N, Espinosa JC, Marín-Moreno A, et al. Protective effect of Val129-PrP against bovine spongiform encephalopathy but not variant Creutzfeldt-Jakob disease. Emerg Infect Dis 2017; 23:1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makarava N, Savtchenko R, Alexeeva I, Rohwer RG, Baskakov IV. Fast and ultrasensitive method for quantitating prion infectivity titre. Nat Commun 2012; 3:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seuberlich T, Zurbriggen A. Distinct molecular signature of bovine spongiform encephalopathy prion in pigs. Emerg Infect Dis 2010; 16:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vidal E, Fernández-Borges N, Pintado B, et al. Bovine spongiform encephalopathy induces misfolding of alleged prion-resistant species cellular prion protein without altering its pathobiological features. J Neurosci 2013; 33:7778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Espinosa JC, Andréoletti O, Castilla J, et al. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J Virol 2007; 81:835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Béringue V, Vilotte JL, Laude H. Prion agent diversity and species barrier. Vet Res 2008; 39:47. [DOI] [PubMed] [Google Scholar]
- 39. Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science 2007; 318:930–6. [DOI] [PubMed] [Google Scholar]
- 40. Martin T, Hughes S, Hughes K, Dawson M. Direct sequencing of PCR amplified pig PrP genes. Biochim Biophys Acta 1995; 1270:211–4. [DOI] [PubMed] [Google Scholar]
- 41. Bett C, Fernández-Borges N, Kurt TD, et al. Structure of the β2-α2 loop and interspecies prion transmission. FASEB J 2012; 26:2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Billeter M, Riek R, Wider G, Hornemann S, Glockshuber R, Wüthrich K. Prion protein NMR structure and species barrier for prion diseases. Proc Natl Acad Sci U S A 1997; 94:7281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sigurdson CJ, Nilsson KP, Hornemann S, et al. A molecular switch controls interspecies prion disease transmission in mice. J Clin Invest 2010; 120:2590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Houston F, Goldmann W, Chong A, et al. Prion diseases: BSE in sheep bred for resistance to infection. Nature 2003; 423:498. [DOI] [PubMed] [Google Scholar]
- 45. Angers RC, Kang HE, Napier D, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 2010; 328:1154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shibuya S, Higuchi J, Shin RW, Tateishi J, Kitamoto T. Codon 219 Lys allele of PRNP is not found in sporadic Creutzfeldt-Jakob disease. Ann Neurol 1998; 43:826–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.