Abstract
Background
During the COVID-19 pandemic, decreased volumes of stroke admissions and mechanical thrombectomy were reported. The study’s objective was to examine whether subarachnoid haemorrhage (SAH) hospitalisations and ruptured aneurysm coiling interventions demonstrated similar declines.
Methods
We conducted a cross-sectional, retrospective, observational study across 6 continents, 37 countries and 140 comprehensive stroke centres. Patients with the diagnosis of SAH, aneurysmal SAH, ruptured aneurysm coiling interventions and COVID-19 were identified by prospective aneurysm databases or by International Classification of Diseases, 10th Revision, codes. The 3-month cumulative volume, monthly volumes for SAH hospitalisations and ruptured aneurysm coiling procedures were compared for the period before (1 year and immediately before) and during the pandemic, defined as 1 March–31 May 2020. The prior 1-year control period (1 March–31 May 2019) was obtained to account for seasonal variation.
Findings
There was a significant decline in SAH hospitalisations, with 2044 admissions in the 3 months immediately before and 1585 admissions during the pandemic, representing a relative decline of 22.5% (95% CI −24.3% to −20.7%, p<0.0001). Embolisation of ruptured aneurysms declined with 1170–1035 procedures, respectively, representing an 11.5% (95%CI −13.5% to −9.8%, p=0.002) relative drop. Subgroup analysis was noted for aneurysmal SAH hospitalisation decline from 834 to 626 hospitalisations, a 24.9% relative decline (95% CI −28.0% to −22.1%, p<0.0001). A relative increase in ruptured aneurysm coiling was noted in low coiling volume hospitals of 41.1% (95% CI 32.3% to 50.6%, p=0.008) despite a decrease in SAH admissions in this tertile.
Interpretation
There was a relative decrease in the volume of SAH hospitalisations, aneurysmal SAH hospitalisations and ruptured aneurysm embolisations during the COVID-19 pandemic. These findings in SAH are consistent with a decrease in other emergencies, such as stroke and myocardial infarction.
Keywords: aneurysm, coil, haemorrhage, infection, subarachnoid
Background
The COVID-19 pandemic led to the rationing of healthcare resources worldwide to accommodate the care of critically ill patients with SARS-CoV-2 infection.1 Changes in prehospital emergency medical service, emergency room care, acute stroke and subarachnoid haemorrhage (SAH) protocols2 3 were reported to conserve resources and to mitigate infection risk to patients and their providers. Decreases in ischaemic stroke admission, rates of intravenous thrombolysis (IVT)4–6 and mechanical thrombectomy (MT) volume7 were reported in several regions in Europe,8 9 Germany,10 China,11 Brazil12 and the USA,13 14 with steeper declines in stroke hospitalisations seen in areas with higher COVID-19 hospitalisation volume.15
However, there is a paucity of information on the impact of the COVID-19 pandemic on SAH admissions. Early regional or single-centre reports from Paris16 and Toronto17 suggest a decrease in aneurysmal SAH volumes, whereas no changes were seen in Berlin.18 We evaluated the impact of COVID-19 on the volumes of SAH admissions and embolisation treatments for patients with ruptured intracranial aneurysms during the height of the first 3 months of the pandemic, defined from 1 March to 31 May 2020.
Study objectives and hypothesis
Our primary hypothesis was that there would be a reduction in SAH hospitalisations and endovascular coil embolisation procedures for ruptured aneurysms during the pandemic, compared with the immediate 3 months prior to the pandemic. Our secondary hypothesis was that there would be a reduction in these volumes compared with a similar calendar period in 2019. The third hypothesis was that the reduction in SAH volume would occur in most centres, including those with low or non-existent COVID-19 hospitalisation burden, but would be more significant in centres with high COVID-19 hospitalisation burden. The fourth hypothesis was that high procedural coiling volume centres would be less impacted by procedural volume changes than low procedural volume centres.
Methods
Study design
This was a cross-sectional, observational, multicentre, retrospective study of consecutive patients hospitalised with SAH, aneurysmal SAH, non-traumatic SAH and ruptured intracranial aneurysm embolisations.
Setting and participants
Of 175 invited sites, 140 comprehensive stroke centres submitted data from 37 countries across six continents with 5571 patients with SAH and 3473 ruptured aneurysm embolisations across the three different study periods. Monthly and weekly volume of SAH, ruptured aneurysm embolisations and COVID-19 admission volume data were collected over three periods of time: 1 March–31 May 2020 (pandemic months), 1 November 2019–29 February 2020 (immediately preceding the pandemic months) and 1 March–31 May 2019 (equivalent period 1 year prior to the pandemic). The period of recruitment was conducted between 26 May and 30 July 2020. The data were collected on Excel (version 16.45) documents.
Data were collected from collaborators of the Society of Vascular and Interventional Neurology, the Middle East North Africa Stoke and Interventional Neurotherapies Organisation, the Japanese Interventional Neurology Society and several academic partners. The following countries were represented (number of centres): USA (45), Japan (30), China (6), Brazil (6), Canada (6), France (4), Australia (3), Korea (3), India (3), Chile (2), Spain (2), Switzerland (2), England (2), Saudi Arabia (2), Turkey (2), Austria (1), Argentina (1), Egypt (1), Germany (1), Vietnam (1), Croatia (1), Greece (1), Indonesia (1), Ireland (1), Israel (1), Italy (1), Lebanon (1), New Zealand (1), Oman (1), Pakistan (1), Portugal (1) Qatar (1), South Africa (1), Thailand (1), Tunisia (1), United Arab Emirates (1) and Uruguay (1).
Study variables and outcome measures
SAH data were obtained by a prospectively maintained aneurysm or stroke databases at each comprehensive stroke centre or by International Classification of Diseases, 10th Revision (ICD-10) codes (primary, secondary or tertiary discharge codes) with verification by a physician or coordinator. The following ICD-10 codes were used: I60 (non-traumatic SAH), I60.0 (non-traumatic SAH from carotid siphon and bifurcation), I60.1 (non-traumatic SAH from middle cerebral artery), I60.2 (non-traumatic SAH from anterior communicating artery), I60.3 (non-traumatic SAH from posterior communicating artery), I60.4 (non-traumatic SAH from basilar artery), I60.5 (non-traumatic SAH from vertebral artery, I60.6 (non-traumatic SAH from other intracranial arteries), I60.7 (non-traumatic SAH from intracranial artery, unspecified) I60.8 (other non-traumatic SAH) and I60.9 (non-traumatic SAH unspecified).
Subgroup analysis of confirmed aneurysmal SAH hospitalisations and non-traumatic SAH were performed. Aneurysmal SAH was defined as SAH related to a ruptured intracranial aneurysm. Non-traumatic SAH was defined as SAH unrelated to traumatic causes but could include SAH secondary to aneurysmal, arteriovenous malformation (AVM), perimesencephalic or other causes. The volume of embolisations of ruptured intracranial aneurysms was also retrieved.
COVID-19 hospitalisation was defined as a patient admitted with COVID-19 diagnosis, inclusive of non-neurological diagnosis. Monthly and weekly volumes of COVID-19 hospitalisation were collected from 1 March to 31 May 2020.
Low, intermediate and high procedural volume centres were categorised according to monthly coiling of ruptured aneurysm volume data received of the 4 months immediately preceding the pandemic (1 November 2019–29 February 2020, inclusive) and divided into tertiles: low volume, <1.25; intermediate volume, >1.25–<3.0; and high volume, >3 coiling cases per month. COVID-19 hospitalisation volumes were based on mean monthly volume data received and were divided into tertiles: low volume, <10.6; intermediate volume, >10.6–<103.6; and high volume, >103.6 hospitalisations per month.
Bias
A second control period (1 March–31 May 2019) was included to account for seasonal variation. To reduce the risk of bias, centres with incomplete data were excluded from the subgroup analysis in which the data were missing.
Statistical analysis
The monthly volumes for the ruptured aneurysm coil embolisation procedure and SAH admissions were compared for the period before (1 year and immediately before) and during the COVID-19 pandemic. The normality of the data was tested with the Shapiro-Wilk test. The data were determined to be non-normal and were therefore presented as median (IQR). The non-parametric Wilcoxon signed-rank test was applied to compare differences in monthly volume between two time periods. The analyses were repeated in the setting of low, intermediate and high COVID-19 and procedural volume hospitals.
We further looked at the percentage change in the number of procedures and SAH admissions, aneurysmal SAH admissions, and non-traumatic SAH admissions before and during the COVID-19 pandemic. For this analysis, we restricted the immediately before group to 3 months before the pandemic (1 December 2019–29 February 2020) to keep it consistent with the COVID-19 group. The 95% CIs for percentage change were calculated using the Wilson procedure without correction for continuity. The differences in the number of procedures and admissions across the two periods were assessed for significance using the Poisson means test. The relative percentage decrease in volume between low-volume, intermediate-volume and high-volume hospitals was tested using the z‐test of proportion.
We performed a supplementary analysis comparing monthly volumes and percentage change in the number of ruptured aneurysm coiling procedures and SAH hospitalisations across different world regions. All data were analysed using SAS V.9.4, and the significance level was set at a p value of <0.05.
This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.19
Findings
A total of 1088, 2044 and 1585 SAH hospitalisations (overall n=4717) and 719, 1170 and 1035 coiling procedures for ruptured aneurysms (overall n=2924) were included across the 3-month prior year periods, 3 months immediately prepandemic and 3 months pandemic, respectively. These were distributed across 140 comprehensive stroke centres, 37 nations and 6 continents. The Shapiro-Wilk test revealed that the normality of the data was non-normal.
Subarachnoid hemorrhage hospitalisation volumes
In the primary analysis, 118 centres submitted data on SAH volume with a total of 2044 admissions in the 3 months immediately before and 1585 admissions during the 3 months of the pandemic, representing a relative volume decline of 22.5% (95% CI −24.3% to −20.7%, p<0.0001). Monthly SAH admission volumes also demonstrated a relative decline before and during the pandemic months (median, 4.5 (IQR 2.5–7.1) vs 3.3 (IQR1.3–6.3); p<0.0001) (table 1 and figure 1).
Table 1.
SAH hospitalisation volumes immediately before and during the COVID-19 pandemic
| Overall volume | Monthly volume | |||||||||
| N | n1 | n2 | Relative (%) change | P value | N | Immediately before n=2838 |
During COVID-19 n=1645 |
Difference* (95% CI) | P value | |
| % (95% CI) | Median (IQR) | |||||||||
| Overall | 118 | 2044 | 1585 | −22.5 (−24.3 to −20.7) | <0.0001 | 124 | 4.5 (2.5–7.1) | 3.3 (1.3–6.3) | −0.88 (−1.1 to −0.58) | <0.0001 |
| Hospital COVID-19 volume† | ||||||||||
| Low | 32 | 432 | 367 | −15.1 (−18.7 to −12.0) | 0.014 | 33 | 3.5 (2.5–6.5) | 3.3 (1.7–6.0) | −0.83 (−1.9 to 0.50) | 0.076 |
| Int | 32 | 589 | 458 | −22.2 (−25.8 to −19.1) | <0.0001 | 34 | 4.9 (3.5–6.8) | 3.7 (1.7–6.0) | −0.83 (−1.9 to −0.17) | 0.001 |
| High | 33 | 731 | 513 | −29.8 (−33.2 to −26.6) | <0.0001 | 36 | 6.0 (3.0–8.4) | 4.2 (2.2–7.2) | −1.0 (−2.0 to −0.67) | <0.0001 |
| Hospital SAH coil embolisation volume‡ | ||||||||||
| Low | 42 | 370 | 293 | −20.8 (−25.2 to −17.0) | 0.002 | 45 | 2.5 (1.3–3.8) | 2.0 (1.0–3.3) | −0.25 (−0.75 to 0.08) | 0.141 |
| Int | 35 | 490 | 385 | −21.4 (−25.3 to −18.0) | 0.0002 | 36 | 4.4 (2.9–5.6) | 3.0 (1.5–4.7) | −1.0 (−1.5 to −0.17) | 0.007 |
| High | 35 | 1014 | 783 | −22.8 (−25.5 to −20.3) | <0.0001 | 36 | 7.3 (5.9–11.6) | 6.7 (4.0–9.3) | −2.0 (−3.1 to −0.75) | <0.0001 |
n1 is based on 3 months before the pandemic (December 2019–February 2020). Immediately before is based on 4 months before the pandemic (November 2019–February 2020). n2 and during COVID-19 are based on March 2020–May 2020.
P value is from Poisson means test (overall volume analysis) and Wilcoxon signed-rank test (monthly volume analysis).
*Difference denotes the median difference between the two time periods.
†P value: low versus Int=0.004, low versus high=<0.0001, Int versus high=0.002.
‡P value: low versus Int=0.831, low versus high=0.429, Int versus high=0.541.
Int, intermediate; N, number of hospitals; n, number of admissions; SAH, subarachnoid haemorrhage.
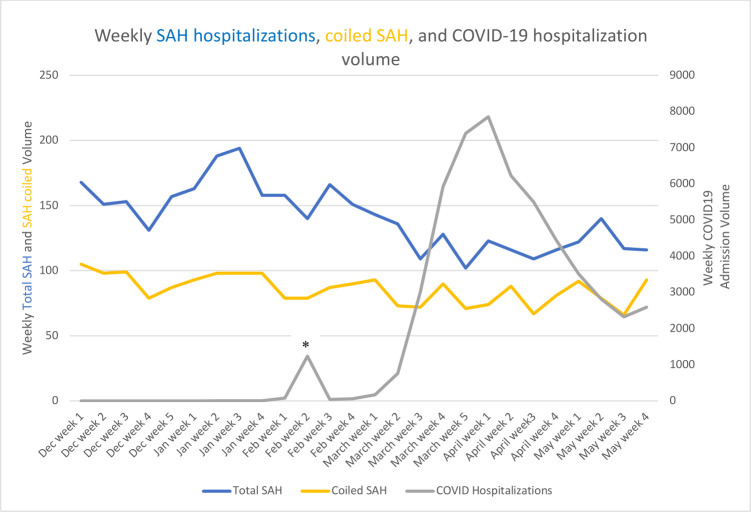
Figure 1.
*Peak of 1235 COVID-19 hospitalisations in the second week of February, predominantly from one hospital in Wuhan, China. SAH, subarachnoid haemorrhage.
In the secondary analysis, 75 centres contributed data with SAH monthly volumes 1 year prior (table 2). There were 1088 before, compared with 900 SAH admissions during the pandemic, representing a 17.3% relative decline (95% CI, −19.6 to −15.2, p<0.0001). The median monthly SAH admission volume declined from a median of 3.0 [IQR, 2.0–6.3] in the corresponding period of the prior year to 2.7 [IQR, 1.3–5.7, p=0.001] over the first 3 months of the pandemic.
Table 2.
SAH volumes 1 year before and during the COVID-19 pandemic
| Overall volume | Monthly volume | |||||||||
| N | n1 | n2 | Relative (%) change | P value | N | 1 year before | During COVID-19 |
Difference* (95% CI) | P value | |
| % (95% CI) | Median (IQR) | |||||||||
| SAH | 75 | 1088 | 900 | −17.3 (−19.6 to −15.2) | <0.0001 | 75 | 3.0 (2.0–6.3) | 2.7 (1.3–5.7) | −0.33 (−1.0 to 0.0) | 0.001 |
| Coil embolisation† | 83 | 719 | 652 | −9.3 (−11.7 to −7.4) | 0.071 | 85 | 1.7 (0.67–3.7) | 1.3 (0.67–2.7) | 0.0 (−0.33 to 0.0) | 0.197 |
n1 and 1 year before are based on 3-month data 1 year before the pandemic (March 2019–May 2019). n2 and during COVID-19 are based on data from March 2020 to May 2020.
P value is from Poisson means test (overall volume analysis) and Wilcoxon signed-rank test (monthly volume analysis).
*Difference denotes the median difference between the two time periods.
†85 centres contributed 728 and 655 patients to 1 year before and during the COVID-19 period in the monthly volume analysis.
n, number of admissions/procedures; N, number of hospitals; SAH, subarachnoid haemorrhage.
In subgroup analysis, 56 centres confirmed aneurysmal SAH admissions data in the 3 months immediately before and during the pandemic. There was a relative decline from 834 to 626 hospitalisations, representing a 24.9% relative decline (95% CI −28.0% to −22.1%, p<0.0001). Additionally, 37 centres confirmed aneurysmal SAH admissions data in the 1-year prior control period, also noted for a relative decline from 435 to 370 hospitalisations, representing a 14.9% relative decline (95% CI −18.6 to −11.9, p=0.022) (table 3).
Table 3.
Aneurysmal SAH, non-traumatic SAH hospitalisations before and during the pandemic
| Immediately before and during the pandemic | 1 year before and during the pandemic | |||||||||
| N | n1 | n2 | Relative change % (95% CI) | P value | N | n1 | n2 | Relative change % (95% CI) | P value | |
| Aneurysmal SAH | 56 | 834 | 626 | −24.9 (−28.0 to −22.1) | <0.0001 | 37 | 435 | 370 | −14.9 (−18.6 to −11.9) | 0.022 |
| Non-traumatic SAH* | 85 | 1451 | 1094 | −24.6 (−26.9 to −22.5) | <0.0001 | 53 | 744 | 628 | −15.6 (−18.4 to −13.1) | 0.002 |
n1 immediately before the pandemic is based on 3-month data from December 2019 to February 2020. n1 1 year before is based on 3-month data from March 2019 to May 2019. n2 is based on 3-month control data during the COVID-19 from March 2020 to May 2020 for both analyses.
P value is from the Poisson means test.
*Non-traumatic SAH include aneurysms and perimesencephalic SAH.
n, number of admissions; N, number of hospitals; SAH, subarachnoid haemorrhage.
Non-traumatic SAH admissions had parallel relative declines both in the immediately before (−24.6%, 95% CI −26.9% to −22.5%, p<0.0001, n=85 centres) and 1-year before periods (−15.6%, 95% CI −18.4% to −13.1%, p=0.002, n=53 centres) (table 3).
Declines in SAH hospitalisation volumes were significant in Asia, with a relative decrease of 24.7% (95% CI −28.0% to −21.7%, p<0.0001, n=47 centres); North America, with a relative decrease of 21.0% (95% CI −24.0% to −18.3%, p<0.0001, n=46 centres); Europe, with a relative decrease of 29.0% (95% CI −35.3% to −23.5%, p=0.001, n=11 centres); South America, with a relative decrease of 21.5% (95% CI −27.4% to −16.6%, p=0.012, n=8 centres). In contrast, no significant change was noted in Oceania or Africa. (online supplemental table 1). Country-specific relative changes in SAH hospitalisation volumes are represented in online supplemental table 3 and online supplemental figure 1).
svn-2020-000695supp001.pdf (3.9MB, pdf)
SAH aneurysm embolisation volumes
In the primary analysis, 125 centres submitted data on ruptured aneurysm embolisation volumes with a total of 1170 procedures in the 3 months immediately before and 1035 procedures performed during the 3 months of the pandemic, representing a relative drop of 11.5% (95% CI −13.5% to −9.8%, p=0.002). Median monthly embolisation volumes demonstrated a relative decline compared with the same periods immediately preceding (median, 1.8 (IQR 1–4) vs 1.7 (IQR 0.67–3.3); p=0.0004) (table 4 and figure 1).
Table 4.
SAH coil embolisation volumes immediately before and during the COVID-19 pandemic
| Overall volume | Monthly volume | |||||||||
| N | n1 | n2 | Relative (%) change | P value | N | Immediately before n=1670 |
During COVID-19 n=1075 |
Difference* (95% CI) | P value | |
| % (95% CI) | Median (IQR) | |||||||||
| Overall | 125 | 1170 | 1035 | −11.5 (−13.5 to −9.8) | 0.002 | 133 | 1.8 (1.0–4.0) | 1.7 (0.67–3.3) | −0.25 (−0.58 to −0.08) | 0.0004 |
| Hospital COVID-19 volume† | ||||||||||
| Low | 39 | 270 | 266 | −1.5 (−3.7 to −0.58) | 0.764 | 40 | 1.5 (0.88–2.5) | 1.0 (0.50–2.8) | −0.29 (−0.67 to 0.08) | 0.294 |
| Int | 33 | 319 | 287 | −10.0 (−13.8 to −7.2) | 0.151 | 35 | 2.5 (1.0–3.8) | 2.0 (1.0–3.0) | −0.25 (−0.75 to 0.0) | 0.041 |
| High | 31 | 329 | 256 | −22.2 (−27.0 to −18.0) | 0.002 | 34 | 2.0 (1.3–5.0) | 2.0 (1.0–4.0) | −0.63 (−1.2 to 0.0) | 0.007 |
| Hospital SAH Coil embolisation volume‡ | ||||||||||
| Low | 46 | 107 | 151 | 41.1 (32.3 to 50.6) | 0.008 | 49 | 0.75 (0.25–1.0) | 0.67 (0.33–1.7) | 0.0 (0.0 to 0.33) | 0.044 |
| Int | 37 | 217 | 192 | −11.5 (−16.5 to −7.9) | 0.178 | 39 | 2.0 (1.8–2.5) | 1.3 (0.67–2.7) | −0.75 (−1.1 to −0.08) | 0.015 |
| High | 42 | 846 | 692 | −18.2 (−20.9 to −15.8) | <0.0001 | 45 | 5.3 (4.0–8.8) | 4.7 (2.7–6.3) | −1.8 (−2.3 to −0.67) | <0.0001 |
n1 is based on 3 months before the pandemic (December 2019–February 2020). Immediately before is based on 4 months before the pandemic (November 2019–February 2020). n2 and during COVID-19 are based on March 2020–May 2020.
P value is from Poisson means test (overall volume analysis) and Wilcoxon signed-rank test (monthly volume analysis).
*Difference denotes the median difference between the two time periods.
†P value: low versus Int≤0.0001, low versus high≤0.0001, Int versus high≤0.0001.
‡P value: low versus Int=n/a; low versus high=n/a; Int versus high=0.019.
Int, intermediate; N, number of hospitals; n, number of procedures; n/a, not applicable; SAH, subarachnoid haemorrhage.
In the secondary analysis, 83 centres contributed data for ruptured aneurysm coiled volumes during the pandemic and 1 year previously. Ruptured aneurysm embolisations also declined numerically between the calendar year, 719 vs 652 procedures, with a 9.3% (95% CI −11.7% to −7.4%, p=0.07) relative drop in volumes (table 2). No significant change was noted in the median monthly volume (p=0.197).
During the pandemic, ruptured aneurysm coiling volume was decreased in Asia with a 20.5% relative decline (95% CI −24.9% to −16.6%, p=0.003, n=52 centres), decreased in Europe with a 15.3% relative decline (95% CI −20.4% to −11.3%, p=0.06, n=14 centres) and increased in Oceania by 77.8% (95% CI 54.8 to 91.0, p=0.06, n=4 centres), whereas no significant change in volume was noted in North America, South America nor Africa (online supplemental table 2). Country-specific relative changes in ruptured aneurysm coiling volumes are represented in online supplemental table 3 and online supplemental figure 2.
COVID-19 hospitalisation volume, SAH hospitalisation and ruptured aneurysm embolisation volumes in relation to the pandemic
Figure 1 depicts the weekly number of SAH hospitalisations, ruptured aneurysm coiling and COVID-19 hospitalisation volumes. Across the tertiles of COVID-19 hospitalisation volume, high-volume COVID-19 centres (−29.8%, 95% CI −33.2% to −26.6%) were significantly more vulnerable to declines in SAH hospitalisation volumes than low-volume COVID-19 centres (−15.1%, 95% CI −18.7% to −12.0%; p<0.0001) (table 1).
Similarly, there was a gradient for greater decrease in ruptured aneurysm embolisation in high-volume COVID-19 centres (−22.2%, 95% CI −27.0% to −18.0%) compared with intermediate-volume (−10.0%, 95% CI −13.8% to −7.2%, p<0.0001) and low-volume (−1.5%, 95% CI −3.7% to −0.6%, p<0.001) COVID-19 centres (table 4).
Ruptured aneurysm procedural volumes, SAH hospitalisation and ruptured aneurysm embolisation volumes in relation to the pandemic
There were declines in SAH hospitalisation volume across the three tertiles of high (−22.8%, 95% CI −25.5% to −20.3%, p<0.0001), intermediate (−21.4%, 95% CI −25.3% to −18.0%, p=0.0002) and low (−20.8% 95% CI −25.2% to −17.0%, p=0.002) SAH procedural volume centres, with no differences in decline seen between the three tertiles (table 1).
Similarly, ruptured aneurysm embolisation volume declines were noted in high (−18.2%, 95% CI −20.9% to −15.8%, p<0.0001) procedural volume centres. However, in hospitals with low tertile procedural volumes, there was an increase noted in the coiling of the ruptured aneurysm during the pandemic of 41% (95% CI 32.3% to 50.6%, p=0.008) (table 4).
Discussion
We noted a decrease in the volume of SAH hospitalisations, aneurysmal SAH hospitalisations and embolisation of ruptured aneurysms during the first 3 months of the COVID-19 pandemic compared with the immediate prior months. Compared with the corresponding period in the prior year, there was a significant reduction in SAH hospitalisation volume, but no change was noted in the number of embolisation procedures for ruptured aneurysms. To our knowledge, this is the first report of a multicentre decrease in volumes for SAH hospitalisations, aneurysmal SAH hospitalisations and embolisation procedures for ruptured intracranial aneurysm during the COVID-19 pandemic. Our findings are similar to reported decreases in SAH city-wide in Paris during a 2-week period of the pandemic16 and decreases in a Toronto hospital,17 whereas Berlin and Joinville, South Brazil, reported no decreases in SAH during the COVID-19 pandemic.12 18
As expected, hospitals with higher tertiles of COVID-19 hospitalisation burden were more vulnerable to the decline in SAH admissions and ruptured aneurysm coiling volume. However, hospitals with lower COVID-19 hospitalisation burden also demonstrated decreases in SAH admissions, suggesting that access to hospital care was likely not a principal factor to explain the decrease.
High and intermediate procedural volume centres were more affected by declines in SAH hospitalisations and ruptured aneurysm embolisation than low-volume SAH coiling centres during the pandemic. In contrast, hospitals with low SAH coiling volumes at baseline demonstrated an increase in the coiling of ruptured aneurysms during the pandemic despite a significant decrease in total SAH admissions within this tertile of hospitals. An increase in ruptured aneurysm embolisations was observed in another recent multicentre study during the COVID-19 pandemic.20 This suggests a shift towards treating more patients with ruptured aneurysms with endovascular techniques during the pandemic, possibly to mitigate risks of perioperative infection to the patient and/or provider.
These findings of decreases in SAH volumes, including embolisation of ruptured aneurysms, are similar to reports of decreases in stroke admissions, intravenous thrombolysis, MT and acute ST-elevation myocardial infarction (STEMI) activations during the COVID-19 pandemic.10 13 21 As postulated with reasons for the decline in stroke admissions in the stroke literature,8 patients with milder presentations of aneurysmal SAH may be afraid to present to a hospital due to fear of contracting SARS-CoV-2 infection.
This analysis’s strength is the aggregate volume of data worldwide across diverse geography, allowing a high volume or sample size. We used two control periods for comparison; the immediately preceding 3 months and the same 3 months a year ago, to account for potential seasonal variations that may occur in the presentation of SAH.22
Study limitations
This study’s limitations are that while our cohort of centres inform an international, multicentre experience, it is not comprehensive without source data from national databases to account for regional differences in health systems of care. The diagnosis of SAH was obtained using ICD-10 codes in some centres. We cannot exclude the possibility of traumatic SAH. To differentiate from this possibility, we performed a subgroup analysis of confirmed aneurysmal SAH and non-traumatic SAH admissions and found similar relative declines in both control periods. Most centres contributing to these data have systems in place to track SAH admissions and coiling volumes; hence, the relative changes in volume from this analysis are likely robust. Details on patient SAH presentation grade, clinical outcomes and clipping volume were not collected as they were outside the scope of the study.
Our study definition of the beginning of the pandemic relates to the WHO designation on 11 March 2020. However, regions affected by the pandemic earlier, such as China, met the nadir of their SAH volumes prior to starting our defined pandemic period. As endovascular coiling remains unavailable in many low-income and lower-income to middle-income countries, specific geographical regions were not well represented (ie, Central Africa) in our study. Another shortcoming in selection bias is that several countries in which endovascular coiling is available were not represented in this study (ie, Eastern Europe, South America, Central America and Asia).
Interpretation
In conclusion, there was a relative decrease in the volume of SAH hospitalisations, aneurysmal SAH hospitalisations and ruptured aneurysm embolisation treatments during the first 3 months studied of the COVID-19 pandemic. There were steeper relative declines in SAH hospitalisations and SAH coiling volume in hospitals with higher COVID-19 volume. Among low-volume coiling SAH hospitals, there was a shift towards an increase in ruptured aneurysm coiling. These findings can inform regional neuroscience centres’ preparedness2 23 24 in the face of a potential second wave or resurgence of COVID-19.
Acknowledgments
The authors thank Judith Clark, RN, Boston Medical Center; Matt Metzinger, MBA, CPHQ; Kamini Patel, RN, MSN, MBA, CPHQ, Jefferson; Janis Ginnane, RN, Emory University Hospital.
Footnotes
Twitter: @NguyenThanhMD, @DiogoHaussen, @Ossama_Mansour, @AbdalkaderMD, @eneri_neuro, @MahmoudNeuro, @AlAlBayati1, @SaimaAh46545828, @neurofox, @DrLe2287, @VietquyNguyen, @bourcierromain1, @Mali27043317, @donfreimd, @PascalJabbourMD, @NabeelHerial, @almuftifawaz, @dgandhimd, @MatoukCharles, @dr_mchen, @VitorMendesPer1, @paddynicholson, @Neurorradio, @raghidkikano, @CerebrovascLab, @AANeurosurgeon, @Operatingnheels, @eacora, @NagelSimon, @HosamJehani, @SunilAShethMD, @JimSiegler, @AjitSPuri1, @GianmarcoBerna5, @neuroabud, @opontesnetoMD, @eytanraz, @ShadiYaghi2, @NeuroINX, @SteveCordina, @ArenillasJF, @DoctorGaldamez, @jordiblascoa, @Teddyyhwu, @MPeyT1, @Robin_Novakovic, @michaelebkelly, @stephen_winters, @StrokeVAN, @VSainiMD, @dyavagal, @italolinfante, @dliebesk, @drviktorszeder, @HMasoud_, @brendan_dr, @alicenomalice, @AmeerEHassan, @MokinMax, @AlexChebl, @OKargiotis, @DrAltschul, @anirudhvk, @pablolavados, @volavarria, @GiseleSampaioS, @artemka__crh, @AdelALHAZZANI, @chungmdphd, @stephanamayer, @johannatfifi, @mihill68, @TudorGJovin, @oozaidat
TNN and RGN contributed equally.
Contributors: TNN and RGN conceived the project. They wrote the first draft of the paper with subsequent input from all coauthors. All coauthors played a major role in data acquisition and revision of the manuscript. MMQ was the lead statistician for this study and performed the analysis. MA prepared the global maps in the supplement.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: TNN: PI CLEAR study (Medtronic). DCH: Stryker, Vesalio, Cerenovus consultant. AEH: consultant and speaker for Medtronic, Stryker, Microvention, Penumbra, Balt, Scientia, Genentech and GE Healthcare. PJ: Medtronic, Microvention, Balt, Cerenovus consultant. SO-G: Medtronic, Stryker consultant. DSL: Cerenovus, Genentech, Stryker, Medtronic consultant. TGJ: advisor/investor for Anaconda, Route92, FreeOx, and Blockade Medical; Medtronic grants, DAWN, AURORA PI (Stryker). WJM: consultant: Rebound Therapeutics, Viseon Imperative Care, Q’Apel, Stryker, Stream Biomedical, Spartan Micro; Investor: Cerebrotech, Endostream, Q’Apel, Viseon, Rebound, and Spartan Micro. RGN: Stryker; Cerenovus/Neuravi; Anaconda, Cerebrotech, Ceretrieve, Vesalio (Advisory Board); Imperative Care.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Anonymised data are available upon reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The institutional review boards (IRBs) from the coordinating sites determined that because the investigators did not have access to protected health information nor any private identifiable information, the study did not meet the definition of human subject research and therefore no informed consent or IRB oversight was required.
References
- 1. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med Overseas Ed 2020;382:2049–55. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen TN, Jadhav AP, Dasenbrock HH, et al. Subarachnoid hemorrhage guidance in the era of the COVID-19 pandemic - An opinion to mitigate exposure and conserve personal protective equipment. J Stroke Cerebrovasc Dis 2020;29:105010. 10.1016/j.jstrokecerebrovasdis.2020.105010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nguyen TN, Abdalkader M, Jovin TG, et al. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the Society of vascular and Interventional Neurology. Stroke 2020;51:1896–901. 10.1161/STROKEAHA.120.030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nogueira RG, Qureshi M, Abdalkader M. Global impact of COVID-19 on stroke care and intravenous thrombolysis. American Academy of Neurology; April 17-22, 2021. [Google Scholar]
- 5. Alonso de Leciñana M, Castellanos M, Ayo-Martín Óscar, et al. Stroke care during the COVID-19 outbreak in Spain: the experience of Spanish stroke units. Stroke Vasc Neurol 2020. 10.1136/svn-2020-000678. [Epub ahead of print: 04 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ortega-Gutierrez S, Farooqui M, Zha A, et al. Decline in mild stroke presentations and intravenous thrombolysis during the COVID-19 pandemic: the Society of vascular and Interventional Neurology multicenter collaboration. Clin Neurol Neurosurg 2020;201:106436. 10.1016/j.clineuro.2020.106436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hajdu SD, Pittet V, Puccinelli F, et al. Acute stroke management during the COVID-19 pandemic: does confinement impact eligibility for endovascular therapy? Stroke 2020;51:2593–6. 10.1161/STROKEAHA.120.030794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: decreased activity, and increased care delays. Stroke 2020;51:2012–7. 10.1161/STROKEAHA.120.030373 [DOI] [PubMed] [Google Scholar]
- 9. Pop R, Quenardelle V, Hasiu A, et al. Impact of the COVID-19 outbreak on acute stroke pathways - insights from the Alsace region in France. Eur J Neurol 2020;27:1783–7. 10.1111/ene.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seiffert M, Brunner FJ, Remmel M, et al. Temporal trends in the presentation of cardiovascular and cerebrovascular emergencies during the COVID-19 pandemic in Germany: an analysis of health insurance claims. Clin Res Cardiol 2020;109:1540–8. 10.1007/s00392-020-01723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke 2020;51:1996–2001. 10.1161/STROKEAHA.120.030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diegoli H, Magalhães PSC, Martins SCO, et al. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke 2020;51:2315–21. 10.1161/STROKEAHA.120.030481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegler JE, Heslin ME, Thau L, et al. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis 2020;29:104953. 10.1016/j.jstrokecerebrovasdis.2020.104953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsiao J, Sayles E, Antzoulatos E, et al. Effect of COVID-19 on emergent stroke care: a regional experience. Stroke 2020;51:e2111–4. 10.1161/STROKEAHA.120.030499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nogueira R, Abdalkader M, Qureshi MM, et al. Global impact of the COVID-19 pandemic on stroke hospitalizations and mechanical thrombectomy volumes. Int J Stroke 2021:174749302199165. 10.1177/1747493021991652 [DOI] [Google Scholar]
- 16. Bernat AL, Giammattei L, Abbritti R, et al. Impact of COVID-19 pandemic on subarachnoid hemorrhage. J Neurosurg Sci 2020;64:409–10. 10.23736/S0390-5616.20.04963-2 [DOI] [PubMed] [Google Scholar]
- 17. Diestro JDB, Li YM, Parra-Fariñas C, et al. Letter to the Editor 'Aneurysmal Subarachnoid Hemorrhage: Collateral Damage of COVID?'. World Neurosurg 2020;139:744–5. 10.1016/j.wneu.2020.05.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hecht N, Wessels L, Werft F-O, et al. Need for ensuring care for neuro-emergencies-lessons learned from the COVID-19 pandemic. Acta Neurochir 2020;162:1795–801. 10.1007/s00701-020-04437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 20. Qureshi AI, Agunbiade S, Huang W, et al. Changes in neuroendovascular procedural volume during the COVID-19 pandemic: an international multicenter study. J Neuroimaging 2021;31:171–9. 10.1111/jon.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Filippo O, D'Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med 2020;383:88–9. 10.1056/NEJMc2009166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishihara H, Kunitsugu I, Nomura S, et al. Seasonal variation in the incidence of aneurysmal subarachnoid hemorrhage associated with age and gender: 20-year results from the Yamaguchi cerebral aneurysm registry. Neuroepidemiology 2013;41:7–12. 10.1159/000345247 [DOI] [PubMed] [Google Scholar]
- 23. Abdalkader M, Sathya A, Malek AM, et al. Roadmap for Resuming elective neuroendovascular procedures following the first COVID-19 surge. J Stroke Cerebrovasc Dis 2020;29:105177. 10.1016/j.jstrokecerebrovasdis.2020.105177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular Neurointerventional procedures: a scientific statement from the American heart association. Circulation 2018;137:e661–89. 10.1161/CIR.0000000000000567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000695supp001.pdf (3.9MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Anonymised data are available upon reasonable request from the corresponding author.