Abstract
Background and aims
COVID-19 pandemic has strained the health infrastructure globally, providing an opportunity to identify cost-effective biomarkers. We aimed to identify simple hematological prognostic markers in hospitalized severe COVID-19 patients with and without diabetes.
Methods
Retrospective study of RT-PCR confirmed hospitalized severe COVID-19 patients (total: n = 154 patients, including diabetic subset n = 57) were analyzed. Clinically applicable cut-offs were derived using receiver operating characteristic (ROC) curve analysis for total leucocyte count (TLC), absolute neutrophil count (ANC), neutrophil lymphocyte ratio (NLR), and derived neutrophil lymphocyte ratio (dNLR) in order to prognosticate the outcome.
Results
Among 154 severe COVID-19 patients, significant association with mortality was seen with respect to TLC(p < 0.001), ANC (p < 0.001), NLR(p < 0.001) and dNLR(p < 0.001). In the total cohort, applicable cut-offs based on ROC curve in predicting outcome were, for TLC 8950 cells/mm3 (area under curve (AUC)-0.764, odds ratio (OR)-7.53), ANC 7679 cells/mm3 (AUC-0.789, OR-8.14), NLR 5.13 (AUC-0.741, OR-4.77), dNLR 3.44 (AUC -0.741, OR-4.43) respectively.In diabetic subset, the cut-offs for TLC was 8950 cells/mm3 (AUC -0.762, OR-14.9), ANC 6510 cells/mm3 (AUC -0.773, OR-16.8), NLR 5.13(AUC -0.678, OR-6) and dNLR 3.25(AUC -0.685, OR-4.7) respectively.
Conclusions
In severe COVID-19 patients irrespective of diabetes, a simple, applicable total leucocyte count cut-off, 8950 cells/mm3 , together with easily derived cut-offs for ANC, NLR, dNLR may serve as cost-effective prognosticators of clinical outcome. A normal TLC may be misleading in the intensive care and the above applicable cut-off for TLC serves as an early warning tool for high-risk identification and better in-hospital management. Even with similar or lower cut-offs, diabetics had a higher mortality.
Keywords: Severe covid-19, Diabetes mellitus, TLC, ANC, NLR, dNLR and CRP
1. Introduction
COVID-19 (coronavirus disease 2019) pandemic has posed a huge economic burden on the health care globally [1,2], especially on the developing nations, but it has provided an opportunity to rediscover/identify simple and cost-effective biomarkers, helping to predict or prognosticate the outcome.
COVID-19 infection begins as an inflammatory disorder that may progress to cytotoxic storm and death. The critical nature of the illness comprising severe pneumonia, acute respiratory distress syndrome and multi-organ failure may result in prolonged hospitalization with poor overall outcomes [3]. Those with co-morbid conditions like diabetes mellitus and the elderly have higher mortality [4]. Analyzing hematological parameters at hospital admission, and their applicable thresholds may serve as effective tools for the clinicians in the early identification of patients at risk of developing severe disease [5].
Certain studies have focused on the hematological parameters like neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), red cell distribution width etc., showing them to be prognostic markers in COVID-19 [6], [7].
In this study, we analyzed retrospective hematological data of the hospitalized severe COVID-19 patients, including a diabetic subset and their relation to the outcome. In addition, we evaluated the role of applicable cut-offs of total leuckocyte count (TLC), absolute neutrophil count (ANC), neutrophil lymphocyte ratio (NLR), absolute lymphocyte count and absolute eosinophil count etc. derived from peripheral blood hemocytometry, as valid prognostic biomarkers helpful in prompt identification of those at risk of poor outcome. This may help in devising early and effective management strategies in the intensive care unit.
2. Materials and methods
We analyzed retrospective data of the hospitalized patients with severe COVID-19 at King George Hospital, Visakhapatnam, Andhra Pradesh, India.
After appropriate Institutional ethics committee clearance, patients admitted with RT-PCR confirmed severe COVID-19, from 1st September to 30th September 2020 were included in our study.
Inclusion criteria: RT-PCR confirmed severe COVID-19 patients [8] were defined as either having:
-
1)
Severe pneumonia (RR > 30/min, respiratory distress with Spo2 <90% at room air, <94% with O2 supplementation)
-
2)
Acute respiratory distress syndrome (ARDS)
-
3)
Septic shock
Exclusion Criteria: Patients with hematological malignancies, immunodeficiency states and those on renal replacement therapy were excluded from the study.
The clinical and laboratory admission data of 154 patients were extracted from the medical case records, which included 57 diabetic patients. Hematological parameters as analyzed by automated cell counter such as hemoglobin, total leucocyte count, platelets were evaluated as simple prognostic markers of outcome in severe COVID-19 patients. Patients with a history of diabetes mellitus and on medications were included in the diabetic subset. Admission plasma glucose of all patients was noted. Absolute neutrophil, lymphocyte, monocyte, eosinophil counts and neutrophil lymphocyte ratio (NLR), derived neutrophil-lymphocyte ratio (dNLR), eosinophil monocyte ratio, platelet lymphocyte ratio (PLR) were calculated from the above hematological data.
All these hematological parameters were correlated with mortality. Their prognostic value as simple, cost-effective tools for determining the outcome of the entire cohort as well as diabetic subset were analyzed. Correlations of these immuno-hematological parameters with inflammatory marker, C-reactive protein (CRP) were done in order to further strengthen their validation as prognostic markers.
2.1. Statistical analysis
Continuous variables were expressed as appropriate means ± standard deviations. Categorical variables were summarized as frequencies and percentages. For categorical variables, Chi-square tests, and for continuous variables, independent T tests were done. Clinically applicable cut-offs of TLC, ANC, NLR and dNLR were derived using receiver operator curve (ROC) analysis and odds ratio (OR) of mortality above the cut-off were calculated. Correlation of hematological parameters with inflammatory protein, CRP were analyzed using Pearson correlation coefficient. Similar analyses were performed among the diabetic subset. P-value <0.05 was recognized as statistically significant. Statistical analysis was performed using SPSS v21.0 software.
3. Results
3.1. Age and mortality
In our study, the total cohort comprised of 154 patients (mean age - 52.73 years), including a diabetic subset of 57 patients (mean age - 59.31years). Among those who expired (n = 43) the mean age was 63.81 years in the total cohort, while it was 48.43 years (n = 111) in those who recovered. The mean age in the diabetic subset among the deceased was 64.95 years (n = 21) while among those who recovered was 56.03 years (n = 36).
A significant association with respect to age and mortality (OR -1.080, p < 0.01) and with recovery (OR-0.926, p < 0.001) was observed both in the total cohort and among diabetics (Table 1 ).
Table 1.
Demographic, hematological parameters and their association with mortality in the total cohort and diabetic subset.
| Total(n = 154) | Diabetic (n = 57) | |||
|---|---|---|---|---|
| Variable | Mean | P-valuea | Mean | P-valuea |
| Age | 52.73 | <0.01 | 59.31 | 0.009 |
| Sex (males) | 67.5% | 0.452 | 73 | 0.358 |
| Hemoglobin | 12.13 | 0.78 | 11.96 | 0.882 |
| Mean corpuscular volume | 79.82 | 0.574 | 81.3 | 0.812 |
| Mean corpuscular hemoglobin | 26.86 | 0.62 | 27.3 | 0.423 |
| Mean corpuscular haemoglobin concentration | 31.96 | 0.207 | 32.05 | 0.855 |
| Packed cell volume | 37.71 | 0.884 | 36.76 | 0.666 |
| Red blood cell count(106/mm3) | 4.88 | 0.602 | 4.47 | 0.236 |
| Platelets | 2.87 × 105 | 0.838 | 3.04 × 105 | 0.804 |
| Total leucocyte count | 10250 | <0.001 | 10500 | 0.002 |
| Absolute neutrophil count | 7881 | <0.001 | 8378 | 0.001 |
| Eosinophils | 263 | 0.051 | 251.3 | 0.194 |
| Monocytes | 394.1 | 0.598 | 385.96 | 0.913 |
| Lymphocytes | 2071 | 0.413 | 1429.2 | 0.803 |
| Neutrophil lymphocyte ratio | 6.514 | <0.001 | 8.92 | 0.043 |
| Derived neutrophil lymphocyte ratio | 4.1 | <0.001 | 5.27 | 0.04 |
| Eosinophil monocyte ratio | 1.53 | 0.792 | 1.17 | 0.264 |
| Platelet lymphocyte ratio | 232.54 | 0.161 | 300 | 0.282 |
| C-reactive protein | 50.65 | 0.004 | 74.72 | 0.045 |
| Random blood sugar | 235 | 0.078 | 291 | 0.236 |
p-value of association between parameters and mortality.
3.2. Gender
In total cohort among 154 patients, mortality was seen in 24% (12/50) females, while in males, the mortality was 29.8% (31/104) (p = 0.452). Among diabetics, mortality was seen in 46.7% (7/15) females, while in males 33.3% (14/42) (p = 0.358) (Table 1).
3.3. Total leucocyte count (TLC)
Our study reported a statistically significant association with a positive correlation between TLC and mortality, seen both in the total cohort (p < 0.001) and in the diabetic subset (p = 0.002). The mean TLC of the patients in the total cohort (n = 154) was 10,250 cells/mm3 while among diabetics (n = 57) it was 10,500 cells/mm [3] (Table 1).
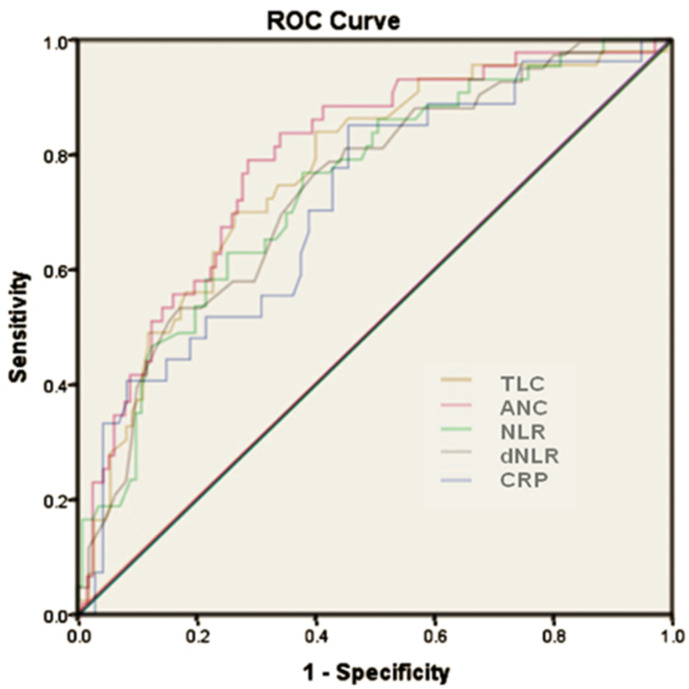
In the total cohort using Younden’s index for deriving the highest sensitivity (83%) and specificity (60%) in the ROC curve, with an area under curve (AUC) of 0.764, an applicable cut-off for TLC was 8950 cells/mm3 with an odds ratio of 7.53 for predicting mortality. In the diabetic subset with a similar cut-off for TLC (AUC-0.762, sensitivity 90% and specificity 60%) the odds ratio for predicting mortality was 14.9. (Fig. 1 , Table 2 ).
Fig. 1.
Receiver operating characteristic curve analysis of total cohort including total leucocyte count (TLC) (area under curve = 0.764), absolute neutrophil count (ANC) (area under curve = 0.789), neutrophil lymphocyte ratio (NLR) (area under curve = 0.741), derived neutrophil lymphocyte ratio (dNLR) (area under curve = 0.741) and c-reactive protein (CRP) (area under curve of 0.718).
Table 2.
Area under curve and applicable cut-offs with sensitivity, specificity, and odds ratio (OR) for mortality derived from the receiver operating characteristics curve for statistically significant hematological variables.
| Variable | Cohort | Area under curve | Cut-off | Sensitivity | Specificity | Odds ratio |
|---|---|---|---|---|---|---|
| Total leucocyte count | Total | 0.764 | 8950 | 83 | 60 | 7.53 |
| Diabetes Mellitus | 0.762 | 8950 | 90 | 61 | 14.9 | |
| Absolute neutrophil count | Total | 0.789 | 7679 | 79 | 71 | 8.14 |
| Diabetes Mellitus | 0.773 | 6510 | 95 | 62 | 16.8 | |
| Neutrophil lymphocyte ratio | Total | 0.741 | 5.1313 | 76 | 62 | 4.77 |
| Diabetes Mellitus | 0.678 | 5.1313 | 85 | 51 | 6 | |
| Derived neutrophil lymphocyte ratio | Total | 0.741 | 3.4466 | 74 | 60 | 4.43 |
| Diabetes Mellitus | 0.685 | 3.2572 | 90 | 51 | 4.7 | |
| C-reactive protein | Total | 0.718 | 19.7 | 85 | 54 | 5.7 |
| Diabetes Mellitus | 0.723 | 52.5 | 72 | 74 | 5.36 |
3.4. Absolute neutrophil count (ANC)
A statistically significant association with a positive correlation between the ANC and mortality is seen in total (p < 0.001) and in diabetic cohorts (p = 0.001). Mean ANC of the patients in the total cohort (n = 154) was 7881 cells/mm3 while among diabetics (n = 57) was 8378 cells/mm [3] (Table 1).
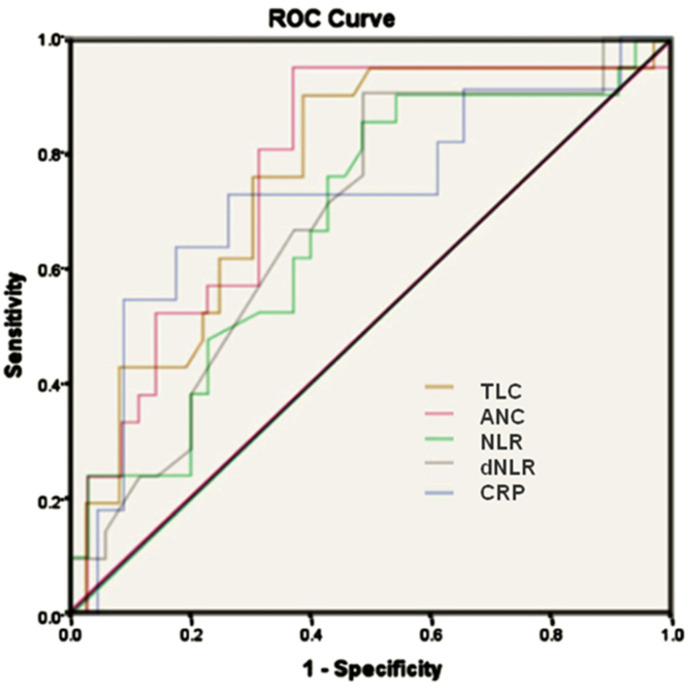
Using Younden’s index for deriving the highest sensitivity (79%) and specificity (71%) in the ROC curve, with an AUC of 0.789, an applicable cut-off for ANC in the total cohort was 7679 cells/mm [3], which had an odds ratio of 8.14 in predicting the mortality (Fig. 1 and Table 2). In the diabetic subset, an ANC cut-off of 6510 cells/mm3 (AUC - 0.773, sensitivity - 95%, specificity - 62%), had a greater odds ratio of 16.8 in predicting the mortality (Table 2 and Fig. 2 ).
Fig. 2.
Receiver operating characteristic curve analysis of total leucocyte count (TLC) (area under curve 0.762), absolute neutrophil count (ANC) (area under curve 0.773), neutrophil lymphocyte ratio (NLR) (area under curve 0.678), derived neutrophil lymphocyte ratio (dNLR) (0.685) and c-reactive protein (CRP) (area under curve 0.723) in diabetic subset.
3.5. Neutrophil lymphocyte ratio (NLR)
A statistically significant association with a positive correlation of the NLR with mortality was seen in the total cohort (OR-4.77, p < 0.001) and in diabetic subset (OR-6.0, p = 0.043). In the total cohort (n = 154) mean NLR of the patients was 6.514, while among diabetics (N = 57) was 8.92 (Table 1). Deriving the highest sensitivity (76%) and specificity (62%) in the ROC curve, with an AUC of 0.741, an applicable cut-off for the NLR in the total cohort was 5.1313 which had an odds ratio of 4.77 in predicting the mortality (Fig. 1 and Table 2). In diabetic subset, AUC for NLR was 0.678, with similar cut-off of NLR, diabetics had a sensitivity of 85% and specificity of 51% and odds ratio of 6 in predicting the mortality (Fig. 2 and Table 2).
3.6. Derived neutrophil lymphocyte ratio (dNLR)
A statistically significant association between dNLR and mortality was seen in the total cohort (OR-4.43, p < 0.001) and in diabetic cohort (OR-4.7, p = 0.04). In the total cohort (n = 154) mean dNLR of the patients was 4.1, while among diabetics (n = 57) was 5.27 (Table 1). Deriving the highest sensitivity (74%) and specificity (60%) in the ROC curve, with an AUC of 0.741, an applicable cut-off for the dNLR in the total cohort was 3.44, which had an odds ratio of 4.43 in predicting the mortality (Fig. 1 and Table 2). In the diabetic subset, dNLR cut-off of 3.25 (AUC - 0.685, sensitivity −90% and specificity - 51%) had a greater odds ratio of 4.7 in predicting the mortality (Fig. 2 and Table 2).
Absolute lymphocyte count, absolute eosinophil count, absolute monocyte count: There was no statistically significant association with mortality and recovery with absolute lymphocyte counts (total, p = 0.352 vs. diabetic, p = 0.803), eosinophil counts (total, p = 0.054 vs. diabetic, p = 0.194) and monocyte counts (total, p = 0.598 vs. diabetic, p = 0.913) (Table 1).
Eosinophil Monocyte Ratio (EOM): No statistically significant association with mortality is seen in both total (p = 0.792) and diabetic subsets (p = 0.264). Platelet Lymphocyte Ratio (PLR) also had no statistically significant association with mortality in both total cohort (p = 0.792) and diabetic subset (p = 0.264) (Table 1).
In our present study, other hematological parameters like hemoglobin, packed cell volume, red blood cell counts, mean corpuscular volume, mean corpuscular hematocrit, mean corpuscular hematocrit concentration, and platelet counts, whether in the total cohort or among diabetics, did not show statistically significant differences with respect to either mortality or recovery (Table 1).
3.7. C-reactive protein (CRP) and mortality
C-reactive protein, an inflammatory marker, has a statistically significant positive correlation with mortality in both groups (p = 0.004). The mean CRP in the total cohort was 50.65 mg/L (higher among those who expired 83.88 mg/L, and 38.68 mg/L among those who survived) (Table 1).
Deriving the highest sensitivity (85%) and specificity (54.6%) in the ROC curve, with an AUC of 0.718, an applicable cut-off for the CRP in the total cohort was 19.7 mg/L, which had an odds ratio of 5.7 in predicting the mortality (Fig. 1 and Table 3 ). In the diabetic subset, CRP has a positive correlation with mortality, which was statistically significant (p = 0.045). The mean CRP in diabetic subset was 74.72 mg/L, while among those who expired, it was 116.3 mg/L, while among those who survived, it was 54.78 mg/L (Table 1)
Table 3.
Correlation between C-reactive protein and hematological parameters.
| PARAMETERS | C-reactive protein in total cohort |
C-reactive protein in diabetic subset |
||
|---|---|---|---|---|
| r value | p value | r value | p value | |
| Total leucocyte count | 0.025 | 0.802 | −0.072 | 0.684 |
| Absolute neutrophil count | 0.090 | 0.370 | −0.040 | 0.821 |
| Neutrophil lymphocyte ratio | 0.344 | <0.001 | .0281 | 0.107 |
| Derived neutrophil lymphocyte ratio | 0.298 | <0.001 | 0.187 | 0.290 |
| Eosinophil monocyte ratio | −0.030 | 0.768 | −0.097 | 0.587 |
| Platelet lymphocyte ratio | 0.422 | 0.365 | 0.373 | 0.543 |
| Platelets | 0.092 | 0.357 | 0.074 | 0.678 |
For diabetic subset a cut-off with AUC of 0.723 with a sensitivity of 72.72% and specificity, 73.91% was 52.5 mg/L. The odds ratio for predicting mortality was 5.36 in diabetic subset (Fig. 2 and Table 3).
3.8. Correlating immuno-hematological parameters with inflammatory protein - CRP
-
1.
CRP vs NLR: In the total cohort, NLR had a highly statistically significant positive correlation with CRP (p < 0.001, Pearson’s correlation coefficient r = 0.358). while in the diabetic subset, it was not statistically significant (p = 0.101, r = 0.286).
-
2.
CRP vs dNLR: dNLR had a statistically significant positive correlation with CRP (p = 0.00, r = 0.319) in the total cohort, while in the diabetic subset, though a positive correlation was seen, it was not statistically significant (p = 0.241, r = 0.207).
-
3.
No statistically significant correlation was seen between CRP and ANC, TLC, eosinophil monocyte ratio, PLR, platelets, and lymphocytes in the total cohort and diabetic subsets (Table 3).
3.9. Admission plasma glucose
Mean admission plasma glucose was 235 mg/dl and 291 mg/dl in the total cohort and diabetic subset, respectively. No significant association between mortality and admission glucose was seen in both the groups (p = 0.078 and p = 0.236). Similarly, no significant correlation between glucose levels and TLC, ANC, NLR, dNLR and CRP was seen in total cohort and diabetic subset (Table 1).
4. Discussion
This study analyzed the immune-hematological biomarker data as prognostic tools in determining the outcome of the hospitalized severe COVID-19 patients. In addition, the correlation between C-reactive protein (CRP) and haemato-cytological markers was assessed to strengthen their validity as predictive tools for the outcome.
In our study, the total cohort comprised of 154 patients, among which the diabetic subset included 57 patients. 43 of 154 patients (27.95%) expired. Among the diabetic subset, 21 of 57 (36.8%) patients expired.
Several laboratory abnormalities were reported in severe and critically ill COVID-19 patients [9], [10], [11]. Henry BM et al. reported that in general, neutrophilia and lymphopenia are associated with disease severity and mortality [12].
Leukocytes provide the first immunological defense against infection in the host, and in this, the neutrophils predominate [13]. Certain investigators attempted to identify inflammatory markers such as NLR, dNLR and PLR derived from hemocytometry and reported optimal thresholds for predicting mortality in COVID-19 patients. In comparison to other studies, we focused on easily accessible peripheral blood indices such as TLC and ANC. In addition, the derived parameters such as NLR, dNLR, PLR, and eosinophil monocyte ratio were also studied. Statistically calculated applicable cut-off based on ROC curves for these indices were analyzed to predict the outcome or mortality in hospitalized severe COVID-19 patients. We postulated that such predictive thresholds might serve as simple bedside cost-effective prognostic biomarkers and offer a huge economic advantage for the health care setting.
More than 50% of the deceased among both groups in our study had leukocytosis and a significant number of patients also had normal leucocyte counts. The mean TLC was 10,250 cells/mm3 in the total cohort (n = 154), and 10,500 cells/mm3 among diabetics (n = 57) respectively.
4.1. Applicable cut-offs for predicting mortality
An important and interesting observation of our study is that, a cut-off of 8950 cells/mm3 even within the normal range of total leucocyte count, predicted poor prognosis in both the total and the diabetic cohorts among severe COVID-19 patients Using Younden’s index for deriving the highest sensitivity (83%) and specificity (60%) in the ROC curve, with an AUC of 0.764, a TLC threshold of 8950 cells/mm3 predicted the mortality with an odds ratio of 7.53 in the total cohort (Fig. 1). While the diabetics had a greater odds ratio of 14.9 (AUC = 0.762, sensitivity = 90%, specificity = 60%), with a similar cut off of 8950 cells/mm [3](Fig. 1). This striking finding can serve as a simple and applicable prognosticator of mortality and help the clinician to be alert and to take appropriate early treatment decisions in improving the clinical outcome in patients. This is further strengthened by our observations regarding the threshold for ANC and prognosis, as discussed below.
Neutrophil mediated phagocytosis and neutrophil extracellular trap formation results in bactericidal and fungicidal actions of neutrophils [14]. However, their role in viral infections remains unclear [15]. Once activated, leucocytes induce cell DNA damage through the release of reactive oxygen species. This, in turn stimulates cellular and humoral response leading to antibody-dependent cell-mediated cytotoxicity [16]. Polymorphonuclear leukocytes can be triggered by virus-related inflammatory factors, such as interleukin-6 and interleukin-8, tumor necrosis factor-alpha (TNF-alfa), granulocyte colony-stimulating factor (G-CSF), and interferon-gamma factors (IFGs), produced by lymphocyte and endothelial cells [17].Post-mortem findings of affected COVID-19 patients demonstrated extensive neutrophil infiltration in pulmonary capillaries with extravasation into the alveolar space [18]. This suggests that inflammatory cell accumulation and endothelitis contribute to "happy hypoxia" as a result of microcirculatory dysfunction [19]. The virus infects T cells in addition to other organs through the CD147-spike protein and angiotensin-converting enzyme 2 (ACE2) receptors [20]. COVID-19 disease results in a decreased population of CD3+, CD4+, and CD8+ T lymphocytes and an increased number of regulatory T cells [21].
In contrast to the combined neutrophilia and lymphocytopenia reported in most other studies [22], our study showed a mean ANC of 7881 cells/mm3 (normal range = 2500–6000) a mean absolute lymphocyte count of 2070 cells/mm3 (normal range = 800–5000). The applicable cut-off for ANC for the total cohort was 7679 cells/mm3 (sensitivity 79%, specificity 71%, and OR - 8.14) in predicting the mortality. This probably explains the poor prognosis in our patients, even when the TLC was in the normal range. This emphasizes that both TLC and ANC together have a positive predictive role in mortality. In addition, diabetics with a lower ANC cut-off of 6510 cells/mm3 had a greater odds ratio of 16.8 in predicting mortality (sensitivity of 95% and specificity of 62%).
Zahorec et al. proposed NLR (NLR = ANC/absolute lymphocyte counts) as a prognostic marker in critically ill patients, which correlated well with APACHE II and SOFA scores [23] and among the avian influenza-infected patients [24]. Ai-Ping et al. reported that NLR of 3.3 had a sensitivity of 88% and specificity of 63.6% in determining prognosis in severe COVID-19 patients. dNLR, calculated using a formula: ANC/(TLC - ANC) has also been projected as another novel prognostic immuno-biomarker. In the same study, dNLR cut-off of 2.8 had a sensitivity of 55% and specificity of 84% in predicting prognosis in severe COVID-19 patients [25].
In our study, the optimal thresholds of NLR and dNLR for predicting mortality in a total cohort using the ROC curve were 5.13(sensitivity = 76% and specificity = 62%, OR = 4.77), 3.44 (sensitivity = 74% and specificity = 51%, OR = 4.43) respectively.
Whereas in the diabetic subset, the applicable thresholds for NLR and dNLR for predicting mortality were 5.13 (sensitivity = 85% and specificity = 51%, OR = 6) and 3.25 (sensitivity = 90% and specificity = 51, OR = 4.7) respectively. An interesting finding of this study is that diabetics with similar or lower thresholds had a greater odds ratio for predicting mortality compared to total cohort.
4.2. Platelet lymphocyte ratio and eosinophil monocyte ratio
Platelet lymphocyte ratio has also been studied as a sensitive indicator of the degree of cytokine storm in COVID-19 [26]. In contrast to this finding, no significant association was seen between platelet lymphocyte ratio and mortality in our study in both total (p = 0.792) and diabetic cohorts (p = 0.264).
When used as a ratio, namely eosinophil monocyte ratio, both eosinophils, and monocytes have been proposed as markers of inflammation in atherosclerotic STEMI patients. Eosinophils release immune-suppressive cytokines like IL-10 and IL-13 which suppress T cells. Monocytes are pro-inflammatory, releasing TNF alpha and IL-6. Thus, a lower eosinophil monocyte ratio has been associated with higher mortality [27]. Hence, we studied the absolute neutrophil count of this marker in predicting mortality in severe COVID -19 patients who in general, have underlying co-morbid conditions. However, no statistically significant association with mortality were seen in both total (p = 0.792) and diabetic subsets (p = 0.264) in our study.
4.3. Correlation with admission plasma glucose
At admission, random plasma glucose levels were not shown to have any significant correlation with immuno-hematological parameters such as TLC, ANC, NLR, dNLR, and CRP in both the groups (p = 0.078 and p = 0.236, respectively) (Table 1). It is to be noted that in the diabetic subset, only those patients with a confirmed history of diabetes and on anti-diabetic medications were included, and we did not compare between diabetics and non-diabetics. HbA1c was not done for all patients due to resource constraints during the COVID-19 pandemic. The mean plasma glucose (235 mg/dl) (Table 1) seen in the total cohort could be explained due to the combination of various factors such as the diabetic subset’s contribution, few cases of de-novo diabetes mellitus, stress hyperglycemia, COVID-19 related hyperglycemia, etc.
In addition, hemoglobin, packed cell volume, red blood cell counts, mean corpuscular volume, mean corpuscular hemoglobin concentration, platelet counts and platelet lymphocyte ratio did not show a statistically significant difference with respect to either mortality or recovery in either total cohort or among diabetics in our patients.
4.4. Optimal thresholds for C-reactive protein in severe COVID-19
C-reactive protein, an acute-phase inflammatory protein, reported to be used as a predictor of severity of COVID-19 infection [28]. Another striking observation in our study is that the diabetic subset had higher admission CRP levels than the total cohort (74.7 mg/L vs 50.65 mg/L). In predicting the mortality, diabetic subset had a higher applicable ROC curve threshold (CRP of 52.5 mg/L, AUC = 0.723 with a sensitivity of 72.72% and specificity 73.91%) compared to the total cohort (CRP of 19.7 mg/l, area under curve 0.718 with a sensitivity of 85% and specificity 54.6%). (Table 1 and Fig. 2). This confirms that CRP is an inflammatory prognostic tool.
4.5. Correlation between CRP and hematological biomarkers
To further validate our observations in better prognostication of the outcome in severe COVID-19 patients, we have evaluated the correlation between these simple blood cell indices and CRP. In the total cohort CRP had a statistically significant association with positive correlation with NLR (p < 0.001, r = 0.358) and with dNLR (p = 0.001, r = 0.319). This highlights the importance of the role of immuno-hematological markers in understanding the underlying pathophysiologic perspective and their role in predicting the mortality and recovery in severe COVID-19 patients.
Focus on the diabetic subset: Diabetes mellitus is an inflammatory disorder. Uncontrolled diabetes mellitus predisposes to severe COVID-19. The consequences of heightened immuno-inflammatory reaction can lead to increased mortality in this subset. In line with this, our study emphasizes that lower or similar applicable cut-offs of TLC, ANC, NLR, dNLR and CRP showed a higher odds ratio in predicting mortality in this group.
The limitations of this study are its retrospective nature and the relatively small sample size. HbA1c at admission would have provided more insight into the association between glycemic status and hematological parameters. Prospective studies with larger patient enrollment can help in establishing their role as predictive biomarkers in severe COVID-19.
5. Conclusion
These statistically proven applicable cut-offs for immuno-hematological biomarkers drawn from simple peripheral blood hemocytometry at hospital admission can serve as applicable guiding tools to the clinicians in early identification of high-risk severe COVID-19 patients with and without diabetes.
Our study highlights that even a normal range total leucocyte count can be deceptive, portending an ominous outcome, and should be viewed critically in severe COVID-19 infection. A simple, applicable total leucocyte count threshold, 8950 cells/mm3, in addition to the other easily derived parameters such as ANC, NLR and dNLR strengthens the positive predictive role in prognosticating the clinical outcome in these patients in a cost-effective manner. The correlation of these biomarkers with CRP emphasizes this point.
We suggest that widespread clinical use of these simple and economic hematological biomarkers may aid in the early and effective implementation of treatment strategies in the hospitalized setting. It improves the clinical outcome and reduces the financial burden on the health care sector in both developing and developed countries.
Authors contributions and acknowledgements
Conceptualization, implementation, data review, manuscript drafting, revision, finalization – JR.
Patient data collection, statistical data analysis, references collation, interdepartmental collaboration – SR, JV and MR.
The authors would like to thank the Principal Dr. PV Sudhakar MBBS MS Mch, Andhra Medical College for his support, Dr. Kiran Pamarthi MD (Asst professor, department of preventive medicine, Andhra Medical College) for inputs in statistical analysis and Dr. Srivalli Madhira MBBS MS for her invaluable insights.
All authors read and approved the final version of the manuscript.
References
- 1.Martin A., Markhvida M., Hallegatte S., Walsh B. Socio-economic impacts of COVID-19 on household consumption and poverty. Econ Disasters Clim Change. 2020 Oct;4(3):453–479. doi: 10.1007/s41885-020-00070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int J Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y., Hou B., Liu J., Chen Y., Zhong P. Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med. 2020;7 doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaiodimos L., Chamorro-Pareja N., Karamanis D., Li W., Zavras P.D., Chang K.M., Mathias P., Kokkinidis D.G. Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. Hormones (Basel) 2020 Oct 29 doi: 10.1007/s42000-020-00246-2. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., Gao Y., Cai L., Wang Z., Yin P., Wang Y. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020 Sep 1;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyit M., Avci E., Nar R., Senol H., Yilmaz A., Ozen M. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. 2021 Feb;1;40:110–114. doi: 10.1016/j.ajem.2020.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C., Zhang H., Cao X., Deng R., Ye Y., Fu Z., Gou L., Shao F., Li J., Fu W., Zhang X. Red cell distribution width (RDW): a prognostic indicator of severe COVID-19. Ann Transl Med. 2020 Oct;8(19) doi: 10.21037/atm-20-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical management protocol: COVID-19 Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division) Version 5. 03.07.20.
- 9.Sayad B., Afshar Z.M., Mansouri F., Rahimi Z. Leukocytosis and alteration of hemoglobin level in patients with severe COVID-19: association of leukocytosis with mortality. Health Sci Rep. 2020 Dec;3(4) doi: 10.1002/hsr2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L., Wang Y., Liang X., Xiao W., Duan G., Yang H. Is neutrophilia associated with mortality in COVID-19 patients? A meta-analysis and meta-regression. Int J Lab Hematol. 2020 Dec;1 doi: 10.1111/ijlh.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziadi A., Hachimi A., Admou B., Hazime R., Brahim I., Douirek F. Lymphopenia in critically ill COVID-19 patients: a predictor factor of severity and mortality. Int J Lab Hematol. 2020 Sep;30 doi: 10.1111/ijlh.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry B.M., Cheruiyot I., Vikse J., Mutua V., Kipkorir V., Benoit J., Plebani M., Bragazzi N., Lippi G. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed: Atenei Parmensis. 2020;91(3) doi: 10.23750/abm.v91i3.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Wang W., Yang F., Xu Y., Feng C., Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal. 2019 Dec 1;17(1):147. doi: 10.1186/s12964-019-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vorobjeva N.V., Pinegin B.V. Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Biochemistry (Mosc) 2014 Dec 1;79(12):1286–1296. doi: 10.1134/S0006297914120025. [DOI] [PubMed] [Google Scholar]
- 15.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014 24th January;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kany S., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019 Jan;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. Cytokine and Growth Factor Reviews. Elsevier Ltd; 2020. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B. Postmortem examination of patients with COVID-19. Jama. 2020 Jun;23;323(24):2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020 Jun;9(6):1383. doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Targeted Ther. 2020 Dec 4;5(1) doi: 10.1038/s41392-020-00426-x. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R., Wang Y., Li J., Han H., Xia Z., Liu F. Decreased T cell populations contribute to the increased severity of COVID-19. Clin Chim Acta. 2020 Sep;1;508:110–114. doi: 10.1016/j.cca.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry B.M., Cheruiyot I., Vikse J., Mutua V., Kipkorir V., Benoit J., Plebani M., Bragazzi N., Lippi G. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed: Atenei Parmensis. 2020;91(3) doi: 10.23750/abm.v91i3.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001 Feb;102(1):5–14. [PubMed] [Google Scholar]
- 24.Zhang Y., Zou P., Gao H., Yang M., Yi P., Gan J., Shen Y., Wang W., Zhang W., Li J., Liu P. Neutrophil–lymphocyte ratio as an early new marker in AIV-H7N9-infected patients: a retrospective study. Therapeut Clin Risk Manag. 2019;15:911. doi: 10.2147/TCRM.S206930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang A.P., Liu J., Tao W., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharm. 2020 Apr 13:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X.M. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 Sep;92(9):1533–1541. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng X., Wang X., Shen L., Yao K., Ge L., Ma J., Zhang F., Qian J., Ge J. Association of eosinophil-to-monocyte ratio with 1-month and long-term all-cause mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Thorac Dis. 2018 Sep;10(9):5449. doi: 10.21037/jtd.2018.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahnach M., Zbiri S., Nejjari S., Ousti F., Elkettani C. C-reactive protein as an early predictor of COVID-19 severity. J Med Biochem. 2020 2nd October;39(4):500. doi: 10.5937/jomb0-27554. [DOI] [PMC free article] [PubMed] [Google Scholar]