Abstract
The emergence of the novel coronavirus, SARS-CoV-2 has pushed forward the world to experience the first pandemic of this century. Any specific drug against this RNA virus is yet to be discovered and presently, the COVID-19 infected patients are being treated symptomatically. During the last few decades, a number of polysaccharides with potential biological activities have been invented from Indian medicinal plants. Many polysaccharides, such as sulfated xylomannan, xylan, pectins, fucoidans, glucans, glucoarabinan, and arabinoxylan from Indian medicinal plants, have been shown to exhibit antiviral and immunomodulating activities. Plant polysaccharides exhibit antiviral activities through interference with the viral life cycle and inhibition of attachment of virus to host cell. Intake of certain immune stimulating plant polysaccharides may also protect from the virus to a certain extent. In process of continuous search for most potent drug, Indian plant polysaccharides may emerge as significant biomaterial to combat COVID-19. This review explores a number of polysaccharides from Indian medicinal plants which showed antiviral and immunomodulating activities. It is aimed to provide an overview about the composition, molecular mass, branching configuration and related bioactivities of polysaccharides which is crucial for their classification as possible drug to induce immune response in viral diseases.
Keywords: COVID-19, SARS-CoV-2, Plant polysaccharides, Antiviral, Immunomodulatory
1. Introduction
COVID-19 is an infectious disease caused by the recently identified novel Coronavirus, SARS-CoV-2. This disease was unknown before the outbreak in Wuhan, Hubei Province, China in December 2019 [1]. Fever, tiredness and coughing are the most common symptoms of COVID-19. Some patients may have body aches, runny nose, loss of smell and taste, sore throat, diarrhoea and in case of serious illness difficulty in breathing may develop [2,3]. Aged people and those with underlying medical problems are more prone to develop serious illness. The World Health Organization (WHO) has declared the COVID-19 as a pandemic on 11 March 2020 signifying its severity and global spread. The pandemic has infected millions of people of more than 200 countries in this short period of time. Human-to-human transmission of the virus via respiratory droplets or via contact is accelerating the rate of infection [4]. India becomes the new global epicenter of COVID pandemic due to recent increase of daily cases of infection. On September 16, according to Johns Hopkins University Centre for Systems Science and Engineering (JHU CCSE), India had a weekly average of over 95,000 new cases each day, higher than the United States and Brazil. For every symptomatic COVID-19 patient, India has thirty asymptomatic patients according to the analysis of the confederation of medical association of Asia and Oceania (CMAAO). Many infected patients remain asymptomatic may be either due to very small viral load or due to better immunity strategy [5]. Several epidemiological models predict that a large percentage of asymptomatic or mildly symptomatic people will help to build up the herd immunity, the major way to control the COVID-19 infection. It is obvious that the present healthcare system of India will be strained if the number of COVID-19 patients suffering from severe to critically ill situation increases continuously. Two antiviral drugs namely, remdesivir and favipravir are reported to reduce viral load but it is not true that all those taking these drugs will recover [6]. According to the very recent report [7], feline coronavirus drug (GC 376) inhibits the main protease of SARS-CoV-2 and blocks virus replication. While several drug trials are ongoing, there is currently no clear evidence that these antiviral drugs can cure COVID-19. Until a vaccine against COVID-19 will be made available, induction of new antiviral drugs for COVID-19 treatment and introduction of immunostimulating materials in regular diet as infection prevention measures will remain at the forefront.
India is sitting on gold mine of well recorded and traditionally well-practiced knowledge of herbal medicine [8,9]. Several systems of traditional medicine such as Ayurveda, Yoga, Unani, Siddha and Homeopathy are being practiced for years together in India [10]. The Ministry of AYUSH, Government of India recommended four medicinal herbs, Tulsi (Ocimum sanctum), Dalchini (Cinnamomum zeylanicum), Sunthi (Zingiber officinale), and Krishna Marich (Piper nigrum) to boost the body's first line of defence against COVID-19 [11]. Tamam, Abd-el-Hamid, Samah, & Marwa [12] reported antiviral and immunomodulatory activity of Cinnamomum zeylanicum oil against Newcastle disease virus (NDV). Aqueous extract of medicinal plants are enriched with several chemical compounds among which polysaccharides are the most active elements [13]. Low toxicity, high molecular mass, branching configuration and conformation of polysaccharides may become crucial for generation of suitable immune response in the incident of pathogenic invasion or other viral diseases. The present review will focus on immunomodulatory and antiviral activities of plant polysaccharides from Indian origin to explore the possibility of emergence of new biomaterial for treatment of COVID-19.
2. Methods
Many currently available literatures were reviewed for COVID-19 infection and their impact on Indian medicinal plants caused by SARS-CoV-2 at the time of writing the paper. The literature search was performed using books, different websites, news bulletins, circulars, professional bodies like WHO, Health Ministry of India etc. The authors have also collected data from different national and international reputed journals. 73 papers have been used in this literature research. Given the nature of the review, no ethical approval was required.
3. SARS-CoV-2 virus and its effect on different parts of human body
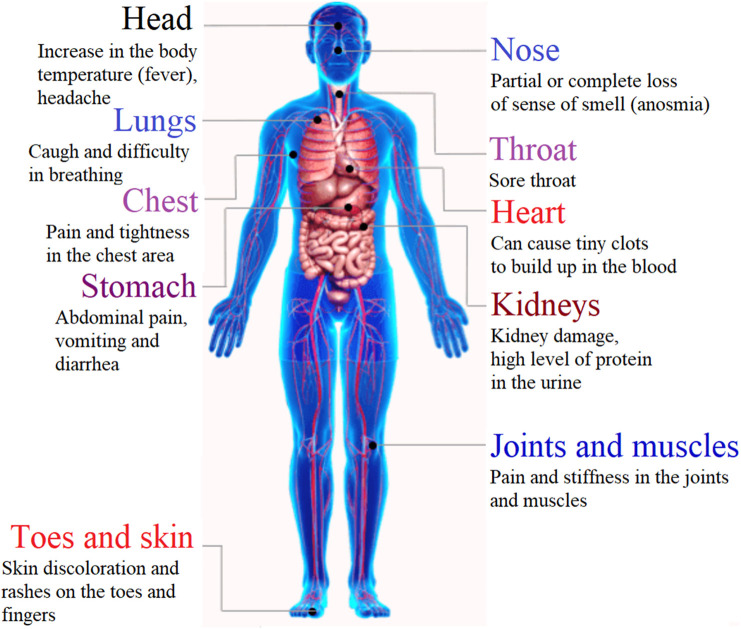
Coronaviruses (CoVs) are enveloped viruses with a single-stranded RNA genome. CoVs have the largest genomes for RNA viruses having the length of genome sizes ranging from 26 to 32 kilobases (kb) [14]. CoVs have been categorized into three groups: α-CoVs, β-CoVs, and γ-CoVs based on genetic and antigenic criteria [15]. The viral disease COVID-19 is caused due to the emergence of the novel SARS-CoV-2 that belonged to β-CoVs genera [16]. Four major structural proteins have been identified in SARS-CoV-2 including spike, nucleocapsid, membrane, and envelope proteins (Fig. 1 ) [17]. The spike proteins of the viruses bind to the angiotensin-converting enzyme 2 (ACE2) receptors present in epithelium in the nose, mouth, lungs and thereby enter into the human body [18]. The viruses affect mainly the upper respiratory tract (sinuses, nose, and throat) and lower respiratory tract (windpipe and lungs) leading to the onset of respiratory tract infections. SARS-CoV-2 has an effect on the major organ of the body including the heart and blood vessels, kidneys, gut, and brain (Fig. 2 ). Anybody from child to elder people may get COVID-19 infection, but serious illness are likely for those having weakened immune system due to one or more pre-existing chronic diseases like HIV, cancer, kidney disease, chronic obstructive pulmonary disease (COPD), coronary artery disease, type 2 diabetes, liver diseases etc. [[19], [20], [21], [22], [23]]. In this context, natural immunity supplements are essential to build resilience in the body against viral infections. According to a recently published report, bowel abnormalities were seen in a number of patients admitted with COVID-19 infections which were more common to those kept in ICUs [24]. Non-digestible carbohydrates which are commonly known as dietary fiber have long been used in the treatment of several gastrointestinal conditions; including assistance to regulate bowel movements [25].
Fig. 1.

Schematic representation of the structure of SARS-CoV-2.
Fig. 2.
COVID-19 affects the major organs of the body.
4. Antiviral activities of some polysaccharides isolated from Indian medicinal plants
Coronavirus infection has been emerged as one of the severe threats to human health [1]. The key to tackling this pandemic is to invent new therapeutic agents having immense antiviral potency and low toxicity. Plant polysaccharides have now become a rich resource of potential drugs due to their antiviral activities against various viruses [26]. Polysaccharides show antiviral activity mainly by inhibition of viral replication and viral binding to cell. The structural complexity and diversity of plant polysaccharides contribute to the antiviral activities in different phases of viral infection processes. Faccin-Galhardi et al. [26] reported that two heteropolysaccharides (P1 and P2) were isolated from water extraction of the leaves of medicinal plant Azadirachta indica and their chemical sulfated derivatives (P1S and P2S) showed anti-polio virus type 1 (PV-1) activity. P1 fraction (molecular mass of 80 kDa) contained arabinose (47%), galactose (24%), glucose (15%), rhamnose (7%), xylose (3%), mannose (3%) and trace amount of fucose (1%) units whereas the P2 fraction contained 51% arabinose, and 26% galactose. The polysaccharides exhibited significant antiviral activity with inhibitory concentrations (IC50) of 80 mg/mL, 37.5 mg/mL, 77.5 mg/mL, and 12.1 mg/mL for P1, P1S, P2 and P2S, respectively. These results indicated that original polysaccharides showed better antiviral activities than their sulfated derivatives. Saha et al. [27] reported that P1 fraction and its sulfated derivatives P1S showed anti-bovine herpesvirus type 1 (BoHV-1) activity. The IC50 value of P1 fraction and its sulfated derivative (P1S) against BoHV-1 are 105.25 and 1440 μg/mL, and 32.12 and > 1600 μg/mL, respectively. Their selectivity indices (S.I.) were 13.6 and > 51.4, respectively. These results also illustrated that P1 fraction was better as antiviral agent than its derivatives. Neutral polysaccharide (RN), an acidic polysaccharide (RA) and a pectic polysaccharide (RP) were isolated from the hot water extract of defatted aerial part of Portulaca oleracea [28]. RN mainly composed of glucose (Glc) (40.1%), mannose (Man) (38.8%), arabinose (Ara) (13.7%) and small amounts of galactose (Gal) (5.3%). RA mainly composed of Ara (23.2%), Gal (67.0%), rhamnose (Rha) (2.8%), xylose (Xyl) (2.9%) and glucuronic acid (GlcA) (4.1%). RP mainly contained galacturonic acid (GalA) (67.8%), Gal (11.3%) and GlcA (10.6%) with small amounts of Ara (5.8%) and Rha (4.3%). Among the three polysaccharides, only RP showed significant anti-HSV-2 activity with selectivity index (SI) of more than 20. According to the report of Mandal et al. [29], two antiviral polysaccharides, namely, F1 and F2 fraction were isolated from water extract of Scinaia hatei. F1 fraction contained mannose and a small amount of xylose, whereas fraction F2 contained xylose only. Modified sulfated F1 fraction had 0.4 sulfate groups per monomer unit and an average molecular weight of 160 kDa. Crude extract ShWE and sulfated F1 exhibited strong antiherpetic activity with inhibitory concentration 50% (IC50) values in the range of 0.6 to 4.6 μg/mL. Sulfated xylomannans was more active than the crude extract ShWE and it also showed higher efficiency than heparin against all HSV strains. Mandal et al. [30] reported three sub-fractions (F1, F2 and F3) from alkali-extract of Scinaia hatei and all of them contained xylose as the major constituent sugar. The apparent molecular mass of purified F2 fraction, a linear polysaccharide having (1 → 4)-linked β-d-xylopyranosyl residues, was calculated to be 120 kDa. Sulfated derivatives (S1, S2, S3 and S4) of F2 fraction showed strong anti-HSV activity with inhibitory concentration 50% (IC50) ranging in between 0.22 and 1.37 μg/mL. Sulfated fucan containing crude extract SmWE and fraction F3 were isolated from aqueous extract of Stoechospermum marginatum and exhibited inhibition to herpes simplex virus [31]. SmWE fraction contained fucose (91%) and a small amount of xylose (3%), mannose (1%), galactose (3%), glucose (2%), whereas fraction F3 contained fucose (96%) and a small amount of xylose (2%), and galactose (2%). SmWE was more active than F3 against HSV-1 (EC50 1.15 and 3.55 μg/mL, respectively) and equally active against HSV-2 (EC50 0.78 μg/mL for SmWE and 0.63 μg/mL for F3). Chattopadhyay et al. [32] reported isolation of crude polysaccharide (GiWE) as well as one pure fraction (F3) from Grateloupia indica. Sulfated GiWE contained mostly galactose (94%) and small amounts of xylose (2%), glucose (3%) and fucose (1%) residues whereas major sub-fraction F3 contained more than 99% galactose neutral sugars. GiWE and F3 were reported to be strong inhibitors of HSV-1 (F) and HSV-2 (MS), with values of IC50 ranging from 0.25 to 0.31 μg/mL. F3 was the major fraction of Sphacelaria indica and had apparent molecular mass of 26 ± 5 kDa [33]. F3 contained fucose (65%), galactose (20%), xylose (13%) and small amount of glucose (1%) and mannose (1%), which also contained 3% (w/w) of uronic acid. The F3 and their chemically sulfated derivatives showed dose-dependent anti-herpes simplex virus type 1 (HSV-1) activities with IC50 having the range of 0.6–10 μg/mL. Sulfated galactan (WE2NGF, Au 2, and AAu 2) were isolated from the cold water extraction of Gracilaria corticata and exhibited antiviral activity against herpes simplex virus types 1 and 2 [34]. WE2NGF shows stronger antiviral activity (IC50 values of 0.19 and 0.24 μg/mL for HSV-1 and HSV-2, respectively) than heparin (IC50: 1.3 and 2.1 μg/mL for HSV-1 and HSV-2, respectively) used as a standard. The fractions Au2 and AAu2 showed less antiviral activity against both herpes virus, with IC50 ranging from 27.5 to 50.0 μg/mL. The antiviral potency of the sulfated polysaccharides depends on the sulfate content, the position of sulfate group, the sugar composition, and the molar mass. Sulfated galactans (GiWE and GiF3 from Grateloupia indica; GcWE and GcF3 from Gracilaria corticata), the fucans (CiWE and CiF3 from Cystoseira indica; SmWE and SmF3 from Stoechospermum marginatum), the xylomannans (ShWE and ShF1 from Scinaia hatei) and the heteropolysaccharide (CrHWE from Caulerpa racemosa) showed antiviral activity against the four serotypes of dengue virus (DENV). The 50% inhibitory concentration (IC50) values of those polysaccharides against DENV-2 were in the range of 0.12–20 μg/mL [35]. A α-(1 → 4)-linked glucan and a fucoidan were isolated from Padina tetrastromatica [36]. Sulfated derivatives of this fucoidan (S1–S3), which contained 0.8–1.2 sulfate groups per monomer unit, showed antiviral activity against HSV-1and HSV-2. Their 50% inhibitory concentration (IC50) values varied in the range 0.74–1.05 and 0.30–0.39 μg/mL against HSV-1 and HSV-2, respectively. It was also examined that the sulfated fucoidans were more efficient herpetic inhibitors than the standard compound heparin. Bioactive polysaccharide (RMP) was isolated from the mangrove plant Rhizophora mucronata and shows potent anti-human immunodeficiency virus activity [37]. RMP contained large amount of neutral sugars and uronic acids. It showed concentration dependent anti-HIV activity with the EC50 value of 4.38 μg/mL. A polysaccharide (RAP) extracted from the leaf of mangrove plant Rhizophora apiculata showed anti-human and simian immunodeficiency virus's activities [38]. It was composed mainly of galactose (47%) and other components such as galactosamine (19%), glucose (17%) and arabinose (17%). RAP showed concentration-dependent inhibition of HIV-1 or HIV-2 or SIV replication within the EC50 concentration range of 6.5 to 40.6 μg/mL. RAP completely blocked the binding of HIV-1 to MT-4 cells at a concentration of 100 μg/mL. RAP also reduced the formation of viral mRNA when added before virus adsorption.
The major factors influencing the antiviral activities of polysaccharides are sugar composition, molecular mass, branching configuration, conformation and their chemical modification. Modern research reveals that antiviral activity is mainly focused on low and high-molecular weight plant polysaccharides. It has been found that the sulfated galactofucan and glucuronomannan showed strong binding ability to SARS-CoV-2 spike glycoproteins in comparison to heparin implying structure specific activity. It was also found that the degree and position of sulfate groups in sulfated polysaccharides influence its binding to the viral proteins, thereby alter antiviral efficacy. Therefore, the above discussions are in agreement with the fact that the antiviral activities of the polysaccharides vary both quantitatively and qualitatively depending on their structural variations. The resources, structural features and antiviral activities of some important medicinal plant polysaccharides of Indian origin were summarized in Table 1, Table 2 .
Table 1.
List of virus.
| Name of virus | Abbreviation |
|---|---|
| Poliovirus type 1 | PV-1 |
| Bovine herpesvirus type 1 | BoHV-1 |
| Herpes simplex virus type 1 | HSV-1 |
| Herpes simplex virus type 2 | HSV-2 |
| Dengue virus | DENV |
| Human immunodeficiency virus | HIV |
| Simian immunodeficiency virus | SIV |
Table 2.
Some important antiviral polysaccharides from Indian medicinal plants.
| Name of plants | Extraction method | Biological agents | Molecular weight (Da) | Antiviral activities | Reference |
|---|---|---|---|---|---|
| Azadirachta indica | Water extract | Heteropolysaccharides (P1 fraction: glucose, rhamnose, xylose, mannose and trace amount of fucose; P2 fraction: arabinose, and galactose) and their sulfated derivatives | 80 × 103 | PV-1 | [26] |
| P1 fraction and its sulfated derivatives | 80 × 103 | BoHV-1 | [27] | ||
| Calendula officinalis | Water extract | Polysaccharides | – | HIV | [39] |
| Grateloupia indica | Water extract | Sulphated F3: galactose. Sulphated GiWE: galactose and small amount xylose, fucose, glucose. |
60 × 103 | HSV | [32] |
| Gracilaria corticata | Cold water extract | Sulfated galactan (WE2NGF, Au 2, AAu 2) | 165,197; 61,990; 54,494 | HSV-1 & HSV-2 | [34] |
| Padina tetrastromatica | Hot water extract | glucan fucoidan and sulfated derivatives (S1–S3) |
50 × 103 | HSV-1 & HSV-2 | [36] |
| Portulaca oleracea |
Hot water extract |
Neutral polysaccharide (glucose, mannose, arabinose and small amounts of galactose) | 8.3 × 103 | HSV-2 | [28] |
| Acidic polysaccharide (arabinose, galactose, rhamnose, xylose and glucuronic acid) | 5.8 × 104 | ||||
| Pectic polysaccharide (galacturonic acid, glucuronic acid, arabinose, and galactose, rhamnose) | 8.7 × 104 | ||||
| Rhizophora mucronata | Water extract | Polysaccharides | – | HIV | [37] |
| Rhizophora apiculata | Water extract | RAP: galactose, galactosamine, glucose, and arabinose. | – | HIV-1, HIV-2, SIV | [38] |
| Scinaia hatei | Alkali extract | Sulfated xylomannan | 160 × 103 | HSV | [29] |
| xylans | 120 × 103 | HSV | [30] | ||
| Sphacelaria indica | Water extract | F3: fucose, galactose, xylose and small amount of glucose and mannose. | (26 ± 5) x103 | HSV-1 | [33] |
| Stoechospermum marginatum | Water extract | Sulfated fucan (F3): fucose, and small amount xylose, galactose. Sulfated fucan (SmWE): fucose and small amount of xylose, mannose, galactose, glucose. |
40 × 103 | HSV-1 & HSV-2 | [31] |
5. Immunomodulatory activities of some important polysaccharides isolated from Indian medicinal plants
Many pathogens invent routes to weaken the activation of leukocytes. For example, the human immunodeficiency virus (HIV) hinders with the Fc-α receptor signaling in human macrophages and inhibits phagocytosis [40]. Under such conditions, an external agent may be helpful to activate the immune system either by activating phagocytes or by directly acting as polyclonal mitogens. Many such agents have been identified from microbial origin like LPS [41], CpGDNA [42], and monophosphoryl lipid A [43]. However, these agents have limited application due to their toxicity. Plant derived polysaccharides have attracted extensive attention as model immunomodulators due to their relatively low toxicity and side effects as compared to those derived from microbial origin [44]. A water soluble (1 → 4)-linked-α-D-glucan having relative molecular weight 70,000 Da was isolated from mature pods (fruits) of Moringa oleifera (sajina) [45]. The glucan showed significant macrophage activation through the release of nitric oxide on mouse monocyte J744.1 cell line. The effect of glucan on macrophage proliferation was not in dose-dependent manner and maximum NO production was noted at 0.1 μg/mL dose of the glucan. In a different report, a heteroglycan was isolated from the aqueous extract of the corm of Amorphophallus campanulatus which contained D-galactose, d-glucose, 4-O-acyl-D-methyl galacturonate, and L-arabinose in a molar ratio 2:1:1:1 [46]. Chemical analysis further revealed that it was composed of 1,3-linked-β-D-Galp, 1,4-linked-α-D-Galp, 1,4-linked-4-O-Ac-α-D-GalpA6Me, 1,3,4-linked-α-D-Glcp and terminal α-L-Araf unit. The heteroglycan showed splenocyte activation in mouse cell culture medium. The effect of the heteroglycan (5–100 μg/mL) was tested on single spleen suspension medium by MTT method. The maximum proliferation index was observed at 50 μg/mL dose of the heteroglycan. Ojha et al. [47] reported a linear heteroglycan (SMPS) from the aqueous extract of the green (unripe) fruits of Solenum melongena (Brinjal). The heteroglycan composed of D-galactose, D-methyl galacturonate, 3-O-acetyl D-methyl galacturonate, and L-arabinose in a molar proportion of nearly 1:1:1:1. Linkage analysis established that the heteroglycan consisted of 1,5-linked-α-L-Araf, 1,2-linked-α-D-GalpA6Me, 1,2-linked-3-O-Ac-α-D-GalpA6Me and 1,4-linked-β-D-Galp. The heteroglycan showed splenocyte and thymocyte activations. Maximum splenocyte proliferation was observed at 20 μg/mL dose of the SMPS while thymocyte showed maximum proliferation at 50 μg/mL. Patra et al. [48] reported an immunoenhancing heteropolysaccharide of relative average molecular weight 2.0 × 105 Da from aqueous extract of the leaves of Catharanthus rosea. The heteropolysaccharide was found to consist of 6-O-methyl-glucose, arabinose, rhamnose, and methyl galacturonate in 1:2:1:2 M ratios. Detailed analysis of structure revealed that 6-O-methyl-glucose and α-L-arabinose remained in the side chain while the backbone of the polysaccharide built of methyl galacturonate and α-L-rhamnose. The immunoenhancing activities of the heteropolysaccharide were assessed through macrophage activation, splenocyte and thymocyte proliferation. On treatment with different concentrations of the polysaccharide on peritoneal macrophages, an enhanced production of NO was observed in a dose dependent manner with optimum production at 100 μg/mL dose of the polysaccharide. Maximum splenocyte and thymocyte proliferation was noted at 50 μg/mL dose of the heteropolysaccharide. Joseph et al. [49] reported an immunomodulatory galctomannan (PSP001) from fruit rind of Punica granatum. Investigation confirmed that the backbone of the galctomannan consisted of (1 → 3)-linked β-d-galactopyranose with side chains containing β-D-mannopyranose and α-D-mannopyranose units. PSP001 showed in vitro growth stimulatory effect on normal human lymphocytes, and a proliferative index of 1.21 ± 0.01 was observed at a concentration of 1000 μg/mL, indicating immunostimulatory activity. A water-soluble pectic polysaccharide (PS-I) was reported from the hot water extract of the pods of green bean (Phaseolus vulgaris), consisted of methyl ester of galacturonic acid, galactose, and arabinose in nearly 2:2:1 M ratio [50]. Structural investigation ascertained that 1,4-linked methyl ester of galacturonic acid constructed the main chain of the pectic polysaccharide while arabinose and galactose remaining in the side chains. In vitro immunomodulatory potency of the pectin was analyzed by MTT colorimetric assay through determination of the proliferation of mice splenocytes and thymocytes. The effect of the pectic polysaccharide on splenocytes and thymocytes proliferation was not in dose-dependent manner and maximum proliferations were observed respectively at 100 μg/mL and 25 μg/mL doses. Another pectic polysaccharide having Mw 1.8 × 105 Da and consisting of D-galactose, 6-O-Me-D-galactose, 3-O-acetyl-D-methyl galacturonate and D-methyl galacturonate in a molar ratio of 1:1:1:1 from immature onion stick (Allium cepa) was reported by Patra et al. [51]. The pectic polysaccharide exhibited in vitro macrophage activation by nitric oxide (NO) production in culture supernatant. NO production decreased gradually upon treatment with increasing concentrations of this pectic polysaccharide. Maximum production of NO (18.5 μM per 5 × 105 macrophages) was observed at 12.5 μg/mL dose of the pectin. Pectin induced proliferation of splenocytes and thymocytes were examined in vitro and the results indicated that 50 μg/mL of the pectin was the optimum dose for increase of splenocyte and thymocyte counts. Mandal et al. [52] reported a gluco-arabinan of average relative Mw 62 kDa from alkaline extract of Caesalpinia bondu. Structural elucidation validated the presence of terminal Glcp, (1 → 4)-Glcp, (1 → 2,3)-Glcp, terminal Araf, (1 → 5)-Araf, (1 → 2,5)-Araf, and (1 → 2,3,5)-Araf within the polysaccharide. Immunomodulating activities (macrophage activation, splenocyte and thymocyte proliferation) of the gluco-arabinan on murine immune system were investigated in vitro. The gluco-arabinan showed maximum splenocyte proliferation at 12.5 μg/mL (1.38 fold higher than control PBS) and maximum thymocyte proliferation at 25 μg/mL (1.41 fold higher than PBS). The gluco-arabinan at 100 μg/mL dose was capable of producing only 8 μM NO which was not significant while considering the macrophage activation. Das et al. [53] reported an arabinoxylan from the aqueous extract of green leaves of Litsea glutinosa. It was a branched polysaccharide having ~1,75,000 Da average relative molecular weight. Structural analysis revealed that xylose remained at the main chain of the polysaccharide and arabinose remained in the side chain. The screening of immunomodulatory properties of the arabinoxylan was examined through splenocyte and thymocyte proliferation assay. The function of the arabinoxylan on activation of innate immune cells was further investigated on peritoneal macrophages. The proliferation of splenocyte, thymocyte and activation of macrophages respectively, at 25 μg/mL, 50 μg/mL and 100 μg/mL doses of arabinoxylan confirmed its role as promising immunotherapeutic agents. Bhatia et al. [54] reported isolation of a sulfated polysaccharide (Porphyran) from hot water extract of Porphyra vietnamensis. It was a linear homopolysaccharide composing 4-O-α-L-galactose-6-sulfate repeating units. Immunostimulatory activities of porphyran were investigated in albino rats and albino mice. Increase in weight of the thymus, spleen and lymphoid organ cellularity was noticed after oral administration of porphyran (200–500 mg/kg). The total lymphocyte and leucocyte count was also increased significantly (P < 0.005). Enhancement in the adhesion of neutrophil to nylon fibres as well as dose-dependent increase in antibody titre values was observed due to porphyran treatment. A potential phagocytic response was observed and significant changes were also noted in the formation of formazone crystals after porphyran administration. The results indicated that the sulfated polysaccharide from P. vietnamenis possessed potential immunomodulatory activities. Aloe vera is a medicinal plant species of the genus Aloe with a long history of traditional therapeutic use around the world. Acemannan, the main bioactive polysaccharides of Aloe vera possesses several pharmacological activities among which immunomodulation are considered as the most vital one. According to the report of Kumar & Tiku [55], radiation-induced mortality of mice was significantly decreased when the mice were treated with acemannan at a dose of 150 mg/kg body weight by oral gavage for 7 days. The findings revealed that 7-days pretreatment or post-treatment with acemannan increased survivability of mice by 60 and 20%. It was further established that acemannan could upregulate the cytokines like TNF-α and IL-1 and also improved peripheral lymphocytes counts, spleen cellularity and spleen index. Moreover, acemannan stimulated the production of macrophage cytokines, nitric oxide release, surface molecule expression and cell morphology in RAW 264.7 cells from a mouse macrophage cell line. Interleukin-6 (IL-6) and (TNF-α) were also produced in a dose-dependent manner with a change in the morphology of the cells. Panda et al. [56] reported a water soluble pectic polysaccharide (PS) of relative Mw 2 × 105 Da from the aqueous extract of the green fruits of Momordica charantia which contained D-galactose and D-methyl galacturonate in 1:4 M ratio. The pectic polysaccharide showed macrophage activation as well as splenocyte and thymocyte proliferation. The optimum dose for macrophage activation and splenocyte proliferation was 200 μg/mL, while the same for thymocyte proliferation was 25 μg/mL. Shalini, Yengkhom, Subramani & Michael [57] reported that the polysaccharide fraction (PF) isolated from Dendrophthoe falcata (DF) leaves exhibited immunostimulatory properties in Oreochromis niloticus. Different groups of experimental fish were fed with three different doses of D. falcata polysaccharide fraction (DFPF) as supplemented diet and non-specific immunological parameters, immune related gene expression were assessed after every schedule of feeding. The DFPF treated groups showed significant upregulation of lysozyme and TNF-α gene expression. Administration of 1% DFPF in the feed for a week afforded protection against the virulent pathogen challenge, with a relative percent survival (RPS) of 100. These results suggested that DFPF could be considered as potential immunostimulant feed supplement in fish aquaculture. The resources, structural features, and immunological activities of some important medicinal plant polysaccharides were summarized in Table 3 .
Table 3.
Some important immunomodulatory pure polysaccharides isolated from Indian medicinal plant.
| Source | Extraction method | Fraction name | Molecular weight (Da) | Monosaccharide composition | Biological agents | Biological activity | Reference |
|---|---|---|---|---|---|---|---|
| Allium cepa | Hot water extract | PS | 1.8 × 105 | D-galactose: 6-O-Me-D-galactose: 3-O-acetyl-D-methyl galacturonate: D-methyl galacturonate = 1:1:1:1 | Pectin | Polysaccharide: macrophage, splenocyte, and thymocyte activations | [51] |
| Aloe vera | Water extract | Acemannan | – | man: glc: gal = 62.9%: 13.1%: 0.6% |
Heteroglycan | Polysaccharide: immunoenhancing | [55] |
| Amorphophallus campanulatus | Hot water extract | PS | 1,80,000 | D-galactose: d-glucose: 4-O-acyl-D-methyl galacturonate: L-arabinose = 2:1:1:1 | Heteroglycan | Polysaccharide: splenocyte proliferation | [46] |
| Caesalpinia bonduc | Alkaline extract | PS-II | 62,000 | glc:ara = 6:7 | Gluco-arabinan | Polysaccharide: splenocyte, thymocyte and macrophage activations | [52] |
| Catharanthus rosea | Hot water extract | PS-I | 2.0 × 105 | 6-O-methyl-glucose: arabinose: rhamnose: methyl galacturonate = 1:2:1:2. | Heteroglycan | Polysaccharide: macrophages, splenocyte and thymocyte activations | [48] |
| Litsea glutinosa | Hot water extract | PS | 1,75,000 | xyl:arab = 1:3 | Arabinoxylan | Polysaccharide: splenocyte, thymocyte and macrophage activations | [53] |
| Moringa oleifera | Hot water extract | – | 70,000 | glc | (1 → 4)-α-D glucan | Polysaccharide: immunoenhancing | [45] |
| Momordica charantia | Hot water extract | PS | 2 × 105 | D-galactose: D-methyl galacturonate = 1:4 | Pectic polysaccharide | Polysaccharide: splenocyte, thymocyte, macrophage activations | [56] |
| Phaseolus vulgaris | Hot water extract | PS-I | 1.8 × 105 | D-galacturonic acid: D-galactose: L-arabinose = 2:2:1 | Heteroglycan | Polysaccharide: splenocyte, thymocyte proliferation | [50] |
| Punica granatum | Water extract | PSP001 | 1.1 × 105 | Galactose, glucose and mannose | Galctomannan | Polysaccharide: growth stimulatory effect | [49] |
| Porphyra vietnamensis | Hot water extract | Porphyran | – | α-L-galactose-6-sulfate | Sulfated polysaccharide | Polysaccharide: immunoenhancing | [54] |
| Solenum melongena | Hot water extract | SMPS | 1.92 × 105 | gal:ara = 3:1 | Heteroglycan | Polysaccharide: splenocyte and thymocyte activations | [47] |
6. Immunomodulatory and antiviral activities of the plant polysaccharides/aqueous extracts of the plants recommended by the Ministry of AYUSH, Government of India
The Ministry of AYUSH, Government of India, in its advisory prescribed the use of several single drugs e.g. Seer (Allium sativum), Zanjabeel (Zingiber officinale), Aslassus (Glycyrrhiza glabra), Kalonji (Nigella sativa), Gilo (Tinospora cordifolia), Behi dana (Cydonia oblonga), Unnab (Zizyphus jujube) etc. against COVID-19 because of their remarkable antiviral and immunomodulating activities [58]. Several reports on the immunomodulatory and antiviral activities either of the aqueous extract or the polysaccharide fraction isolated from aqueous extract of these medicinal plants are available in the literature. Weber et al. [59] reported in vitro antiviral effect of Allium sativum aqueous extract. Activity was determined against both RNA and DNA enveloped and nonenveloped viruses. Herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), parainfluenza virus type 3 (Para-3), vaccinia virus (VV), and vesicular stomatitis virus (VSV) were the five selected viruses. HSV-1 strain KOS and HSV-2 strain 333 were grown in Vero cells, while Para-3 strain C-243 was grown in HeLa cells. In addition, VSV strain Indiana and VV strain Elstree were cultured in both cell lines. Cytotoxicity data suggested that Garlic extract was virucidal to each of the five virus strains. At the highest concentration (1000 mg/mL) tested, infectivity of all five viruses was substantially reduced. Garlic extract was most virucidal against VSV. HSV-1 and HSV-2 were almost comparable in their sensitivity followed by Para-3 and VV in decreasing order. Although VSV (RNA virus) was most susceptible to the garlic extract, there was no general pattern to conclude that RNA viruses being more sensitive than DNA viruses. Kaushik et al. [60] reported anti-chikungunya activities of Zingiber officinale (Ginger) rhizome aqueous extract in the animal cell culture model. Median tissue culture infective dose (TCID50) of Chikungunya virus (CHIKV) and Maximum non-toxic dose (MNTD) of Z. officinale extract were determined in Vero cell line. Maximum non-toxic dose of Z. officinale plant extract was found 62.5 μg/mL. Cell viability was increased to 51.05% and 35.10% when Vero cells were pre-treated respectively, with MNTD and half of MNTD of Z. officinale extract. Similarly, in co-treatment, when MNTD, half of MNTD of Z. officinale and Median tissue culture infective dose CHIKV were inoculated simultaneously, then the viability of Vero cell-line was increased by 52.90% and 49.02% respectively. Therefore, the rhizome extracts of Z. officinale had high potential to treat CHIKV. A number of components have been isolated from the roots of Glycyrrhiza glabra, including triterpene, pectins, saponin, flavonoids, simple sugars, polysaccharides, mineral amino acids, salts, asparagines etc. [61]. A phytopharmaceutical formulation containing an extract of Glycyrrhiza glabra root was investigated for immunostimulating potential [62]. In the in vitro phagocytosis test, it showed a 44–53% stimulating effect at a concentration of 100 μg/mL. Chemoluminescence assay at a concentration of 1.25 μg/mL exhibited a moderate enhancing effect while at a concentration of 100 μg/mL, a notable stimulating activity (30–50%) was found in the T-lymphocyte CD69 bioassay. The potential immunomodulatory effects of aqueous extract from Nigella sativa were examined in splenocyte proliferation; macrophage and NK cell activations in BLAB/c and c57/BL6 cells [63]. Results of the experiments revealed that the aqueous extract of Nigella sativa significantly enhances splenocyte proliferation in a dose-dependent manner. The secretion of IL-6, TNF-α and production of NO in macrophages were significantly suppressed by the aqueous extract of N. sativa. Chintalwar et al. [64] reported an acidic arabinogalactan of average relative molecular mass 2.2 × 106 from the stems of Tinospora cordifolia. The polysaccharide was composed of galacturonic acid (35%), galactose (32%), arabinose (31%) and very small amount of rhamnose (1.4%). Partial acid hydrolysis revealed that galactoses were the part of main chain while the side chains were mainly composed of arabinose and galacturonic acid. Methylation analysis revealed presence of terminal arabinose, terminal galactose, 1,5-linked arabinose, 1,4-linked galactose, 1,6-linked galactose and 1,3,6-linked galactose. Experiments further confirmed the backbone of the polysaccharide was constructed by 1,4-linked galactose and some 1,3,6-linked galactose. The polysaccharide showed mitogenic activity through activation of polyclonal murine B-cells which was an indicator of immunostimulant activity. Later on, Raghu et al. [65] reported the detail molecular actions associated with the acidic arabinogalactan (G1–4A) induced in vitro and in vivo immunomodulation. Administration of the acidic arabinogalactan to mice led to an increase in the numbers of T cells, B cells and macrophages. In vivo proliferation of B cells and degradation of IκB-α suggested that TLR-4 was a receptor for G1–4A on B cells. Reports further revealed that the activation of RAW 264.7 macrophages by G1–4A was dependent on NF-κB-mediated signals. The effect of the same acidic polysaccharide on functional maturation of murine bone marrow derived dendritic cells was investigated by Pandey, Shankar & Sainis [66]. The arabinogalactan enhanced surface expression of CD40, CD80, CD86, MHCII and splenic dendritic cells. It could also induce allostimulatory activity on T cell and increase of IL-12 and TNF-α secretion by bone marrow derived dendritic cells were also observed. Thus, the acidic arabinogalactan were capable of modulating both innate and adaptive immune responses. Gupta, Rajan & Kulkarni [67] reported up regulation of expression of TNF-α, IL-β, IL-6, IL-12, IL-10 and IFN-γ in RAW 264.7 cell line and peritoneal macrophages after treatment with the same acidic arabinogalactan. Nitric oxide levels were also enhanced along with up-regulation of NOS2 expression in murine macrophages due to treatment of G1–4A. Moreover, treatment of the acidic arabinogalactan up-regulated the surface expression of MHC-II and CD-86 and also activated p38, ERK and JNK MAPKs in macrophages. Treatment of aqueous extract of Cordia myxa fruit in the BALB/c mice showed enhancement of mitotic index (MI) of bone marrow and spleen cells due to immunization effect [68]. The immunomodulatory properties of the extract from Cydonia oblonga were explored to inhibit the production of IL-8 and TNF- α from human mast cells. Chi et al. [69] reported isolation of polysaccharide conjugates (JPC) from the fruits of Ziziphus jujube. Monosaccharide analysis of JPC showed that it was composed of Man, Rib, GlucA, GalcA, Glu, Xyl, Gal and Ara in the molar percentages of 5.3%, 3.1%, 3.6%, 11.4%, 13.4%, 14.5%, 23.4%, and 25.1%, respectively. Oral administration of JPC in Chronic Fatigue Syndrome (CFS) rats led to T lymphocyte proliferation, elevated CD4+/CD8+ ratio and improved activities of natural killer (NK) cells. Elevation of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) levels in serum post JPC treatment were also reported. The results and findings discussed so far reveal antiviral and immune stimulating properties of different Indian plant polysaccharides, which may also help to treat COVID-19 infected people.
7. Mechanism of actions
The mechanism of antiviral activities of polysaccharides is largely related to its specific structure and virus type. Coronaviruses (CoVs) are enveloped viruses with a positive-stranded RNA genome. Four structural proteins namely, spike, nucleocapsid, membrane, and envelope have been identified in SARS-CoV-2. The CoVs enter into host cells by a complex process, initiated by the specific interaction between the virus and host-cell-surface receptors, involving the fusion of the envelope with the host cell membrane arbitrate by viral S proteins. Polysaccharides can inhibit the virus in different pathways, such as- (i) Polysaccharides, mainly sulfated polysaccharides, through interaction with the surface of virus by negative charge, may either inhibit the transmittable capacity of the virus, or kill the virus. (ii) Sulfated polysaccharides, by virtue of its strong poly anionic activities can stop virus adsorption or invasion by blocking the positive charge on the cell surface. (iii) These can also directly inhibited viral transcription and replication on host cells. (iv) After viral invasion in host body, the plant polysaccharides might trigger the host NK cells and macrophages and thereby induce production of immune cytokines, and indirectly exert antiviral effects by activating innate immunity. These are also capable of generating innate immune responses through production of NO; up regulation of expression of TNF-α and interleukins (IL-6, IL-12) in macrophages via attachment to TLR-2, activation of NF-κB and MAPK cells for development of innate immune response in host cells. Possible mechanism of action of antiviral and immunomodulatory plant polysaccharides against COVID-19 is shown in Fig. 3 .
Fig. 3.

Possible mechanism of action of antiviral and immunomodulatory plant polysaccharides against COVID-19.
8. Summary and perspectives
Currently, people have no other option than to survive with SARS-CoV-2 until development and application of suitable vaccine to the entire population. The immediate challenge for the world and also for India is to curb the spread of COVID-19. Indian herbal medicines with 1000 years' experience in the prevention of pandemic and endemic infectious diseases may have significant role in this COVID pandemic. During the last few decades, a number of polysaccharides with potential therapeutic properties have been identified from Indian plants. Many plant polysaccharides, such as xylan, acemannan, sulfated fucans, sulfated xylomannan, fucoidans, pectins, glucans, porphyran, glucoarabinan, and arabinoxylan have antiviral and immunomodulating activities and their potential for therapeutic applications is drawing attention of the researchers all over the world. Plant polysaccharides with specific structures or certain molecular weights exhibit different antiviral and immunomodulatory activities. The viral infection can be inhibited by the plant polysaccharides through interference with the viral life cycle or by improving the host immune response. Intake of certain immunomodulating plant polysaccharides may also protect from the virus to a certain extent. Use of polysaccharides in vaccine production already reported as they are cost-effective, easily available and have no side-effect [[70], [71], [72], [73]]. So, antiviral Indian medicinal plant polysaccharides may be functional for recovery of asymptomatic and mild-symptomatic patients with COVID-19 infection. Scientific community should focus on the development of plant polysaccharide-based drugs to deal with this COVID pandemic.
Funding
None.
CRediT authorship contribution statement
Ipsita K. Sen: Writing, Reviewing and Editing.
Indranil Chakraborty: Reviewing and Editing.
Amit Kumar Mandal: Reviewing.
Sunil K. Bhanja: Reviewing.
Sukesh Patra: Reviewing.
Prasenjit Maity: Writing, Reviewing, Editing, and Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to Prof. Syed Sirajul Islam, former professor, Department of Chemistry and Chemical Technology, Vidyasagar University, West Bengal, India.
References
- 1.Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci. Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.F., Yuan S., Kok K.H., K.K. To, Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long Q.X., Tang X.J., Shi Q.L., Li Q. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 6.Pan X., Dong L., Yang L., Chen D., Peng C. Potential drugs for the treatment of the novel coronavirus pneumonia (COVID-19) in China. Virus Res. 2020;286:198057. doi: 10.1016/j.virusres.2020.198057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuong W., Khan M.B., Fischer C., Artyunova E., et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey N.K., Kumar R., Tripathi P. Global promotion of herbal medicine: India's opportunity. Curr. Sci. 2004;86:37–41. [Google Scholar]
- 9.Joshi V.K., Joshi A., Dhiman K.S. The ayurvedic pharmacopoeia of India, development and perspectives. J. Ethnopharmacol. 2017;197:32–38. doi: 10.1016/j.jep.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Adhikari P.P., Paul S.B. History of Indian traditional medicine: a medical inheriterence. Asian J. Pharm. Clin. Res. 2018;11:421–426. [Google Scholar]
- 11.AYUSH Ayush health promotion product' for commercial manufacturing by Ayurveda, Siddha and Unani drug manufacturersreg. 2020. http://www.ccras.nic.in/sites/default/files/Notices/25042020_Letter_to_States_UTs_for_Ayush_Kwath.pdf (Accessed 24th April, 2020)
- 12.Tamam S.M., Abd el Hamid M.S., Samah M.H., Marwa A.N. The anti-viral and immunomodulatory activity of Cinnamon zeylanicum against “NDV” Newcastle disease virus in chickens. IJSBAR. 2017;32(2):251–262. [Google Scholar]
- 13.Maity G.N., Maity P., Dasgupta A., Acharya K., Dalai S., Mondal S. Structural and antioxidant studies of a new arabinoxylan from green stem Andrographis paniculata (Kalmegh) Carbpol. 2019;212:297–303. doi: 10.1016/j.carbpol.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 14.Estes M.K., Lemon S.M., et al. In: Virus Taxonomy: Classification and Nomenclature of Viruses Seventh Report of the International Committee on Taxonomy of Viruses. Van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., editors. Academic Press; San Diego: 2000. Coronaviridae; pp. 835–849. ISBN 0123702003. [Google Scholar]
- 15.Pradesh U., Upadhayay P.D.D., Vigyan P.C. Coronavirus infection in equines: a review. Asian J. Anim. Vet. Adv. 2014;9:164–176. [Google Scholar]
- 16.Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu R., Shi M., Li J., Song P., Li N. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System. Front. Bioeng. Biotechnol. 2020;8:1–6. doi: 10.3389/fbioe.2020.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh A., Gupta R., Mishra A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: guidelines for physicians. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:273–276. doi: 10.1016/j.dsx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joob B., Wiwanitkit V. SARS-CoV-2 and HIV. J. Med. Virol. 2020 doi: 10.1002/jmv.25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang W., Guan W., Chen R., Wang W., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–336. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G., et al. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020;167:105941. doi: 10.1061/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P.P., et al. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 24.Bhayana R., Som A., Matthew D.L., Denston E.C., et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297(1):207–215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Salhy M., Ystad S.O., Mazzawi T., Guundersen D. Dietary fiber in irritable bowel syndrome. Int. J. Mol. Med. 2017;40:607–613. doi: 10.3892/ijmm.2017.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faccin-Galhardi L.C., Yamamoto K.A., Ray S., Ray B., et al. The in vitro antiviral property of Azadirachta indica polysaccharides for poliovirus. J. Ethnopharmacol. 2012;142:86–90. doi: 10.1016/j.jep.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Saha S., Galhardi L.C., Yamamoto K.A., Linhares R.E., Bandyopadhyay S.S., Sinha S., et al. Water extracted polysaccharides from Azadirachta indica leaves: Structural features, chemical modification and the anti-bovine herpesvirus type 1 (BoHV-1) activity. Int. J. Biol. Macromol. 2010;47(5):640–645. doi: 10.1016/j.ijbiomac.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Ding C.X., Hayashi K., Lee J.B., Hayashi T. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea. Chem. Pharm. Bull. 2010;58:507–510. doi: 10.1248/cpb.58.507. [DOI] [PubMed] [Google Scholar]
- 29.Mandal P., Pujot C.A., Carlucci M.J., Chattopadhyaya K., Damonte E.B., Ray B. Anti-herpetic activity of a sulfated xylomannon from Scinaia hatei. Phytochem. 2008;31:2193–2199. doi: 10.1016/j.phytochem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Mandal P., Pujot C.A., Damonte E.B., Ghosh T., Ray B. Xylans from Scinaia hatei: Structural features, sulfation and anti-HSV activity. Int. J. Biol. Macromol. 2010;46:173–178. doi: 10.1016/j.ijbiomac.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Adhikari U., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochem. 2006;67:2474–2482. doi: 10.1016/j.phytochem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay K., Mateu C.G., Mandal P., Pujol C.A., Damonte E.B., Ray B. Galactan sulfate of Grateloupia indica: Isolation, structural features and antiviral activity. Phytochem. 2007;68:1428–1435. doi: 10.1016/j.phytochem.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay S.S., Navid M.H., Ghosh T., Schnitzler P., Ray B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochem. 2011;72:276–283. doi: 10.1016/j.phytochem.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Mazumder S., Ghosal P.K., Pujol C.A., Carlucci M.J., Damonte E.B., Ray B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta) Int. J. Biol. Macromol. 2002;31:87–95. doi: 10.1016/s0141-8130(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 35.Pujola C.A., Ray S., Ray B., Damontea E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012;51:412–416. doi: 10.1016/j.ijbiomac.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar P., Pujol C.A., Damonte E.B., Ghosh T., Ray B. Polysaccharides from Padina tetrastromatica: Structural features, chemical modification and antiviral activity. Carbpol. 2010;80:513–520. [Google Scholar]
- 37.Premanathan M., Kathiresan K., Yamamoto N., Nakashima H. In vitro anti-human immunodeficiency virus activity of polysaccharide from the Rhizophora mucronata Poir. Biosci. Biotechnol. Biochem. 1999;63:1187–1191. doi: 10.1271/bbb.63.1187. [DOI] [PubMed] [Google Scholar]
- 38.Premanathan M., Arakaki R., Izumi H., Kathiresan K., Nakano M., Yamamoto N., Nakashima H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antivir. Res. 1999;44:113–122. doi: 10.1016/s0166-3542(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D., Arya V., Kaur R., Bhat Z.A., Gupta V.K., Kumar V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012;45:165–184. doi: 10.1016/j.jmii.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Kedzierska K., Ellery P., Mak J., Lewin S.R., Crowe S.M., Jaworowski A. HIV-1 down-modulates γ signaling chain of FcγR in human macrophages: a possible mechanism for inhibition of phagocytosis. J. Immunol. 2002;168:2895–2903. doi: 10.4049/jimmunol.168.6.2895. [DOI] [PubMed] [Google Scholar]
- 41.Skidmore B.J., Chiller J.M., Morrison D.C., Weigle W.O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J. Immunol. 1975;114:770–775. [PubMed] [Google Scholar]
- 42.Yi A.K., Tuetken R., Redford T., Waldschmidt M., Kirsch J., Krieg A.M. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J. Immunol. 1998;160:4755–4761. [PubMed] [Google Scholar]
- 43.Martin M., Michalek S.M., Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 2003;71:2498–2507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schepetkin I.A., Quinn M.T. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Mondal S., Chakraborty I., Pramanik M., Rout D., Islam S.S. Structural studies of an immunoenhancing polysaccharides isolated from mature pods (fruits) Moringa oleifera, sajina. Med. Chem. Res. 2004;13(6/7):390–400. [Google Scholar]
- 46.Das D., Mondal S., Roy S.K., Maiti D., Bhunia B., Maiti T.K., Islam S.S. Isolation and characterization of a heteropolysaccharide from the corm of Amorphophallus campanulatus. Carbohydr. Res. 2009;344:2581–2585. doi: 10.1016/j.carres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Ojha A.K., Chandra K., Ghosh K., Bhunia B., Maiti T.K., Islam S.S. Structural analysis of an immunoenhancing heteropolysaccharide isolated from the green (unripe) fruits of Solenum melongena (Brinjal) Carbohydr. Res. 2009;344:2357–2363. doi: 10.1016/j.carres.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Patra S., Maity K.K., Bhunia S.K., Dey B., Das D., Mondal S., Bhunia B., Maiti T.K., Islam S.S. Structural characterization of an immunoenhancing heteropolysaccharide isolated from hot water extract of the fresh leaves of Catharanthus rosea. Carbpol. 2010;81:584–591. [Google Scholar]
- 49.Joseph M.M., Aravind S.R., Varghese S., Mini S., Sreelekha T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012;5:489–496. doi: 10.3892/mmr.2011.638. [DOI] [PubMed] [Google Scholar]
- 50.Patra P., Das D., Behera B., Maiti T.K., Islam S.S. Structure elucidation of an immunoenhancing pectic polysaccharide isolated from aqueous extract of pods of green bean (Phaseolus vulgaris) Carbpol. 2012;87:2169–2175. [Google Scholar]
- 51.Patra P., Sen I.K., Bhanja S.K., Nandi A.K., Samanta S., Das D., Devi K.S.P., Maity T.K., Islam S.S. Pectic polysaccharide from immature onion stick (Allium cepa): structural and immunological investigation. Carbpol. 2013;92:345–352. doi: 10.1016/j.carbpol.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Mandal E.K., Mandal S., Maity S., Behera B., Maiti T.K., Islam S.S. Structural studies of an immunostimulating gluco-arabinan from seeds of Caesalpinia bonduc. Carbpol. 2013;92:704–711. doi: 10.1016/j.carbpol.2012.08.093. [DOI] [PubMed] [Google Scholar]
- 53.Das D., Maiti S., Maity T.K., Islam S.S. A new arabinoxylan from green leaves of Litsea glutinosa (Lauraeae): structural and biological studies. Carbpol. 2013;92:1243–1248. doi: 10.1016/j.carbpol.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 54.Bhatiaa S., Ratheea P., Sharmab K., Chaugulec B.B., Kard N., Berad T. Immuno-modulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013;57:50–56. doi: 10.1016/j.ijbiomac.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S., Tiku A.B. Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food Agric. Immunol. 2016;27:72–83. [Google Scholar]
- 56.Panda B.C., Mondal S., Devi K.S.P., Maiti T.K., et al. Pectic polysaccharide from the green fruits of Momordica charantia (Karela): Structural characterization and study of immunoenhancing and antioxidant properties. Carbohydr. Res. 2015;401:24–31. doi: 10.1016/j.carres.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Shalini K.S., Yengkhom O., Subramani P.A., Michael R.D. Polysaccharide fraction from the Indian mistletoe, Dendrophthoe falcata (L.f.) Ettingsh enhances innate immunity and disease resistance in Oreochromis niloticus (Linn.) Fish Shellfish Immunol. 2019;88:407–414. doi: 10.1016/j.fsi.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 58.AYUSH Ministry of Ayush, Government of India. Guidelines for Unani practitioners for COVID 19. 2020. www.ayush.gov.in'docs'unani-guidelines
- 59.Weber N.D., Andersen’ D.O., North J.A., Murray’ B.K., Lawson L.D., Hughes B.G. In vitro virucidal effects of Allium sativum (Garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 60.Kaushik S., Jangra G., Kundu V., Yadav J.P., Kaushik S. Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. Virus Dis. 2020;31:270–276. doi: 10.1007/s13337-020-00584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffmann D. 2nd ed. Element; Shaftesbury: 1990. The New Holistic Herbal. [Google Scholar]
- 62.Wagner H., Jurcic K. Immunological studies of Revitonil®, a phytopharmaceutical containing Echinacea purpurea and Glycyrrhiza glabra root extract. Phytomed. 2002;9:390–397. doi: 10.1078/09447110260571616. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chintalwar G., Jain A., Sipahimalani A., Banerji A., Sumariwall P., Ramakrishnan R., Sainis K. An immunologically active arabinogalactan from Tinospora cordifolia. Phytochem. 1999;52:1089–1093. doi: 10.1016/s0031-9422(99)00386-6. [DOI] [PubMed] [Google Scholar]
- 65.Raghu R., Sharma D., Ramakrishnan R., Khanam S., Chintalwar G.J., Sainis K.B. Molecular events in the activation of B cells and macrophages by a non-microbial TLR4 agonist, G1-4A from Tinospora cordifolia. Immunol. Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Pandey V.K., Shankar B.S., Sainis K.B. G1-4 A, an arabinogalactan polysaccharide from Tinospora cordifolia increases dendritic cell immunogenicity in a murine lymphoma model. Int. Immunopharmacol. 2012;14:641–649. doi: 10.1016/j.intimp.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Gupta P.K., Rajan M.G.R., Kulkarnia S. Activation of murine macrophages by G1-4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. Int. Immunopharmacol. 2017;50:168–177. doi: 10.1016/j.intimp.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Ali W.R., Al-Asady Z.T., Ibrahim A.A.J. Immunomodulatory of Cordia myxa (L.) aqueous extract fruit in immunized mice with hydatid cyst fluid. J. Nat. Sci. Res. 2015;5:0921–2225. [Google Scholar]
- 69.Chi A., Kang C., Zhang Y., Tang L., Guo H., Li H., Zhang K. Immunomodulating and antioxidant effects of polysaccharideconjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbpol. 2015;122:189–196. doi: 10.1016/j.carbpol.2014.12.082. [DOI] [PubMed] [Google Scholar]
- 70.Han B., Xu K., Liu Z., Ge W., Shao S., Li P., Zhang Z. Oral yeast-based DNA vaccine confers effective protection from Aeromonas hydrophila infection on Carassius auratus. Fish Shellfish Immunol. 2019;84:948–954. doi: 10.1016/j.fsi.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 71.Liu Z., Zhou G., Ren C., Xu K., Yan Q., Li X., Zhang Z. Oral administration of myostatin-specific recombinant Saccharomyces cerevisiae vaccine in rabbit. Vaccine. 2016;34:2378–2382. doi: 10.1016/j.vaccine.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 72.Lindsey B.B., Armitage E.P., Kampmann B., de Silva T.I. The efficacy, effectiveness, and immunogenicity of influenza vaccines in Africa: a systematic review. Lancet Infect. Dis. 2019;19(4):110–119. doi: 10.1016/S1473-3099(18)30490-0. [DOI] [PubMed] [Google Scholar]
- 73.Moreno-Mendieta S., Guillén D., Hernández-Pando R., Sanchez S., Rodriguez-Sanoja R. Potential of glucans as vaccine adjuvants: a review of the α-glucans case. Carbpol. 2017;165:103–114. doi: 10.1016/j.carbpol.2017.02.030. [DOI] [PubMed] [Google Scholar]