Summary
Background
Despite substantial decreases in recent decades, acute gastroenteritis causes the second greatest burden of all infectious diseases worldwide. Noroviruses are a leading cause of sporadic cases and outbreaks of acute gastroenteritis across all age groups. We aimed to assess the role of norovirus as a cause of endemic acute gastroenteritis worldwide.
Methods
We searched Embase, Medline, and Global Health databases from Jan 1, 2008, to March 8, 2014, for studies that used PCR diagnostics to assess the prevalence of norovirus in individuals with acute gastroenteritis. We included studies that were done continuously for 1 year or more from a specified catchment area (geographical area or group of people), enrolled patients who presented with symptoms of acute gastroenteritis, and used PCR-based diagnostics for norovirus on all stool specimens from patients with acute gastroenteritis. The primary outcome was prevalence of norovirus among all cases of gastroenteritis. We generated pooled estimates of prevalence by fitting linear mixed-effect meta-regression models.
Findings
Of 175 articles included, the pooled prevalence of norovirus in 187 336 patients with acute gastroenteritis was 18% (95% CI 17–20). Norovirus prevalence tended to be higher in cases of acute gastroenteritis in community (24%, 18–30) and outpatient (20%, 16–24) settings compared with inpatient (17%, 15–19, p=0·066) settings. Prevalence was also higher in low-mortality developing (19%, 16–22) and developed countries (20%, 17–22) compared with high-mortality developing countries (14%, 11–16; p=0·058). Patient age and whether the study included years of novel strain emergence were not associated with norovirus prevalence.
Interpretation
Norovirus is a key gastroenteritis pathogen associated with almost a fifth of all cases of acute gastroenteritis, and targeted intervention to reduce norovirus burden, such as vaccines, should be considered.
Funding
The Foodborne Disease Burden Epidemiology Reference Group (FERG) of WHO and the Government of the Netherlands on behalf of FERG.
Introduction
Despite substantial decreases in recent decades, acute gastroenteritis (diarrhoea and vomiting) causes the second greatest burden of all infectious diseases, estimated at 89·5 million disability-adjusted life-years (DALYs) and 1·45 million deaths worldwide every year.1,2 Low-income countries bear the brunt of severe outcomes of acute gastroenteritis, with more than 25% of deaths in children younger than 5 years being attributable to acute gastroenteritis in Africa and southeast Asia.3,4 Although some general prevention measures are broadly effective against enteric infections, targeted control programmes need an understanding of the relative burden of individual pathogens.
Noroviruses are a leading cause of sporadic cases and outbreaks of acute gastroenteritis across all age groups.5–7 A systematic review8 of the scientific literature published between January, 1990, and February, 2008, estimated that 12% of all cases of sporadic acute gastroenteritis in children younger than 5 years and older children and adults were associated with norovirus. The review identified 31 unique datasets from 22 countries, but only 11 datasets were from low-income country settings. Thus, the role of norovirus in the causes of acute gastroenteritis in high-burden, low-income countries could not be established.
With the increasing use of molecular diagnostics worldwide, the number of studies assessing the causal role of norovirus in sporadic acute gastroenteritis in both high-income and low-income countries has increased substantially in the past 5 years. The availability of these new data prompted us to update previous estimates of the prevalence of norovirus in patients with acute gastroenteritis. This work was part of a broader effort commissioned by the Foodborne Disease Burden Epidemiology Reference Group (FERG) of WHO to estimate the global burden of foodborne disease.
In addition to the study of the overall frequency of norovirus in cases of acute gastroenteritis, we aimed to assess prevalence by age group, disease severity, and level of under-5 mortality in the country where the study was done. In view of the diversity of norovirus genotypes, and the known occurrence of global pandemics of norovirus associated with the emergence of novel norovirus strains,9–15 we assessed differences in burden in pandemic versus non-pandemic seasons.
Methods
Search strategy and selection criteria
We did a systematic review16 of Embase, Medline, and Global Health databases of studies published between Jan 1, 2008, and March 8, 2014, with the search terms “norovirus,” “Caliciviridae,” and related terms. The appendix shows the full search strategy. Two independent reviewers screened titles and abstracts for relevance. Original articles were obtained and assessed in detail for inclusion. Studies had to meet all three of the following criteria for inclusion: done continuously for 1 year or more from a specified catchment area (geographical area or group of people); enrolled patients who presented with symptoms of acute gastroenteritis; used PCR-based diagnostics for norovirus on all stool specimens from patients with acute gastroenteritis. We excluded studies that did not report the number of patients with acute gastroenteritis, the number of norovirus-positive cases, or percentages that allowed these raw numbers to be calculated. We also excluded reports that did not have abstracts in English. We added data for the period before January, 2008, from the previous systematic review by Patel and colleagues,8 and extracted data for additional variables as needed.
Data extraction and bias assessment
The following information was extracted for each study when provided: first author, title, journal, year of publication, country, start date, end date, diagnostic method used, case definition of acute gastroenteritis, number of cases tested, number of cases positive for norovirus (by genogroup when presented), number of controls (ie, patients without acute gastroenteritis) tested (if included), and number of positive controls (ie, patients without acute gastroenteritis with norovirus detected in their stool). Data for age and setting (community, outpatient, or inpatient) were also extracted; we stratified data from studies that reported multiple settings or age groups.
We included specimens with both norovirus and another pathogen detected (eg, rotavirus) as norovirus positives, so as not to introduce a bias against studies that did not test specimens for other pathogens. For studies that tested only for norovirus among patients who were alternate pathogen negative (eg, bacteria-negative and rotavirus-negative), we used the total number of cases of acute gastroenteritis, rather than alternate pathogen-negative cases, as the denominator, thereby conservatively assuming that norovirus coinfections with other pathogens were negligible.
Data were stratified by age, setting of surveillance, development index, and strain-year (pandemic vs non-pandemic). After stratification, some studies provided data for more than one strata (eg, two age groups reported), so the total number of data points sometimes exceeded the number of studies. For age, we grouped studies into three categories: younger than 5 years, 5 years and older, and mixed (studies that did not report age stratified at age 5 years). We considered other age groupings (appendix), but because of inconsistencies in each study’s inclusion criteria and the age stratifications in which they were reported, we restricted the main analysis to these three age groups.
We stratified data into four setting categories as an indicator of severity: inpatient, including emergency department or patients in hospital (severe disease); outpatient, including outpatients at hospitals, primary care clinics, or dispensaries (moderate disease); community, including community cohort studies (mild disease); and other settings, including studies in which setting was not described or in which a mixture of settings was included but stratified data were not reported. Community-based studies capture the full range of disease severity; however, because most individuals with norovirus do not seek care, and care-seeking is associated with severity, we assumed that community cases of acute gastroenteritis were, on average, less severe than were individuals with acute gastroenteritis who were medically attended.
As an indicator of development and epidemiological context, we used WHO mortality stratum.17 This development index is “defined by geography, state of economic and demographic development, and mortality patterns” of children and adults.17 WHO produces three broad groupings of development: high-mortality developing, low-mortality developing, and developed. We grouped data into one of these three strata on the basis of the country in which cases were enrolled.
We also hypothesised that norovirus prevalence might have increased in years when a new variant of genotype GII.4 emerged. A study was designated as including a pandemic period if the study dates included Dec 1, 2002, to Jan 31, 2003, or Dec 1, 2006, to Jan 31, 2007. This definition was selected to maximise the chance of detection of an increase in prevalence concurrent with the new variant. Shifts in the prevalent GII.4 virus have happened about seven times during the past 2 decades; we selected the two periods of geographically widespread reports of heightened activity.13,14,18
Statistical analysis
We generated pooled estimates of prevalence by fitting linear mixed-effect meta-regression models. Prevalence was the outcome, and we used the restricted maximum-likelihood estimator for residual heterogeneity, a random effect for study, and the Knapp-Hartung adjustment for random effects. We fitted four separate models for age, setting, development index, and pandemic years. The significance of these variables was tested in the bivariate models on the basis of the F statistic (omnibus test of a variable under the Knapp-Hartung adjustment). We reported the amount of residual heterogeneity with the t2 statistic, and used the Q statistic to test for residual heterogeneity.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We identified 5399 articles. Of these, 2941 were unique records, from which 2533 records were excluded based on their titles and abstracts (figure 1). We assessed the eligibility of 408 full-text articles. From these, 64 did not report usable data (eg, full time-series of data not presented, no denominator data presented), 43 reported outbreak or laboratory data alone (rather than endemic disease surveillance data), 43 were in a foreign language and either did not have an English abstract or had insufficient information in an English abstract to meet the inclusion criteria, 30 did not use PCR diagnostics, 28 were less than one consecutive year long, three were already identified by Patel and colleagues, nine articles could not be obtained, and 38 were excluded for other miscellaneous reasons (eg, reported traveller’s diarrhoea, discussed laboratory methods, reported other caliciviruses). Thus, 150 articles met the inclusion criteria and full data were extracted. Of these, one article only presented data for a specific genogroup, and was excluded in the overall analysis, and two articles were excluded for presenting data already accounted for in another, more inclusive article.
Figure 1:
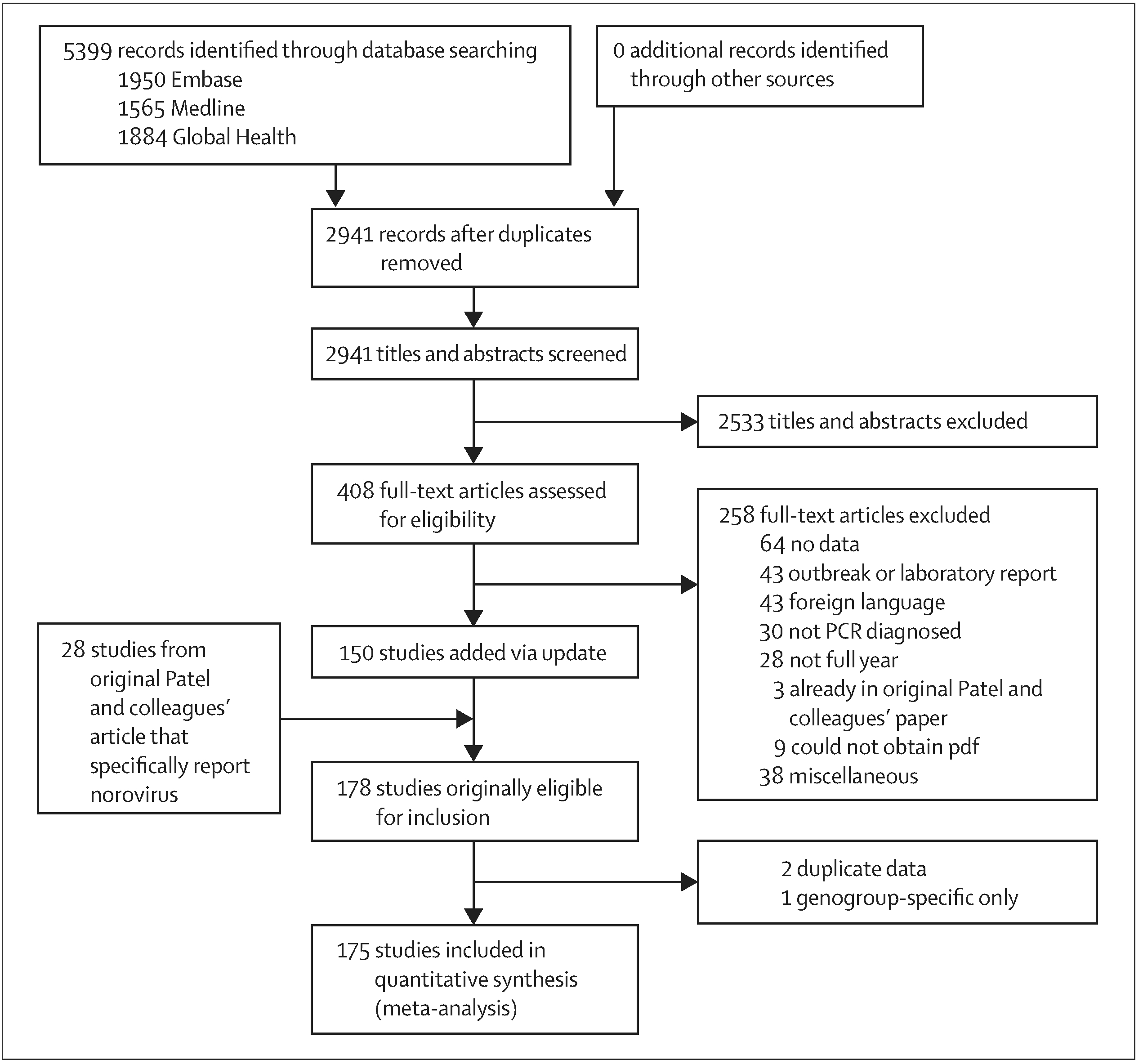
Study profile
Studies originally identified by Patel and colleagues were appended to our search. However, three of the 31 studies reported human calicivirus prevalence, and did not distinguish norovirus from sapovirus. These were excluded, leaving 28 additional articles (appendix). Therefore, our updated review included 175 studies (table). 20 of these studies also included data for healthy controls.
Table:
Distribution of data among various strata
| Number of studies (N=175) | Median number cases tested per study (N=457; range) | Casestested (N=187 336) | |
|---|---|---|---|
| Age | |||
| <5 years | 78 | 431 (44–10 600) | 69 040 |
| ≥5 years | 20 | 369 (23–2382) | 14 749 |
| Mixed | 94 | 522 (30–10 030) | 103 547 |
| Setting | |||
| Inpatient | 113 | 434 (23–10 600) | 106 499 |
| Outpatient | 35 | 421 (44–5091) | 28 249 |
| Community | 16 | 479 (30–4727) | 14 490 |
| Other | 23 | 528 (49–10 030) | 38 098 |
| Development index | |||
| High mortality developing | 26 | 241 (44–1941) | 15 113 |
| Low mortality developing | 79 | 411 (47–10 600) | 93 658 |
| Developed | 70 | 570 (23–6231) | 78 565 |
| Included pandemic period | |||
| Yes | 61 | 465 (30–7002) | 95 398 |
| No | 114 | 457 (30–7002) | 91 938 |
The final dataset included data from 48 countries, with generally only a few studies from each represented country (figure 2). More studies from Japan (13) and China (31) than other countries were included, but these tended to be small studies. Only 26 of the 175 studies were from high-mortality developing indexed countries. Most of these data either represented patients younger than 5 years, or of mixed ages, and were from inpatient settings (severe disease). 32 of the 175 studies allowed for vomiting-only (without diarrhoea) in the case definition of acute gastroenteritis. Most studies were case-only; 20 (11%) presented data for healthy controls and six (3%) were based on community cohorts with prospective follow-up.
Figure 2:
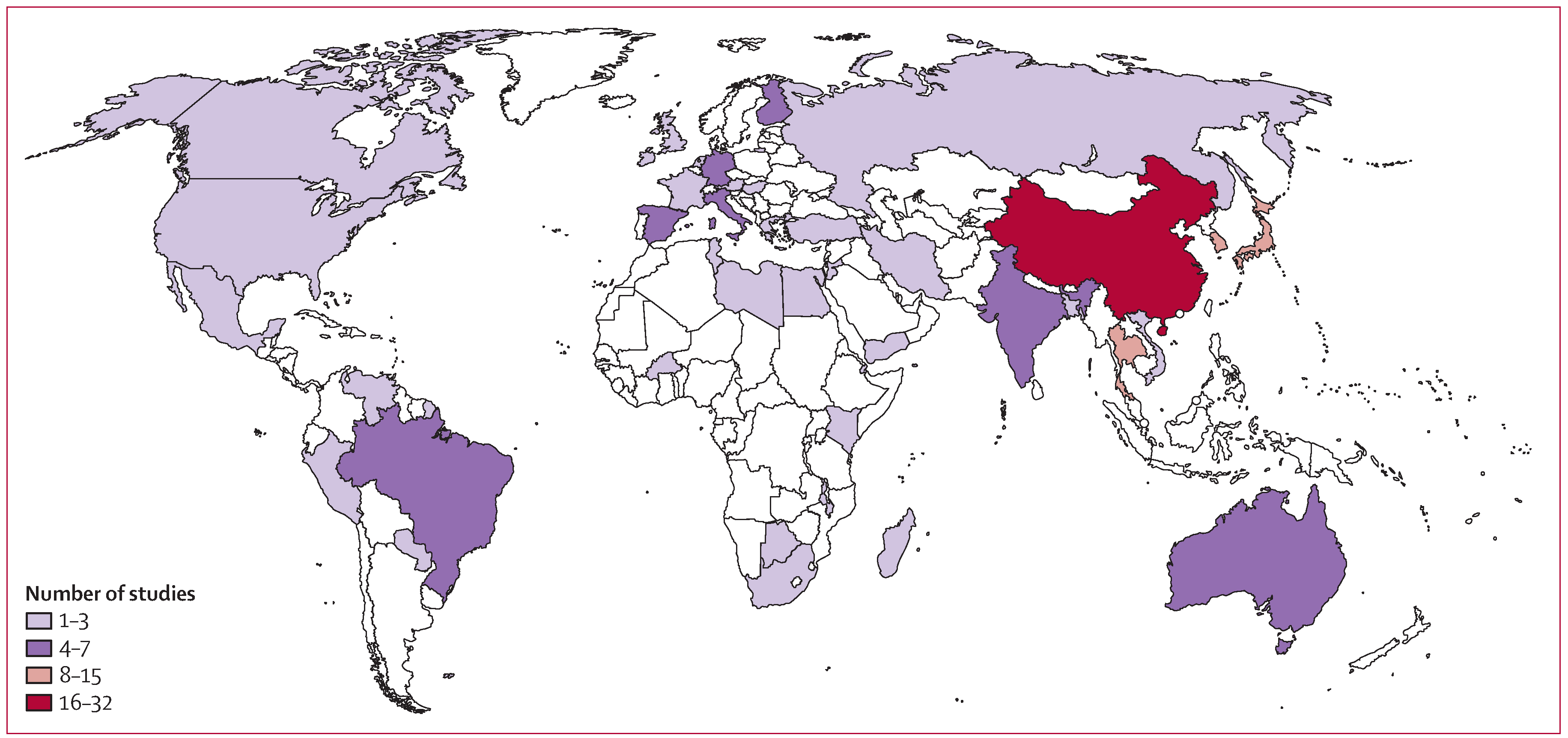
Heat map of unique datasets from each country
Of 175 studies, norovirus prevalence in cases of acute gastroenteritis was estimated at 18% (95% CI 17–20; t2=0·013; p<0·001 test for heterogeneity). By age, prevalence was similar in patients with acute gastroenteritis younger than 5 years (18%; 15–20) 5 years or older (18%; 13–24), and of mixed ages (19%, 17–21; p=0·758; heterogeneity p<0·001). By setting, prevalence was higher in cases of acute gastroenteritis in the community (24%, 18–30) and outpatient settings (20%, 16–24) compared with inpatient settings (17%, 15–19; p=0·066; heterogeneity p<0·001). Prevalence was also higher in low-mortality developing (19%, 16–22) and developed countries (20%, 17–22) compared with high-mortality developing countries (14%, 11–16; p=0·058; heterogeneity p<0·001). Prevalence was similar in studies that included pandemic periods (19%, 16–22) compared with those that did not (18%, 16–20; p=0·59; heterogeneity p<0·001; figure 3 and appendix).
Figure 3: Forest plots of proportion of patients positive for norovirus with acute gastroenteritis and individuals without acute gastroenteritis.
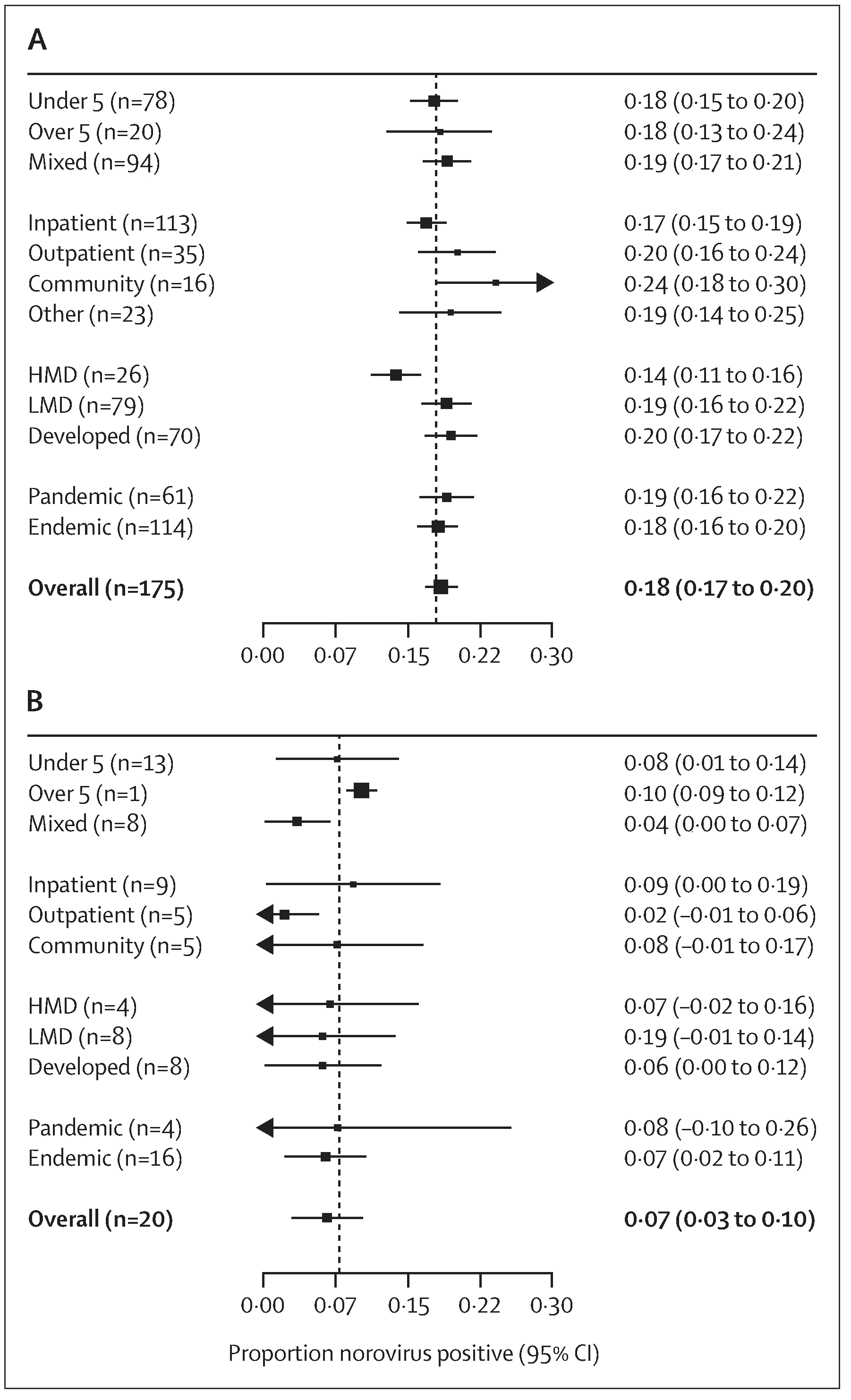
(A) Patients with acute gastroenteritis and (B) individuals without acute gastroenteritis. n values show the number of unique studies included in each stratum. Because studies report different numbers of strata, stratum-specific n values do not sum to overall n value. Taiwan and Hong Kong are not officially WHO member states. For this analysis, we regarded Taiwan a low-mortality developing location, and Hong Kong a developed location. HMD=high-mortality developing. LMD=low-mortality developing.
In the 20 studies that included data for both cases of acute gastroenteritis and a healthy control group, norovirus was detected in 20% (16–24, heterogeneity p<0·001) of cases of acute gastroenteritis and 7% (3–10; heterogeneity p<0·001) of controls. In this small subset, we found no significant difference in prevalence amongst controls by age, setting, development index, or strain-year (figure 3).
Of the 175 unique studies, 79 did not provide a clear definition of acute gastroenteritis. Of these, prevalence of norovirus was 17% (95% CI 15–19; heterogeneity p<0·0001). Norovirus can cause vomiting without diarrhoea. Of the studies that provided a clear definition of acute gastroenteritis, 64 did not allow vomiting-only in their definition of acute gastroenteritis; these studies had a norovirus prevalence of 20% (17–23; heterogeneity p<0·0001). The remaining 32 studies with a reported definition of acute gastroenteritis that did allow vomiting-only for enrolment (diarrhoea not a requirement) had a norovirus prevalence of 19% (15–23; heterogeneity p<0·0001). When analysis was restricted to studies that lasted 1 year, the overall norovirus prevalence estimate did not markedly change (17%, 5–20; heterogeneity p<0·001), n=59 studies).
Discussion
The rapidly expanding scientific literature on norovirus shows that this pathogen contributes substantially to the global burden of acute gastroenteritis across all settings and age groups. Our systematic review and meta-analysis included more than five times as many studies from almost twice as many countries than did a similar review8 based on the data available less than 5 years ago. This increased body of evidence leads us to an updated and more precise estimate that norovirus is associated with 18% (95% CI 17–20) of all cases of acute gastroenteritis.
We noted a gradient of decreasing prevalence from community to outpatient to inpatient groups, which supports the notion that norovirus is a more common cause of mild acute gastroenteritis. However, although norovirus proportionally makes a greater contribution to acute gastroenteritis in community and outpatient settings, outcomes including hospitalisation and death arise, with roughly 70 000 and 800, respectively, every year in the USA.19,20 Thus, the sheer frequency of illness results in a large burden of severe disease.
Norovirus was found in a lower proportion of cases of acute gastroenteritis in high-mortality countries (about 14%), than in lower mortality settings (about 20%). This finding should not be interpreted as norovirus causing a smaller burden in these settings. Rather, overall incidence of acute gastroenteritis is increased because of a wider diversity of bacterial and parasitic pathogens in low-income settings, so that among cases of acute gastroenteritis, norovirus might be less prevalent. Thus, low prevalence in low-income settings might suggest a more prominent role for other pathogens that are largely controlled through water and sanitation improvements in high-income settings. Few studies have been done in high-mortality settings, and more data from these areas would help improve our understanding of the precise role of norovirus. Our findings are broadly consistent with those of Lanata and colleagues,21 which showed that norovirus was associated with 13·8% (11·8–17·6) of children younger than 5 years needing hospitalisation, behind only rotavirus (38·3%) and enteropathogenic Escherichia coli (15·3%). In some countries that have introduced universal rotavirus vaccination, such as the USA, norovirus is the most common cause of medically-attended gastroenteritis in children.22,23
Norovirus prevalence was similar in studies that included pandemic years (2002–03 and 2006–07) compared to those that did not. Novel variants of GII.4 viruses emerged during these periods and increases in outbreak activity have been recognised in many countries.13,14 This part of our analysis was limited in a number of ways. We used a non-specific outcome (norovirus cases amongst all cases of gastroenteritis) and a non-specific categorisation, whereby all studies that included the 2002–03, or 2006–07, winters were considered pandemic.
Noroviruses can commonly be detected in healthy controls.24–26 Of the 20 studies included in our analysis that tested a control group, norovirus could be detected in about 7% of healthy individuals, less than half the detection rate in corresponding case groups. Possible reasons for presence of virus in stools of individuals without diarrhoea include long-term shedding from a previous symptomatic episode, true asymptomatic infection, and transient passage of ingested non-replicating virus through the gut.27 Burden of disease studies should carefully consider attribution in view of detection in controls. The recent Global Enteric Multicenter Study,24 the largest systematic assessment of the cause of childhood diarrhoea in developing countries, generally noted norovirus in controls at similar frequency as in cases of moderate to severe diarrhoea. These findings are not consistent with those of our Article in which case prevalence was higher than control prevalence. Additionally, we posit that simple subtraction or ratios of prevalence in cases and controls might underestimate the role of norovirus, especially in settings where exposures and reinfection arise frequently.28
This systematic review has some important limitations. First, age grouping was highly variable in published reports, which made it difficult to categorise study populations into finite age categories. We were restricted to two broad age groups stratified at age 5 years, and were unable to estimate prevalence separately in older children, younger adults, and elderly people. We tried an alternative age stratification scheme and noted similar results (appendix). We would have liked to quantify the role of norovirus in older ages (eg, >65 years); however, few studies reported data specifically for this age category. Second, some individuals with norovirus do not have diarrhoea and present with vomiting only, with or without other symptoms such as fever or abdominal cramps. However, most studies included in this Article did not capture vomiting-only cases or did not provide a definition of acute gastroenteritis (143 of 175 [82%] studies). Therefore, extrapolations from the burden of diarrhoeal disease to number of norovirus cases probably underestimates the burden of norovirus because this method would not account for vomiting-only norovirus cases. Third, categorisation of studies on the basis of three mortality strata probably does not fully capture the variation of water and sanitation access, health-care availability, and local epidemiology. The fourth limitation is general publication bias and incomplete geographical representation. Although this Article includes many more locations than included by Patel and colleagues,8high-mortality developing countries are still underrepresented (26 of 175 studies). A clear data gap remains for norovirus prevalence in developing country settings; high quality studies in those settings are crucial to improve burden of disease estimates. Fifth, the emergence of new GII.4 variants has prompted more intense study, so the pandemic years of 2002–03 and 2006–07 might be over-represented, potentially leading to a small overestimate of the overall prevalence. Finally, the Q statistic for all models was highly significant, suggesting that other factors that we did not consider might affect norovirus prevalence.
In conclusion, the findings of this systematic review show that norovirus is a key pathogen worldwide, causing both mild and severe acute gastroenteritis across age ranges. Partly because of diagnostic challenges, norovirus has not been included in some key burden of disease studies.1,2 The prevalence estimates from this study can be used to develop estimates of the global burden of norovirus gastroenteritis, including foodborne disease. To that end, studies are needed that consider norovirus in the context of other key enteric pathogens (eg, like Lanata and colleagues21) and that attribute to specific modes of transmission, including foodborne disease. Other data sources, including outbreak surveillance risk-factor studies and expert solicitation, will be needed. This knowledge could be used to target future interventions and inform vaccine development.29
Supplementary Material
Acknowledgments
This work was funded by the Foodborne Disease Burden Epidemiology Reference Group (FERG) of the WHO, and by the Government of the Netherlands on behalf of FERG. Members of the FERG Task Force reviewed this work before publication, but the final document was submitted at the sole discretion of the authors. We thank Marieke Bierhoff and Molly Steele for their assistance with the systematic literature review. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Sharia M Ahmed, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Aron J Hall, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Anne E Robinson, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Linda Verhoef, National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands.
Prasanna Premkumar, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Umesh D Parashar, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Marion Koopmans, National Institute for Public Health and the Environment (RIVM), Bilthoven, Netherlands; Erasmus University Medical Center Rotterdam, Rotterdam, Netherlands.
Benjamin A Lopman, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Rollins School of Public Health, Emory University, Atlanta, GA, USA.
References
- 1.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–223. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PloS One 2012; 7: e29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–61. [DOI] [PubMed] [Google Scholar]
- 5.de Wit MA, Koopmans MP, Kortbeek LM, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol 2001; 154: 666–74. [DOI] [PubMed] [Google Scholar]
- 6.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health 2012; 12: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 2008; 14: 1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho EC, Cheng PK, Wong DA, Lau AW, Lim WW. Correlation of norovirus variants with epidemics of acute viral gastroenteritis in Hong Kong. J Med Virol 2006; 78: 1473–79. [DOI] [PubMed] [Google Scholar]
- 10.Bull RA, Eden JS, Rawlinson WD, White PA. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog 2010; 6: e1000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debbink K, Lindesmith LC, Donaldson EF, et al. Emergence of new pandemic GII.4 Sydney norovirus strain correlates with escape from herd immunity. J Infect Dis 2013; 208: 1877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu S, Regev D, Watanabe M, et al. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog 2013; 9: e1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 2004; 363: 682–88. [DOI] [PubMed] [Google Scholar]
- 14.Siebenga JJ, Vennema H, Zheng DP, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis 2009; 200: 802–12. [DOI] [PubMed] [Google Scholar]
- 15.Kroneman A, Vega E, Vennema H, et al. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 2013; 158: 2059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaglehole R, Alec I, Prentice T. The world health report 2003: shaping the future. Geneva: World Health Organization, 2003. [Google Scholar]
- 18.Debbink K, Lindesmith LC, Donaldson EF, Baric RS. Norovirus immunity and the great escape. PLoS Pathog 2012; 8: e1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai R, Hembree CD, Handel A, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis 2012; 55: 189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19: 1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8: e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo HL, Neill FH, Estes MK, et al. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J Pediatric Infect Dis Soc 2013; 2: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne DC, Vinje J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in US children. N Engl J Med 2013; 368: 1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382: 209–22. [DOI] [PubMed] [Google Scholar]
- 25.Trainor E, Lopman B, Iturriza-Gomara M, et al. Detection and molecular characterisation of noroviruses in hospitalised children in Malawi, 1997–2007. J Med Virol 2013; 85: 1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur J Clin Microbiol Infect Dis 2007; 26: 311–23. [DOI] [PubMed] [Google Scholar]
- 27.Levine MM, Robins-Browne RM. Factors that explain excretion of enteric pathogens by persons without diarrhea. Clin Infect Dis 2012; 55: 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopman B, Simmons K, Gambhir M, Vinje J, Parashar U. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol 2014; 179: 507–12. [DOI] [PubMed] [Google Scholar]
- 29.Richardson C, Bargatze RF, Goodwin R, Mendelman PM. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines 2013; 12: 155–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


