Abstract
Background:
Noroviruses are increasingly recognized as a major cause of sporadic and epidemic acute gastroenteritis (AGE). Although there have been multiple studies published on norovirus epidemiology in Latin America, no comprehensive assessment of the role of norovirus has been conducted in the region. We aim to estimate the role of norovirus in the Latin American region through a systematic review and meta-analysis of the existing literature.
Methods:
We carried out a literature search in MEDLINE, SciELO and LILACS. We included papers that provided information on the prevalence or incidence of norovirus (including seroprevalence studies and outbreaks), with a recruitment and/or follow-up period of at least 12 months and where the diagnosis of norovirus was confirmed by reverse transcription polymerase chain reaction. The data were pooled for meta-analysis to estimate the prevalence of norovirus AGE and norovirus asymptomatic infection with 95% confidence intervals (CIs).
Results:
Thirty-eight studies were included in the review. Overall, the prevalence of norovirus among AGE cases was 15% (95% CI: 13–18). By location, it was 15% in the community (95% CI: 11%–21%), 14% in outpatient settings (95% CI: 10%–19%) and 16% in hospital locations (95% CI: 12%–21%). The prevalence of norovirus among asymptomatic subjects was 8% (95% CI: 4–13). Norovirus GII.4 strains were associated with 37%–100% of norovirus AGE cases, but only 7% of norovirus asymptomatic detections.
Conclusions:
Noroviruses are associated with almost 1 out of every 6 hospitalizations because of acute diarrhea in children younger than 5 years of age in Latin America.
Keywords: norovirus, human caliciviruses, acute gastroenteritis, Latin America, diarrheal disease
Despite recent advances, infectious acute gastroenteritis (AGE) continues to be an important cause of morbidity and mortality among children less than 5 years of age. An estimated 1.7 billion AGE episodes occurred in 2010, of which 36 million progressed to severe disease, causing 700,000 deaths.1 AGE remains the second leading cause of death in this age group after pneumonia.1 Greater than half of severe AGE episodes in children less than 5 years of age can be attributed to rotavirus, enteropathogenic Escherichia coli, human caliciviruses (which includes norovirus and sapovirus) and enterotoxigenic E.coli.2
Noroviruses, part of the Caliciviridae family, are increasingly recognized as a major cause of sporadic cases and outbreaks of AGE in all age groups. A recent review estimated that 24% of all community AGE cases, 20% of those attending outpatient clinics with AGE and 17% of those hospitalized for AGE are associated with norovirus.3 In the US alone, it is estimated that 20 million cases occur every year resulting in 71,000 hospitalizations, 400,000 emergency room visits and 1.8 million primary care visits.4
Noroviruses were the leading cause of AGE requiring hospitalization after rotavirus in children.5–8 However, in countries that have introduced rotavirus vaccines, noroviruses are becoming the leading cause of hospitalization for AGE in the pediatric population.9–11 Asymptomatic infection also occurs and noroviruses can be shed for weeks following infection, both in symptomatic and asymptomatic subjects.12 While asymptomatic infections are relatively common,13 their significance and importance in transmission are uncertain.
Although there have been several studies on norovirus in different Latin American countries, there has not been a systematic review conducted to estimate its role regionally. Accordingly, we conducted a systematic literature review and meta-analysis to estimate the role of norovirus infection in Latin America. Specifically, we aimed to describe the prevalence of norovirus AGE, the prevalence of asymptomatic norovirus infection, the incidence of norovirus AGE, the seroprevalence of norovirus infection and the prevalence of norovirus associated with AGE outbreaks.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
We carried out a literature search in MEDLINE (via PubMed), SciELO and LILACS using the terms norovirus* OR calicivirus* AND “country.” We carried out the search individually for each Latin American country following the list of Pan American Health Organization membership nations.14 There were no language limitations or other filters applied to the search. We applied the following inclusion criteria:
The study must include primary data.
- The study must provide data on at least one of the following:
- Prevalence of norovirus infection among AGE cases, defined as the number of AGE cases that have a stool sample positive for norovirus by RT-polymerase chain reaction (RT-PCR) divided by the total number of AGE cases tested.
- Incidence of norovirus-associated AGE, defined as the number of AGE cases that have a stool sample positive for norovirus by RT-PCR in a defined time period and population divided by the total population initially at risk (or by the sum of the person time of observation contributed by each population subject).
- Prevalence of norovirus infection among asymptomatic subjects, defined as the number of subjects without AGE symptoms that present a stool sample positive for norovirus by RT-PCR.
- Seroprevalence of norovirus infection, defined as the number of subjects with detectable norovirus antibodies in their serum divided by the total number of subjects tested.
- Prevalence of norovirus AGE outbreaks, defined as the number of AGE outbreaks attributed to norovirus in a defined area or region, divided by the total number of AGE outbreaks in the area or region under surveillance.
Recruitment or follow-up of at least 12 months (not applicable for seroprevalence studies).
We excluded the following: (1) studies that did not report the total number of patients with AGE, the number of norovirus-positive cases or percentages that allowed these raw numbers to be calculated; (2) studies where only samples negative for other pathogens were tested for norovirus and did not provide the total number of samples tested; (3) studies providing data on the prevalence of norovirus AGE outbreaks, which did not provide the total number of AGE outbreaks in the region or area under surveillance and (4) studies for which we could not obtain the full text electronically. Study selection and data extraction were carried out by a single reviewer.
Data Extraction and Bias Assessment
Data were extracted and stored in an MS Excel grid, purposefully developed for this review. The following data were extracted: first author, title, journal, year of publication, country, start and end year of data collection, study design, case definition of AGE, diagnostic method used, sample size (number of cases tested), number of cases positive for norovirus (by genotype when available), number of asymptomatic subjects tested (if included), number of asymptomatic subjects positive for norovirus, age and location. In addition, information was collected on the rotavirus vaccine introduction status of the country at the time of the data collection.15 When available, the data were stratified by age group and location for those studies reporting data for more than one age group and/or location, as well as by rotavirus vaccine introduction status.
All norovirus-positive samples were included even in the presence of other pathogens to avoid introducing a bias when comparing with studies that tested only for norovirus and in which coinfection with other pathogens was not assessed. For studies that assessed norovirus only among samples negative for other pathogens, we considered the total number of samples tested as denominator.
We stratified data into 4 different locations as a proxy for severity: community, outpatient, hospital (including emergency department visits) and other/not specified (for studies that did not specify location or where data were given for more than one location without stratification or other locations, eg, day care centers). Where possible, we also stratified the data by norovirus genotype.
Meta-analysis
A meta-analysis was performed for the prevalence of norovirus AGE and for the prevalence of asymptomatic norovirus infection. We decided not to perform a meta-analysis for the other outcomes given the low number of studies available and/or the heterogeneity of study methods and outcomes/summary statistics reported between studies.
The meta-analysis was stratified where possible by location, age group, genotype and rotavirus vaccine introduction status. We generated pooled estimates of the prevalence of norovirus AGE and asymptomatic infection with 95% confidence intervals (CIs) by fitting random-effects meta-analysis regression models using the restricted maximum likelihood method. We used the Freeman–Tukey double arcsine transformation for normalization and variance stabilization,16 and back-transformation of the estimated average using the harmonic mean of the sample sizes.17 We calculated the I2 statistic as a measure of the proportion of the overall variation that was attributable to between-study heterogeneity. All analyses were conducted with the statistical software R version 3.2.0 (R Core team, Vienna, Austria) using the metafor package.18,19
RESULTS
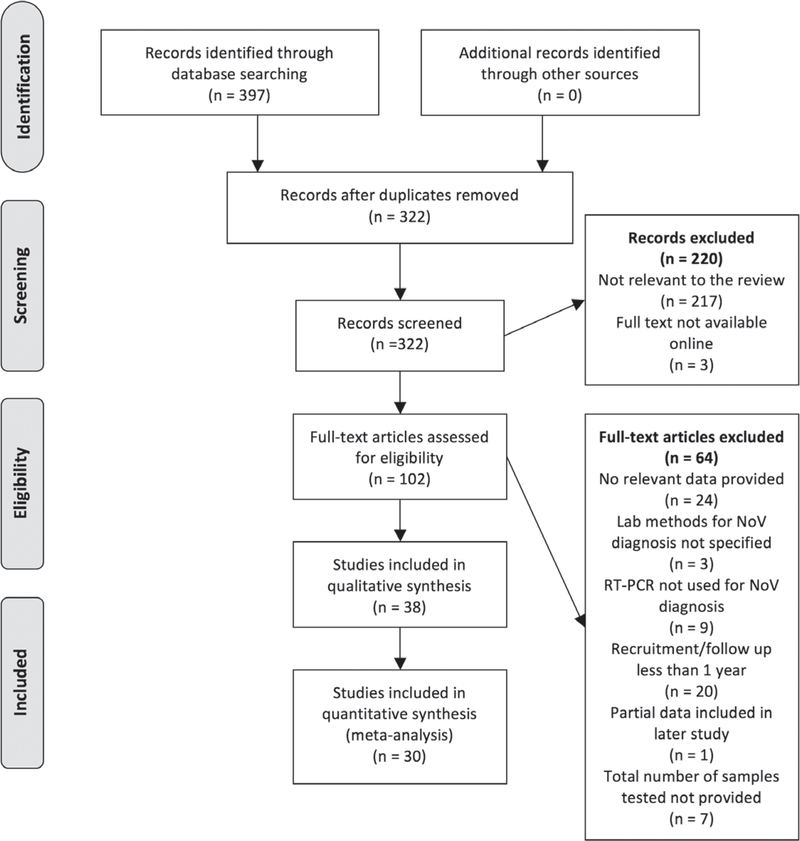
We carried out the database search on March 17, 2015, identifying 397 records. After duplicates were removed, we were left with 322 unique records for screening. We excluded 217 records after title and abstract screening for not being related to the purpose of this review. A further 3 records were excluded because it was not possible to obtain the full text online. We assessed 102 full text articles for eligibility, of which 38 were selected to be included in the review (Fig. 1). Of these, 30 were included in the meta-analysis.
FIGURE 1.
Study selection flow diagram (PRISMA). PRISMA indicates Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses.
Among the 38 articles selected, 29 provided data on the prevalence of norovirus AGE, 3 provided data on the incidence of norovirus AGE, 13 on the prevalence of asymptomatic norovirus infection, 6 on seroprevalence and 2 on outbreaks. The study characteristics and references are presented in the Table (Supplemental Digital Content 1, http://links.lww.com/INF/C552).
Prevalence of Norovirus Infection Among AGE Cases
We identified 29 studies providing data on the prevalence of norovirus disease from 11 countries. The data collection of these studies took place between 1989 and 2012, with most of the data collected in the period 2004–2012. All studies except for one provided data only for children, with most studies focusing in children less than 5 years of age. Overall, the prevalence of norovirus AGE ranged from 2% (A7) to 36% (A4). By location, the prevalence of norovirus AGE ranged from 7% (A19) to 23% (A23) in the community (N = 8), 7% (A6) to 24% (A5) in outpatient locations (N = 7) and 2% (A7) to 36% (A4) in hospital locations (N = 19) (Table 1). (Nota bene: N refers to the number of studies.)
TABLE 1.
Prevalence of Norovirus Infection Among AGE Cases in Latin America per Location and Age Group
| Location | Age Group | Number of Studies | Sample Size | Prevalence Range (%) | References |
|---|---|---|---|---|---|
| All locations | <6 mo | 6 | 758 | 7–25 | A2, A9, A11, A15, A25, A30 |
| 6–11 mo | 9 | 1198 | 9–39 | A2, A9, A11, A15, A21, A24, A25, A27, A30 | |
| 12–23 mo | 7 | 1061 | 6–40 | A2, A9, A11, A15, A25, A27, A30 | |
| 24–59 mo | 9 | 923 | 0–20 | A2, A9, A11, A15, A21, A24, A25, A27, A30 | |
| >59 mo | 1 | 14 | 14 | A24 | |
| All ages | 29 | 14,781 | 2–36 | A1–A15, A17–A30 | |
| Community | <12 mo | 3 | 101 | 21–35 | A21, A24, A27 |
| 12–23 mo | 1 | 102 | 23 | A27 | |
| 24–59 mo | 3 | 390 | 3–20 | A21, A24, A27 | |
| >59 mo | 1 | 14 | 14 | A24 | |
| All ages | 8 | 3606 | 7–23 | A1, A12, A19, A21, A23, A24, A26, A27 | |
| Outpatient | <6 mo | 2 | 130 | 14–25 | A15, A25 |
| 6–11 mo | 2 | 208 | 15–29 | A15, A25 | |
| 12–23 mo | 2 | 248 | 12–23 | A15, A25 | |
| 24–59 mo | 2 | 153 | 4–14 | A15, A25 | |
| >59 mo | - | - | - | ||
| All ages | 7 | 3283 | 7–24 | A5, A6, A15, A17, A18, A22, A25 | |
| Hospital | <6 mo | 6 | 630 | 8–25 | A2, A9, A11, A15, A25, A30 |
| 6–11 mo | 6 | 889 | 9–39 | A2, A9, A11, A15, A25, A30 | |
| 12–23 mo | 6 | 711 | 6–40 | A2, A9, A11, A15, A25, A30 | |
| 24–59 mo | 6 | 380 | 0–14 | A2, A9, A11, A15, A25, A30 | |
| >59 mo | - | - | - | ||
| All ages | 19 | 8055 | 2–36 | A2–A4, A6–A11, A13–A15, A18, A20, A22, A25, A28, A29, A30 | |
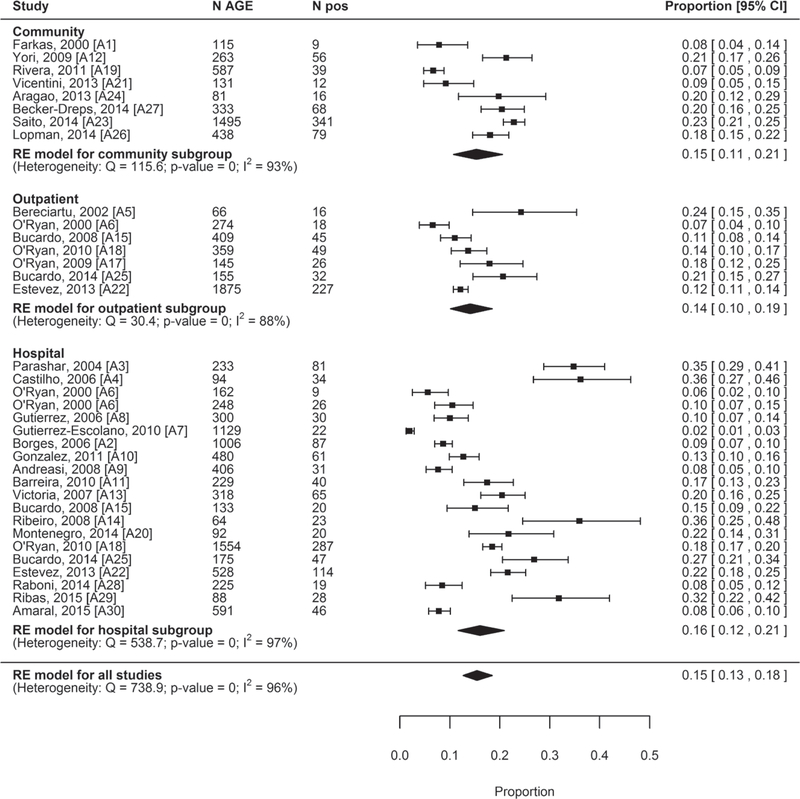
All 29 studies were included in the meta-analysis to estimate the pooled prevalence of norovirus infection among AGE cases. The overall pooled prevalence was 15% (95% CI: 13%–18%). By location, it was 15% in the community (95% CI: 11%–21%); 14% in outpatient locations (95% CI: 10%–19%) and 16% in hospital locations (95% CI: 12%–21%) (Table 2; Fig. 2).
TABLE 2.
Meta-analysis Results
| Number of Studies |
Mean Prevalence (95% CI) |
|||||
|---|---|---|---|---|---|---|
| Location | Total | Pre-RV Vaccine | Post-RV Vaccine | All | Pre-RV Vaccine | Post-RV Vaccine |
| Overall | 29 | 21 | 8 | 15% (13–18) | 15% (12–19) | 16% (12–22) |
| Community | 8 | 3 | 5 | 15% (11–21) | 11% (4–21) | 18% (14–23) |
| Outpatient | 7 | 6 | 1 | 14% (10–19) | 13% (9–17) | 21% (15–27) |
| Hospital | 19 | 16 | 3 | 16% (12–21) | 17% (12–22) | 13% (4–26) |
Pooled estimates of norovirus infection prevalence among AGE cases overall, per location and per rotavirus vaccine introduction.
RV indicates rotavirus.
FIGURE 2.
Forest plots of the proportion of AGE cases positive for norovirus stratified by location (community, outpatient and hospital), ordered by last year of data collection.
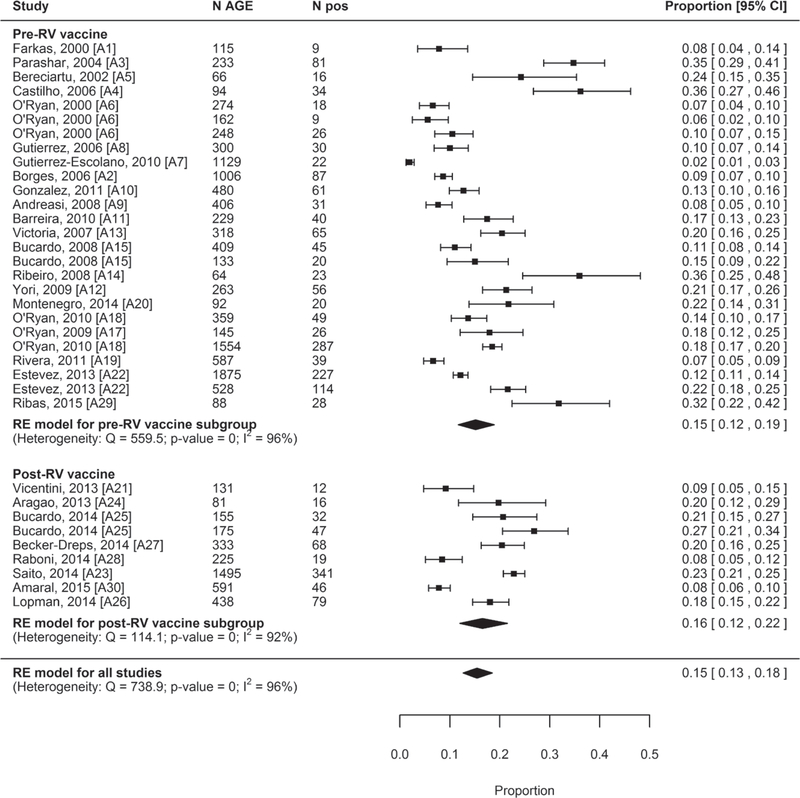
The pooled prevalence was 15% (95% CI: 12–19) for studies from countries that had not introduced rotavirus vaccination at the time of data collection (N = 21), and 16% (95% CI: 12–22) for studies from countries that had already introduced rotavirus vaccination at the time of data collection (N = 8) (Fig. 3).
FIGURE 3.
Forest plots of the proportion of AGE cases positive for norovirus, overall and stratified by RV vaccine introduction, ordered by last year of data collection. RV indicates rotavirus.
Fourteen of the studies provided genogroup and/or genotype information. Overall, GI noroviruses were found in 0%–25% of all cases (A4, A7, A10, A11, A13, A15, A21–A28), whereas GII noroviruses were found in 75%–100% of all cases (A4, A7, A10, A11, A13, A15, A21–A28). GII.4 strains were identified in 37%–100% of all cases (N = 13) (A4, A7, A10, A11, A13, A15, A21–A26, A28).
Incidence of Norovirus-associated AGE
Three studies from 3 different countries provided data on the incidence of norovirus disease. The data collection took place between 2007 and 2012. All these studies were carried out in children younger than 5 years of age in community locations. The overall norovirus AGE incidence in these studies ranged from 17 to 23 cases of norovirus disease per 100 person-years (A26, A27) (see Table 3).
TABLE 3.
Incidence of Norovirus Infection in the Community in Latin America
Prevalence of Norovirus Infection Among Asymptomatic Subjects
We found 13 studies providing data on the prevalence of asymptomatic norovirus infection from 6 countries, with data collected between 1989 and 2012. All studies were conducted in children younger than 15 years of age. The prevalence in the different studies ranged from 0% (A6, A24) to 36.4% (A4). By location, the prevalence of asymptomatic infection ranged from 0% (A24) to 18% (A26) in the community (N = 8) and from 0% (A6) to 36.4% (A4) in hospital locations (N = 5) (Table 4). Only 2 studies provided data on genotypes, reporting norovirus GII in 67%–100% of the infections (A11, A16). Among norovirus GII infections, over 90% were caused by non-GII.4 genotypes (A11, A16).
TABLE 4.
Prevalence of Asymptomatic Norovirus Infection in Latin America Overall and by Location
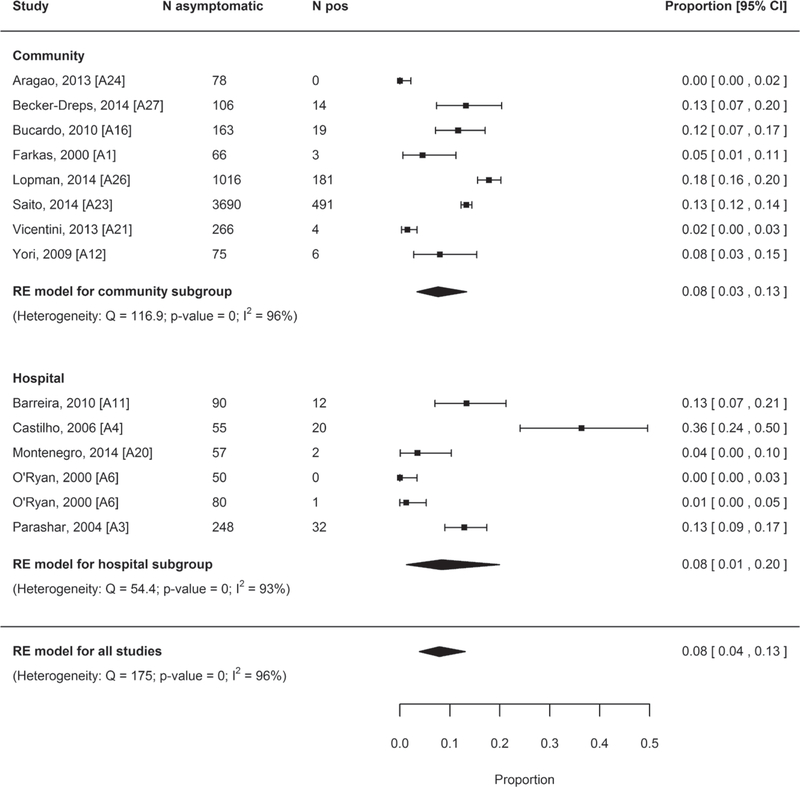
The 13 studies were included in the meta-analysis. The pooled prevalence of norovirus infection among asymptomatic subjects was 8% (95% CI: 4–13) (Fig. 4). The mean prevalence was 8% (95% CI: 3–15) for studies carried out before rotavirus vaccine introduction (N = 8) and 7% (95% CI: 1–17) for studies carried out after the introduction (N = 5).
FIGURE 4.
Forest plots of the proportion of asymptomatic individuals positive for norovirus, overall and stratified by RV vaccine introduction. RV indicates rotavirus.
Seroprevalence of Norovirus Infection
Six studies provided data on seroprevalence of norovirus infection from 4 countries, with data collected between 1970 and 2002. These studies tested for GI.1 antibodies, GII.3 antibodies or both. Three studies were conducted in children younger than 5 years of age, one included adults up to 45 years, and two included all ages. One study did not specify the genotype/s that were tested for. The seroprevalence of norovirus infection overall ranged from 2% to 87% (N = 6) (A31–A36). For GI.1, it ranged from 2% to 77% (N = 4) (A31–A33, A35) and for GII.3 from 16% to 57% (N = 2) (A33, A34). Age group specific data are presented in Table 5.
TABLE 5.
Seroprevalence of Norovirus Infection in Latin America per Genotype and Age Group
| Genotype | Age Group (yr) | Number of Studies | Sample Size | Seroprevalence Range (%) | References |
|---|---|---|---|---|---|
| Overall | All | 6 | 3550 | 2–87 | A31–A36 |
| GI.1 | 0–5 | 3 | 930 | 71–75 | A31, A32, A35 |
| 6–10 | 1 | 54 | 87 | A31 | |
| 11–20 | 1 | 123 | 70 | A31 | |
| 21–30 | 1 | 116 | 72 | A31 | |
| 30+ | 1 | 127 | 85 | A31 | |
| Total | 4 | 1380 | 2–77 | A31, A32, A33, A35 | |
| GII.3 | 1–4 | 1 | 133 | 34 | A34 |
| 5–14 | 1 | 261 | 56 | A34 | |
| 15+ | 1 | 111 | 81 | A34 | |
| Total | 2 | 2359 | 16–57 | A33, A34 | |
| NS | <2 | 1 | 52 | 58 | A36 |
NS indicates not specified.
Prevalence of Norovirus AGE Outbreaks
We identified 2 studies providing data on the prevalence of norovirus AGE outbreaks from 2 countries, with data collection between 2000 and 2009. The prevalence of norovirus among AGE outbreaks reported was 4 and 45% (A37–A38).
DISCUSSION
In this review and meta-analysis, we report the first systematic synthesis of epidemiologic studies of norovirus for the Latin American region. We found norovirus to be a major cause of AGE in Latin America, associated with 15% (95% CI: 13–18) of all cases.
The overall prevalence of norovirus infection among AGE cases in Latin America is in line with a recent global systematic review and meta-analysis of noroviruses, which found noroviruses to be associated with 18% of AGE cases.3 While the general design of our review is similar to the one by Ahmed et al,3 there are some important differences: we did not stratify per development index or strain year, and the scope of our review is wider, including not only the prevalence of norovirus but also incidence, seroepidemiology and outbreaks. We also did not stratify in the meta-analysis by age group, because all studies published except for one included only pediatric population younger than 15 years of age. Recent studies from Finland, the US and Nicaragua have shown an increase in the prevalence of norovirus after the introduction of rotavirus vaccination in those countries.10,11,20 We did not see a pattern of increasing norovirus prevalence in locations with rotavirus vaccination. Only 2 studies provided data for rotavirus vaccination coverage in the study population. Even if rotavirus vaccine was introduced in the country, vaccination coverage may have been low in the study population and account for the lack of rotavirus vaccine impact on the prevalence of norovirus infection we observed. In addition, data collection for several of these studies were initiated only 1–2 years after rotavirus vaccine introduction, precluding a robust before/after comparison.
The community incidence of norovirus AGE among children less than 5 years of age ranged from 17 to 23 cases per 100 person-years. This is in line with estimates from the United Kingdom, of 21 per 100 person-years in the same age group.21 Norovirus asymptomatic infection is common among children in Latin America. We estimated the prevalence of norovirus asymptomatic infection at 8% (95% CI: 4–13); this is similar to the 7% (95% CI: 3–10) reported by Ahmed et al.3
The few seroprevalence studies available confirm that norovirus exposure is very common in Latin America, with up to 77% of the population exposed to GI.1 and up to 57% to GII.3. As expected, seroprevalence generally increased progressively with age as reported in other regions.22,23 These seroprevalence studies were performed decades ago, so the most common current serogroup circulating worldwide (GII.4) was not studied.
Noroviruses are the leading cause of outbreak-associated gastroenteritis worldwide, causing 50% of all-cause and more than 90% nonbacterial epidemic gastroenteritis.24 Only 2 studies provided data on the prevalence of norovirus infection among AGE outbreaks in Latin America with very different results. Surveillance data from Brazil found only 4% of all AGE outbreaks to be associated with norovirus, compared with 45% in Chile. This discrepancy could be explained by differences in the focus and coverage of the 2 surveillance systems, as it has been reported previously for European countries.25
Like in most studies elsewhere, GII was dominant compared with GI in Latin America, with GII associated with over 75% of norovirus AGE cases. GII.4 strains were associated with 37%–100% of all norovirus AGE cases. However, only 2 studies provided data for norovirus genotyping. Hoa Tran et al26 reported in a review of pediatric norovirus AGE that GII noroviruses were associated with 96% of cases, whereas GII.4 strains were associated with 70% of all norovirus AGE cases. In contrast, the majority of asymptomatic infections were caused by non-GII.4 strains in our review. The relevance of this may need further evaluation because the total number of samples tested was small (N = 22). Novel GII.4 variants emerge globally every 2–4 years, apparently in response to population immunity.27 Increases of norovirus disease activity have been reported from a number of countries, most clearly in March 2002 and July 2006, though it would be unlikely to detect such an impact in a nonsensitive literature review as conducted here, where studies typically covered many seasons.
Our study had several limitations. Although our initial objective was to evaluate the role of norovirus infection in Latin America for all ages, the lack of studies in adults largely limited our conclusions to the pediatric population. Only 1 study included subjects 15 years of age and older, and most restricted recruitment to children younger than 5 years of age. We found considerable heterogeneity in results of individual studies. This may reflect real differences in norovirus prevalence between different countries and/ or regions. However, results may have been influenced by a variety of factors including differences in laboratory testing, use of different AGE case definitions, use of different definitions of asymptomatic subject (healthy vs. non-AGE) and symptom-free period (varying from 2 weeks to 2 months), as well as differences in health care access and utilization between countries. Some studies only tested for norovirus among specimens negative for another pathogen, so coinfections may have been underestimated. In addition, we included all studies that provided data for 12 or more months, but without restricting to multiples of 12 months to ensure full year recruitments. This could have overestimated the prevalence of norovirus infection among AGE cases if winter months were over-represented, although we think this is unlikely since only 8 out of the 29 studies included had recruitments that were not multiples of 12 months. Among those, only 1 had recruitment duration below 24 months. Finally, screening and data extraction were carried out by a single reviewer. However, given the nature of this review where only descriptive data were considered (as opposed to comparative effectiveness reviews) with simple and straightforward eligibility criteria, we considered that the impact on the quality of the review would be minimal.
In conclusion, noroviruses are a leading cause of AGE in Latin America, in line to what has been reported globally. Noroviruses are associated with 15% of all AGE cases, and GII.4 strains predominate. Asymptomatic infection is common with an apparent predominance of non-GII.4 strains. Serologic studies point to universal exposure, but current serologic survey methods are insufficient to identify exposure to specific strains. More studies are needed to fully define the burden of norovirus infection in terms of total cases, medical care-seeking and attributable deaths in children and, especially, in adults, for whom data are very scarce. AGE outbreak reporting with norovirus diagnostics, including molecular epidemiology, is also lacking for many countries in the region.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Kaat Bollaerts and Dr. Tom Cattaert for their support with data analysis.
M.O. has received funding from Takeda for ongoing norovirus epidemiologic studies in Chile, not included in this review, and has been a consultant for norovirus vaccine development. M.R.M. has received consulting fees from Takeda, not related to this review. The authors have no conflicts of interest to disclose.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, or the US Department of Health and Human Services.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiegering V, Kaiser J, Tappe D, et al. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15:e401–e407. [DOI] [PubMed] [Google Scholar]
- 6.Tran A, Talmud D, Lejeune B, et al. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010;48:1943–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junquera CG, de Baranda CS, Mialdea OG, et al. Prevalence and clinical characteristics of norovirus gastroenteritis among hospitalized children in Spain. Pediatr Infect Dis J. 2009;28:604–607. [DOI] [PubMed] [Google Scholar]
- 8.Räsänen S, Lappalainen S, Salminen M, et al. Noroviruses in children seen in a hospital for acute gastroenteritis in Finland. Eur J Pediatr. 2011;170:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010;48:1673–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemming M, Räsänen S, Huhti L, et al. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockx B, De Wit M, Vennema H, et al. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis. 2002;35:246–253. [DOI] [PubMed] [Google Scholar]
- 13.Phillips G, Tam CC, Rodrigues LC, et al. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiol Infect. 2010;138:1454–1458. [DOI] [PubMed] [Google Scholar]
- 14.Pan American Health Organization (PAHO). Countries and centres. Available at http://www.paho.org/hq/index.php?option=com_wrapper&view=wrapper&Itemid=2005&lang=en. Accessed March 17, 2015.
- 15.PATH. Rotavirus vaccine access and delivery - Country introduction maps and spreadsheet. December 8, 2014. Avalaible at: http://sites.path.org/rotavirusvaccine/country-introduction-maps-and-spreadsheet/ - spreadsheet. Accessed March 17, 2015. [Google Scholar]
- 16.Freeman MF, Tukey JW. {Transformations related to the angular and the square root}. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 17.Miller JJ. The inverse of the Freeman – Tukey double arcsine transformation. Am Stat. 1978;32:138–138. [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing (2015). Vienna, Austria: R Foundation for Statistical Computing. Available at: http://www.r-project.org/. [Google Scholar]
- 19.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36:1–48. [Google Scholar]
- 20.Bucardo F, Reyes Y, Svensson L, et al. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS One. 2014;9:e98201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol. 2010;171:1014–1022. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S, Fujiwara N, Takeda N, et al. Seroepidemiological study of norovirus infection in Aichi Prefecture, Japan. Microbiol Immunol. 2009;53:356–359. [DOI] [PubMed] [Google Scholar]
- 23.Menon VK, George S, Aladin F, et al. Comparison of age-stratified seroprevalence of antibodies against norovirus GII in India and the United Kingdom. PLoS One. 2013;8:e56239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MM, Hall AJ, Vinjé J, et al. Noroviruses: a comprehensive review. J Clin Virol. 2009;44:1–8. [DOI] [PubMed] [Google Scholar]
- 25.Kroneman A, Verhoef L, Harris J, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol. 2008;46:2959–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoa Tran TN, Trainor E, Nakagomi T, et al. Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. J Clin Virol. 2013;56:185–193. [DOI] [PubMed] [Google Scholar]
- 27.Lindesmith LC, Beltramello M, Donaldson EF, et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 2012;8:e1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.