Abstract
Context.
Papillary thyroid carcinoma (PTC) is an uncommon tumor in the pediatric population. A limited number of studies have examined genetic mutations affecting the mitogen-activated protein kinase (MAPK) pathway in the pediatric population.
Objective.
To examine mutations affecting this pathway in PTC in our pediatric population and compare the BRAF V600E mutation rates in pediatric and adult tumors.
Design.
Eighty-four patients, including 14 pediatric and 70 adult, with PTC were tested for the BRAF V600E mutation by using real-time polymerase chain reaction and sequencing. Additionally, we examined the rate of RAS point mutations with real-time polymerase chain reaction and rearrangements of RET/PTC1 and RET/PTC3 in the pediatric group with fluorescence in situ hybridization. Clinical and histologic data were compared as well.
Results.
Of 77 tumors that had an interpretable result, the BRAF V600E mutant was identified in 4 of 13 pediatric patients (31%) and 43 of 64 adult patients (67%), which was a significant difference (using Fisher exact test, P = .03). One pediatric and 6 adult cases did not reveal an interpretable result with melting curve analysis. One of these cases harbored a rare 3–base pair deletion mutation (c.1799_1801delTGA). Mutations in RAS genes were not seen in any pediatric tumors. One tumor with a RET/PTC1 rearrangement and another with RET/PTC3 were identified in the pediatric population (15%).
Conclusions.
The rate of the BRAF V600E mutation in the pediatric population is significantly lower than that seen in the adult population. Mutations in RAS do not contribute significantly to pediatric PTC. This experience from our institution adds to the growing body of knowledge regarding tumor genetics in pediatric PTC.
Papillary thyroid carcinoma (PTC) is an uncommon tumor in the pediatric population. Despite having an excellent prognosis with long-term mortality rates of less than 1%,1,2 pediatric patients with PTC tend to present with advanced disease often with increased rates of extrathyroidal tumor extension, lymphovascular invasion, and distant metastases.1,3-5
The distinct clinical nature of papillary thyroid cancer in the pediatric population has prompted interest in the molecular tumorigenesis. The mitogen-activated protein kinase (MAPK) pathway includes components of particular interest, including BRAF, RAS, and RET. Between 37% and 52% of PTCs have been linked to the specific BRAF mutation V600E, and clinical correlations support that this foretells a more aggressive disease course in adults.6-9 The incidence of this BRAF mutation in the pediatric population is thought to be lower than in adults with rates of 0% to 37%,10-15 though 1 recent series16 revealed a rate of 63%. The rearrangement mutations of RET are also considered a major contributor to PTC tumorigenesis, but rates of this mutation have fluctuated widely in reported series.17 RET/PTC rearrangements have been associated with young age18 and radiation exposure.19,20 Mutations in RAS genes (HRAS, KRAS, and NRAS) characterize only 10% to 20% of PTCs and are seen in approximately 40% to 50% of follicular thyroid carcinomas.21 Several studies11,22-24 have suggested that mutation in RAS genes comprise a negligible portion, if any, of pediatric PTCs. Given the common signaling pathway of these oncogenes, constitutive activation in any one member has been considered a mutually exclusive event.25-27 However, more recent studies12,28 have identified that co-mutation does occur.
This study compares the incidence of BRAF V600E in PTC from our adult and pediatric populations. We concomitantly analyzed rates of RET/PTC rearrangements and RAS mutations in the pediatric population. The clinical and histopathologic characteristics of these tumors are compared as well.
PATIENTS AND METHODS
Patients and Chart Review
This study was approved by the University of Wisconsin Institutional Review Board. The specimen database from the University of Wisconsin Hospital and Clinics in Madison, Wisconsin, was retrospectively queried for thyroidectomy specimens with the diagnosis of ″papillary thyroid carcinoma″ made between January 1, 2008, and October 31, 2012. We excluded cases with primary (largest) tumor diameter below 0.4 cm and non-PTC. Two study groups were established: a pediatric group (age 18 years and younger) and an adult group (age greater than 18 years). We selected all pediatric cases from the query and compiled an adult group through random selection of resulted cases from the database query. Chart review was conducted of each patient to elucidate multiple clinical variables in addition to demographics. The presence of inflammatory thyroid disease, additional malignancies, and history of exposure to radiation was all determined. When evaluating for nonthyroid malignancy, we excluded cutaneous squamous cell carcinoma and basal cell carcinoma from data collection. Family history for thyroid and nonthyroid malignancy was evaluated and extended only to first-degree relatives. Staging and other prognostic parameters including tumor size, focality, extrathyroidal extension, lymphovascular invasion, lymph node metastases, and the presence of distant metastases were recorded.
Histopathology
Hematoxylin-eosin–stained slides of tumors within thyroidectomy specimens were reviewed by 2 of the authors (R.V.L. and R.J.G.). Each tumor was morphologically characterized. Follicular variant of PTC was designated in tumors harboring nuclear features of PTC with follicles encompassing more than 95% of the tumor. Tall cell variant was designated in tumors composed of more than 35% tall cells, containing eosinophilic cytoplasm and exhibiting a height greater than 2 times the width of the cell. Cases were otherwise categorized as ″classic″ if they had some degree of papillary formation and did not meet criteria for other morphologic types. Tumors were designated encapsulated if they had a noninfiltrated, fibrous capsule completely encircling them.
Molecular Analyses
Nucleic Acid Extraction and Polymerase Chain Reaction.—
Formalin-fixed, paraffin-embedded tissue was deparaffinized with vigorous vortexing in CitriSolv (2 × 1 mL; Fisher Scientific, Pittsburgh, Pennsylvania) and then in 100% ethanol (2 × 1 mL). Microdissection of tissue was used when tumors constituted less than 10% of the cross-sectional area of a chosen block or when substantial lymphocytic infiltrate was present. Tissue was dried briefly in a 56°C bead bath. Subsequent tissue digestion and DNA extraction were performed according to manufacturer’s specifications by using the MagNA Pure LC and the MagNA Pure LC DNA Isolation Kit II (Roche, Indianapolis, Indiana). Screening for BRAF V600E (c.1799G>A) was accomplished by real-time coamplification at lower denaturation temperature-polymerase chain reaction (COLD-PCR) followed by melting curve analysis on the LightCycler 2.0 (Roche) using fluorescent energy transfer probes. BRAF variants were verified by Sanger DNA sequencing using an ABI 3500 (LifeTechnologies, Carlsbad, California).
The DNA from pediatric tumors was also evaluated for RAS mutation as previously reported by using real-time LightCycler PCR (Roche) with melting curve analysis.29 Screened mutations included HRAS codon 61, NRAS codon 61, and KRAS codons 12 and 13.
Fluorescence In Situ Hybridization.—
Unstained slides were prepared from the tissue blocks used for DNA extraction. Tissue was assessed for the presence of common RET rearrangements by using fluorescence in situ hybridization (FISH). This procedure has previously been published.29 Screening examined the most common RET rearrangements, RET/PTC1 and RET/PTC3, by using dual-color interphase FISH.
Statistical Analyses
Comparison studies used the Wilcoxon rank sum test, χ2 test, and Fisher exact test. The significance threshold level for the P value was less than .05.
RESULTS
Patients
A total of 84 patients were identified for study, including 14 pediatric patients and 70 adult patients. Clinical characteristics of the patients are summarized in Table 1. The pediatric group had a mean age of 14 years (range, 8–18 years), and the adult group had a mean age of 48 years (range, 20–83 years). The entire group of patients was predominantly female (2.2:1) with the pediatric group having a similar rate (1.8:1) to the adult group (2.3:1). Twenty five of 70 adults (36%) had a family history of malignancy, while no pediatric patients had such history (P = .01). No significant differences in Graves disease or Hashimoto thyroiditis were detected. Radiation exposure history was noted in 5 of 70 adult patients (7.1%) and no pediatric patients, but this was not a significant difference (P = .59).
Table 1.
Clinical Characteristics
| Total | Pediatric | Adult | P Value | |
|---|---|---|---|---|
| Total cases | 84 | 14 | 70 | |
| Age | ||||
| Mean (standard deviation) | 42 (20) | 13 (3.4) | 48 (17.0) | |
| Median (interquartile range) | 41 (27–60) | 14 (12–17) | 48 (35–63) | |
| Sex (female/male) | 58/26 (2.2:1) | 9/5 (1.8:1) | 49/21 (2.3:1) | .76 |
| Graves thyroiditis, No. (%) | 7 (8.3) | 2 (14) | 5 (7.1) | .60 |
| Hashimoto thyroiditis, No. (%) | 11 (13) | 3 (21) | 8 (11) | .37 |
| Concurrent nonthyroid malignancy, No. (%) | 14 (17) | 1 (7.1) | 13 (19) | .45 |
| Family history nonthyroid malignancy, No. (%) | 25 (30) | 0 (0) | 25 (36) | .01 |
| Family history thyroid carcinoma, No. (%) | 2 (2.4) | 1 (7.1) | 1 (1.4) | .31 |
| Radiation exposure, No. (%) | 5 (6) | 0 (0) | 5 (7.1) | .59 |
Disease Characteristics
Disease characteristics are described in Table 2. Pediatric tumors were not significantly different from adult tumors with respect to size, rate of multifocality, or rates of extrathyroidal extension. Four of 14 pediatric tumors (29%) exhibited lymphovascular invasion as compared to 3 of 70 adult tumors (4.3%) (P = .01). Nine of 14 pediatric patients (64%) and 19 of 70 adult patients (27%) had positive lymph nodes detected (P = .01). Distant metastases were identified in none of the pediatric cases and 3 of 70 (4.3%) of the adult cases, a difference that was not significant (P > .99). An adult with concomitant small cell lung carcinoma died at 77 months of follow-up and was the only death in this study group. The average follow-up period was 32 months in the pediatric patient group and 31 months in the adult group. There were no differences found between pediatric and adult patients relative to histologic types (see Table 3).
Table 2.
Disease Characteristics
| Total | Pediatric | Adult | P Value | |
|---|---|---|---|---|
| Total cases | 84 | 14 | 70 | |
| Tumor diameter, cm | ||||
| Mean (standard deviation) | 2.2 (1.8) | 2.3 (2.0) | 2.2 (1.8) | .82 |
| Median (interquartile range) | 1.7 (1.0–2.8) | 1.9 (1.0–2.9) | 1.7 (1.0–2.5) | |
| Multifocal, No. (%) | 41 (49) | 7 (50) | 34 (49) | |
| Extrathyroidal extension, No. (%) | 14 (17) | 3 (21) | 11 (16) | .69 |
| Lymphatic/vascular invasion, No. (%) | 7 (8.3) | 4 (29) | 3 (4.3) | .01 |
| Positive lymph nodes, No. (%) | 28 (33) | 9 (64) | 19 (27) | .01 |
| Distant metastases, No. (%) | 3 (3.6) | 0 (0) | 3 (4.3) | >.99 |
| Follow-up period, mo | ||||
| Mean (standard deviation) | 31 (18) | 32 (19) | 31 (18) | .78 |
| Median (interquartile range) | 26 (19–43) | 32 (16–44) | 26 (20–41) | |
| Positive for BRAF V600E, No. (%) | 47 (61)a | 4 (31) | 43 (67) | .03 |
Percents shown among 77 tumors (13 pediatric and 64 adult patient tumors) with an interpretable result.
Table 3.
Histologic Patterns
| Histologic Types | Total | Pediatric | Adult |
|---|---|---|---|
| Total cases | 84 | 14 | 70 |
| Classic type, No. (%) | 59 (70) | 9 (64) | 50 (71) |
| Follicular variant, No. (%) | 16 (19) | 4 (29) | 12 (17) |
| Solid variant, No. (%) | 6 (7.1) | 0 (0) | 6 (8.6) |
| Diffuse sclerosing variant, No. (%) | 1 (1.2) | 1 (7.1) | 0 (0) |
| Tall cell variant, No. (%) | 1 (1.2) | 0 (0) | 1 (1.4) |
| Unclassifiable morphology, No. (%) | 1 (1.2) | 0 (0) | 1 (1.4) |
Mutation Analysis
The BRAF point mutation V600E was identified in 4 of 13 pediatric patient tumors, yielding a clear result on PCR (31%). This rate was significantly lower than in the adult population in which 43 tumors (67%, P= .03) were identified as positive for this mutation out of 64 that had an interpretable result. The pediatric cases are summarized in Table 4. A single pediatric result revealed an unusually low melting curve temperature. Subsequent sequencing of the BRAF region identified a 3—base pair deletion mutation (c.1799_1801delTGA). If this mutation is counted with the classic mutation (for a rate of 38%), the pediatric BRAF mutation rate is still significantly less than that of the adult population (P = .04). Within the individual groups, no associations were evident between BRAF mutation status and any of the clinical characteristics analyzed. Across both groups, patients with tumors positive for the V600E mutation were more likely to have a family history of cancer (P = .04), but this association was not independently significant when accounting for the older age in mutation-positive patients (P = .16). Patients who were mutation positive were less likely to have Hashimoto thyroiditis (P = .07) and had smaller tumor diameter (2.1 cm versus 2.6 cm, P = .08). Point mutations in RAS were not present in any of the pediatric patient tumors. Two rearrangements with RET were detected among 13 tumors (15%), one involving PTC1 and another involving PTC3. One of the 13 patient samples did not yield an interpretable result with FISH analysis. The patient with the RET/PTC3 rearrangement also harbored a BRAF V600E mutation.
Table 4.
Relationship Between Patient Clinicopathologic Characteristics and BRAF Mutation Status
| Total (N = 77) |
Pediatric (N = 13) |
Adult (N = 64) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BRAF V600E | − (%) | + (%) | P Value | − (%) | + (%) | P Value | − (%) | + (%) | P Value |
| Total cases, No. (%) | 30 (39) | 47 (61) | 9 (69) | 4 (31) | 21 (33) | 43 (67) | |||
| Age, mean (standard deviation), Range, y | 34 (20) | 46 (19) | .02 | 14 (3.7) | 14 (2.9) | >.99 | 43 (17) | 49 (16) | .25 |
| 8–75 | 10–83 | 8–18 | 10–17 | 20–75 | 20–83 | ||||
| Sex (male), No. (%) | − 19 (63) | 33 (70) | .53 | 6 (67) | 2 (50) | >.99 | 13 (62) | 31 (72) | .41 |
| + 11 (37) | 14 (30) | 3 (33) | 2 (50) | 8 (38) | 12 (28) | ||||
| Graves thyroiditis, No. (%) | − 28 (93) | 43 (92) | >.99 | 8 (89) | 4 (100) | >.99 | 20 (95) | 39 (91) | >.99 |
| + 2 (6.7) | 4 (8.5) | 1 (11) | 0 (0) | 1 (4.8) | 4 (9.3) | ||||
| Hashimoto thyroiditis, No. (%) | − 23 (77) | 43 (92) | .07 | 6 (67) | 4 (100) | .50 | 17 (81) | 39 (91) | .42 |
| + 7 (23) | 4 (8.5) | 3 (33) | 0 (0) | 4 (19) | 4 (9.3) | ||||
| Concurrent nonthyroid malignancy, No. (%) | − 26 (87) | 39 (83) | .76 | 8 (89) | 4 (100) | >.99 | 18 (86) | 35 (81) | >.99 |
| + 4 (13) | 8 (17) | 1 (11) | 0 (0) | 3 (14) | 8 (19) | ||||
| Family history nonthyroid malignancy, No. (%) | − 25 (83) | 29 (62) | .04 | 9 (69) | 4 (31) | >.99 | 16 (76) | 25 (58) | .16 |
| + 5 (17) | 18 (38) | 0 (0) | 0 (0) | 5 (24) | 18 (42) | ||||
| Family history thyroid carcinoma, No. (%) | − 30 (100) | 45 (96) | .52 | 9 (100) | 3 (75) | .31 | 21 (100) | 42 (98) | >.99 |
| + 0 (0) | 2 (4.3) | 0 (0) | 1 (25) | 0 (0) | 1 (2.3) | ||||
| Tumor size (standard deviation), cm | 2.6 (1.8) | 2.1 (1.9) | .08 | 2.7 (2.4) | 1.7 (1.2) | .70 | 2.6 (1.6) | 2.1 (1.9) | .10 |
| Extrathyroidal extension, No. (%) | − 26 (87) | 39 (83) | .76 | 7 (78) | 3 (75) | >.99 | 19 (91) | 36 (84) | .71 |
| + 4 (13) | 8 (17) | 2 (22) | 1 (25) | 2 (9.5) | 7 (16) | ||||
| Lymphatic-vascular invasion, No. (%) | − 26 (87) | 45 (96) | .20 | 6 (67) | 3 (75) | >.99 | 20 (95) | 42 (98) | >.99 |
| + 4 (13) | 2 (4.3) | 3 (33) | 1 (25) | 1 (4.8) | 1 (2.3) | ||||
Abbreviations: +, presence of given variable or condition; −, absence of given variable or condition.
Among the 77 patients in the study with a definitive BRAF mutation status, the classic type of carcinoma was associated with BRAF V600E mutation with 43 of 47 mutation-positive tumors (91%) displaying a classic-type morphology, while only 2 of 47 mutation-positive tumors (4%) displayed a follicular variant morphology, a difference that was significant (P < .001). The presence of BRAF V600E mutation, among both adults and children, was not associated with increased tumor size, rate of lymphovascular invasion, rate of lymph node positivity, or extrathyroidal extension (see Table 4).
COMMENT
The results of this study illustrate that while the BRAF V600E mutation is present in a substantial portion of adult PTCs, it is significantly less prevalent in PTC from pediatric patients. The rate of 31% in the pediatric patients in this study is within the range reported among most similar studies, but is somewhat on the high side (see Table 5). The relationship of BRAF mutation and age is apparent even within the adult group. This mutation is seen in 8 of 17 younger adults (47%; 19–35 years of age) and in 35 of 47 middle-aged and elderly adults (74%; 36–83 years of age) (P = .03). A potential bias exists when compared to the pediatric PTC rate from other studies in that the COLD-PCR methodology used here is a fairly sensitive assay. Our assay limit of detection is at least 5% of mutant copies in a sample of extracted DNA from paraffin-embedded tissue.
Table 5.
Summary of Studies Examining BRAF Mutation in Pediatric Papillary Thyroid Carcinoma
| Source, y | Patient Population |
Patient Age, y |
BRAF Prevalence, No. (%) |
Detection Method |
Mutation-Associated Clinical and Disease Characteristics in Pediatric Patients |
|---|---|---|---|---|---|
| Nikiforova et al,15 2004 | Belarusian, post-Chernobyl | 6–20 | 0/34 (0) | PCR with FMCA | Not examined |
| Ukrainian, post-Chernobyl | 12–31 | 2/21 (9.5) | |||
| Kumagai et al,11 2004 | Japanese | <15 | 1/29 (3.4) | PCR with sequencing | Not examineda |
| Ukrainian, post-Chernobyl | <15 | 0/15 (0) | |||
| Penko et al,10 2005 | USA | ≤21 | 0/14 (0) | PCR with sequencing | Not examined |
| Rosenbaum et al,14 2005 | USA | 10–17 | 4/20 (20)b | Mutectorc Primer extension | Not examined |
| Sassolas et al,12 2012 | France | 8–35 | 21/103 (20) | PCR with sequencing | No association between BRAF mutation and aggressive characteristics |
| Givens et al,13 2014 | USA | 2.8–17.4 | 7/19 (37) | PCR with pyro-sequencing | Follicular variant associated with absence of BRAF V600E;no association with mutation and lymphatic or distant metastases, age, tumor size, extrathyroidal extension, lymphovascular invasion, MACIS score, or microcarcinoma |
| Henke et al,16 2014 | USA | <22 | 17/27 (63) | PCR with RFLP | Male sex associated with presence of BRAF V600E; no association with mutation and survival, extent of disease at diagnosis, tumor size, capsular invasion, vascular invasion, soft tissue invasion, margin status, or laterality |
| Gertz et al, 2015 (this study) | USA | ≤18 | 4/13 (31) | PCR with FMCA | Follicular variant associated with absence of BRAF V600E, classic-type histologic profile associated with presence of BRAF V600E;not associated with tumor size, lymphovascular invasion, lymph node metastases, or extrathyroidal extension |
Abbreviations: FMCA, fluorescence melting curve analysis; MACIS, metastasis, age at diagnosis, completeness of resection, invasion, size of the tumor; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism.
Not examined, exclusive of adult patients.
Follicular variant–type tumors excluded.
Trigen Laboratories, Sayreville, New Jersey.
No differences in clinical behavior were detected between BRAF mutant-positive and mutant-negative cases; however, the study was not powered to detect such differences. The tumors of pediatric patients examined in our study had comparatively more aggressive clinical behavior than those of adults, as is classically seen in other studies.5,30 This is suggested by significantly increased rates of positive lymph nodes and lymphovascular invasion seen in the younger patient group. However, pediatric patients were more likely to have lymph nodes submitted for pathologic examination (P=.04), suggesting that more extensive operative management was conducted on the pediatric patients. When comparing patients with at least 1 lymph node examined, we see lymph node metastases in 9 of 12 pediatric tumors (75%) and 19 of 32 adult tumors (59%), which was not a significant difference (P = .27). This may represent a selection bias with increased discovery of metastases, with pediatric patients receiving more extensive surgery; however, lymph node dissections are often performed in the context of palpable nodes. In fact, the most frequent presenting symptom for pediatric patients with PTC is palpable lymph nodes, while for adults it is goiter.4 Additionally, the higher rate of lymphovascular invasion in our pediatric group would be relatively unrelated to this bias and still supports a more aggressive nature in pediatric tumors. None of our pediatric patients had distant metastases, though the study population is too small for a valid comparison to the adult rate. Furthermore, the follow-up period in our study is too limited to adequately assess long-term outcomes including disease recurrence.
Histologic profile between the study groups was not significantly different. Most tumors presented a classic-type histology pattern in both groups. Follicular variant was noted in 4 of 14 pediatric patients (29%), which is comparable to the 10% to 37% suggested in the literature.10-13,16,30 The rate of follicular variant morphology is increasing, according to a recent study,29 though this may be linked to higher rates of RAS mutations, which were not detected in any of our pediatric patients. Solid variant carcinoma, which has been associated with young age,18 was not seen in any pediatric patients. This histologic profile has also been seen with radiation exposure, which was not reported in any of the pediatric patients in this study. This variant was seen in 6 adult patients (ages 48–79 years), none of which had a reported history of radiation exposure.
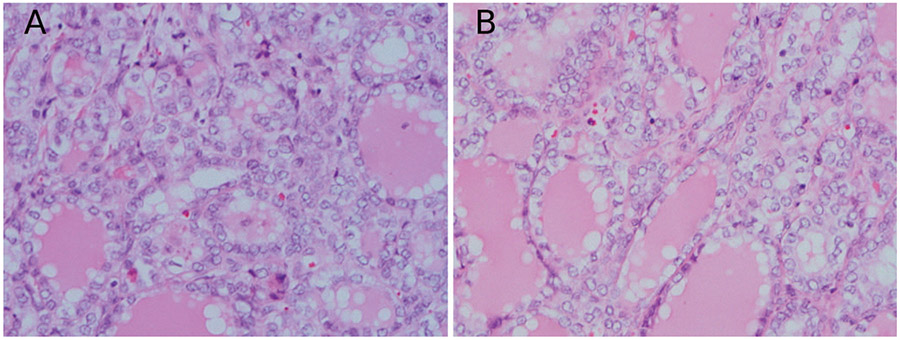
One of the pediatric patients harbored an unusual deletion mutation of BRAF that has been previously identified.6,9,31,32 The histologic appearance of this tumor is illustrated in the Figure, A and B. The classic c.1799T>A (V600E) mutation represents approximately 97% of BRAF alterations in PTC, while this mutant represents approximately 0.2% to 0.5% of PTCs.6,9 To the best of our knowledge, the patient in this cohort represents the youngest reported to have such a mutation. Hou and colleagues32 identified kinetic properties of the product of this BRAF mutation that resemble the V600E mutant with constitutive activation. Non-V600E BRAF mutated tumor cells will not stain with antibodies directed at V600E mutant BRAF on paraffin-embedded tissue.33 Further study on these rare variants, specifically on their impact on tumor behavior and implications in the realm of targeted therapies, may be warranted if molecular typing is to become a significant part of the management of patients with PTC.
A and B, Papillary carcinoma with 3-bp deletion in BRAF (hematoxylin-eosin, original magnification ×40 [A and B]).
We identified 2 of 13 patients (15%) with RET rearrangements. Of particular interest is the presence of concomitant mutation of BRAF and a RET rearrangement detected in 1 of the tumors. Although previously thought to be mutually exclusive tumorigenic events, some evidence suggests that additional mutations may signify progression of the tumor to a more aggressive type.34 Rearrangements of RET have classically been associated with radiation and young age; at least 1 rigorous study suggests that this may not be the case,35 and the quoted prevalence of these mutations may be heavily influenced by the type of detection method.36 A potential association with radiation may have some relevance in the context of the reported decreasing rate of this mutation.29 Our study confirms the finding in a previous series that RAS mutation does not occur with significant frequency in pediatric PTC. As previously mentioned, this event plays a more significant role in follicular thyroid carcinoma, which is rare in pediatric patients.37
Though young patients with PTC generally have an excellent prognosis, there are a number of considerations that warrant continued study of this disease entity. The current treatment for this disease renders patients completely dependent on thyroid replacement therapy for most of their expected lifespan. This is particularly concerning since this subpopulation has a proportionately high number of female patients who may be subject to hormone management with potential complications in subsequent pregnancies. Ideally, treatments allowing subtotal thyroidectomy and retention of thyroid function would be preferred. In a large retrospective study of pediatric patients with PTC who had extended follow-up, partial thyroidectomies carried larger rates of disease recurrence than bilateral thyroidectomies.1 Further understanding of the genetics of pediatric PTC may help stratify patients and identify subgroups who could safely be treated with subtotal thyroidectomy. Tumor genetics of course can present opportunities for targeted therapy, which could be used as adjuvant therapy for individuals with very limited disease treated with subtotal thyroidectomy or in the rare, unfortunate patients with recalcitrant, advanced disease. Most of the recent attention toward molecular marker therapy guidance has been focused on the BRAF mutant V600E, given its high incidence and correlation with aggressive disease and with the potential to predict response to I-131 therapy.38,39 Improved diagnostics are another way that molecular markers can aid in patient care, particularly with the amenability of molecular techniques to fine-needle aspiration specimens. This has been examined as a useful application in the fine-needle aspiration of pediatric thyroid nodules.40
In summary, the experiences from our institution substantiate findings in similar studies and support a significantly lower rate of the BRAF V600E mutation in PTC in pediatric patients than in adults. Mutations in RAS genes do not contribute significantly to PTC tumorigenesis in this small group of pediatric patients. Large, long-term studies integrating clinicopathologic data with comprehensive molecular profiling are needed to refine and improve the care of pediatric patients with PTC.
Acknowledgments
The authors wish to thank the University of Wisconsin Translation Research Initiatives in Pathology Laboratory, in part supported by the University of Wisconsin Department of Pathology and Laboratory Medicine, in Madison, and University of Wisconsin Carbone Cancer Center grant P30 CA014520, for use of its facilities and services. We also wish to thank Chong Zhang, MS, for her assistance with the initial statistics and study design. Funding was provided by the Department of Pathology and Laboratory Medicine at the University of Wisconsin, Madison.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Presented in part as a poster at the United States and Canadian Academy of Pathology (USCAP) annual meeting; March 3, 2014; San Diego, California.
Contributor Information
Ryan J. Gertz, Department of Pathology, MD Anderson Cancer Center, Houston, Texas; Department of Pathology, OhioHealth Riverside Methodist Hospital, Columbus, Ohio..
Yuri Nikiforov, Division of Molecular Genomic Pathology, University of Pittsburgh, Pennsylvania.
William Rehrauer, Department of Pathology and Laboratory Medicine, University of Wisconsin, Madison.
Lee McDaniel, School of Public Health, Louisiana State University, New Orleans.
Ricardo V. Lloyd, Department of Pathology and Laboratory Medicine, University of Wisconsin, Madison.
References
- 1.Hay I, Gonzalez-Losada T, Reinalda M, Honetschlager J, Richards M, Thompson G. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34(6):1192–1202. [DOI] [PubMed] [Google Scholar]
- 2.Shayota B, Pawar S, Chamberlain R. MeSS: a novel prognostic scale specific for pediatric well-differentiated thyroid cancer: a population-based, SEER outcomes study. Surgery. 2013;154(3):429–435. [DOI] [PubMed] [Google Scholar]
- 3.Miccoli P, Minuto M, Ugolini C, et al. Papillary thyroid cancer: pathological parameters as prognostic factors in different classes of age. Otolaryngol Head Neck Surg. 2008;138(2):200–203. [DOI] [PubMed] [Google Scholar]
- 4.Zohar Y, Strauss M, Laurian N. Adolescent versus adult thyroid carcinoma. Laryngoscope. 1986;96(5):555–559. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman D, Hay I, Gough I, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104(6):1157–1166. [PubMed] [Google Scholar]
- 6.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(11):4085–4090. [DOI] [PubMed] [Google Scholar]
- 7.Elisei R, Ugolini C, Viola D, et al. BRAF V600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. [DOI] [PubMed] [Google Scholar]
- 8.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF V600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer. Cancer. 2012;118(7):1764–1773. [DOI] [PubMed] [Google Scholar]
- 9.Barollo S, Pezzani R, Cristiani A, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid. 2014;24(5):809–819. [DOI] [PubMed] [Google Scholar]
- 10.Penko K, Livezey J, Fenton C, et al. BRAF mutations are uncommon in papillary thyroid cancer of young patients. Thyroid. 2005;15(4):320–325. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai A, Namba H, Saenko V, et al. Low frequency of BRAF T1796A mutations in childhood thyroid carcinomas. J Clin Endocrinol Metab. 2004;89(9):4280–4284. [DOI] [PubMed] [Google Scholar]
- 12.Sassolas G, Hafdi-Nejjari Z, Ferraro A, et al. Oncogenic alterations in papillary thyroid cancers of young patients. Thyroid. 2012;22(1):17–26. [DOI] [PubMed] [Google Scholar]
- 13.Givens D, Buchmann L, Agarwal A, Grimmer J, Hunt J. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope. 2014;124(9):E389–E393. [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum E, Hosler G, Zahurak M, Cohen Y, Sidransky D, Westra W. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol. 2005;18(7):898–902. [DOI] [PubMed] [Google Scholar]
- 15.Nikiforova M, Ciampi R, Salvatore G, et al. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett. 2004;209(1):1–6. [DOI] [PubMed] [Google Scholar]
- 16.Henke L, Perkins S, Pfeifer J, et al. BRAF V600E mutational status in pediatric thyroid cancer. Pediatr Blood Cancer. 2014;61(7):1168–1172. [DOI] [PubMed] [Google Scholar]
- 17.Romei C, Elisei R. RET/PTC translocations and clinico-pathological features in human papillary thyroid carcinoma. Front Endocrinol. 2012;3(54):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikiforov Y, Rowland J, Bove K. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57(9):1690–1694. [PubMed] [Google Scholar]
- 19.Leeman-Neill R, Brenner A, Little M, et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119(10):1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricarte-Filho J, Li S, Garcia-Rendueles M, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2012;123(11):4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikiforov Y Recent developments in the molecular biology of the thyroid. In: Lloyd R, ed. Endocrine Pathology: Differential Diagnosis and Molecular Advances. 2nd ed. New York: Springer; 2010:245–246. [Google Scholar]
- 22.Nikiforov Y, Nikiforova M, Gnepp D, Fagin J. Prevalence of mutations of ras and p53 in benign and malignant thyroid tumors from children exposed to radiation after the Chernobyl nuclear accident. Oncogene. 1996;13(4):687–693. [PubMed] [Google Scholar]
- 23.Suchy B, Waldmann V, Klugbauer S, Rabes H. Absence of RAS and p53 mutations in thyroid carcinoma of children after Chernobyl in contrast to adult thyroid tumours. Br J Cancer. 1998;77(6):952–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauws E, Tummers R, Ris-Stalpers C, de Vijlder J, Voute T. Absence of activating mutations in ras and gsp oncogenes in a cohort of nine patients with sporadic pediatric thyroid tumors. Med Pediatr Oncol. 2001;36(6):630–634. [DOI] [PubMed] [Google Scholar]
- 25.Kimura E, Nikiforova M, Zhu Z. High prevalence of BRAF mutations in thyroid cancer: genetic evidence of constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63(7):1454–1457. [PubMed] [Google Scholar]
- 26.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22(29):4578–4580. [DOI] [PubMed] [Google Scholar]
- 27.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-BRAF linear signalling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115(4):1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Guerra A, Zeppa P, Bifulco M, Vitale M. Concomitant BRAF V600E mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. 2014;24(2):254–259. [DOI] [PubMed] [Google Scholar]
- 29.Jung CK, Little M, Lubin J, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014; 99(2):E276–E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collini P, Massimino M, Leite SF, et al. Papillary thyroid carcinoma of childhood and adolescence: a 30-year experience at the Istituto Nazionale Tumori in Milan. Pediatr Blood Cancer. 2006;46(3):300–306. [DOI] [PubMed] [Google Scholar]
- 31.Jang M-A, Lee S-T, Oh YL, et al. Identification of a rare 3 bp BRAF gene deletion in a thyroid nodule by mutant enrichment with 3′modified oligonucleotides polymerase chain reaction. Ann Lab Med. 2012;32(3):238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou P, Liu D, Xing M. Functional characterization of the T1799-1801 del and A1799-1816ins BRAF mutations in papillary thyroid cancer. Cell Cycle. 2007;6(3):377–379. [DOI] [PubMed] [Google Scholar]
- 33.Ilie M, Lassalle S, Long-Mira E, et al. Diagnostic value of immunohistochemistry for the detection of the BRAFV600E mutation in papillary thyroid carcinoma: comparative analysis with three DNA-based assays. Thyroid. 2014;24(5):858–866. [DOI] [PubMed] [Google Scholar]
- 34.Zou M, Baitei E, Alzahrani A, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014;24(8):1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elisei R, Romei C, Vorontsova T, et al. RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant, and benign. J Clin Endocrinol Metab. 2001;86(7):3211–3216. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Z, Ciampi R, Nikiforova M, Gandhi M, Nikiforov Y. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91(9):3603–3610. [DOI] [PubMed] [Google Scholar]
- 37.Enomoto K, Enomoto Y, Uchino S, Yamashita H, Noguchi S. Follicular thyroid cancer in children and adolescents: clinicopathologic features, long-term survival, and risk factors for recurrence. Endocr J. 2013;60(5):629–635. [DOI] [PubMed] [Google Scholar]
- 38.Miccoli P, Basolo F. BRAF mutation status in papillary thyroid carcinoma: significance for surgical strategy. Langenbecks Arch Surg. 2014;399(2):225–228. [DOI] [PubMed] [Google Scholar]
- 39.Yang K, Wang H, Liang Z, Liang J, Li F, Lin Y. BRAF V600E mutation associated with non-radioiodine-avid status in distant metastatic papillary thyroid carcinoma. Clin Nucl Med. 2014;39(8):675–679. [DOI] [PubMed] [Google Scholar]
- 40.Monaco S, Pantanowitz L, Khalbuss W, et al. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathol. 2012;120(5):342–350. [DOI] [PubMed] [Google Scholar]