Abstract
Aims
Cardiohepatic interactions have been a focus of attention in heart failure (HF). The model for end‐stage liver disease excluding international normalized ratio (MELD‐XI) score has been shown to be useful for predicting poor outcomes in patients with acute decompensated HF (ADHF). Furthermore, the fibrosis‐4 (FIB‐4) index, a simple marker to assess liver fibrosis, predicts adverse prognoses in patients with HF as well. However, there is little information available on the prognostic significance of the combination of the MELD‐XI score and FIB‐4 index in patients with ADHF and its association with left ventricular ejection fraction (LVEF) subgroup.
Methods and results
We prospectively studied 466 consecutive patients who were admitted for ADHF [HF with reduced LVEF (LVEF < 40%): n = 164, HF with mid‐range LVEF (40% ≤ LVEF < 50%): n = 104, and HF with preserved LVEF (LVEF ≥ 50%): n = 198]. We calculated the MELD‐XI score and FIB‐4 indices at discharge. The primary endpoint was all‐cause death (ACD). During the mean follow‐up period of 2.8 years, 143 patients had ACD. In the multivariate Cox analysis, the MELD‐XI score and FIB‐4 index were independently associated with ACD. Patients were stratified into the following three groups according to the median value of MELD‐XI score (=11) and FIB‐4 index (=2.13): Group 1 had both a low MELD‐XI score and a low FIB‐4 index; Group 2 had either a high MELD‐XI score (MELD‐XI score ≥11) or a high FIB‐4 index (FIB‐4 index ≥2.13); and Group 3 had both a high MELD‐XI score and a high FIB‐4 index. Kaplan–Meier analysis revealed that Group 2 and Group 3 had a significantly greater risk of ACD than Group 1 [Group 2 vs. Group 1: adjusted hazard ratio, 2.48 (95% confidence interval: 1.75–3.53), P < 0.0001; Group 3 vs. Group 1: adjusted hazard ratio, 7.03 (95% confidence interval: 3.95–13.7), P < 0.0001]. In addition, the patients with both a higher MELD‐XI score and FIB‐4 index showed a significantly higher risk of ACD also in the patients with HF with reduced LVEF, HF with mid‐range LVEF, and HF with preserved LVEF (all P < 0.0001).
Conclusions
The combination of MELD‐XI score and FIB‐4 index may be useful for stratifying patients at risk for ACD in patients with ADHF, irrespective of LVEF.
Keywords: Acute decompensated heart failure, MELD‐XI, FIB‐4, Liver fibrosis, Liver dysfunction
Introduction
Despite advances in modern cardiology, acute decompensated heart failure (ADHF) has been an increasing clinical and public health problem associated with unacceptably high mortality and morbidity. 1 Therefore, it is important to identify high‐risk patients in the management of heart failure (HF).
Systemic venous congestion due to HF is known to cause multiple end‐organ dysfunction or tissue damage. It has also been reported that reduced arterial perfusion and passive congestion cause liver dysfunction and fibrosis in HF. 2 , 3 Liver function test abnormalities have been shown to be commonly observed and to predict worse clinical outcome in patients with ADHF. 4 , 5 Liver dysfunction and fibrosis in patients with HF, a condition known as cardiohepatic syndrome, 2 , 3 , 6 has a strong impact on clinical outcome and risk of death. The model for end‐stage liver disease excluding international normalized ratio (MELD‐XI) score, a robust scoring system of liver dysfunction in patients with advanced liver disease, 7 , 8 has been reported to predict poor outcomes in patients with ADHF. 9 , 10 , 11 The fibrosis‐4 (FIB‐4) index, a simple marker to assess liver fibrosis, 12 , 13 is also reported to be useful for prognosis predictions in patients with HF. 14 , 15 , 16 However, there is little information available on the combination of prognostic values of MELD‐XI score and FIB‐4 index in patients with ADHF.
A new category of patients with HF with mid‐range ejection fraction (HFmrEF) has recently been defined, separate from reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). 17 Although the prognostic value of the MELD‐XI score and FIB‐4 index has been reported in the previous studies, it remains to be fully elucidated whether the MELD‐XI score and FIB‐4 index are useful in assessing risk in patients with ADHF with each left ventricular ejection fraction (LVEF) subgroup. Therefore, the aim of this study was to investigate the epidemiology and the prognostic value of the MELD‐XI score and FIB‐4 index in patients with ADHF, not only in total cohort but also in each LVEF category.
Methods
Subject and study protocol
We analysed data from the Osaka Prefectural Acute Heart Failure Syndrome Registry (UMIN 000015246), which is a single‐centre, observational, prospective cohort study. 18 , 19 We enrolled 507 consecutive patients with ADHF who were discharged with survival in a prospective study that was conducted between October 2011 and November 2019 (Figure 1 ). ADHF was diagnosed based on clinical signs and symptoms according to the Framingham Heart Study criteria. Patients were excluded from this study if they had chronic liver disease (n = 8; portal hypertension, cirrhosis, hepatic tumours, bile duct disease, etc.), were on maintenance haemodialysis (n = 18), and had severe valvular or coronary artery disease requiring urgent surgery during hospitalization (n = 7). Patients who were lost to follow‐up after discharge (n = 8) were also excluded. Chronic liver disease was defined based on clinical history of decompensated cirrhosis. After excluding these patients, we studied 466 patients with ADHF (mean age 74 ± 13, 270 men and 196 women). This study was approved by the Osaka General Medical Center's Review Committee (approval number: UMIN 000015246). All patients provided written informed consent for participation in this study. The investigation conforms to the principles outlined in the Declaration of Helsinki.
Figure 1.
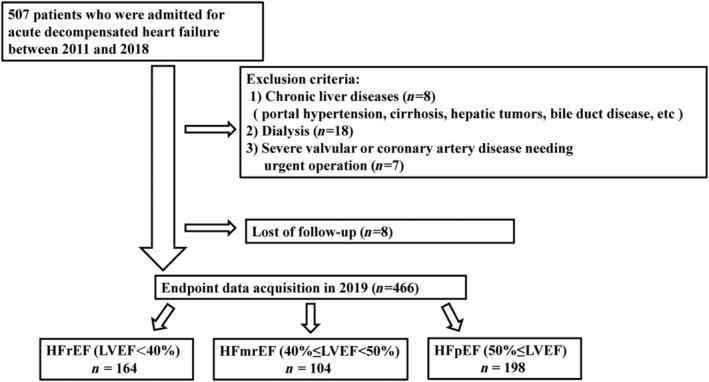
Flow of patients through the study. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction.
Measurement
Baseline data of age, sex, New York Heart Association functional class, body mass index, medical history, venous blood sampling, echocardiographic data, and current medications were collected during hospitalization. All patients underwent testing for hepatitis B surface antigen and hepatitis C antibodies, and their medical histories were checked for chronic liver disease (cirrhosis, hepatic tumours, etc.). Echocardiography was performed by an experienced echocardiographer using the standard techniques. The echocardiographic parameters measured included the left ventricular end‐diastolic dimension, left ventricular end‐systolic dimension, and left atrial dimension. LVEF was measured using the modified Simpson's method. Blood sampling for assessment of complete blood count, serum sodium, chloride, potassium, creatinine, blood urea nitrogen, albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholinesterase, and plasma brain natriuretic peptide levels was obtained just before discharge in the clinically stable phase. The MELD‐XI and FIB‐4 indices were calculated at discharge, as well. The MELD‐XI score and FIB‐4 index were calculated using the following formula, as previously reported: MELD‐XI score = 11.76 × ln (creatinine [mg/dL]) + 5.11 × ln (total bilirubin [mg/dL]) + 9.44, 8 , 20 where ln means the natural logarithm. If a patient had a creatinine or bilirubin level lower than 1.0 mg/dL, the value of 1.0 mg/dL was entered into the calculations to prevent negative logarithmic values in the formula, and the calculated score was rounded. Thus, the minimum possible MELD score was 9.0. The FIB‐4 index = (age (years) × AST [U/L])∕(platelet count [103/μL] × square root of ALT [U/L]). 12
Clinical outcome
After discharge, all patients were followed up in our HF unit at least every 1 or 2 months. Survival dates were obtained by direct contact with patients by their physicians at the hospital in an outpatient setting, by telephone interview with their families, or by mail by dedicated coordinators and investigators. The primary endpoint of this study was all‐cause death (ACD). Secondary endpoints were cardiac death and non‐cardiac death.
Statistical analysis
Normally distributed data were expressed as mean ± standard deviation, and non‐distributed data are presented as medians with inter‐quartile ranges. Categorical variables are expressed as numbers and percentages. Patients were stratified by LVEF subgroup. The statistical significance of differences was analysed using one‐way analysis of variance for parametric continuous variables and the Kruskal–Wallis rank‐sum test for non‐parametric continuous variables. The χ 2 test was used to compare differences in categorical variables. The event‐free survival rates were calculated using the Kaplan–Meier method, and the differences in survival rates were compared between groups with the log‐rank test. The prognostic value of the baseline characteristics was assessed with a Cox proportional hazards regression analysis. The Cox multivariate model included established confounders, such as age, sex, LVEF, haemoglobin level, serum sodium level, serum chloride level, serum uric acid level, and plasma brain natriuretic peptide level. These potential confounding variables were selected from well‐known HF risk models. The predictive value of the MELD‐XI score, FIB‐4 index, and the combination of MELD‐XI score and FIB‐4 index for the primary and secondary endpoints were compared using receiver operating characteristic (ROC) curve analysis, and results are expressed in terms of the area under the curve and 95% confidence interval (CI) for this area. A value of P < 0.05 was considered statistically significant. All statistical analysis, except for ROC curve analysis, was performed using JMP Version 13 (SAS Institute Inc., Cary, NC, USA). ROC curve analysis was performed using MedCalc statistical software Version 19.3.1 (MedCalc Software Ltd, Ostend, Belgium).
Results
During the follow‐up period of 2.8 ± 1.5 years, 143 patients had ACD (HFrEF n = 49, HFmrEF n = 34, and HFpEF n = 60). Cardiac death occurred in 62 patients: 34 patients had pump failure death, and 28 patients had sudden cardiac death. Non‐cardiac death occurred in 81 patients (pneumonia, n = 20; cancer, n = 15; infection/sepsis, n = 9; old age, n = 7; renal failure, n = 6; stroke, n = 5; gastrointestinal bleeding, n = 4; and other causes of death, n = 15).
Baseline patient characteristics among patients with heart failure with reduced ejection fraction, heart failure with mid‐range ejection fraction, and heart failure with preserved ejection fraction
The baseline clinical characteristics of the 466 patients with ADHF were stratified by LVEF subgroup and are shown in Table 1 . The mean age was 74 years, and 58% were male. There were 164 patients with HFrEF (LVEF < 40%), 104 patients with HFmrEF (40% ≤ LVEF < 50%), and 198 patients with HFpEF (LVEF ≥ 50%). Patients with HFrEF and HFmrEF were more frequently male and had a higher rate of ischaemic origin and beta‐blocker use than those with HFpEF. Patients with HFrEF were less likely to have atrial fibrillation and hypertension and were younger than those with HFmrEF and HFpEF. Patients with HFpEF were more anaemic than those with HFrEF and HFmrEF. There was no difference in the MELD‐XI score among patients in the three groups. The FIB‐4 index was significantly lower in patients with HFrEF than in patients with HFmrEF and HFpEF. There was a weak correlation between the MELD‐XI score and FIB‐4 index (r = 0.182, P < 0.0001). No patients were on sacubitril/valsartan because the drug had not yet been approved for clinical use in Japan.
Table 1.
Baseline characteristics at discharge in patients with acute decompensated heart failure stratified by LVEF subgroups
| Overall (n = 466) | HFrEF (n = 164) | HFmrEF (n = 104) | HFpEF (n = 198) | P‐value | |
|---|---|---|---|---|---|
| Clinical data | |||||
| Age (years) | 74 ± 13 | 68 ± 14 | 75 ± 13 | 78 ± 9 | <0.001 |
| Gender (male, %) | 58 | 71 | 66 | 42 | <0.001 |
| BMI | 21.4 ± 4.3 | 21.5 ± 4.7 | 21.4 ± 3.8 | 21.2 ± 4.2 | 0.886 |
| NYHA Class I/II/III (%) | 30/53/17 | 30/56/14 | 42/32/26 | 24/61/15 | 0.258 |
| SBP (mmHg) | 115 ± 18 | 109 ± 18 | 116 ± 14 | 119 ± 18 | <0.001 |
| Heart rate (b.p.m.) | 67 ± 11 | 67 ± 11 | 67 ± 11 | 66 ± 11 | 0.588 |
| Ischaemic origin (%) | 26 | 34 | 37 | 14 | <0.001 |
| Atrial fibrillation (%) | 47 | 34 | 52 | 57 | <0.001 |
| Hypertension (%) | 81 | 72 | 80 | 90 | <0.001 |
| Diabetes mellitus (%) | 43 | 40 | 47 | 42 | 0.387 |
| Dyslipidaemia (%) | 50 | 50 | 44 | 53 | 0.518 |
| Prior HF hospitalization (%) | 19 | 24 | 17 | 16 | 0.551 |
| Medications | |||||
| ACEI or ARB (%) | 55 | 57 | 54 | 54 | 0.789 |
| Beta‐blocker (%) | 89 | 95 | 96 | 81 | <0.001 |
| Loop diuretics (%) | 86 | 85 | 85 | 88 | 0.991 |
| Spironolactone (%) | 34 | 37 | 38 | 31 | 0.343 |
| Tolvaptan (%) | 6 | 7 | 2 | 7 | 0.529 |
| Digitalis (%) | 3 | 3 | 4 | 3 | 0.698 |
| Statin (%) | 36 | 40 | 36 | 33 | 0.526 |
| Echocardiography | |||||
| LVEF (%) | 47 ± 15 | 31 ± 7 | 45 ± 3 | 61 ± 8 | <0.001 |
| LVDd (mm) | 53 ± 10 | 61 ± 8 | 53 ± 7 | 47 ± 7 | <0.001 |
| LVDs (mm) | 41 ± 11 | 51 ± 9 | 41 ± 6 | 31 ± 6 | <0.001 |
| LAD (mm) | 43 ± 7 | 44 ± 8 | 43 ± 7 | 43 ± 7 | 0.142 |
| Laboratory data | |||||
| Haemoglobin (g/dL) | 11.8 ± 2.2 | 12.5 ± 2.4 | 12.0 ± 2.0 | 11.2 ± 1.9 | <0.001 |
| Platelet count (× 103/μL) | 208 ± 77 | 197 ± 80 | 207 ± 74 | 219 ± 76 | <0.001 |
| Sodium (mEq/L) | 138 ± 3 | 138 ± 4 | 138 ± 3 | 139 ± 4 | 0.205 |
| Chloride (mEq/L) | 101 ± 5 | 101 ± 5 | 101 ± 5 | 101 ± 5 | 0.445 |
| Potassium (mEq/L) | 4.2 ± 0.5 | 4.3 ± 0.5 | 4.3 ± 0.5 | 4.1 ± 0.6 | 0.211 |
| Creatinine (mEq/L) | 1.13 (0.87–1.62) | 1.11 (0.87–1.57) | 1.15 (0.91–1.57) | 1.11 (0.86–1.64) | 0.979 |
| BUN (mg/dL) | 25 (18–36) | 23 (16–33) | 25 (18–35) | 26 (19–39) | 0.056 |
| Uric acid (mg/dL) | 7.0 (5.8–8.5) | 7.1 (5.7–8.4) | 6.6 (5.4–8.5) | 7.0 (6.0–8.7) | 0.3724 |
| Albumin (g/dL) | 3.5 (3.1–3.7) | 3.6 (3.1–3.9) | 3.4 (3.1–3.8) | 3.4 (3.1–3.7) | 0.068 |
| Total bilirubin (mg/dL) | 0.6 (0.5–0.8) | 0.7 (0.5–1.0) | 0.6 (0.5–0.7) | 0.6 (0.4–0.8) | <0.001 |
| AST (U/L) | 23 (18–30) | 25 (18–32) | 22 (19–31) | 23 (18–29) | 0.882 |
| ALT (U/L) | 17 (11–27) | 19 (13–33) | 17 (12–26) | 16 (11–23) | 0.199 |
| BNP (mg/dL) | 209 (104–435) | 277 (131–510) | 218 (113–475) | 171 (77–333) | <0.001 |
| Cholinesterase (U/L) | 225 ± 76 | 234 ± 83 | 223 ± 77 | 216 ± 67 | <0.001 |
| MELD‐XI score | 11 (9–15) | 11 (9–15) | 11 (9–15) | 11 (9–15) | 0.861 |
| FIB‐4 index | 2.13 (1.47–2.97) | 1.98 (1.24–3.21) | 2.20 (1.49–3.04) | 2.18 (1.60–2.82) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; FIB‐4, fibrosis‐4; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LAD, left atrial dimension; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; MELD‐XI, model for end‐stage liver disease excluding international normalized ratio; NYHA, New York Heart Association; SBP, systolic blood pressure.
Prognostic analysis in overall cohort
In overall cohort, multivariate Cox proportional hazards analysis revealed that the MELD‐XI score and FIB‐4 index were significantly and independently associated with ACD after adjusting for the established confounders (Table 2 ). Moreover, the MELD‐XI score was also an independent predictor of cardiac death (Table 3 ). The FiB‐4 index showed an independent association with both cardiac and non‐cardiac death (Table 4 ).
Table 2.
Univariate and multivariate Cox analysis for the prediction of all‐cause death in patients with ADHF
| Unadjusted | Adjusted model a | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall cohort | ||||
| MELD‐XI score | 1.0827 (1.0553–1.1090) | <0.0001 | 1.0725 (1.0319–1.1137) | 0.0003 |
| FIB‐4 index | 1.3249 (1.2273–1.4218) | <0.0001 | 1.2173 (1.1077–1.3278) | <0.0001 |
| Patients with HFrEF | ||||
| MELD‐XI score | 1.1243 (1.0739–1.1721) | <0.0001 | 1.0876 (1.0130–1.1646) | 0.0179 |
| FIB‐4 index | 1.2765 (1.2680–1.4242) | <0.0001 | 1.2095 (1.0126–1.4165) | 0.0248 |
| Patients with HFmrEF | ||||
| MELD‐XI score | 1.1661 (1.0961–1.2358) | <0.0001 | 1.2101 (1.1137–1.3156) | <0.0001 |
| FIB‐4 index | 1.4184 (1.1736–1.6793) | 0.0001 | 1.2685 (0.9941–1.5867) | 0.0441 |
| Patients with HFpEF | ||||
| MELD‐XI score | 1.1350 (1.0956–1.1730) | <0.0001 | 1.0435 (0.9765–1.1151) | 0.2085 |
| FIB‐4 index | 1.3618 (1.2020–1.5204) | <0.0001 | 1.3454 (1.1521–1.5473) | <0.0001 |
ADHF, acute decompensated heart failure; CI, confidence interval; FIB‐4, fibrosis‐4; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; MELD‐XI, model for end‐stage liver disease excluding international normalized ratio.
Adjusted for age, sex, left ventricular ejection fraction, haemoglobin level, serum sodium level, serum chloride level, serum uric acid level, and plasma brain natriuretic peptide level at discharge.
Table 3.
Univariate and multivariate Cox analysis for the prediction of cardiac death in patients with ADHF
| Unadjusted | Adjusted model a | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall cohort | ||||
| MELD‐XI score | 1.1032 (1.0632–1.1417) | <0.0001 | 1.0975 (1.0391–1.1571) | 0.0007 |
| FIB‐4 index | 1.3227 (1.1778–1.4657) | <0.0001 | 1.1764 (1.0175–1.3375) | 0.0189 |
| Patients with HFrEF | ||||
| MELD‐XI score | 1.1429 (1.0737–1.2095) | <0.0001 | 1.1163 (1.0154–1.2217) | 0.0185 |
| FIB‐4 index | 1.2712 (1.0690–1.4677) | 0.0026 | 1.1279 (0.8791–1.4471) | 0.3439 |
| Patients with HFmrEF | ||||
| MELD‐XI score | 1.1581 (1.0468–1.2690) | 0.0023 | 1.1281 (0.9938–1.2804) | 0.0623 |
| FIB‐4 index | 1.2706 (0.8728–1.7034) | 0.1509 | 1.0259 (0.6672–1.4689) | 0.8949 |
| Patients with HFpEF | ||||
| MELD‐XI score | 1.0693 (1.0072–1.1271) | 0.0181 | 1.0687 (0.9649–1.1836) | 0.2023 |
| FIB‐4 index | 1.3999 (1.1607–1.6393) | 0.0001 | 1.3287 (1.0467–1.6371) | 0.0110 |
ADHF, acute decompensated heart failure; CI, confidence interval; FIB‐4, fibrosis‐4; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; MELD‐XI, model for end‐stage liver disease excluding international normalized ratio.
Adjusted for age, sex, left ventricular ejection fraction, haemoglobin level, serum sodium level, serum chloride level, serum uric acid level, and plasma brain natriuretic peptide level at discharge.
Table 4.
Univariate and multivariate Cox analysis for the prediction of non‐cardiac death in patients with ADHF
| Unadjusted | Adjusted model a | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall cohort | ||||
| MELD‐XI score | 1.0656 (1.0269–1.1017) | 0.0004 | 1.0496 (0.9941–1.1065) | 0.0753 |
| FIB‐4 index | 1.3268 (1.1942–1.4583) | <0.0001 | 1.2401 (1.0959–1.4032) | 0.0006 |
| Patients with HFrEF | ||||
| MELD‐XI score | 1.1018 (1.0246–1.1716) | 0.0041 | 1.0375 (0.9318–1.1511) | 0.4926 |
| FIB‐4 index | 1.2867 (1.0650–1.5074) | 0.0040 | 1.2716 (0.9820–1.5922) | 0.0463 |
| Patients with HFmrEF | ||||
| MELD‐XI score | 1.1742 (1.0836–1.2644) | <0.0001 | 1.2706 (1.1338–1.4309) | <0.0001 |
| FIB‐4 index | 1.4867 (1.1768–1.8295) | 0.0004 | 1.3938 (1.0201–1.8648) | 0.0285 |
| Patients with HFpEF | ||||
| MELD‐XI score | 1.0211 (0.9577–1.0773) | 0.4828 | 1.0252 (0.9336–1.1207) | 0.5900 |
| FIB‐4 index | 1.3312 (1.1151–1.5439) | 0.0005 | 1.3324 (1.0708–1.6107) | 0.0052 |
ADHF, acute decompensated heart failure; CI, confidence interval; FIB‐4, fibrosis‐4; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; MELD‐XI, model for end‐stage liver disease excluding international normalized ratio.
Adjusted for age, sex, left ventricular ejection fraction, haemoglobin level, serum sodium level, serum chloride level, serum uric acid level, and plasma brain natriuretic peptide level at discharge.
Patients were stratified into the following three groups according to the median value of MELD‐XI score (=11) and FIB‐4 index (=2.13): Group 1 had both a low MELD‐XI score and a low FIB‐4 index; Group 2 had either a high MELD‐XI score (MELD‐XI score ≥11) or a high FIB‐4 index (FIB‐4 index ≥2.13); and Group 3 had both a high MELD‐XI score and a high FIB‐4 index. Kaplan–Meier analysis revealed that patients with both a high MELD‐XI score and a high FIB‐4 index and those with either a high MELD‐XI score or a high FIB‐4 index had a significantly greater risk of ACD than those with a low MELD‐XI score and a low FIB‐4 index [50% vs. 28% vs. 10%, respectively; Group 3 vs. Group 1: adjusted hazard ratio (HR), 7.03 (95% CI: 3.95–13.7), P < 0.0001; Group 2 vs. Group 1: adjusted HR, 2.48 (95% CI: 1.75–3.53), P < 0.0001]. There was also a significant difference in the risk of cardiac death and non‐cardiac death among the three groups (Figure 2 ).
Figure 2.
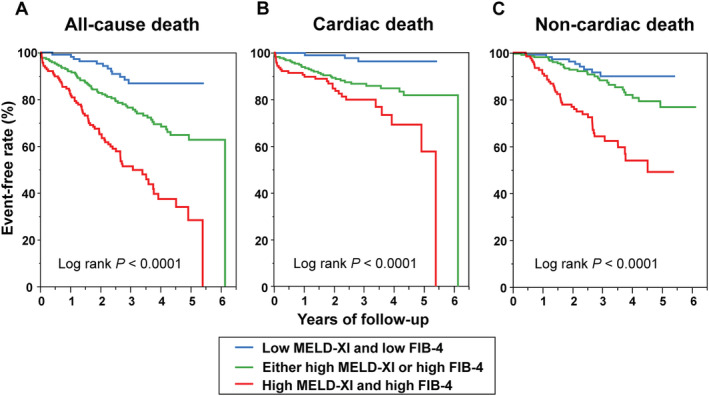
(A) All‐cause, (B) cardiac, and (C) non‐cardiac death free rate curves in patients with acute decompensated heart failure stratified by the median value of model for end‐stage liver disease excluding international normalized ratio score (MELD‐XI) and fibrosis‐4 index (FIB‐4) (overall cohort). Median value of MELD‐XI and FIB‐4 was 11 and 2.13, respectively.
Receiver operating characteristic curve analysis showed that combination of MELD‐XI score and FIB‐4 index had higher C‐statistic values for ACD, cardiac death, and non‐cardiac death than those calculated for MELD‐XI score or FIB‐4 index in isolation (Figure 3 ).
Figure 3.
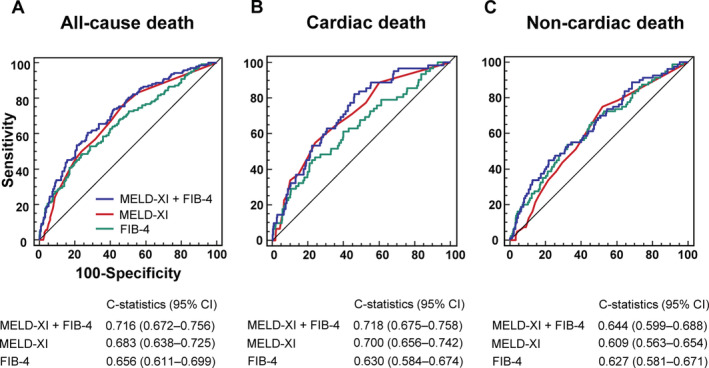
The receiver operating characteristic curve analysis for the prediction of (A) all‐cause, (B) cardiac, and (C) non‐cardiac death. CI, confidence interval; FIB‐4, fibrosis‐4 index; MELD‐XI, model for end‐stage liver disease excluding international normalized ratio score.
Prognostic analysis in each left ventricular ejection fraction subgroup
In multivariate Cox proportional hazards analysis, MELD‐XI score was independently associated with ACD in patients with HFrEF and HFmrEF, but not in patients with HFpEF (P for interaction = 0.0065). Conversely, FIB‐4 index was associated with ACD regardless of LVEF category (P for interaction = 0.8713, Table 2 ). Although MELD‐XI score in patients with HFrEF and FIB‐4 index in patients with HFpEF were associated with cardiac death, association between cardiac death and MELD‐XI score or FIB‐4 index was not affected by LVEF category (P for interaction = 0.2013 and 0.9681, respectively, Table 3 ). MELD‐XI score was associated with non‐cardiac death only in patients with HFmrEF (P for interaction = 0.0408), while FIB‐4 index showed a significant association with non‐cardiac death in patients with HFrEF, HFmrEF, and HFpEF (P for interaction = 0.8324, Table 4 ).
Kaplan–Meier analysis showed that patients with both a high MELD‐XI score and high FIB‐4 index and those with either a high MELD‐XI score or high FIB‐4 index had a significantly greater risk of ACD than those with a low MELD‐XI score and low FIB‐4 index in the groups with HFrEF [47% vs. 28% vs. 10%, respectively, Group 3 vs. Group 1: adjusted HR, 6.11 (95% CI: 2.52–18.2), P < 0.0001; Group 2 vs. Group 1: adjusted HR, 2.65 (95% CI: 1.73–4.13), P < 0.0001], HFmrEF [58% vs. 29% vs. 6%, respectively, Group 3 vs. Group 1: adjusted HR, 7.72 (95% CI: 1.56–19.8), P = 0.0077; Group 2 vs. Group 1: adjusted HR, 3.26 (95% CI: 1.71–6.24), P < 0.0001], and HFpEF [48% vs. 25% vs. 12%, respectively, Group 3 vs. Group 1: adjusted HR, 4.76 (95% CI: 2.16–12.6), P < 0.0001; Group 2 vs. Group 1: adjusted HR, 3.41 (95% CI: 1.90–6.42), P < 0.0001]. There was also a significant difference in the risk of cardiac death and non‐cardiac death among the three groups, except for the risk of cardiac death in patients with HFmrEF (Figure 4 ).
Figure 4.
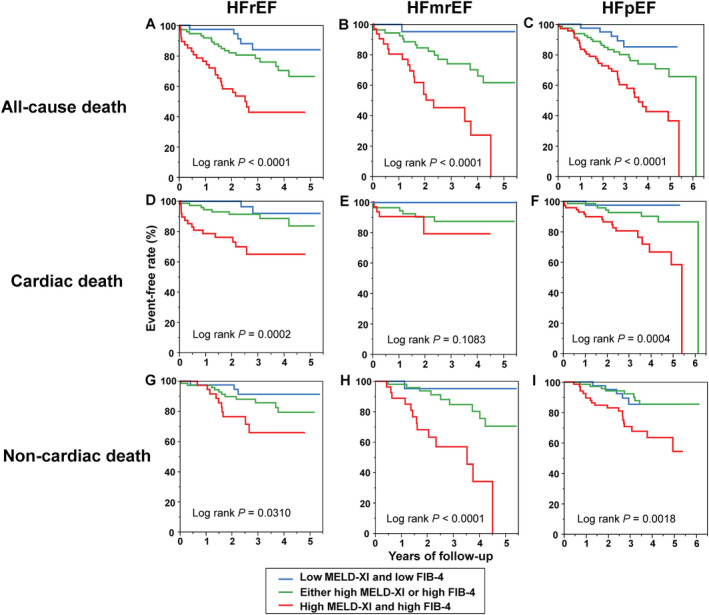
(A–C) All‐cause, (D–F) cardiac, and (G–I) non‐cardiac death free rate curves in patients with acute decompensated heart failure with heart failure with reduced ejection fraction (HFrEF), heart failure with mid‐range ejection fraction (HFmrEF), and heart failure with preserved ejection fraction (HFpEF) stratified by the median value of model for end‐stage liver disease excluding international normalized ratio score (MELD‐XI) and fibrosis‐4 index (FIB‐4). In patients with HFrEF, median value of MELD‐XI and FIB‐4 was 11 and 2.18, respectively. In patients with HFmrEF, median value of MELD‐XI and FIB‐4 was 11 and 2.20, respectively. In patients with HFpEF, median value of MELD‐XI and FIB‐4 was 11 and 1.98, respectively.
Discussion
In the present study, the MELD‐XI score and FIB‐4 index were shown to be independently associated with the risk of ACD, and the patients with both a higher MELD‐XI score and FIB‐4 index had a significantly higher risk of ACD. Moreover, the MELD‐XI score and FIB‐4 index were also shown to be related to the risk of cardiac death and non‐cardiac death, and the patients with both a higher MELD‐XI score and FIB‐4 index showed a significantly higher risk of cardiac death and non‐cardiac death. As shown in the ROC curve analysis, combination of MELD‐XI score and FIB‐4 index had higher C‐statistic values for ACD, cardiac death, and non‐cardiac death than those calculated for MELD‐XI score or FIB‐4 index alone. Risk stratification using the MELD‐XI score and FIB‐4 index was also useful when the patients were analysed in the each LVEF subgroup. To the best of our knowledge, this is the first study to show that the combination of MELD‐XI score and FIB‐4 index may be useful for stratifying patients at risk for ACD in patients with ADHF, irrespective of LVEF subgroup.
Liver dysfunction and cardiac dysfunction are thought to interact mutually. Cardiac dysfunction, especially in the ADHF phase, causes not only elevated venous pressure but also reduced liver blood flow and arterial flow. Increased hepatic venous pressure causes hepatocyte atrophy and oedema of the peripheral area in the liver, which provokes liver damage. The mechanism is known as hypoxic hepatopathy. 21 , 22 Hypoxic hepatopathy leads to acute hepatocellular necrosis and causes liver stiffness. 23 It was reported that elevated AST, one of the components of the FIB‐4 index, is a sensitive marker for hepatocellular necrosis and liver fibrosis. 23 Stagnant flow caused by increased hepatic venous pressure produces thrombosis within the liver lobules, which affects platelet count. 14 Furthermore, several bilirubin metabolic processes, a component of the MELD‐XI score, are attenuated by hepatocellular hypoxia. 24 , 25 The pathogenesis of hypoxic hepatopathy might result in a weak correlation between the FIB‐4 index and the MELD‐XI score. On the other hand, elevation of the MELD‐XI score, which is a score that allows one to evaluate the functions of the two organs critical to ADHF progression (the kidneys and liver), may result from abnormalities of either bilirubin, creatinine, or both, which suggests that the MELD‐XI score and the FIB‐4 index may provide independent prognostic information.
Recently, there has been an increase in patients with HF who have multiple co‐morbidities, such as hypertension and diabetes mellitus, which may affect clinical outcomes. Thus, identifying high‐risk patients is clinically important in the management of HF. Cardiohepatic syndrome is one of the more important co‐morbidities in patients with HF. Although liver biopsies are the gold standard for the evaluation of liver fibrosis, they are invasive and carry a risk of complications. Transient elastography is also used to evaluate liver dysfunction and fibrosis. Furthermore, transient elastography predicts adverse prognoses in patients with HF. 26 In the usual clinical setting, as an alternative simple, quick, and non‐invasive marker for the measurement of liver dysfunction and fibrosis, the MELD‐XI score and FIB‐4 index may be useful for patients with HF, although there exists no direct evidence that supports significant correlation between these two indices and the liver damage. Several previous studies have demonstrated that a higher MELD‐XI score and FIB‐4 index were associated with adverse events, mainly in patients with chronic HF. 11 , 16 In addition, the MELD‐XI score has been shown to be useful for the prediction of prognosis in patients with ADHF. 9 , 10 , 11 Our study has expanded on these findings by showing that the combination of the MELD‐XI score and FIB‐4 index is a useful tool for risk stratification also in patients with ADHF.
Several limitations of this study should be acknowledged. First, it was a single‐centre observational study. The small and empirically chosen population sample size was a significant limitation, and the follow‐up period was relatively short. Second, although patients with ADHF with documented liver disease were excluded, we could not completely deny the presence of liver disease. Third, as we included patients with ADHF who survived to discharge, association between the MELD‐XI score and FIB‐4 index and the in‐hospital mortality could not be examined. Fourth, there were no prespecified time points for follow‐up. Fifth, confirmation of the endpoints might not have been sufficient, because we could not use the national reporting databases. Lastly, because the MELD‐XI score includes serum creatinine level and strongly correlates with indices of renal function, it remains unclear if the MELD‐XI score has an additional value over serum creatinine level or renal function.
In conclusion, the combination of MELD‐XI score and FIB‐4 index may be useful for stratifying patients at risk for ACD in patients with ADHF, irrespective of LVEF. Further larger studies are needed to confirm our findings.
Conflict of interest
None declared.
Funding
No funding was received that was applicable to this study.
Acknowledgement
We thank Dr Yohei Sotomi (Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Japan) for his statistical advice.
Kawahira, M. , Tamaki, S. , Yamada, T. , Watanabe, T. , Morita, T. , Furukawa, Y. , Kawasaki, M. , Kikuchi, A. , Kawai, T. , Seo, M. , Nakamura, J. , Kayama, K. , Kimura, T. , Ueda, K. , Sakamoto, D. , Kogame, T. , Ito, S. , Chang, Y. , and Fukunami, M. (2021) Prognostic value of impaired hepato‐renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Failure, 8: 1274–1283. 10.1002/ehf2.13195.
References
- 1. Rocha BML, Menezes Falcao L. Acute decompensated heart failure (ADHF): a comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol 2016; 223: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol 2011; 20: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poelzl G, Ess M, Mussner‐Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest 2012; 42: 153–163. [DOI] [PubMed] [Google Scholar]
- 4. Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, Maggioni AP, Swedberg K, Konstam MA, Zannad F, Gheorghiade M, EVEREST trial investigators . Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail 2012; 14: 302–311. [DOI] [PubMed] [Google Scholar]
- 5. Samsky MD, Dunning A, DeVore AD, Schulte PJ, Starling RC, Tang WH, Armstrong PW, Ezekowitz JA, Butler J, McMurray JJ, Teerlink JR, Voors AA, Metra M, Mentz RJ, O'Connor CM, Patel CB, Hernandez AF. Liver function tests in patients with acute heart failure and associated outcomes: insights from ASCEND‐HF. Eur J Heart Fail 2016; 18: 424–432. [DOI] [PubMed] [Google Scholar]
- 6. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 7. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 8. Heuman DM, Mihas AA, Habib A, Gilles HS, Stravitz RT, Sanyal AJ, Fisher RA. MELD‐XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl 2007; 13: 30–37. [DOI] [PubMed] [Google Scholar]
- 9. Biegus J, Zymlinski R, Sokolski M, Siwolowski P, Gajewski P, Nawrocka‐Millward S, Poniewierka E, Jankowska EA, Banasiak W, Ponikowski P. Impaired hepato‐renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail 2016; 18: 1518–1521. [DOI] [PubMed] [Google Scholar]
- 10. Konno R, Tatebe S, Sugimura K, Satoh K, Aoki T, Miura M, Suzuki H, Yamamoto S, Sato H, Terui Y, Miyata S, Adachi O, Kimura M, Saiki Y, Shimokawa H. Prognostic value of the model for end‐stage liver disease excluding INR score (MELD‐XI) in patients with adult congenital heart disease. PLoS One 2019; 14: e0225403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biegus J, Demissei B, Postmus D, Cotter G, Davison BA, Felker GM, Filippatos G, Gimpelewicz C, Greenberg B, Metra M, Severin T, Teerlink JR, Voors AA, Ponikowski P. Hepatorenal dysfunction identifies high‐risk patients with acute heart failure: insights from the RELAX‐AHF trial. ESC Heart Fail 2019; 6: 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, Nash Clinical Research N. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McPherson S, Hardy T, Dufour JF, Petta S, Romero‐Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017; 112: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver stiffness assessed by Fibrosis‐4 index predicts mortality in patients with heart failure. Open Heart 2017; 4: e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yokokawa T, Sugimoto K, Yoshihisa A, Goto T, Misaka T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y. The fibrosis‐4 index is useful for predicting mortality in patients with pulmonary hypertension due to left heart disease. Int Heart J 2019; 60: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 18. Kondo T, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Ozaki T, Sato Y, Seo M, Ikeda I, Fukuhara E, Abe M, Nakamura J, Sakata Y, Fukunami M. Serial change in serum chloride during hospitalization could predict heart failure death in acute decompensated heart failure patients. Circ J 2018; 82: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 19. Seo M, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kawai T, Abe M, Nakamura J, Yamamoto K, Kayama K, Kawahira M, Tanabe K, Kimura T, Ueda K, Sakamoto D, Sakata Y, Fukunami M. Prognostic significance of serum cholinesterase in patients with acute decompensated heart failure: a prospective comparative study with other nutritional indices. Am J Clin Nutr 2019; 110: 330–339. [DOI] [PubMed] [Google Scholar]
- 20. Hakui H, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kondo T, Ishimi M, Sato Y, Seo M, Ozaki T, Ikeda I, Fukuhara E, Sakata Y, Fukunami M. Usefulness of cardiac metaiodobenzylguanidine imaging to improve prognostic power of the model for end‐stage liver disease scoring system in patients with mild‐to‐moderate chronic heart failure. Am J Cardiol 2016; 117: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 21. Iwasaki Y, Tomiyama H, Shiina K, Matsumoto C, Kimura K, Fujii M, Takata Y, Yamashina A, Chikamori T. Liver stiffness and arterial stiffness/abnormal central hemodynamics in the early stage of heart failure. Int J Cardiol Heart Vasc 2018; 20: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takahashi T, Watanabe T, Shishido T, Watanabe K, Sugai T, Toshima T, Kinoshita D, Yokoyama M, Tamura H, Nishiyama S, Arimoto T, Takahashi H, Yamanaka T, Miyamoto T, Kubota I. The impact of non‐alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels 2018; 33: 733–739. [DOI] [PubMed] [Google Scholar]
- 23. Biegus J, Hillege HL, Postmus D, Valente MA, Bloomfield DM, Cleland JG, Cotter G, Davison BA, Dittrich HC, Fiuzat M, Givertz MM, Massie BM, Metra M, Teerlink JR, Voors AA, O'Connor CM, Ponikowski P. Abnormal liver function tests in acute heart failure: relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail 2016; 18: 830–839. [DOI] [PubMed] [Google Scholar]
- 24. Shorey J, Schenker S, Combes B. Effect of acute hypoxia on hepatic excretory function. Am J Physiol 1969; 216: 1441–1452. [DOI] [PubMed] [Google Scholar]
- 25. Abe S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Liver dysfunction assessed by model for end‐stage liver disease excluding INR (MELD‐XI) scoring system predicts adverse prognosis in heart failure. PLoS One 2014; 9: e100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hopper I, Kemp W, Porapakkham P, Sata Y, Condon E, Skiba M, Farber L, Porapakkham P, Williams TJ, Menahem S, Roberts S, Krum H. Impact of heart failure and changes to volume status on liver stiffness: non‐invasive assessment using transient elastography. Eur J Heart Fail 2012; 14: 621–627. [DOI] [PubMed] [Google Scholar]


