Abstract
Aims
Biomarkers reflecting myocardial fibrosis and inflammation have been individually associated with left ventricular hypertrophy (LVH) and diastolic dysfunction (DD). However, the added value of a fibrosis‐inflammation multimarker approach in a populational setting is yet to be studied. We evaluated the value of a multimarker approach to detect LVH and DD in a large population‐based cohort.
Methods and results
In a prespecified analysis (BioSe‐PreIC study) of the 4th visit of the STANISLAS cohort (1705 subjects, 47 ± 14 years, 47.4% men), we evaluated the ability of brain natriuretic peptide (BNP), Galectin‐3 (GAL3), N‐terminal propeptide of procollagen type III (P3NP), and soluble ST2 to predict LVH (LV mass > 116/100 g/m2 for men/women) and DD using discrimination (C‐index) and reclassification analysis (NRI).
Participants with LVH and/or DD had significantly higher levels of BNP, GAL3, and ST2. Overall, the predictive value of clinical variables for LVH and/or DD was good (C‐index ranging from 0.76 to 0.82) and the addition of BNP, Gal3, P3NP, and ST2 moderately but significantly improved predictive value (delta C‐index = 0.03, P = 0.03 for LVH and 0.01, P = 0.01 for DD) and reclassification (NRI = 25.3, P = 0.02 for LVH and NRI = 32.7 for DD, P < 0.0001). Gal3, P3NP, and ST2 significantly improved predictive value (delta C‐index = 0.01, P = 0.01) and reclassification (NRI = 31.3, P < 0.0001) for DD of top of clinical variables and BNP.
Conclusions
As the measurement of Gal3, P3NP, and ST2 results in marginal (even if significant) increase in the prediction of DD/LVH on top of routine evaluation, their systematic use should not be promoted in unselected healthy individuals to screen for preclinical DD. Further research is needed to determine whether a more personalized medicine approach combing proteomic and clinical scoring can amplify the added value of biomarkers to identify preclinical DD.
Keywords: Biomarkers; Heart failure, diastolic; Echocardiography, Doppler; Brain natriuretic peptide; Collagen; Left ventricle; Systolic function
Background
Early identification of patients with cardiac abnormalities [Stage B—structural heart disease in the absence of clinical signs or symptoms of heart failure (HF)] represents a major challenge for research and clinical practice. 1 Recent guidelines emphasized the importance of the detection of preclinical diastolic dysfunction (DD) using conventional echocardiographic algorithms as they identify higher risk of developing overt HF. 2 , 3 Yet, a widespread systematic use of echocardiography as a screening tool cannot be routinely used in patients without symptoms primarily because of the lack of machine/physician time and high cost. Brain natriuretic peptide (BNP) alone has limited specificity to detect cardiac structural/functional disease in Stage B. 4 Whether a fibrosis‐inflammation multimarker approach can improve detection of LV remodelling and preclinical DD in a populational setting remains uncertain.
Aims
We aimed to evaluate the ability of four a priori selected serum biomarkers [BNP, Galectine‐3 (GAL3), N‐terminal propeptide of procollagen type III (P3NP), and ST2] to detect structural and/or functional cardiac disease [left ventricular hypertrophy (LVH) and/or DD] in a populational setting.
Methods
The STANISLAS Cohort has been described previously. 5 Briefly, it is a single‐centre familial longitudinal cohort which includes 1006 families (4295 subjects) from the Nancy region of France recruited in 1993–1995. From 2011 to 2015, a total of 1705 subjects aged 20 to 75 years underwent their 4th examination using high‐quality echocardiographic imaging at our department. In this prespecified analysis (BioSe‐PreIC study funded in 2012—specifically targeted to test the prespecified hypothesis above) of the 4th visit of the STANISLAS populational cohort (Lorraine, France), 5 we include all patients who attended the 4th visit (1705 subjects, 47 ± 14 years, 47.4% men) with available echocardiographic examinations (performed using Vivid 9, General Electric Medical Systems, Horten, Norway).
All cardiac chamber volumes and mass measures were measured according to current guidelines indexed to body surface area. 6 DD was graded according to the 2009 guidelines. 7 Briefly, as previously used by our group, two qualifying steps were applied: the first is based on e′ and left atrial volume index, while the second is based on E/A, E/e′, and deceleration time. 8 We assessed the additional value of biomarkers on top of the HOMAGE score 9 recently validated as an accurate predictive tool for HF. We measured BNP, GAL3, and ST2 using the OLINK Proseek® Multiplex. The assays use a proximity extension assay (PEA) technology, where oligonucleotide‐labelled antibody probe pairs bind to their respective targets and give rise to new DNA amplicons with each ID‐barcoding their respective antigens. The amplicons are subsequently quantified using a Fluidigm BioMark™ HD real‐time PCR platform. The good correlations of PEA‐based approach for the quantification of circulating plasma natriuretic peptides have been reported previously. 10 P3NP was measured by an ELISA (CISBIO, Saclay, France; dynamic range 2.2 to 30 μg/L; inter‐assay coefficient of variation 8%). The ability of biomarkers to predict LVH (LV mass > 116/100 g/m2 for men and women) and DD were evaluated using discrimination (C‐index), integrated discrimination improvement and reclassification analysis (NRI).
Results
The DD and LVH prevalence were 18% and 14%, respectively. Patients with LVH and/or DD were older and had higher body mass index and systolic blood pressure (Table 1 ). All four biomarkers were significantly higher in patients with DD and/or LVH. GAL3 had the highest discrimination value for DD (C‐index of GAL3 0.64 vs. 0.62 for BNP, 0.55 for ST2, and 0.54 for P3NP) whereas BNP and ST2 had the highest discrimination value for LVH (C‐index 0.68 and 0.62, respectively). Overall, the discrimination value of the HOMAGE score (age, body mass index, systolic blood pressure, heart rate, serum creatinine, smoking, diabetes mellitus, history of coronary artery disease, and use of antihypertensive medication) for LVH and/or DD was good (C‐index ranging from 0.76 to 0.82) (Figure 1 ), and the addition of BNP, Gal3, P3NP, and ST2 significantly but moderately improved discrimination value (delta C‐index = 2.6%, P = 0.03 for LVH and 1.2%, P = 0.01 for DD) and reclassification (NRI = 25.3, P = 0.02 for LVH and NRI = 32.7, P < 0.0001).
Table 1.
Subjects characteristics, circulating biomarkers, and imaging phenotype according to the presence of diastolic dysfunction and/or left ventricular hypertrophy
| Whole cohort | Individuals 50 or older | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Overall (n = 1705) | No DD/LVH (n = 1,313) | DD and/or LVH (n = 350) | P value | No DD/LVH (n = 633) | DD and/or LVH (n = 331) | P value |
| Clinical variables | |||||||
| Female gender | 878 (51.5%) | 681 (51.9%) | 175 (50.0%) | 0.55 | 315 (49.8%) | 165 (49.8%) | 1.00 |
| Age (years) | 48.9 ± 14.1 | 45.7 ± 13.8 | 60.2 ± 7.4 | <0.0001 | 59.2 ± 4.0 | 61.5 ± 5.0 | <0.0001 |
| BMI (kg/m2) | 26.0 ± 4.8 | 25.4 ± 4.5 | 27.8 ± 5.2 | <0.0001 | 26.4 ± 4.4 | 27.8 ± 5.2 | <0.0001 |
| Diabetes | 70 (4.1%) | 45 (3.4%) | 23 (6.6%) | 0.014 | 38 (6.0%) | 23 (7.0%) | 0.58 |
| Coronaropathy | 42 (2.5%) | 24 (1.8%) | 18 (5.2%) | 0.002 | 15 (2.4%) | 18 (5.5%) | 0.015 |
| Hypertension | 337 (19.8%) | 192 (14.7%) | 135 (38.8%) | <0.0001 | 163 (25.8%) | 133 (40.4%) | <0.0001 |
| SBP (mmHg) | 126 ± 16 | 124 ± 15 | 132 ± 18 | <0.0001 | 128 ± 16 | 133 ± 19 | <0.0001 |
| Biology and biomarkers | |||||||
| Creatinine (μmol/L) | 72.20 ± 14.11 | 71.66 ± 13.73 | 73.83 ± 15.48 | 0.012 | 72.73 ± 15.42 | 73.70 ± 15.44 | 0.16 |
| Natriuretic peptides B (BNP) | 1.43 ± 0.60 | 1.35 ± 0.50 | 1.71 ± 0.81 | <0.0001 | 1.52 ± 0.56 | 1.73 ± 0.81 | 0.003 |
| NT‐pro BNP | 3.58 ± 1.02 | 3.48 ± 0.96 | 3.95 ± 1.13 | <0.0001 | 3.80 ± 0.90 | 3.99 ± 1.10 | 0.031 |
| Galectin‐3 (Gal‐3) | 6.31 ± 0.34 | 6.28 ± 0.33 | 6.43 ± 0.33 | <0.0001 | 6.38 ± 0.32 | 6.44 ± 0.33 | 0.030 |
| ST2 protein (ST2) | 4.57 ± 0.61 | 4.54 ± 0.61 | 4.68 ± 0.62 | <0.0001 | 4.63 ± 0.54 | 4.68 ± 0.61 | 0.10 |
| P3NP (ng/mL) | 6.20 ± 2.09 | 6.14 ± 1.90 | 6.34 ± 2.72 | 0.082 | 6.07 ± 1.73 | 6.36 ± 2.77 | 0.047 |
| Echocardiography | |||||||
| LVEF (%) | 65 ± 7 | 65 ± 6 | 65 ± 8 | 0.81 | 66 ± 6 | 65 ± 8 | 0.029 |
| Indexed LVM (g/m2) | 76.2 ± 19.2 | 72.6 ± 15.7 | 90.0 ± 24.8 | <0.0001 | 76.4 ± 15.5 | 89.8 ± 24.5 | <0.0001 |
| Indexed LAV (mL/m2) | 22.8 ± 7.3 | 22.1 ± 6.7 | 25.6 ± 8.7 | <0.0001 | 22.5 ± 7.2 | 25.6 ± 8.9 | <0.0001 |
| E/A | 1.19 ± 0.42 | 1.27 ± 0.43 | 0.91 ± 0.26 | <0.0001 | 1.04 ± 0.30 | 0.89 ± 0.23 | <0.0001 |
| Mean E′:lateral septal (cm/s) | 11.4 ± 3.2 | 12.3 ± 2.8 | 7.9 ± 1.9 | <0.0001 | 10.4 ± 1.6 | 7.7 ± 1.6 | <0.0001 |
| E/E′ | 6.48 ± 1.85 | 6.02 ± 1.41 | 8.17 ± 2.32 | <0.0001 | 6.54 ± 1.41 | 8.24 ± 2.33 | <0.0001 |
| DT (ms) | 210.4 ± 53.3 | 207.3 ± 51.8 | 220.9 ± 56.3 | <0.0001 | 218.9 ± 52.9 | 223.3 ± 56.5 | 0.23 |
BMI, body mass index; BNP, brain natriuretic peptide; DT, deceleration time; LAV, left atrial volume; LVH, left ventricular hypertrophy; LVM, left ventricular mass; NT‐pro BNP, N‐terminal pro brain natriuretic peptide; SBP, systolic blood pressure.
FIGURE 1.
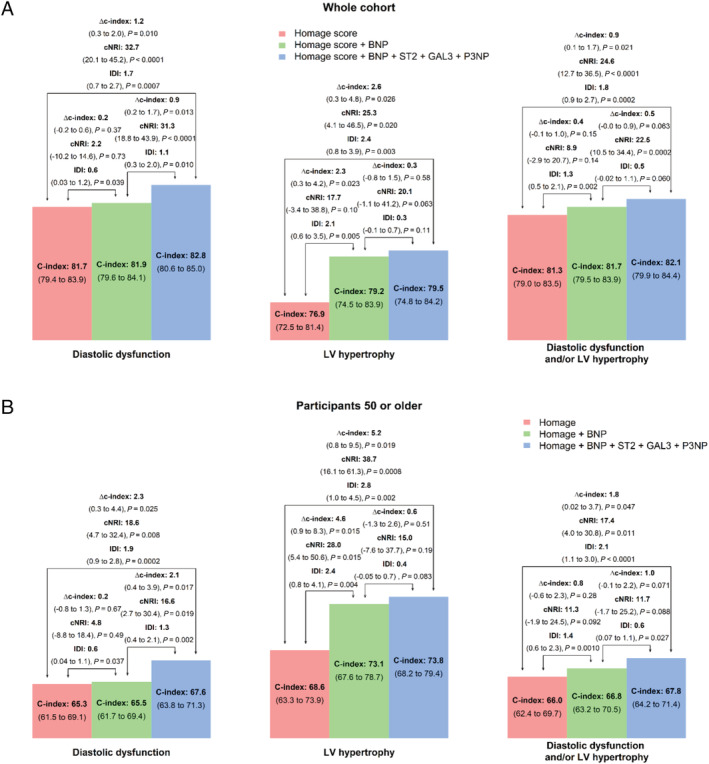
Discriminant value of brain natriuretic peptide (BNP), Galectin‐3 (GAL3), N‐terminal propeptide of procollagen type III (P3NP), and ST2 for left ventricular hypertrophy and/or diastolic dysfunction in the whole cohort (Panel A) and in patients 50 or older (Panel B).
In patients 50 or older, the predictive value of clinical variables were lower (all C‐index <0.70) whereas the added value of biomarkers remained significant (delta C‐index = 0.05, P = 0.02 for LVH and 0.02, P = 0.03 for DD).
In both the whole population and participants 50 or older, the addition of GAL3, ST2, and P3NP significantly increased the discrimination for DD on top of the HOMAGE score and BNP (P = 0.01 and P = 0.02, respectively), whereas BNP did not significantly increase the discrimination for DD on top of the HOMAGE score.
Considering N‐terminal pro BNP in place of BNP retrieved similar results (data not shown).
Conclusions
We provide evidence for the additional value of several circulating biomarkers, related to different biological pathway involved in HF, to predict LV remodelling and/or DD in a populational setting.
Natriuretic peptides are well‐established biomarkers for prognosis in HF patients but are currently not recommended as a screening tool in asymptomatic individuals especially in a population at low risk to develop overt HF. Importantly, in the results presented herein, BNP did not increase discrimination for DD.
In our study, we intended to improve the accuracy of the biological screening for functional/structural abnormality using additional biomarkers selected a priori based on their association with mechanistic pathways leading to HF. 11 We showed herein a significant association of these additional biomarkers, exploring fibrosis (Gal 3 and N‐terminal propeptide of procollagen type III) and inflammation (ST2), with structural and functional cardiac changes at a very early stage of the disease process. These results extend our understanding as these biomarkers were previously mostly studied in patients with overt HF.
The increase in myocardial Gal‐3 expression has been previously related to LVH and incident HF with preserved EF. 12 In our analysis, among the four biomarkers tested, Gal‐3 (C‐index 0.64) appears to be the most promising biomarkers to detect preclinical DD.
In the results reported herein, ST2 had some predictive value for LVH (C‐index 0.62). ST2 is related to myocardial inflammation, which can lead to myocardial fibrosis 13 and increase the risk for DD and incident HF with preserved EF. 14 Our results confirm the results of smaller studies reporting improved prediction of LVH and/or DD in preclinical HF patients with the isolated use of ST2. 15 , 16
We demonstrated significant differences in P3NP levels in participants >50 with and without cardiac abnormalities (P = 0.04) in a populational setting. This extends previous data showing that P3NP have diagnostic/prognostic value in patients with HFpEF 17 or elderly individuals. 18
The superiority of a multi‐biomarker approach on top of a mono‐marker (BNP) strategy has been reported for the detection of LVH, 19 but scarce evidence exist for the detection of DD. In our study, we show that a screening based on four biomarkers results in marginal but significant increase in the prediction of both LVH and DD on top of the HOMAGE score and that this significant increase persists when considering individuals aged 50 or older (as C‐index changes related to the addition of biomarkers increased while the predictive value of the HOMAGE score dropped in this subset). Importantly, the achieved increase appeared more important for DD than for LVH, possibly because of greater DD variability in this populational pre‐disease setting than LVH. In addition, the ageing process could increase DD and LVH variability to a greater extent in older individuals. In addition, our results show that the addition of GAL3, ST2, and P3NP have additive value on top of the HOMAGE score and BNP to detect DD.
Importantly, we used PEA to quantify natriuretic peptides, GAL3 and ST2. We have evidence of the adequacy of PEA‐based approach for natriuretic peptides, 10 but the quantification of GAL3 and ST2 with ELISA may have improved the value of these biomarkers to identify preclinical HF.
These findings suggest that a screening using four biological biomarkers improves our prediction for LVH and/or DD in populational settings, but that the achieved prediction remains fairly low. GAL3 for DD and ST2 for LVH appeared the most informative biomarkers. Approaches based on integrative approaches using more biological biomarkers, possibly combined with imaging biomarkers, are warranted to better identify individuals at risk for HF. Future studies should determine whether an approach based on GAL3 for DD and ST2 measurements in clinical settings, using predetermined thresholds and routine biological methods, have an impact on our detection of DD/LVH in routine practice.
Conflict of interest
None declared.
Funding
The STANISLAS study was sponsored by Nancy CHRU and supported by a public grant overseen by the French National Research Agency (ANR) as part of the second ‘Investissements d'Avenir’ programme (reference: ANR‐15‐RHUS‐0004). The BioSE‐PreIC study was funded by the "programme hospitalier de recherche clinique" (PHRCI 13‐084).
Acknowledgements
Subjects were included in the STANISLAS COHORT, which was supported by grants from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Inter‐régional 2008–2013) and sponsored by the CHU Nancy, F‐54000, Nancy, France.
The authors deeply thank the entire Clinical Investigation Centre staff all of whom are involved in the daily management of the STANISLAS cohort.
Huttin, O. , Kobayashi, M. , Ferreira, J. P. , Coiro, S. , Bozec, E. , Selton‐Suty, C. , Filipetti, L. , Lamiral, Z. , Rossignol, P. , Zannad, F. , and Girerd, N. (2021) Circulating multimarker approach to identify patients with preclinical left ventricular remodelling and/or diastolic dysfunction. ESC Heart Failure, 8: 1700–1705. 10.1002/ehf2.13203.
References
- 1. Echouffo‐Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta‐analysis. JACC Heart Fail 2016; 4: 237–248. [DOI] [PubMed] [Google Scholar]
- 2. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011; 306: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 4. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR, American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research . Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017; 135: e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira JP, Girerd N, Bozec E, Mercklé L, Pizard A, Bouali S, Eby E, Leroy C, Machu JL, Boivin JM, Lamiral Z. Cohort profile: rationale and design of the fourth visit of the STANISLAS cohort: a familial longitudinal population‐based cohort from the Nancy region of France. Int J Epidemiol 2018; 47: 395–395j. [DOI] [PubMed] [Google Scholar]
- 6. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 7. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 2009; 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 8. Huttin O, Fraser AG, Coiro S, Bozec E, Selton‐Suty C, Lamiral Z, Frikha Z, Rossignol P, Zannad F, Girerd N. Impact of changes in consensus diagnostic recommendations on the echocardiographic prevalence of diastolic dysfunction. J Am Coll Cardiol 2017; 69: 3119–3121. [DOI] [PubMed] [Google Scholar]
- 9. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang W, Zhang Z, Latini R, Masson S, Agabiti N, Sever P, Delles C. Risk for incident heart failure: a subject‐level meta‐analysis from the Heart “OMics” in AGEing (HOMAGE) Study. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2017; 6: e005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arrigo M, Vodovar N, Von Moos S, Masson E, Segerer S, Cippà PE, Mebazaa A. High accuracy of proximity extension assay technology for the quantification of plasma brain natriuretic peptide. J Clin Lab Anal 2018; 32: e22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira JP, Verdonschot J, Collier T, Wang P, Pizard A, Bär C, Björkman J, Boccanelli A, Butler J, Clark A, Cleland JG, Delles C, Diez J, Girerd N, González A, Hazebroek M, Huby AC, Jukema W, Latini R, Leenders J, Levy D, Mebazaa A, Mischak H, Pinet F, Rossignol P, Sattar N, Sever P, Staessen JA, Thum T, Vodovar N, Zhang ZY, Heymans S, Zannad F. Proteomic bioprofiles and mechanistic pathways of progression to heart failure. Circ Heart Fail 2019; 12: e005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Boer RA, Edelmann F, Cohen‐Solal A, Mamas MA, Maisel A, Pieske B. Galectin‐3 in heart failure with preserved ejection fraction. Eur J Heart Fail 2013; 15: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 13. Shah KB, Kop WJ, Christenson RH, Diercks DB, Henderson S, Hanson K, Li SY, deFilippi CR. Prognostic utility of ST2 in patients with acute dyspnea and preserved left ventricular ejection fraction. Clin Chem 2011; 57: 874–882. [DOI] [PubMed] [Google Scholar]
- 14. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 15. Daniels LB, Clopton P, Iqbal N, Tran K, Maisel AS. Association of ST2 levels with cardiac structure and function and mortality in outpatients. Am Heart J 2010; 160: 721–728. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y‐C, Yu C‐C, Chiu F‐C, Tsai C‐T, Lai L‐P, Hwang J‐J, Lin JL. Soluble ST2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients. J Card Fail 2013; 19: 163–168. [DOI] [PubMed] [Google Scholar]
- 17. Martos R, Baugh J, Ledwidge M, O'Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail 2009; 11: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, Tracy RP. The relationship between serum markers of collagen turnover and cardiovascular outcome in the elderly: the Cardiovascular Health Study. Circ Heart Fail 2011; 4: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zile MR, Desantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, Mehurg SM, Spinale FG. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail 2011; 4: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


