Abstract
Aims
The CANVAS Program identified the effect of canagliflozin on major adverse cardiovascular events (MACE) differed according to whether participants were using diuretics at study commencement. We sought to further evaluate this finding related to baseline differences, treatment effects, safety, and risk factor changes.
Methods and results
The CANVAS Program enrolled 10 142 participants with type 2 diabetes mellitus and high cardiovascular risk. Participants were randomized to canagliflozin or placebo and followed for a mean of 188 weeks. The primary outcome was major cardiovascular events, a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included multiple cardiovascular, renal, and safety events. In this post hoc subgroup analysis, participants were categorized according to baseline use of any diuretic. The effect on outcomes was compared using Cox proportional hazards models, while risk factor changes were compared using mixed‐effect models. At baseline, 4490 (44.3%) participants were using a diuretic. Compared with those not using a diuretic, participants using a diuretic were more likely to be older (mean age ± standard deviation, 64.3 ± 8.0 vs. 62.5 ± 8.3), be female (38.9% vs. 33.4%), and have heart failure (19.6% vs. 10.3%) (all P difference < 0.0001). The effect of canagliflozin on major cardiovascular events was greater for those using diuretic at baseline than for those who were not [adjusted hazard ratio 0.65 (95% confidence interval 0.54–0.78) vs. adjusted hazard ratio 1.13 (95% confidence interval 0.93–1.36), P heterogeneity < 0.0001]. Changes in most risk factors, including blood pressure, body weight, and urine albumin‐to‐creatinine ratio, were similar between groups (all P difference > 0.11), although the effect of canagliflozin on haemoglobin A1c reduction was slightly weaker in participants using compared with not using diuretics at baseline (−0.52% vs. −0.64%, P heterogeneity = 0.0007). Overall serious adverse events and key safety outcomes, including adverse renal events, were also similar (all P heterogeneity > 0.07).
Conclusions
Participants on baseline diuretics derived a greater benefit for major cardiovascular events from canagliflozin, which was not fully explained by differences in participant characteristics nor risk factor changes.
Keywords: Diuretics, Canagliflozin, CANVAS Program, Sodium‐glucose cotransporter 2 inhibitor (SGLT2i)
Introduction
Sodium‐glucose cotransporter 2 inhibitors (SGLT2i) cause clinically meaningful reductions in cardiovascular and renal morbidity and mortality in people with type 2 diabetes (T2DM), 1 , 2 , 3 , 4 , 5 established cardiovascular disease (CVD), chronic kidney disease, and albuminuria. 4 , 6 Individuals at high risk of CVD, but without established disease, are also likely to derive a benefit from this drug class, particularly in regard to primary and secondary prevention of heart failure (HF). 7
The primary CANVAS Program analyses identified heterogeneity in major adverse cardiovascular event (MACE) reduction with canagliflozin treatment depending on participant use of baseline diuretic therapy. 8 The mechanism for this difference is unclear but may be due to the natriuretic properties of SGLT2i 9 and potential for additive benefits with concomitant use of diuretic and SGLT2i therapy on endothelial function, 10 , 11 , 12 microvascular perfusion, 13 sequential nephron blockade, or a combination thereof. On the other hand, the heterogeneity of treatment effect could be due to baseline differences among patients taking diuretics, including baseline hypertension, CVD, or HF. Understanding the safety profile of SGLT2i among patients taking baseline diuretics is another important consideration for clinicians.
Accordingly, we sought to more comprehensively post hoc analyse the effects of canagliflozin on key clinical and safety outcomes in the CANVAS Program, stratified by diuretic use at study baseline. To better understand the observed heterogeneity and consideration of clinical implications of this heterogeneity, particularly potential changes in risk factors, we sought to compare the characteristics of participants by baseline diuretics use and the effects of canagliflozin compared with placebo on MACE, adverse events (AEs), and cardiovascular risk factors in each subgroup.
Methods
Program design and participants
The detailed protocols, statistical analysis plans, and main results of the CANVAS Program have been published. 8 , 14 , 15 In brief, the CANVAS Program consisted of two double‐blinded, placebo‐controlled randomized trials, CANVAS and CANVAS‐Renal (CANVAS‐R), which assessed the cardiovascular and renal efficacy and safety of canagliflozin in participants with T2DM and high cardiovascular risk. The trials were scheduled for joint closeout once at least 688 cardiovascular events and a minimum of 78 weeks of follow‐up accrued for the last randomized participant, which occurred in February 2017. The ethics committee at every centre approved the trial protocols (ClinicalTrials.gov NCT01032629 and NCT01989754). The investigation conformed with the principles outlined in the Declaration of Helsinki. All participants provided written informed consent.
CANVAS Program participants had T2DM [glycated haemoglobin (HbA1c) ≥ 7.0% and ≤10.5% and estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73 m2]. Participants were either aged ≥30 years with a history of symptomatic atherosclerotic vascular disease or aged ≥50 years with ≥2 risk factors for CVD. Risk factors included duration of T2DM of at least 10 years, systolic blood pressure (SBP) > 140 mmHg while receiving one or more antihypertensive agents, current smoker, microalbuminuria or macroalbuminuria, or high‐density lipoprotein cholesterol (HDL‐C) < 1 mmol/L. 8 Prevalent HF was not an exclusion criterion for the CANVAS Program.
Participants included in this post hoc subgroup analysis were categorized according to diuretic use recorded at baseline by study investigators. In this analysis, we classified diuretic categories into (i) any loop diuretics (furosemide, bumetanide, torsemide, piretanide, and ethacrynic acid), (ii) thiazide, thiazide‐like, or other diuretics (any thiazides, altizide, chlorthalidone, clopamide, indapamide, metolazone, xipamide, and metipamide), and (iii) mineralocorticoid receptor antagonists (MRAs; spironolactone and eplerenone). However, in CANVAS‐R trial, diuretic use was categorized as loop or non‐loop without further non‐loop‐specific drug data. To further explore the association between baseline diuretic use and cardiovascular, renal, and safety outcomes, participants were also stratified according to baseline atherosclerotic cardiovascular disease (ASCVD) or HF, in separate and combined analyses.
Randomized treatment and follow‐up
Participants in CANVAS were randomly assigned in a 1:1:1 ratio to canagliflozin 100 mg, canagliflozin 300 mg, or matching placebo, while participants in CANVAS‐R were randomly assigned in a 1:1 ratio to canagliflozin or matching placebo, initially 100 mg with an optional increase to 300 mg from Week 13. Participants and trial and sponsor staff were blinded to individual treatment allocations. Background glycaemic and cardiovascular therapies were managed according to best practice. Follow‐up visits were scheduled at least three times in the first year and every 6 months thereafter, with alternating telephone and face‐to‐face follow‐up. Primary and secondary outcome events and serious adverse events (SAEs) were evaluated at every follow‐up visit.
Outcomes
The primary outcome for the CANVAS Program was the rate of MACE, a composite of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke. Secondary outcomes included individual rates of cardiovascular death, nonfatal MI, nonfatal stroke, fatal or nonfatal MI, fatal or nonfatal stroke, cardiovascular death or hospitalization for heart failure (HHF), HHF, and all‐cause mortality. Effects on kidney function were assessed using a composite renal outcome (defined as a 40% reduction in eGFR requirement for renal replacement therapy, or renal death) and progression of albuminuria (defined as >30% increase in albuminuria and a change from either normoalbuminuria to microalbuminuria or macroalbuminuria or from microalbuminuria to macroalbuminuria). CVD risk factors were also analysed, including changes in SBP, diastolic blood pressure, pulse, body weight, HbA1c, haematocrit, urinary albumin‐to‐creatinine ratio (UACR), and uric acid.
Both serious and non‐serious AEs were collected and reported in the CANVAS trial until January 2014, as mandated by regulatory agencies for initial approval of canagliflozin. After January 2014, only SAEs, AEs leading to study drug discontinuation, and selected AEs of interest were collected in the CANVAS trial. This streamlined the AE collection approach used for the entirety of CANVAS‐R. Accordingly, due to the differences in AE recording between CANVAS and CANVAS‐R, an integrated analysis for some outcomes was not possible, and CANVAS trial data are reported alone. All SAEs are reported for the entire CANVAS Program.
Statistical analysis
Categorical variables are presented as patient numbers with corresponding percentages, and continuous variables are presented as means with standard deviations (SDs) or medians with interquartile ranges. Baseline characteristics between baseline diuretic and non‐diuretic groups were compared by χ 2 test or generalized Cochran–Mantel–Haenszel test for categorical variables or Wilcoxon two‐sample test or Wilcoxon rank‐sum test for continuous variables.
Post hoc efficacy analyses were based upon the full integrated dataset and the intention‐to‐treat principle using all follow‐up time on or off study treatment, with the comparison between all participants assigned to canagliflozin (regardless of drug dose) and all participants assigned to placebo. Analyses were based on the occurrence of the first event under investigation. Annualized incidence rates per 1000 patient‐years of follow‐up were calculated for all outcomes, in addition to hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox regression models with stratification according to trial (CANVAS or CANVAS‐R) and baseline history of CVD. The heterogeneity of the treatment effect across subgroups defined by baseline diuretic use was examined by including a treatment–diuretic interaction term in the respective Cox proportional hazards model. A two‐sided P‐value of <0.05 for the interaction term was deemed likely to reflect a difference beyond chance. Where evidence for interaction was observed, multivariable models were fit with adjustment for baseline characteristics that were different between the subgroups (age, gender, race, smoking history, diabetes duration, history of HF, baseline weight, baseline SBP, baseline HbA1c, baseline eGFR, baseline UACR, baseline lipid, and cardiovascular medications, including statins, antithrombotics, renin–angiotensin–aldosterone system inhibitors, and beta‐blockers).
Changes by treatment group in intermediate markers of cardiovascular risk, including blood pressure, pulse, body weight, and HbA1c, were assessed by repeated measures from baseline across the entire follow‐up period. The average change in these continuous outcomes from baseline by canagliflozin treatment and the difference in treatment effect between those on and not on diuretics at baseline were analysed using mixed‐effect models for repeated measures including all data up to Week 312 and covariates for study, visit, treatment, baseline measures, treatment‐by‐visit, and baseline‐by‐visit interactions. An interaction term for treatment–diuretic use at baseline was included in the model to test for heterogeneity between groups. Due to the highly skewed distribution of UACR data, UACR values were log‐transformed, and the geometric mean of post‐baseline UACR was estimated. Changes in albuminuria were calculated as the ratio of the geometric mean of post‐randomization UACR changes on canagliflozin was compared with placebo in those using vs. not using diuretic therapy at baseline.
For safety outcomes, an on‐treatment analysis was performed using only events that occurred among participants who had a safety outcome while they were receiving canagliflozin or placebo, or within 30 days after discontinuation of drug or placebo. The exception was for amputation and fracture outcomes, where analyses included participants who received at least one dose of canagliflozin or placebo and had an event at any time during follow‐up.
We present a complete case analysis and did not perform imputation to account for missing data. Analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC, USA) and SAS Enterprise Guide Version 7.1 (SAS Institute).
Results
Baseline characteristics
The CANVAS Program randomized 10 142 participants [CANVAS (n = 4330) and CANVAS‐R (n = 5812)] who had a mean follow‐up time of 188 weeks. The flow chart of participants included in this analysis is shown in Supporting Information, Figure S1 . The mean age of participants was 63.3 years, 64.2% were men, mean duration of diabetes was 13.5 years, and 65.6% had a history of CVD.
Baseline characteristics of participants by use of any diuretics at baseline are presented in Table 1 . Within the diuretic and non‐diuretic groups, baseline participant characteristics were balanced by randomization status to canagliflozin or placebo (Table S1 ). Different characteristics of participants by use of loop diuretics, thiazides, or MRAs based on available data are summarized in Table S2 . Nearly half (n = 4490; 44.3%) of study participants were using a diuretic at baseline, with no significant difference observed in the frequency of diuretic use between treatment groups (43.8% on canagliflozin, 45.0% on placebo; P = 0.23). This included 3182 (31.4%) using a non‐loop diuretic, 1350 (13.3%) using thiazide, thiazide‐like, or other diuretic, 1308 (12.9%) participants using a loop diuretic, and 52 (0.5%) participants using an MRA (Table S3 ).
Table 1.
Characteristics of participants in the CANVAS Program, stratified by baseline use of any diuretic a
| Baseline any diuretic use (n = 4490) | Baseline no diuretic use (n = 5652) | P‐value b | |
|---|---|---|---|
| Age, years, mean (SD) | 64.3 (8.0) | 62.5 (8.3) | <0.0001 |
| Male, no. (%) | 2744 (61.1) | 3765 (66.6) | <0.0001 |
| Race, no. (%) | <0.0001 g | ||
| White | 3743 (83.4) | 4201 (74.3) | |
| Asian | 348 (7.8) | 936 (16.6) | |
| Black or African American | 167 (3.7) | 169 (3.0) | |
| Other/missing c | 232 (5.2) | 346 (6.1) | |
| Current smoker, no. (%) | 664 (14.8) | 1142 (20.2) | <0.0001 |
| History of hypertension, no. (%) | 4335 (96.5) | 4790 (84.7) | <0.0001 |
| Duration of diabetes, years, mean (SD) | 14.2 (7.9) | 13.1 (7.6) | <0.0001 |
| History of heart failure, no. (%) | 878 (19.6) | 583 (10.3) | <0.0001 |
| History of atrial fibrillation, no. (%) | 401 (8.9) | 212 (3.8) | <0.0001 |
| History of myocardial infarction, no. (%) | 1335 (29.7) | 1621 (28.7) | 0.25 |
| Microvascular disease history, % | |||
| Retinopathy | 1047 (23.3) | 1082 (19.1) | <0.0001 |
| Nephropathy | 931 (20.7) | 843 (14.9) | <0.0001 |
| Neuropathy | 1510 (33.6) | 1600 (28.3) | <0.0001 |
| Atherosclerotic vascular disease history, no. (%) d | |||
| Coronary | 2607 (58.1) | 3114 (55.1) | 0.003 |
| Cerebrovascular disease | 977 (21.8) | 981 (17.4) | <0.0001 |
| Peripheral | 993 (22.1) | 1120 (19.8) | 0.005 |
| Any | 3290 (73.3) | 4034 (71.4) | 0.03 |
| Cardiovascular disease history e , no. (%) | 2943 (65.6) | 3713 (65.7) | 0.88 |
| History of coronary revascularization, no. (%) | 1613 (35.9) | 1951 (34.5) | 0.14 |
| History of CABG, no. (%) | 403 (15.6) | 416 (12.9) | 0.004 |
| History of amputation, no. (%) | 110 (2.4) | 128 (2.3) | 0.54 |
| Weight, kg, mean (SD) | 94.3 (21.2) | 86.8 (18.9) | <0.0001 |
| Body mass index, kg/m2, mean (SD) | 33.4 (6.2) | 30.8 (5.4) | <0.0001 |
| Systolic blood pressure, mmHg, mean (SD) | 138.2 (16.3) | 135.4 (15.2) | <0.0001 |
| Diastolic blood pressure, mmHg, mean (SD) | 77.6 (10.0) | 77.8 (9.4) | 0.16 |
| Glycated haemoglobin, %, mean (SD) | 8.2 (0.9) | 8.3 (0.9) | 0.05 |
| Total cholesterol, mmol/L, mean (SD) | 4.3 (1.2) | 4.4 (1.1) | 0.0001 |
| Triglycerides, mmol/L, mean (SD) | 2.1 (1.4) | 2.0 (1.4) | 0.0005 |
| LDL cholesterol, mmol/L, mean (SD) | 2.2 (0.9) | 2.3 (0.9) | <0.0001 |
| HDL cholesterol, mmol/L, mean (SD) | 1.2 (0.3) | 1.2 (0.3) | 0.0008 |
| eGFR, mL/min/1.73 m2, mean (SD) | 71.9 (19.7) | 80.1 (20.4) | <0.0001 |
| UACR, mg/g, median (IQR) f | 13.5 (6.9–53.2) | 11.5 (6.5–34.9) | <0.0001 i |
| Normoalbuminuria, no. (%) | 2938 (66.2) | 4069 (72.7) | <0.0001 |
| Microalbuminuria, no. (%) | 1096 (24.7) | 1170 (20.9) | |
| Macroalbuminuria, no. (%) | 402 (9.1) | 358 (6.4) | |
| Drug therapy, no. (%) | |||
| Insulin | 2526 (56.3) | 2569 (45.5) | <0.0001 |
| Sulfonylurea | 1768 (39.4) | 2593 (45.9) | <0.0001 |
| Metformin | 3329 (74.1) | 4496 (79.6) | <0.0001 |
| GLP‐1 receptor agonist | 202 (4.5) | 205 (3.6) | 0.03 |
| DPP‐4 inhibitor | 591 (13.2) | 670 (11.9) | 0.05 |
| Thiazolidinedione | 195 (4.3) | 297 (5.3) | 0.03 |
| Statin | 3549 (79.0) | 4051 (71.7) | <0.0001 |
| Antithrombotic h | 3454 (76.9) | 4017 (71.1) | <0.0001 |
| RAAS inhibitor | 3995 (89.0) | 4121 (72.9) | <0.0001 |
| Beta‐blocker | 2742 (61.1) | 2679 (47.4) | <0.0001 |
| Calcium blocker | 1931 (43.0) | 1512 (26.8) | <0.0001 |
CANVAS, CANagliflozin cardioVascular Assessment Study; CANVAS‐R, CANagliflozin cardioVascular Assessment Study—Renal; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; RAAS, renin–angiotensin–aldosterone system; SD, standard deviation; UACR, urine albumin‐to‐creatinine ratio.
One participant was randomized at two different sites, and only the first randomization is included in the intention‐to‐treat analysis set.
P‐value for comparison between total participants with diuretics at baseline and total participants without diuretics at baseline.
Includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, and unknown.
Some patients had more than one type of atherosclerotic vascular disease.
A history of cardiovascular disease was defined as a history of symptomatic atherosclerotic vascular disease (coronary, cerebrovascular, or peripheral).
Values for albuminuria categories calculated based on N of 4436 with diuretics and N of 5597 without diuretics and 10 033 for the total population for the CANVAS Program.
P‐value corresponds to generalized Cochran–Mantel–Haenszel test for no general association.
Includes antiplatelets and anticoagulants.
P‐value corresponds to Wilcoxon rank‐sum test of equal media.
Participants using a diuretic were older and more often female, were less frequently current smokers, had a longer duration of T2DM, and were more likely to have established hypertension, HF, and microvascular or cerebrovascular disease as compared with those without baseline diuretic therapy (all P < 0.0001). There was no significant difference in the proportion of participants with a history of MI or CVD between groups, but patients on baseline diuretics had lower eGFR [mean (SD): 71.9 (19.7) vs. 80.1 (20.4) mL/min/1.73 m2, P < 0.0001], higher SBP [mean (SD): 138.2 (16.3) vs. 135.4 (15.2) mmHg, P < 0.0001], and higher UACR [median (interquartile range): 13.5 (6.9–53.2) vs. 11.5 (6.5–34.9), P < 0.0001]. Participants on baseline diuretic therapy used more cardiovascular medicines (including statins, antithrombotic agents, beta‐blockers, and renin–angiotensin–aldosterone system inhibitors) and insulin (all P < 0.0001). Conversely, a lower proportion of those on diuretics used oral sulfonylurea, metformin, and thiazolidinediones.
Among 1468 (32.7%) participants with available data about the indication for diuretic administration from the CANVAS trial (Table S4 ), most (1155, 78.7%) participants were prescribed diuretics for hypertension, while 89 (6.1%) for HF and 74 (5.0%) for oedema.
Cardiovascular and renal outcomes
The effects of canagliflozin vs. placebo on cardiovascular and renal outcomes in subgroups stratified by baseline diuretic use are presented in Figure 1 . Canagliflozin treatment was associated with greater risk reduction in MACE among participants using diuretics compared with those not using diuretics [HR 0.66 (95% CI 0.56–0.79) vs. HR 1.11 (95% CI 0.93–1.34), P heterogeneity < 0.0001]. The observed difference was driven by nonfatal MI [HR 0.58 (95% CI 0.43–0.77) vs. HR 1.25 (95% CI 0.92–1.69), P heterogeneity = 0.001], although the pattern was similar for cardiovascular death (P heterogeneity = 0.06) and nonfatal stroke (P heterogeneity = 0.07). There was no heterogeneity between groups using vs. not using diuretics for HHF (P heterogeneity = 0.58), albuminuria (P heterogeneity = 0.62), the renal composite outcome (P heterogeneity = 0.23), or all‐cause mortality (P heterogeneity = 0.37).
Figure 1.
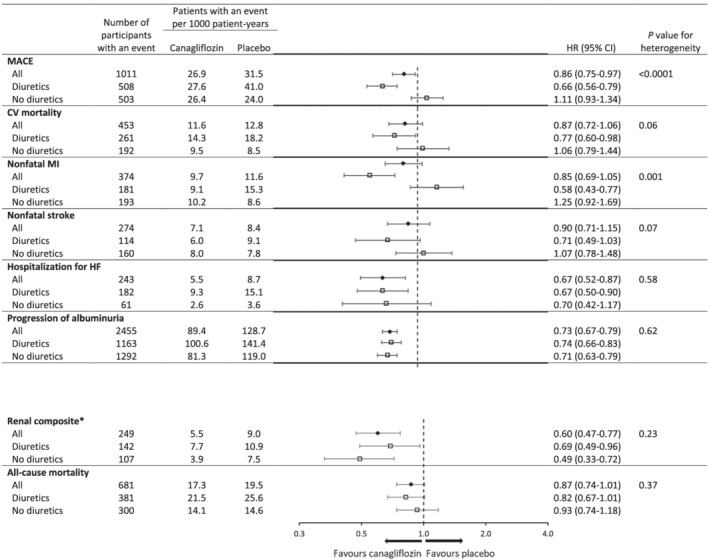
Effects of canagliflozin on cardiovascular and renal outcomes in participants in the CANVAS Program, stratified by baseline use of any diuretic. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with the use of Cox regression models, with stratification according to trial and history of cardiovascular disease for all canagliflozin groups combined versus placebo. CV, cardiovascular; HF, heart failure; MACE, major adverse cardiovascular event, including death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke; MI, myocardial infarction. *40% reduction in estimated glomerular filtration rate, end‐stage kidney disease, or death from renal causes.
The effects of canagliflozin vs. placebo on MACE in subgroups, stratified by diuretic type at baseline, are presented in Figure 2 . A similar pattern in terms of a greater relative risk reduction for MACE among patients on baseline diuretics was observed for baseline loop and thiazide, thiazide‐like, and other diuretics, but this observation was statistically significant only for participants on baseline thiazide, thiazide‐like, and other diuretics [HR 0.61 (95% CI 0.47–0.80) vs. HR 0.94 (95% CI 0.81–1.08), P heterogeneity = 0.006]. The CIs around the effect estimates for those participants using an MRA at baseline were too wide to draw definitive conclusions. There was no heterogeneity on cardiovascular, renal, and safety outcomes among patients with and without baseline ASCVD (Figure S2 ) or HF (Figure S3 ) separately, as well as in a combined analysis (Figure S4 ).
Figure 2.
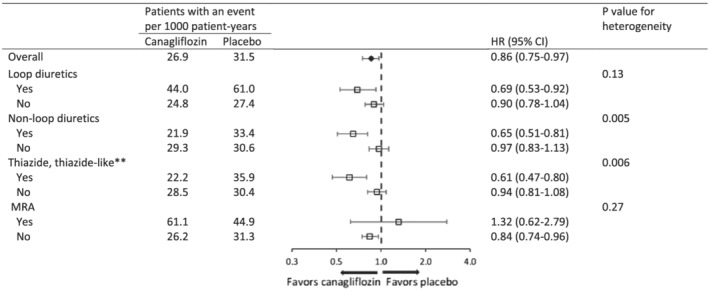
Effects of canagliflozin on major adverse cardiac events in participants in the CANVAS Program, stratified by baseline use of diuretic classes*. *In CANVAS Program, any diuretics were categorized as loop or non‐loop diuretics. In CANVAS trial, non‐loop diuretics were further categorized with specific drug classes. **Includes thiazide, thiazide‐like, or other diuretics.
The adjusted analysis for the outcome of MACE did not attenuate the heterogeneity observed among participants using and not using baseline diuretics [adjusted HR 0.65 (95% CI 0.54–0.78) vs. adjusted HR 1.13 (95% CI 0.93–1.36), P heterogeneity < 0.0001; Table S5 ]. In the adjusted models, a greater relative risk reduction from canagliflozin therapy was observed among those on baseline diuretics compared with those not on baseline diuretic therapy for cardiovascular death [adjusted HR 0.74 (95% CI 0.58–1.94) vs. adjusted HR 1.12 (95% CI 0.83–1.52), P heterogeneity = 0.04] and nonfatal stroke [adjusted HR 0.66 (95% CI 0.45–0.97) vs. adjusted HR 1.10 (95% CI 0.79–1.53), P heterogeneity = 0.04]. Estimates of heterogeneity for the other outcomes were unchanged in adjusted models.
Cardiovascular risk factors
The effects of canagliflozin on changes in intermediate markers of cardiovascular risk factors by diuretic subgroup are reported in Figure 3 and Table S6 . Greater reductions in pulse (−0.50 vs. 0.03 b.p.m., P heterogeneity = 0.02) and uric acid (−26.4 vs. −20.1 μmol/L, P heterogeneity = 0.02) were observed with canagliflozin treatment vs. placebo in those on baseline diuretic therapy compared with those not on diuretic therapy. Reductions in SBP (−3.98 and −3.88 mmHg; P heterogeneity = 0.86), DBP (−1.58 vs. −1.26 mmHg; P heterogeneity = 0.19), body weight (−1.56 vs. −1.63 kg; P heterogeneity = 0.26), haematocrit (0.03% vs. 0.03%; P heterogeneity = 0.93), and UACR (−20% vs. −16%; P heterogeneity = 0.11) were similar between diuretic subgroups. Canagliflozin reduced HbA1c to a modestly lower amount in participants on baseline diuretics compared with those not on diuretics (−0.52% vs. −0.64%; P heterogeneity = 0.0007).
Figure 3.
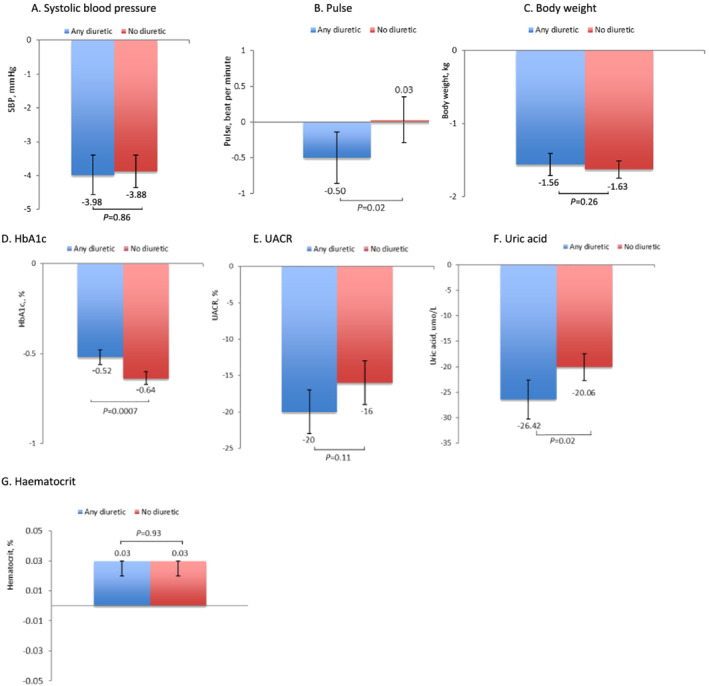
Effect of canagliflozin* on (A) systolic blood pressure; (B) pulse; (C) body weight; (D) haemoglobin A1c; (E) urinary albumin‐to‐creatinine ratio; (F) serum uric acid; and (G) haematocrit in participants in the CANVAS Program, stratified by baseline use of any diuretic. *Calculated as mean change from baseline across the entire follow‐up period. The average change in these continuous outcomes from baseline by canagliflozin treatment and the difference in treatment effect between those on and not on diuretics at baseline were analysed using mixed‐effect models for repeated measures including all data up to Week 312 and covariates for study, visit, treatment, baseline measures, treatment‐by‐visit, and baseline‐by‐visit interactions.
Adverse events
Serious adverse outcomes, AEs leading to discontinuation, AEs of interest in the CANVAS Program, and other selected AEs in the CANVAS trial alone are reported in Figure 4 . There was no difference in AEs between subgroups defined by baseline diuretic use (all P heterogeneity > 0.05), including for all SAEs, AEs leading to discontinuation, or serious renal safety outcomes, including acute kidney injury.
Figure 4.
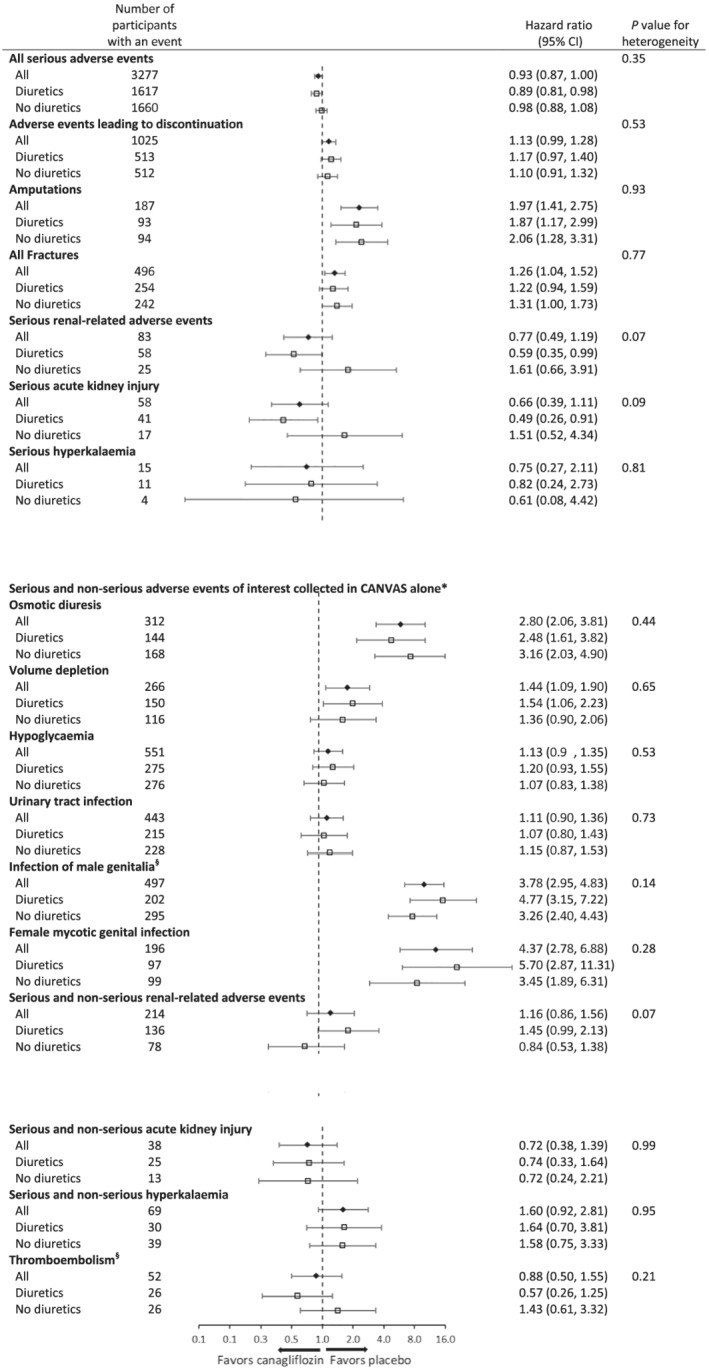
Adverse events in the CANVAS Program participants stratified by baseline use of any diuretic. CANVAS indicates Canagliflozin Cardiovascular Assessment Study; CI, confidence interval. *The annualized event rates are reported with data from CANVAS alone through 7 January 2014, because after this time, only serious adverse events or adverse events leading to discontinuation were collected. In CANVAS‐R, only serious adverse events or adverse events leading to discontinuation were collected. Owing to the differences between the two trials in methods of collection of the data, an integrated analysis of these adverse events is not possible. §Data collected in CANVAS and CANVAS‐R.
Discussion
Participants with T2DM on baseline diuretic therapy experienced a greater relative risk reduction in MACE from canagliflozin therapy than those not on baseline diuretics, which was largely driven by differences in the rate of nonfatal MI. This was not explained by a greater reduction in any intermediate markers of cardiovascular health, such as body weight, SBP, pulse, UACR, or HbA1c. Further, the adjusted models suggest that differences in baseline participant characteristics between those on and not on diuretics were not responsible for the differences in outcomes that have been observed across diuretic subgroups.
Heterogeneous effects of SGLT2i in patient using vs. not using diuretics were not observed in the only other large SGLT2i study—the EMPA‐REG OUTCOME Trial 1 —which reported data for this subgroup. However, EMPA‐REG only included patients with high risk of cardiovascular events, which was defined as pre‐existing atherosclerotic CVD (76% with coronary artery disease), which may have contributed to this differential finding, as similar rate of baseline diuretic use was present (43%). Neither DECLARE TIMI‐58 3 nor CREDENCE 6 trial reported cardiovascular outcomes for this subgroup. However, DECLARE TIMI‐58 demonstrated a greater effect of dapagliflozin on cardiovascular death among individuals with baseline HF with reduced ejection fraction [HR 0.55 (95% CI 0.34–0.90)] compared with those without HF with reduced ejection fraction [HR 1.08 (95% CI 0.89–1.31), P heterogeneity = 0.01]. 16 DECLARE TIMI‐58 also showed that dapagliflozin had a smaller effect on a composite renal‐specific outcome (i.e. eGFR decrease ≥40% to <60 mL/min/1.73 m2; end‐stage renal disease; or renal death) among individuals taking diuretics at baseline [HR 0.72 (95% CI 0.54–0.95) vs. HR 0.36 (95% CI 0.25–0.50), P heterogeneity = 0.002]. 17 On the other hand, there was no heterogeneity observed for the composite cardiorenal outcome (i.e. eGFR decrease ≥40% to <60 mL/min/1.73 m2; end‐stage renal disease; or renal or cardiovascular death) based on baseline diuretic status. Recently, the DAPA‐HF trial in patients with HF and reduced ejection fraction 18 demonstrated that the efficacy and safety of SGLT2i dapagliflozin were consistent across subgroups in relation to background diuretic treatment. 19 Compared with placebo, there was no heterogeneity found across each of diuretic subgroups regarding the risk of the primary endpoint [HR for no diuretic, diuretic dose equivalent to furosemide <40, 40, and >40 mg daily at baseline separately: 0.57 (95% CI 0.36–0.92), 0.83 (95% CI 0.63–1.10), 0.77 (95% CI 0.60–0.99), and 0.78 (95% CI 0.63–0.97), respectively; P for interaction = 0.61].
The observed differences in outcomes in CANVAS for post hoc subgroups using vs. not using diuretics at baseline generated several hypotheses about possible mechanisms of effect. For example, it could be hypothesized that the observed results were driven by clinically diagnosed or sub‐clinical HF, but a 2019 meta‐analysis of SGLT2i has found no evidence of heterogeneity of effect by baseline HF status. 6 It could also be postulated that differences in patient characteristics such as higher baseline SBP, longer duration of diabetes, higher smoking rates, and differential use of other therapies might explain the observed heterogeneity. However, while there was a strong rationale for expecting such differences to drive variation in overall risk between subgroups, there was no strong reason for anticipating that these differences would drive variation in the effects of the drug on clinical outcomes, which is supported by the stability of the results after multivariable adjustment. While it might be expected that the findings may be due to difference in changes in cardiovascular risk factor over the study period, including blood pressure, risk factors changes were similar between groups, except for modest differences in pulse, HbA1c, and uric acid.
A potential renal mechanism, mediated via diuretic and natriuretic effects, 20 may partially explain the observed subgroup difference seen in this analysis, including for nonfatal MI. SGLT2 inhibition exerts a number of effects on body sodium and fluid, which are distinct and may be complementary to other diuretics given differential targets on the nephron. These drug classes may have differential effects on interstitial vs. intravascular compartments; for example, loop diuretics may reduce both interstitial and intravascular volume, 21 while SGLT2i may promote natriuresis and volume effects mainly via selectively reducing interstitial oedema rather than intravascular volume. This potentially limits adverse reflex neurohumoral stimulation that occurs in response to intravascular volume contraction, 10 but further research is needed to better understand these effects. Meanwhile, it may be possible that sequential nephron blockade associated with use of multiple diuretic classes could lead to more effective reductions in left ventricular end‐diastolic pressure (LVEDP) without as much reflex neurohumoral stimulation and thus lower risk of nonfatal MI. Furthermore, there is a possible link between coronary microvascular dysfunction and left ventricular diastolic function in patients with diabetes 22 or HF with preserved ejection fraction. 23 Therefore, canagliflozin with diuretics might reduce chance for MI via lowering LVEDP. Additionally, elevated LVEDP is associated cross‐sectionally with increased high‐sensitivity C‐reactive protein (CRP), 24 such as activating hypoxic stress, which has been shown to induce cardiomyocyte production of interleukin‐6, 25 which, in turn, could stimulate the liver to produce CRP, which may be a trigger for MI events. The association between elevated LVEDP and elevated CRP and other inflammatory markers is likely bidirectional, so reducing LVEDP with SGLT2i may be able to break this vicious cycle. The combination of SGLT2i with other diuretics, with their contrasting mechanisms and multifactorial effects, may potentially explain the observed benefits. The effects of canagliflozin on MACE were most robust for patients taking baseline thiazide, thiazide‐like, or other diuretics, but the direction and magnitude of effect were similar for patients taking loop diuretics. Moreover, loop diuretic prescription can be considered as a positive factor, given the large effect size of this drug class in prospective trials. 26 The association of loop diuretic prescription and outcomes, as reported from OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) registry, further shows a lower risk of 30 day all‐cause mortality associated with loop diuretic use compared with non‐use [HR 0.73 (95% CI 0.57–0.94), P = 0.016]. Patients in the loop diuretic group also had a significantly lower risk of 30 day HF readmission [HR 0.79 (95% CI 0.63–0.99), P = 0.037]. 27 The implications of these differences are uncertain but may be due, at least in part, to limited power to detect differences of effect by diuretic class. In addition, the key reason for inconsistency with other diuretics in this post hoc analysis might be due to the very small number of participants on MRAs at baseline. Based on limited data available from patients on MRAs, it is difficult to provide robust evidence to evaluate the combined effect of canagliflozin and MRAs on cardiovascular outcomes.
These post hoc observations may also reflect a chance finding without external validation. The large sample size, high level of statistical significance of the observed heterogeneity (P < 0.001), and similar directions of effect for this finding across each of the MACE components argue against chance as an explanation.
Importantly, there was no signal of harm with combined canagliflozin and diuretic therapy, with no increase in SAEs nor AEs leading to discontinuation of the study drug in this subgroup. AEs related to osmotic diuresis or volume depletion, including hypotension, were not increased in those on baseline diuretic use, which has been a key concern in clinical practice. In addition, there was no signal of greater renal events or worsening of renal function in the patients randomized to canagliflozin who were taking diuretics at baseline, including acute kidney injury. These findings should give clinicians greater confidence in the safety of prescribing SGLT2i in those on concomitant diuretic therapy.
Key limitations to the study are that the subgroup was defined based on diuretic therapy at baseline, not throughout the trial, and specific diuretics were only available in CANVAS. Meanwhile, more detailed on‐treatment use of any diuretics, such as discontinuation, assessing type, and dose of treatment, were not in time captured. It is possible that participants may have discontinued or reduced their diuretic therapy during the trial, and this was not systematically captured. Further, it is possible, though probably unlikely, that changes in diuretic use may have impacted upon the difference in outcomes that were observed across subgroups. While we fitted adjusted models to control for differences in participants' baseline characteristics, measurement of each may have been imprecise, and residual confounding may still have persisted. Additionally, echocardiographic data for assessing the severity of HF in both groups were not systematically collected in the CANVAS Program. Lastly, these analyses were performed post hoc, and thus, the results should be viewed with caution and should be considered hypothesis generating.
In summary, participants in the CANVAS Program on baseline diuretic therapy derived a greater relative risk reduction from canagliflozin treatment for MACE than those not on baseline diuretics. This finding was not fully explained by differences in participants' baseline characteristics nor differential effects on intermediate markers of cardiovascular health. Moreover, there was no increase in renal nor total AEs identified from canagliflozin treatment in those on baseline diuretic therapy, demonstrating that concomitant use of canagliflozin and diuretics is safe. Future analyses of other large trial datasets will be important to determine the robustness of these results in different populations.
Conflict of interest
J.Y., C.A., Y.H., and C.L. are employees of the George Institute. C.A. is supported by an NHMRC/MRFF Priority Fellowship and a NSW Health EMC Grant. B.L.N. is supported by an Australian National Health and Medical Research Council Postgraduate Scholarship and a University Postgraduate Award from the University of New South Wales; he has received travel support from Janssen. H.J.L.H. has served as a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, and Mitsubishi Tanabe and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. K.W.M. has received research support from Afferent, Amgen, Apple Inc., AstraZeneca, Cardiva Medical Inc., Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, National Institutes of Health (NIH), Novartis, Sanofi, St. Jude, and Tenax and has served as a consultant (speaker fees for CME events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol Myers Squibb, Elsevier, GlaxoSmithKline (GSK), Johnson & Johnson, MedErgy, Medscape, Mitsubishi, Myokardia, NIH, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing, and UCSF. C.P.C. has received research grants from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Merck, Janssen, and Takeda and has received consulting fees from Aegerion, Alnylam, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Corvidia, GSK, Innovent, Eisai, Eli Lilly, Kowa, Merck, Pfizer, Regeneron, and Sanofi. G.A.F. reports receiving research support from the co‐funded National Health and Medical Research Council and Heart Foundation (Australia) Practitioner Fellowship and the Heart Research Australia and compensation from Janssen for serving on the Adjudication Panel of the CANVAS Program. V.P. has received fees for advisory boards, steering committee roles, or scientific presentations from AbbVie, Astellas, Astra Zeneca, Bayer, Baxter, BMS, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Sanofi, Servier, Vifor, and Tricida. M.J.J. is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Baxter, Amgen, Eli Lilly, and Merck Sharp & Dohme; serves on a steering committee sponsored by CSL; has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, and Vifor; and has spoken at scientific meetings sponsored by Janssen, with any consultancy, honoraria, or travel support paid to her institution. B.N. is supported by an Australian National Health and Medical Research Council Principal Research Fellowship, holds a research grant for this study from Janssen, and has held research grants for other large‐scale cardiovascular outcome trials from Roche, Servier, and Merck Schering Plough; and his institution has received consultancy, honoraria, or travel support for contributions he has made to advisory boards and/or the continuing medical education programmes of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier. M.H. has received grant support from the World Heart Federation via Boehringer Ingelheim and Novartis; the American Heart Association, Verily, and AstraZeneca; and the American Medical Association for work unrelated to this paper. He has plans for patents for heart failure fixed‐dose combination therapy products, including diuretics. He notes institutional relationships through his appointment at The George Institute with AbbVie, Actelion, and Janssen. None of the authors has any competing interests related to this study.
Funding
These studies were supported by Janssen Research & Development, LLC.
Author contributions
J.Y., C.A., B.N., H.L.H., K.W.M., C.P.C., S.S.K., A.S.B., S.J.S., Y.H., C.L., G.A.F., V.P., M.J., B.N., and M.D.H. contributed to the design and conduct of the study and the interpretation of the data. J.Y., C.A., and M.D.H. contributed to the analysis and interpretation of data. J.Y., C.A., and M.D.H. drafted the manuscript. All authors critically revised the manuscript and gave final approval. J.Y., C.A., and M.D.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Figure S1. Flow chart of analysis among participants in the CANVAS Program stratified by baseline use of any diuretic.
Figure S2. Effects of canagliflozin on cardiovascular and renal outcome in participants in the CANVAS Program, stratified by baseline use of any diuretic and by ASCVD.
Figure S3. Effects of canagliflozin on cardiovascular and renal outcome in participants in the CANVAS Program, stratified by baseline use of any diuretic and by HF.
Figure S4. Effects of canagliflozin on cardiovascular and renal outcomes in participants in the CANVAS Program, stratified by baseline use of any diuretic and by ASCVD or HF.
Table S1. Characteristics of participants in the CANVAS Program according to randomized group status, stratified by baseline use of any diuretic.a
Table S2. Characteristics of participants in the CANVAS Program according to randomized group status, stratified by baseline use of any diuretic.a
Table S3. Classification of participants in the CANVAS Program according to baseline diuretic class use, stratified by randomization group.
Table S4. Available clinical reasons for diuretic administration in patients in the CANVAS Program.
Table S5. Unadjusted and adjusted effects of canagliflozin on cardiovascular outcomes in participants in the CANVAS Program, stratified by baseline use of any diuretic.
Table S6. Effects of canagliflozin on intermediate markers among participants in the CANVAS Program, stratified by baseline use of any diuretic.
Acknowledgements
The authors thank all investigators, study teams, and patients for participating in these studies. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Yu, J. , Arnott, C. , Neuen, B. L. , Heersprink, H. L. , Mahaffey, K. W. , Cannon, C. P. , Khan, S. S. , Baldridge, A. S. , Shah, S. J. , Huang, Y. , Li, C. , Figtree, G. A. , Perkovic, V. , Jardine, M. J. , Neal, B. , and Huffman, M. D. (2021) Cardiovascular and renal outcomes with canagliflozin according to baseline diuretic use: a post hoc analysis from the CANVAS Program. ESC Heart Failure, 8: 1482–1493. 10.1002/ehf2.13236.
Clinical Trial Registration: URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01032629, NCT01989754.
References
- 1. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 4. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet (London, England) 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 5. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diab Endocrinol 2019; 7: 845–854. [DOI] [PubMed] [Google Scholar]
- 6. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 7. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B. Sodium‐glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. J Am Heart Assoc 2020; 9: e014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 2099. [DOI] [PubMed] [Google Scholar]
- 9. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134: 752–772. [DOI] [PubMed] [Google Scholar]
- 10. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018; 61: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 11. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol 2017; 16: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK‐mediated inhibition of mitochondrial fission. Redox Biol 2018; 15: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR, on behalf of the CANVAS Program collaborative group Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS‐R trials. Diabetes Obes Metab 2017;19:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, Erondu N, Desai M, Shaw W, Vercruysse F, Yee J, Deng H, de Zeeuw D, on behalf of the CANVAS‐R Trial Collaborative Group Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study‐Renal (CANVAS‐R): a randomized, placebo‐controlled trial. Diabetes Obes Metab 2017;19:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 17. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617. [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 19. Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, Böhm M, Boulton DW, Chopra VK, DeMets DL, Docherty KF, Dukát A, Greasley PJ, Howlett JG, Inzucchi SE, Katova T, Køber L, Kosiborod MN, Langkilde AM, Lindholm D, Ljungman CEA, Martinez FA, O'Meara E, Sabatine MS, Sjöstrand M, Solomon SD, Tereshchenko S, Verma S, Jhund PS, McMurray JJV. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation 2020; 142: 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verma S, McMurray JJV, Cherney DZI. The metabolodiuretic promise of sodium‐dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol 2017; 2: 939–940. [DOI] [PubMed] [Google Scholar]
- 21. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018; 20: 479–487. [DOI] [PubMed] [Google Scholar]
- 22. Kawata T, Daimon M, Miyazaki S, Ichikawa R, Maruyama M, Chiang SJ, Ito C, Sato F, Watada H, Daida H. Coronary microvascular function is independently associated with left ventricular filling pressure in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2015; 14: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R‐S, Beussink‐Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS‐HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah SJ, Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, Foster E, Chatterjee K, Michaels AD. High‐sensitivity C‐reactive protein and parameters of left ventricular dysfunction. J Card Fail 2006; 12: 61–65. [DOI] [PubMed] [Google Scholar]
- 25. Yamauchi‐Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T. Hypoxic stress induces cardiac myocyte‐derived interleukin‐6. Circulation 1995; 91: 1520–1524. [DOI] [PubMed] [Google Scholar]
- 26. Faris RF, Flather M, Purcell H, Poole‐Wilson PA, Coats AJ. Diuretics for heart failure. Cochrane Database Syst Rev 2012: Cd003838. [DOI] [PubMed] [Google Scholar]
- 27. Faselis C, Arundel C, Patel S, Lam PH, Gottlieb SS, Zile MR, Deedwania P, Filippatos G, Sheriff HM, Zeng Q, Morgan CJ, Wopperer S, Nguyen T, Allman RM, Fonarow GC, Ahmed A. Loop diuretic prescription and 30‐day outcomes in older patients with heart failure. J Am Coll Cardiol 2020; 76: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of analysis among participants in the CANVAS Program stratified by baseline use of any diuretic.
Figure S2. Effects of canagliflozin on cardiovascular and renal outcome in participants in the CANVAS Program, stratified by baseline use of any diuretic and by ASCVD.
Figure S3. Effects of canagliflozin on cardiovascular and renal outcome in participants in the CANVAS Program, stratified by baseline use of any diuretic and by HF.
Figure S4. Effects of canagliflozin on cardiovascular and renal outcomes in participants in the CANVAS Program, stratified by baseline use of any diuretic and by ASCVD or HF.
Table S1. Characteristics of participants in the CANVAS Program according to randomized group status, stratified by baseline use of any diuretic.a
Table S2. Characteristics of participants in the CANVAS Program according to randomized group status, stratified by baseline use of any diuretic.a
Table S3. Classification of participants in the CANVAS Program according to baseline diuretic class use, stratified by randomization group.
Table S4. Available clinical reasons for diuretic administration in patients in the CANVAS Program.
Table S5. Unadjusted and adjusted effects of canagliflozin on cardiovascular outcomes in participants in the CANVAS Program, stratified by baseline use of any diuretic.
Table S6. Effects of canagliflozin on intermediate markers among participants in the CANVAS Program, stratified by baseline use of any diuretic.


