Abstract
Aims
Our objectives were to validate a murine model of chronic cardiotoxicity induced by Doxorubicin (Dox) and Trastuzumab (Trast) and to test the potential cardio‐protective effect of metoprolol.
Methods and results
Male C57Bl6 mice were intraperitoneally injected during 2 weeks with Dox (24 mg/kg) or saline, and then with Trast (10 mg/kg) or saline for two more weeks. Half of the mice received metoprolol (100 mg/kg). Cardiotoxicity was defined by a decline in left ventricular ejection fraction (LVEF) ≥ 10 points. At Day 42, Dox + Trast‐treated mice exhibited a 13‐points decline in LVEF (74 ± 2.6% vs. 87 ± 0.8% for control mice, P < 0.001) and a severe cardiac atrophy (heart weight: 105 ± 2.7 mg vs. 119 ± 3.9 mg for control mice, P < 0.01). This cardiac atrophy resulted from an excess of cardiac necrosis (assessed by plasma cardiac troponin I level: 3.2 ± 0.4 ng/L vs. 1.3 ± 0.06 ng/L for control mice, P < 0.01), an increase in apoptosis (caspase 3 activity showing a six‐fold increase for Dox + Trast‐treated mice vs. controls, P < 0.001), and cardiomyocyte atrophy (myocyte size: 0.67 ± 0.08 μm2 vs. 1.36 ± 0.10 μm2 for control mice, P < 0.001). In addition, Dox + Trast‐treated mice were shown to have an increased cardiac oxidative stress (164 ± 14 dihydroethidine‐marked nuclei per area vs. 56 ± 9.5 for control mice, P < 0.01) and increased cardiac fibrosis (the semi‐quantitative fibrosis score was three‐fold higher for Dox + Trast‐treated mice as compared with controls, P < 0.01). Metoprolol was not able to prevent either the decrease in LVEF or the severe cardiac atrophy, the cardiac necrosis, and the cardiac remodelling induced by chemotherapies.
Conclusion
A murine model of chronic cardiotoxicity induced by Dox and Trast was characterized by a decrease in cardiac function, a cardiac apoptosis and necrosis leading to cardiomyocyte atrophy. Metoprolol did not prevent this cardiotoxicity.
Keywords: Cardiotoxicity, Metoprolol, Doxorubicin, Trastuzumab, Cardiac atrophy
Introduction
Trastuzumab (Trast) associated to doxorubicin (Dox) has been shown to improve the median survival in patients with epidermal growth factor receptor type 2 positive breast cancer. 1 However, the incidence of cardiotoxicity (defined by a decrease in the left ventricular ejection fraction (LVEF) < 50% with a 10% decrease from baseline 2 ) when these two chemotherapies are used together can reach 20%. 3 Anthracyclines (such as Dox) are well known to exert a dose‐dependent cardiotoxic action due to reactive oxygen species generation, 4 oxidative stress, topoisomerase‐IIb inhibition, 5 and mitochondrial damage leading to cardiac cell death and cardiac dysfunction. 6 Trast, a humanized monoclonal antibody directed against epidermal growth factor/HER2 inhibiting homeostasis and cardiomyocyte survival, is responsible for a reversible cardiotoxicity. 7 The early identification of anthracycline and antiHER2‐induced cardiotoxicity is based on the presence of cardiac plasma biomarkers (troponin I) 8 , 9 and imaging assessment: longitudinal global strain 10 and LVEF. The effects of beta‐adrenergic blockade on anthracycline‐related cardiomyopathy are not clear. A systematic preventive approach using standard heart failure treatment did not improve outcome in cancer patients treated with anthracycline and Trast. 11 Avila et al. 12 were also not able to show any protective effect of carvedilol to prevent anthracycline‐induced chronic cardiotoxicity.
Our aim is first to describe and validate the cardiac molecular and functional phenotype of a murine model of chronic cardiotoxicity induced by Dox and Trast, and then to test the potential protective effect of the metoprolol (β1‐blocker) on cardiac function in this murine model of chronic cardiotoxicity.
Methods
Animals and experimental protocol
Male C57Bl6 wild‐type mice (n = 42) (Janvier laboratory, Le Genest‐Saint‐Isle, France) aged 8 to 10 weeks were randomly divided into five groups (Figure 1 ). In the first group (Dox + Trast, n = 11), Dox (Accord, Healthcare, France SAS) was administered by six intraperitoneal injections during 2 weeks (total dose: 24 mg/kg), and after 1 week, Trast (Herceptin, Roche) was administered by six intraperitoneal injections during 2 weeks (total dose: 10 mg/kg). In the second group (Dox, n = 8), Dox was administered by six intraperitoneal injections for 2 weeks (total dose: 24 mg/kg), and after 1 week, mice were injected with saline for two more weeks. In the third group (saline, n = 9), control mice were injected with saline, simulating the combined Dox and Trast. In addition, 14 mice were treated with metoprolol 13 [Sigma Aldrich, PHR 1076, 100 mg/kg in drinking water from Day 10 before the onset of the protocol with Dox + Trast (n = 8) or saline (n = 6)]. The drinking water was weekly refreshed.
Figure 1.
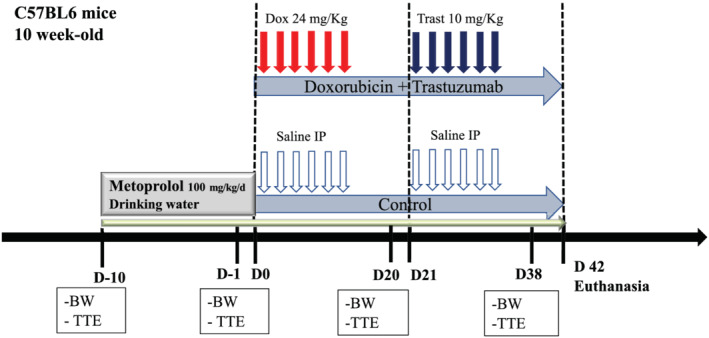
Plan of the experimental protocol for a chronic chemotherapy‐induced cardiomyopathy with beta‐blockers. Ten‐week‐old C57Bl6 male mice were evaluated 2 days before the onset of chemotherapy, with measurements of body weight (BW) and cardiac parameters by transthoracic echocardiography (TTE). This evaluation was repeated on Days 21 and 38. Doxorubicin (Dox, n = 11) or saline (n = 9) was IP injected every 2 days (from Days 0 to 14), and 1 week later, mice received IP of either trastuzumab (Trast) or saline again for 2 weeks. In addition, 14 mice were treated with metoprolol (100 mg/kg) in the drinking water from Day 10 prior to the onset of the protocol with Dox + Trast (n = 8) or saline (n = 6) to the end. BW, body weight; TTE, transthoracic echocardiography.
All animal experiments were approved by the local animal ethics committee. Protocols complied with national and international laws and policies. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1985).
Echocardiography
Transthoracic echocardiography (TTE) was performed using the ACUSON S3000 (Siemens Healthcare, Germany) and a 14 MHz linear transducer with simultaneous electrocardiographic recording. Analyses were performed on mice anaesthetised with intraperitoneal 80 mg/kg Ketamine (Ketamine Panpharma, 50 mg/5 mL). Each mouse was anaesthetised with intraperitoneal 80 mg/kg Ketamine at Day 0, Day + 20, and Day + 38 in order to perform echocardiography at the same time. Targeted heart rate was between 400 and 650 beats per minute, as recommended by Scherrer‐Crosbie and Kurt. 14 In the metoprolol subgroup, mice were also anaesthetised to undergo TTE just before starting drinking water + metoprolol, which corresponds to 10 days before chemotherapy. Two‐dimensional short‐axis M‐mode echocardiography 15 was performed to measure left ventricular (LV) end‐systolic diameter, LV end‐diastolic diameter, LV septal and posterior wall thickness in diastole and systole, and LV end‐systolic and end‐diastolic volumes to calculate LVEF according to Quinones et al. 16 Heart rate was also assessed during TTE. Data were collected and blindly analysed. At least three measures on three cardiac cycles were performed, and final analysis was made with the means of these values.
Blood and tissue analysis
Retro orbital blood sampling (anticoagulant: heparin) was performed in anaesthetised mice. Lethal anaesthesia was performed by pentobarbital injection (40 mg/kg; CEVA, Libourne, France). The hearts were then arrested in diastole by KCl (30 mmol/L), quickly rinsed in cold saline, and blotted. Body, heart, lung and liver weights, and tibia lengths were recorded. The heart was transversally divided into two parts: the base part was embedded into Tissue‐Tek O.C.T. (Sakura Finetek, Villeneuve d'Ascq, France) and frozen in liquid nitrogen for further histological and immunohistochemical analyses, and the apical part was snap frozen in liquid nitrogen. All samples were stored at −80°C until further analyses.
For the evaluation of intracardiac oxidative stress, the cross sections (cryostat) of the ventricles were dried. Dihydroethidine (DHE, Sigma‐Aldrich, D7008) has the property to be oxidized in the presence of superoxide anions to form ethidium, a molecule that then intercalates in DNA to render it fluorescent. The final solution [185 μL of 1 mM DHE in 50 mL of phosphate‐buffered saline (final concentration: 37 μM)] was deposited (1 mL) on each slide in a dark room. The slides were then placed in a humid chamber for 30 min. Then, three washes of 3 min each with phosphate‐buffered saline were carried out. The slides were mounted with Vectashield + DAPI mounting medium (Vector Laboratory, USA), left for 15 min at 20°C and observed using an epifluorescence microscope at ×20 magnification.
For the evaluation of cardiac fibrosis, equatorial cryostat sections (7 μm) of the ventricles were stained with the collagen‐specific picrosirius red stain (0.5% in saturated picric acid). Stained sections were observed under polarized light, and acquired images were used for the measure of collagen area/total ventricular surface ratio on at least five fields per section, three sections per heart, and n > 5 animals per group. All images were acquired using a Leica microscope (Leica Microsystems, Rueil Malmaison, France) and recorded for further analyses. All the measures were performed in a blinded fashion using the IPLab software (BD Biosciences, San Jose, CA). Images were assembled using Photoshop (Adobe Systems, San Jose, CA).
For cAMP quantification, tissues were sonicated in 0.1 M Tris–HCl (pH 7.4), 0.1% IGEPAL CA‐630 (Sigma‐Aldrich), and protein inhibitor cocktail (complete, EDTA‐free, Roche Diagnostics) and centrifuged at 1000g for 10 min at 4°C. Cell lysates were stored at −80°C until use. The cAMP content was determined using the DetectX cyclic AMP EIA Kit (Arbow Assays, Eisenhower Place Ann Arbor, MI). Protein concentrations were measured using the Pierce BCA Protein Assay Kit (Thermo‐Fischer, ref 23227). 17
Gene expression analysis
For quantitative reverse transcription polymerase chain reaction analyses, total RNA extraction from the LVs, reverse transcription, and quantitative polymerase chain reaction were performed as described in Azibani et al. 18 MicroRNA (mRNA) levels for genes of interest were normalized to the GAPDH mRNA levels and expressed as the relative change compared with the control samples. Western blot and immunofluorescence analyses were performed as published. 19
Plasma samples
Plasma levels of cardiac troponin I, myeloperoxidase were measured by sandwich immunoassay methods using commercially available electro‐chemiluminescent detection system, plates, and reagents (V‐PLEX kits, Meso‐Scale Discovery, Gaithersburg, USA) as per manufacturer's instructions. Briefly, 20 μL of plasma were loaded per well in the Meso‐Scale Discovery plate. Plates were analysed using the SECTOR Imager 2400. Caspase‐3 activity was determined with the substrate DEVD‐AFC in the presence or absence of the caspase‐3 inhibitor Ac‐DEVD‐CHO (Calbiochem) as described by the manufacturer (Abcam). The caspase‐3 activity was calculated by subtracting the activity in the presence of Ac‐DEVD‐CHO from the activity in its absence.
Data analysis
Results are presented as means ± SEM, computed from the average measurements obtained from each group of animals. Interaction between chemotherapies and treatment was performed by a two‐way ANOVA analysis followed by a Bonferroni post hoc test. Shapiro–Wilk test was used to assess normality. The Mann–Whitney U test was used to assess statistical differences between two experimental conditions.
The sample size was calculated using ‘The Cohen's d calculation’. The primary outcome was defined by a decrease of 10 points in LVEF. The standard deviation of LVEF in most of studies evaluating the LVEF decrease effect with Dox was 3 to 5. 20 Taking into account a P < 0.05 and a statistical power of 90%, the sample size was seven mice per group. 21 Statistical analysis was performed using GraphPad Prism 7. P < 0.05 was considered statistically significant.
Results
Validation of a chronic murine model of cardiac dysfunction induced by doxorubicin and trastuzumab
As shown in Figure 2 A,B , the LV function assessed by the LVEF declined rapidly after the onset of chemotherapy to reach 74 ± 2.7% vs. 87 ± 0.8% in controls, P < 0.001 at Day 38. This severe decrease of cardiac function in Dox + Trast‐treated mice occurred despite the absence of ventricular dilation (end diastolic LV diameters: 3.37 ± 0.08 mm vs. 3.28 ± 0.06 mm for Dox + Trast‐treated and control mice, respectively, P > 0.05).
Figure 2.
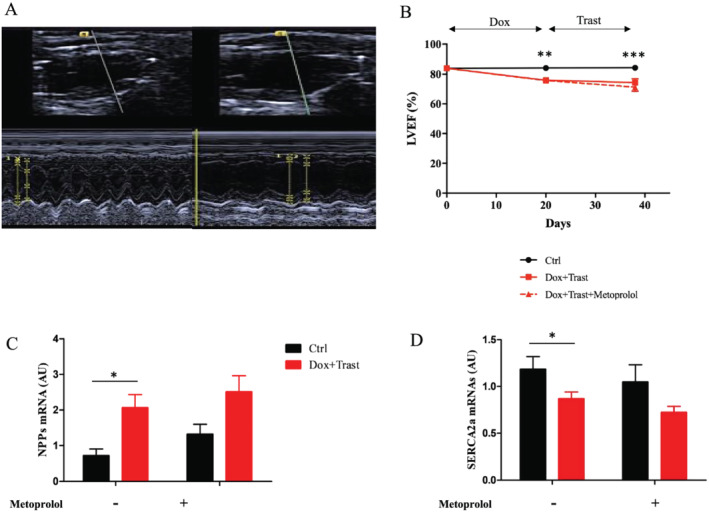
Effects of metoprolol on chemotherapy‐induced cardiac dysfunction. (A) Typical echo image of LV dysfunction without any LV dilation at Day 38 after chemotherapies were initiated. (B) The measure of LVEF with echocardiography from baseline to Day 38 indicates a rapid decline in LVEF, which is not prevented by metoprolol treatment (Ctrl with and without Meto n = 15, Dox + Trast n = 11, Dox + Trast + Meto n = 8, **P < 0.01, ***P < 0.001 by two‐way using ANOVA test followed by Bonferroni post hoc test). (C–D) Quantification of natriuretic peptides (NPPs) (C) and SERCA2a (D) mRNAs in ventricular tissues. Note the significant increase of NPPs after chemotherapy. The decrease in SERCA2a mRNA level is a sign of heart failure. Values are means ± SEM, *P < 0.05, **P < 0.01 by two‐way using ANOVA test followed by Bonferroni post hoc test. (Ctrl n = 8; Ctrl + Meto n = 5, Dox + Trast n = 10, Dox + Trast + Meto n = 7).
Mice treated with chemotherapies were characterized by a marked decrease of the heart weight (−10%, P < 0.001, Table 1 ). The LV mass calculated from echocardiography was reduced in Dox + Trast‐treated mice (68 ± 3.9 mg/mm2 vs. 80 ± 5.8 mg/mm2 for controls, P < 0.05, Table 1 ).
Table 1.
Anatomo‐pathological, echocardiographic data
| Characteristic | Ctrl (n = 9) | Ctrl + Meto (n = 6) | Dox + Trast (n = 11) | Dox + Trast + Meto (n = 8) |
|---|---|---|---|---|
| Anatomical parameters | ||||
| Body weight (g) | 27 ± 0.8 | 27 ± 1.0 | 24 ± 0.5*** | 19 ± 0.9 $$$ |
| Heart weight (mg) | 119 ± 3.9 | 126 ± 5.5 | 105 ± 2.7* | 86 ± 4.2 $$$ |
| Heart weight/Tibia length (mg/mm) | 5.8 ± 0.2 | 6.5 ± 0.3 | 5.3 ± 0.2 | 4.6 ± 0.2 $$$ |
| Heart weight/Body weight | 4.2 ± 0.07 | 4.6 ± 0.08 | 4.5 ± 0.09 | 4.5 ± 0.11 |
| End diastolic left ventricular diameter (mm) | 3.3 ± 0.06 | 3.6 ± 0.06 | 3.4 ± 0.08 | 3.5 ± 0.09 |
| Echocardiographic data | ||||
| LV mass (mg/mm2) | 80 ± 5.8 | 100 ± 3.0 | 68 ± 3.9** | 76 ± 4.3 $$ |
| Shortening fraction (%) | 55 ± 0.9 | 50 ± 1.8 | 40 ± 1.3*** | 41 ± 2.0 $ |
| LVEF (%) | 87 ± 0.8 | 81 ± 2.1 | 74 ± 2.7*** | 71 ± 2.7 $ |
| Heart rate (beats per min) | 614 ± 15 | 571 ± 37 | 598 ± 15 | 322 ± 29 $$$ |
| Intracardiac AMPc (pmol/mg proteins) | 7.7 ± 0.7 | 2.2 ± 0.3 | 56 ± 5.3*** | 20 ± 0.5 $$ |
LV, Left ventricle; LVEF, left ventricular ejection fraction.
Ctrl vs. Dox + Trast P < 0.05,
P < 0.01,
P < 0.001 using two‐way ANOVA test followed by Bonferroni post hoc test.
Ctrl + Meto vs. Dox + Trast + Meto P < 0.05,
P < 0.01,
P < 0.001 using two‐way ANOVA test followed by Bonferroni post hoc test.
In parallel, cardiac remodelling was assessed by a decrease of SERCA2a cardiac transcripts and protein expression (Figures 2D and S1 ) and an increase of both BNP cardiac transcripts (Figure 2 C,D ) and plasma cardiac troponin I (3.2 ± 0.4 ng/L vs. 1.3 ± 0.06 ng/L for controls, P < 0.01, Figure 3 A ).
Figure 3.
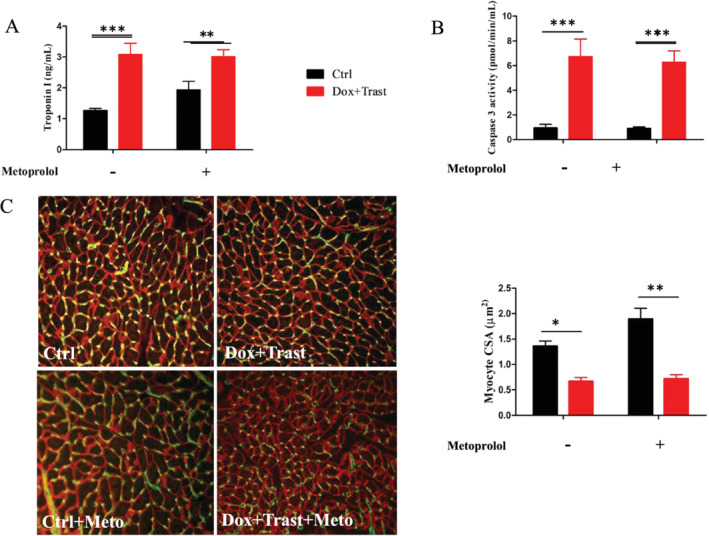
Chemotherapy‐induced cardiac injury and cardiac atrophy. (A) Plasma Troponin I at Day 42 (ng/mL, Ctrl n = 8, Ctrl + Meto n = 6, Dox + Trast n = 11, Dox + Trast + Meto n = 6). (B) Plasma caspase 3 activity (pmol/min/mL, Ctrl n = 8, Ctrl + Meto n = 6, Dox + Trast n = 11, Dox + Trast + Meto n = 6). (C) Vinculin (red) and caveolin (green) immunostaining. Analysis of myocyte cross sectional areas at Day 42 in control and chemotherapy‐treated mice (×20, Ctrl n = 5, Ctrl + Meto n = 5, Dox + Trast n = 5, Dox + Trast + Meto n = 5). Values are means ± SEM. **P < 0.01, ***P < 0.001 using two‐way ANOVA test followed by Bonferroni post hoc test.
Cardiac atrophy could be explained by a significant cardiomyocyte necrosis as assessed by the plasma cardiac troponin I level (a three‐fold increase in Dox + Trast‐treated mice as compared with control mice, Figure 3 A ) but also by an increase of cardiac cell apoptosis as assessed by the six‐fold higher plasma caspase 3 activity in mice treated with chemotherapies (6.2 ± 0.92 pmol/min/mL vs. 0.96 ± 0.11 pmol/min/mL, P < 0.001, Figure 3 B ) and also by an increase of caspase 3 activity protein expression (Figure S2 ).
Finally, the cardiac hypotrophy was also secondary to cardiomyocyte atrophy. Indeed, using vinculin immunostaining, we measured the average cross‐sectional area of cardiomyocytes in the LV sub‐endocardial area: the cardiomyocyte size of Dox + Trast‐treated mice was smaller than the one of control mice (0.67 ± 0.08 μm2 vs. 1.36 ± 0.10 μm2, P < 0.001, Figure 3 C ).
Table S1 shows that despite no effect of Trast when added to Dox regarding LVEF, Troponin I levels were increased in Dox + Trast mice compared with Dox alone (P < 0.01). The intracardiac oxidative stress was also increased in Dox + Trast mice vs. Dox alone as assessed by both DHE staining (P = 0.04) and plasmatic myeloperoxidase dosage (P = 0.02). Plasmatic caspase 3 activity was also increased in Dox + Trast mice vs. Dox alone (P = 0.03).
Effects of metoprolol in this chronic cardiotoxicity model
The efficiency of beta‐blockade induced by metoprolol was assessed by the decrease in heart rate (457 ± 45 vs. 605 ± 11 bpm in mice with and without metoprolol respectively, P = 0.01, Mann Whitney test). This efficiency was confirmed by the decline in intracardiac AMPc concentration: 13 ± 9 pmol/mg protein vs. 38 ± 13 pmol/mg protein in mice with and without metoprolol, respectively, P < 0.01 (Table 1 ).
In Dox + Trast‐treated mice, metoprolol did not prevent either the decrease in LVEF (Table 1 ) or the cardiomyocyte atrophy, the cross‐sectional area being 0.71 ± 0.08 μm2 in mice treated with Dox + Trast and metoprolol vs. 0.67 ± 0.08 μm2 in mice receiving chemotherapies only (P > 0.05).
In mice receiving chemotherapies and metoprolol, natriuretic peptide mRNAs levels were twice as high as compared with those who had not received chemotherapies. Similarly, myocardial expression of SERCA2a mRNAs remained low (Figure 2 D ).
We also investigated whether an excess of cardiac oxidative stress could partly explain the impairment of cardiac function in mice receiving chemotherapies. The DHE staining revealed a three‐fold increase in the level of cardiac oxidative stress for Dox + Trast‐treated mice as compared with controls (Figure 4 A,B ): 164 ± 14 vs. 56 ± 9.5 DHE marked nuclei per area in Dox + Trast and control mice, respectively, P < 0.01. Treating with metoprolol did not prevent this excess of cardiac oxidative stress, which was confirmed by the higher myeloperoxidase plasma levels in Dox + Trast‐treated mice, whether or not the animals were pre‐treated with metoprolol (Figure 4 C ).
Figure 4.
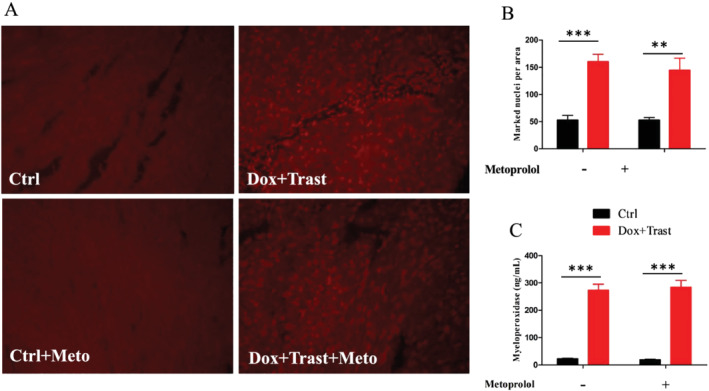
Chemotherapy‐induced cardiac oxidative stress. (A) Intracardiac DHE staining (×20) and marked nuclei per area (Ctrl n = 5, Ctrl + Meto n = 5, Dox + Trast n = 5, Dox + Trast + Meto n = 5). (B) Plasma myeloperoxidase (ng/mL, Ctrl n = 8, Ctrl + Meto n = 6, Dox + Trast n = 11, Dox + Trast + Meto n = 6). Values are means ± SEM. **P < 0.01 using two‐way ANOVA test followed by Bonferroni post hoc test.
Moreover, we observed an important impairment of cardiac metabolism as assessed by intracardiac expression of AMPK mRNA without any protective effect of metoprolol (Figure S3 ). The cardiac expression of sirtuin‐3 mRNA, a cardiac metabolic sensor, was significantly decreased by metoprolol and chemotherapy administration (Figure S4 ). Sirtuin‐3 is known to be activated through the PKA pathway. Beta‐1 adrenergic blockade could then blind the Sirtuin 3 activation leading to a decrease of the protective sirtuin‐3 pathway controlling oxidative stress, mitochondrial function and fibrosis (Figure 6 ).
Figure 6.
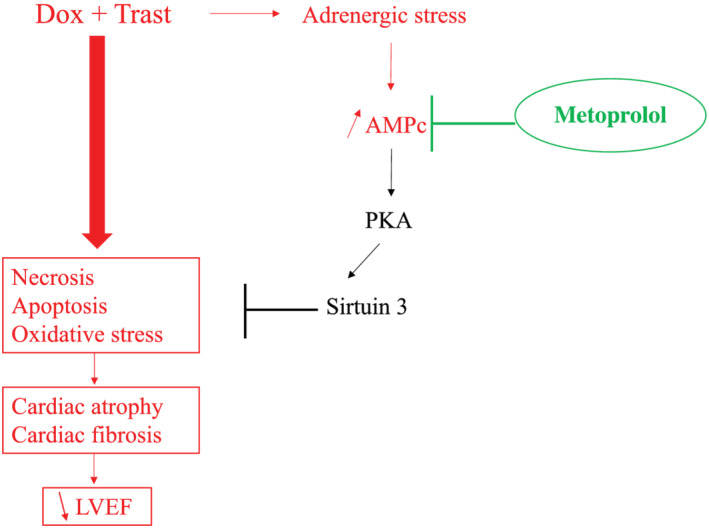
Metoprolol is ineffective to prevent chronic cardiotoxicity induced by Dox + Trast. Dox, Doxorubicin; LVEF, left ventricular ejection fraction; PKA, Protein Kinase A; Trast, Trastuzumab.
Finally, the chronic exposure to Dox and Trast led to an increased cardiac fibrosis as assessed by the Red Sirius staining (the semi‐quantitative fibrosis score was 1.6 ± 0.36% vs. 0.53 ± 0.10% of region of interest for controls, P < 0.01, Figure 5 A,B ). Moreover, the intracardiac connective tissue growth factor mRNA was increased two‐fold in Dox + Trast vs. control mice, P < 0.01 (Figure 5 C ). Metoprolol did not prevent this cardiac fibrosis.
Figure 5.
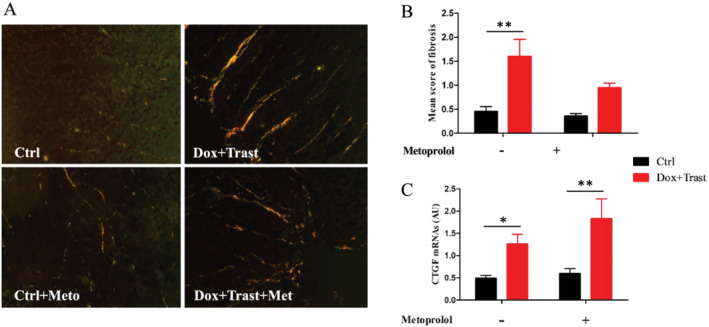
Chemotherapy‐induced cardiac fibrosis. (A) Representative cardiac section stained with Sirius Red (×20), and score graph of cardiac fibrosis (Ctrl n = 5, Ctrl + Meto n = 5, Dox + Trast n = 5, Dox + Trast + Meto n = 5). (B) Intracardiac CTGF (Connective tissue growth factor) expression (Ctrl n = 9, Ctrl + Meto n = 4, Dox + Trast n = 10, Dox + Trast + Meto n = 6). Values are means ± SEM. **P < 0.01 using two‐way ANOVA test followed by Bonferroni post hoc test.
Discussion
Our murine model of chronic and sequential cardiotoxicity induced by Dox and Trast was characterized by both cardiac dysfunction and an increase in plasma levels of cardiac troponin I. We used this chemotherapy combination because of its extensive use in the current treatment of breast cancer. This model has been previously used to show the synergistic effects of these two chemotherapies on the decrease of ventricular function. 20 We clearly showed that metoprolol, administrated before the chemotherapies, failed to prevent this cardiotoxicity.
The effectiveness of a cardioprotective strategy using conventional heart failure therapies (beta‐blockers and/or ACE inhibitors) remains controversial. Some studies 22 have shown the promising effect of carvedilol in a cohort of patients treated by a high dose of anthracycline with antioxidant properties and protective effect on the LVEF decline. However, a systematic preventive treatment with metoprolol 11 does not seem to improve cardiac function in patients undergoing potential cardiotoxic chemotherapies. Our hypothesis was built on the potential property of metoprolol to control the adrenergic stress caused by anthracyclines to avoid a severe synergistic cardiac dysfunction with Trast. Indeed, Sysa‐Shah et al. 23 found a possible co‐regulation between the ErbB2/4 and the β‐adrenergic pathways. They proposed that beta‐blockers would reduce the cardiac expression of the survival Neuregulin1/ErBb/pERK axis and thus make the myocardium less vulnerable to an aggression induced by anthracycline. The present study indicates that metoprolol did not prevent the cardiac dysfunction induced by doxorubicin and Trast and especially did not reverse the cardiac atrophy caused by a decrease in cardiomyocyte size and an increase in cell death (apoptosis and necrosis) as already mentioned by Sweeney et al. 24 Neilan et al. 25 have described the link between the decrease of indexed LV mass and cardiovascular events in 91 patients treated with anthracyclines. This particular phenotype of cardiac atrophy and dysfunction has been well described in a recent study 26 in a subacute model of cardiotoxicity induced by doxorubicin with the role of the ubiquitin ligase, muscle ring finger‐1, upregulated in the hearts of mice treated with doxorubicin. We did not find the same results in our chronic model of cardiotoxicity, very likely because we performed a chronic cardiotoxicity model. At Day 42, the cardiac expression of muscle ring finger‐1 was decreased in Dox + Trast‐treated mice as compared with controls (Figure S4 ).
The blockade of the beta‐1 adrenergic pathway (with metoprolol) could even be deleterious. Indeed, we observed that metoprolol induced a decrease of the intracardiac Sirtuin 3 expression. Many animal models had shown the protective effect of Sirtuin 3 on oxidative stress, mitochondrial function, 27 cardiac fibrosis and cardiac function, 28 especially in doxorubicin‐induced cardiomyopathy 29 (Figure 6 ).
Limitations
One of the limits of our model is that, like in most of the studies, chemotherapies have been injected into healthy mice without tumours. The tumour may affect the sensitivity to cardiotoxicity. 24 However, chemotherapies indeed induced a severe cardiac dysfunction, and it was then possible to test the effect of metoprolol.
Moreover, the anaesthetic agent used in this study could also be discussed. Because ketamine is sometimes more cardio‐depressant than isoflurane, especially when associated with xylazine, xylazine was not used in our model to induce anaesthesia. However, its use is validated for carrying out echocardiography. In addition, sedation was very light (dose of intraperitoneal ketamine: 80 mg/kg). When anaesthetic agents are used for carrying out echocardiography in mice, the most important recommendation for the operator is to obtain a cardiac frequency between 400 and 650 beats per min in order to be able to interpret the echocardiographic results. 30 In this study, all mice had cardiac frequencies between 400 and 650 beats per min (except for those treated with metoprolol).
The number of mice appeared to differ among the groups. Due to the limited total number of mice available and the high risk of mortality, we included more animals in the groups receiving chemotherapy. The power analysis revealed that for a 10 points difference in LVEF, each group should include at least seven mice. Three mice died from hypothermia and/or anaesthesia: one in the Dox + Trast group, one in the Control group, and one in the Dox + Trast + Metoprolol group.
The choice of the metoprolol as beta‐blocker could be discussed. We chose metoprolol because more data are available on its use in mice and because it had been tested in large clinical trials. 11 Carvedilol, because of its interesting antioxidant properties, may have led to different results, but it recently failed to show any cardioprotective effect in patients in the CECCY trial. 12 Recently, Guglin et al. 31 have shown a better survival without cardiotoxicity in cancer patients treated with Trast and who received carvedilol versus placebo, especially in patients who also had received anthracyclines. However, carvedilol was not effective in preventing a decline of LVEF in those patients.
The reason why beta blockers do not seem to be effective in chemotherapy‐induced heart failure while they are in the large majority of heart failure in animals and humans is not clear. It may be related to the specific phenotype of this form of heart failure with atrophy and cardiomyocyte loss rather than hypertrophy or to other unknown reasons.
The MANTICORE 101‐Breast trial 32 that assessed pharmacological prevention of Trast‐cardiotoxicity found no differences between the groups: perindopril and bisoprolol were able to attenuate LVEF decline in these patients but did not prevent LV remodelling that was the primary outcome of the study. The CECCY trial 12 that evaluated the potential beneficial effects of carvedilol in breast cancer employed carvedilol in anthracycline cardiotoxicity, but no differences were found in ejection fraction between treatment and placebo even though the authors found a significant reduction in troponin levels and LV diastolic dysfunction. In contrast, Guglin and collaborators showed that carvedilol prevents cardiotoxicity in women treated with Trast + anthracyclines, in agreement with experimental findings concerning a bidirectional cross‐regulation between ErbB2 and β‐adrenergic signalling pathways 23 with specific involvement of the β2 adreno‐receptor. Sysa‐Shah has also shown that catecholamines (which increase with the onset of heart dysfunction and with anthracycline treatment) can enhance ErbB2 expression in cardiomyocytes, hence making these cells more ‘targetable’ by Trast and thus vulnerable to the drug toxicity. This would explain why β1‐blockade alone is not sufficient to protect women on Trast. On the other hand, while carvedilol was not beneficial in patients treated with anthracyclines alone, 12 Sysa‐Shah et al. 23 and Guglin et al. 31 support carvedilol in the Trast setting. Importantly, the SAFE‐HEaRt trial 33 employs carvedilol as the β‐blocker of choice, in patients with heart dysfunction treated with HER2‐targeted therapies, providing insightful safety data and relevant clinical findings in Cardio‐Oncology.
In conclusion, this murine model of chronic cardiotoxicity induced by a sequential doxorubicin and Trast is characterized by a decrease of cardiac function explained by a cardiac atrophy related to cardiac apoptosis, necrosis, and fibrosis accompanying by cardiomyocyte atrophy. Metoprolol treatment before and during the chemotherapy failed to prevent the occurrence of a severe and chronic cardiotoxicity
Conflict of interest
None declared.
Supporting information
Figure S1. Intracardiac SERCA2a expression at Day 42 (Western Blot) (Ctrl n=4,Ctrl+Meto n=3, Dox+Trast n=5, Dox+Trast+Meto n=4) Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S2. Intracardiac Caspase 3 acitivity expression at Day 42 (Western Blot) (Ctrl n=4, Ctrl+Meto n=3, Dox+Trast n=5, Dox+Trast+Meto n=4)490 Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S3. Effect of chemotherapy on cardiac metabolism. A. Intracardiac AMPK expression (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5). B. Intracardiac Sirtuin 3 expression (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5). Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S4. Murf1 cardiac expression at day 42 (Western Blot). Values are means ±SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5).
Table S1. Comparative data between the Dox vs Dox + Trast groups.
Nicol, M. , Sadoune, M. , Polidano, E. , Launay, J. M. , Samuel, J. L. , Azibani, F. , and Cohen‐Solal, A. (2021) Doxorubicin‐induced and trastuzumab‐induced cardiotoxicity in mice is not prevented by metoprolol. ESC Heart Failure, 8: 928–937. 10.1002/ehf2.13198.
Feriel Azibani and Alain Cohen‐Solal contributed equally to this work.
References
- 1. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan‐Chiu E, Martino S, Paik S, Kaufman PA, Swain SM. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005; 353: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 2. Čelutkienė J, Pudil R, López‐Fernández T, Grapsa J, Nihoyannopoulos P, Bergler‐Klein J, Cohen‐Solal A, Farmakis D, Tocchetti CG, von Haehling S, Barberis V, Flachskampf FA, Čeponienė I, Haegler‐Laube E, Suter T, Lapinskas T, Prasad S, de Boer RA, Wechalekar K, Anker MS, Iakobishvili Z, Bucciarelli‐Ducci C, Schulz‐Menger J, Cosyns B, Gaemperli O, Belenkov Y, Hulot JS, Galderisi M, Lancellotti P, Bax J, Marwick TH, Chioncel O, Jaarsma T, Mullens W, Piepoli M, Thum T, Heymans S, Mueller C, Moura B, Ruschitzka F, Zamorano JL, Rosano G, Coats AJS, Asteggiano R, Seferovic P, Edvardsen T, Lyon AR. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio‐Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail 2020; 22: 1504–1524. [DOI] [PubMed] [Google Scholar]
- 3. Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KAB, Davis RL, Habel LA, Yood MU, Mccarty C, Magid DJ, Wagner EH, Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012; 104: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 1983; 43: 460–472. [PubMed] [Google Scholar]
- 5. Zhang S, Liu X, Bawa‐Khalfe T, Lu L‐S, Lyu YL, Liu LF, Yeh ETH. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med 2012; 18: 1639–1642. [DOI] [PubMed] [Google Scholar]
- 6. Lenneman CG, Sawyer DB. Cardio‐oncology: an update on cardiotoxicity of cancer‐related treatment. Circ Res 2016; 118: 1008–1020. [DOI] [PubMed] [Google Scholar]
- 7. Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med 2012; 367: 2150–2153. [DOI] [PubMed] [Google Scholar]
- 8. Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high‐dose chemotherapy. J Am Coll Cardiol 2000; 36: 517–522. [DOI] [PubMed] [Google Scholar]
- 9. Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nolè F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol Off J Am Soc Clin Oncol 2010; 28: 3910–3916. [DOI] [PubMed] [Google Scholar]
- 10. Negishi K, Negishi T, Haluska BA, Hare JL, Plana JC, Marwick TH. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur Heart J Cardiovasc Imaging 2014; 15: 324–331. [DOI] [PubMed] [Google Scholar]
- 11. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J 2016; 37: 1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi‐dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M. Carvedilol for prevention of chemotherapy‐related cardiotoxicity. CECCY Trial J Am Coll Cardiol 2018; 71: 2281–2290. [DOI] [PubMed] [Google Scholar]
- 13. Thireau J, Karam S, Roberge S, Roussel J, Aimond F, Cassan C, Gac A, Babuty D, Le Guennec JY, Lacampagne A, Fauconnier J. Β‐adrenergic blockade combined with subcutaneous B‐type natriuretic peptide: a promising approach to reduce ventricular arrhythmia in heart failure? Heart Br Card Soc 2014; 100: 833–841. [DOI] [PubMed] [Google Scholar]
- 14. Scherrer‐Crosbie M, Kurtz B. Ventricular remodeling and function: insights using murine echocardiography. J Mol Cell Cardiol 2010; 48: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zacchigna S, Paldino A, Falcão‐Pires I, Daskalopoulos EP, Dal Ferro M, Vodret S, Lesizza P, Cannatà A, Miranda‐Silva D, Lourenço AP, Pinamonti B, Sinagra G, Weinberger F, Eschenhagen T, Carrier L, Kehat I, Tocchetti CG, Russo M, Ghigo A, Cimino J, Hirsch E, Dawson D, Ciccarelli M, Oliveti M, Linke WA, Cuijpers I, Heymans S, Hamdani N, de Boer M, Duncker DJ, Kuster D, van der Velden J, Beauloye C, Bertrand L, Mayr M, Giacca M, Leuschner F, Backs J, Thum T. Toward standardization of echocardiography for the evaluation of left ventricular function in adult rodents: a position paper of the ESC Working Group on Myocardial Function. Cardiovasc Res 2021; 117: 43–59. [DOI] [PubMed] [Google Scholar]
- 16. Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two‐dimensional echocardiography. Circulation 1981; 64: 744–753. [DOI] [PubMed] [Google Scholar]
- 17. Bainor A, Chang L, McQuade TJ, Webb B, Gestwicki JE. Bicinchoninic acid (BCA) assay in low volume. Anal Biochem 2011; 410: 310–312. [DOI] [PubMed] [Google Scholar]
- 18. Azibani F, Benard L, Schlossarek S, Merval R, Tournoux F, Fazal L, Polidano E, Launay JM, Carrier L, Chatziantoniou C, Samuel JL. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension (Dallas Tex 1979) 2012; 59: 1179–1187. [DOI] [PubMed] [Google Scholar]
- 19. Caillard A, Sadoune M, Cescau A, Meddour M, Gandon M, Polidano E, Delcayre C, da Silva K, Manivet P, Gomez AM, Cohen‐Solal A, Vodovar N, Li Z, Mebazaa A, Samuel JL. QSOX1, a novel actor of cardiac protection upon acute stress in mice. J Mol Cell Cardiol 2018; 119: 75–86. [DOI] [PubMed] [Google Scholar]
- 20. Milano G, Raucci A, Scopece A, Daniele R, Guerrini U, Sironi L, Cardinale D, Capogrossi MC, Pompilio G. Doxorubicin and trastuzumab regimen induces biventricular failure in mice. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2014; 27: 568–579. [DOI] [PubMed] [Google Scholar]
- 21. Rosner B. Fundamentals of Biostatistics. 7th ed. Boston: Brooks/Cole; 2011. [Google Scholar]
- 22. Bosch X, Rovira M, Sitges M, Domènech A, Ortiz‐Pérez JT, de Caralt TM, Morales‐Ruiz M, Perea RJ, Monzó M, Esteve J. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013; 61: 2355–2362. [DOI] [PubMed] [Google Scholar]
- 23. Sysa‐Shah P, Tocchetti CG, Gupta M, Rainer PP, Shen X, Kang B‐H, Belmonte F, Li J, Xu Y, Guo X, Bedja D, Gao WD, Paolocci N, Rath R, Sawyer DB, Naga Prasad SV, Gabrielson K. Bidirectional cross‐regulation between ErbB2 and β‐adrenergic signalling pathways. Cardiovasc Res 2016; 109: 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sweeney M, Yiu A, Lyon AR. Cardiac atrophy and heart failure in cancer. Card Fail Rev 2017; 3: 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neilan TG, Coelho‐Filho OR, Pena‐Herrera D, Shah RV, Jerosch‐Herold M, Francis SA, Moslehi J, Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol 2012; 110: 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willis MS, Parry TL, Brown DI, Mota RI, Huang W, Beak JY, Sola M, Zhou C, Hicks ST, Caughey MC, D'Agostino RB Jr, Jordan J, Hundley WG, Jensen BC. Doxorubicin exposure causes subacute cardiac atrophy dependent on the striated muscle‐specific ubiquitin ligase MuRF1. Circ Heart Fail 2019; 12: e005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese Jr RV, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty‐acid oxidation by reversible enzyme deacetylation. Nature 2010; 464: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer‐Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, Odening KE, Kammerer B, Hein L, Bode C, Bugger H. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 2015; 110: 36. [DOI] [PubMed] [Google Scholar]
- 29. Pillai VB, Kanwal A, Fang YH, Sharp WW, Samant S, Arbiser J, Gupta MP. Honokiol, an activator of Sirtuin‐3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin‐induced cardiomyopathy in mice. Oncotarget 2017; 8: 34082–34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindsey ML, Bolli R, Canty JM, Du X‐J, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 2018; 314: H812–H838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guglin M, Krischer J, Tamura R, Fink A, Bello‐Matricaria L, McCaskill‐Stevens W, Munster PN. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 2019; 73: 2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA, Paterson DI. Multidisciplinary approach to novel therapies in cardio‐oncology research (MANTICORE 101‐Breast): a randomized trial for the prevention of trastuzumab‐associated cardiotoxicity. J Clin Oncol Off J Am Soc Clin Oncol 2017; 35: 870–877. [DOI] [PubMed] [Google Scholar]
- 33. Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, Gallagher C, Pohlmann PR, Nunes R, Herbolsheimer P, Warren R, Srichai MB, Hofmeyer M, Cunningham A, Timothee P, Asch FM, Shajahan‐Haq A, Tan MT, Isaacs C, Swain SM. Prospective evaluation of the cardiac safety of HER2‐targeted therapies in patients with HER2‐positive breast cancer and compromised heart function: the SAFE‐HEaRt study. Breast Cancer Res Treat 2019; 175: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Intracardiac SERCA2a expression at Day 42 (Western Blot) (Ctrl n=4,Ctrl+Meto n=3, Dox+Trast n=5, Dox+Trast+Meto n=4) Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S2. Intracardiac Caspase 3 acitivity expression at Day 42 (Western Blot) (Ctrl n=4, Ctrl+Meto n=3, Dox+Trast n=5, Dox+Trast+Meto n=4)490 Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S3. Effect of chemotherapy on cardiac metabolism. A. Intracardiac AMPK expression (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5). B. Intracardiac Sirtuin 3 expression (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5). Values are means ± SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test.
Figure S4. Murf1 cardiac expression at day 42 (Western Blot). Values are means ±SEM. *p<0.05 using Anova‐2 way test followed by Bonferroni post hoc test (Ctrl n=6, Ctrl+Meto n=5, Dox+Trast n=9, Dox+Trast+Meto n=5).
Table S1. Comparative data between the Dox vs Dox + Trast groups.


