Abstract
Aims
Sacubitril/valsartan (sac/val) has shown superior effect compared with blockade of the renin–angiotensin–aldosterone system in heart failure with reduced ejection fraction. We aimed to investigate effects of sac/val compared with valsartan in a pressure overload model of heart failure with preserved ejection fraction (HFpEF).
Methods and results
Sprague–Dawley rats underwent aortic banding or sham (n = 16) surgery and were randomized to sac/val (n = 28), valsartan (n = 29), or vehicle (n = 26) treatment for 8 weeks. Sac/val reduced left ventricular weight by 11% compared with vehicle (P = 0.01) and 9% compared with valsartan alone (P = 0.04). Only valsartan reduced blood pressure compared with sham (P = 0.02). Longitudinal early diastolic strain rate was preserved in sac/val compared with sham, while it was reduced by 23% in vehicle (P = 0.03) and 24% in valsartan (P = 0.02). Diastolic dysfunction, measured by E/e'SR, increased by 68% in vehicle (P < 0.01) and 80% in valsartan alone (P < 0.001), while sac/val showed no increase. Neither sac/val nor valsartan prevented interstitial fibrosis. Although ejection fraction was preserved, we observed mild systolic dysfunction, with vehicle showing a 28% decrease in longitudinal strain (P < 0.01). Neither sac/val nor valsartan treatment improved this dysfunction.
Conclusions
In a model of HFpEF induced by cardiac pressure overload, sac/val reduced hypertrophy compared with valsartan alone and ameliorated diastolic dysfunction. These effects were independent of blood pressure. Early systolic dysfunction was not affected, supporting the notion that sac/val has the largest potential in conditions characterized by reduced ejection fraction. Observed anti‐hypertrophic effects in preserved ejection fraction implicate potential benefit of sac/val in the clinical setting of hypertrophic remodelling and impaired diastolic function.
Keywords: Cardiac pressure overload, Sacubitril/valsartan, Heart failure, Therapy, Hypertrophy, Diastolic dysfunction
Introduction
Heart failure represents a large unmet medical need due to high morbidity and mortality. It is divided into three main forms: heart failure with reduced ejection fraction (HFrEF), heart failure with mid‐range ejection fraction, and heart failure with preserved ejection fraction (HFpEF). 1 Although several drugs are used for the treatment of heart failure, the beneficial effect of these medications has mainly been demonstrated in HFrEF, underscoring differences in aetiology. Among the newer drugs for heart failure, we find sacubitril/valsartan (sac/val), a first in class angiotensin receptor blocker neprilysin inhibitor (ARNi). This drug targets the renin–angiotensin–aldosterone system (RAAS), while aiming to potentiate favourable effects of other endogenous systems, including the natriuretic peptides (NPs). Sac/val was included in treatment guidelines for heart failure after a large trial of HFrEF patients showed 20% reduction in cardiovascular mortality compared with angiotensin‐converting enzyme inhibition. 2 , 3 The follow‐up trial in HFpEF patients, PARAGON‐HF, narrowly missed its primary endpoint, while sub‐analyses indicated a possible treatment effect in the lower ranges of ejection fraction (EF), leaving uncertainty regarding the use of ARNi in patients with HFpEF. 4 Such lack of clear treatment benefit has been seen in several previous HFpEF trials and could be linked to inherent heterogeneity of the condition. To address this problem, pre‐clinical studies can induce distinct pathological stimuli and thereby help elucidate drug effects in specific pathological conditions, thus informing future clinical investigations.
Cardiac pressure overload is one such risk factor for HFpEF, 5 driving development of hypertrophy and fibrosis, as seen in aortic stenosis 6 and hypertension. 7 NPs are known to possess anti‐fibrotic and blood pressure‐lowering properties, counteracting the effects of RAAS, suggesting a possible therapeutic role of NPs in this condition. 8 Indeed, previous pre‐clinical trials demonstrated anti‐remodelling properties of sac/val after myocardial infarction, 9 providing a backdrop for investigations by Burke et al. into the effects of sac/val in a cardiac pressure overload model of HFrEF in mice. They found that fibrosis, hypertrophy, and systolic dysfunction were more ameliorated by sac/val compared with valsartan alone and related these beneficial effects to restoration of PKG signalling. 10 However, Maslov et al. did not find superior reduction of hypertrophy nor preservation of systolic function in a supra‐renal banding model of pressure overload in rats, 11 highlighting the need for further studies to investigate the effects of sac/val in the setting of pressure overload. Therefore, the objective of the current study was to investigate the differential effects of sac/val and valsartan alone after ascending aortic banding (AB) in rats. Utilizing advanced magnetic resonance imaging (MRI) techniques, we aimed to characterize effects on cardiac dysfunction and remodelling. We hypothesized that combining angiotensin receptor blockade and neutral endopeptidase inhibition (NEPi) would yield superior anti‐remodelling effects and positively impact cardiac function as compared with valsartan alone.
Materials and methods
Surgical induction of pressure overload
A more extensive description of the surgical model can be found in Supporting Information, Data S1 . Cardiac pressure overload was induced by AB in male Sprague–Dawley rats through placement of a suture around the ascending aorta. Sham animals underwent the same procedure, apart from constriction. All experiments were performed in accordance with approvals from the Norwegian committee for approval of animal use in research (FOTS approval number 7644).
Stratification and randomization
Aortic banding induces a pressure gradient, which can be approximated through measuring trans‐stenosis flow velocity. Therefore, rats underwent echocardiography on the first post‐operative day and were stratified according to estimated gradient. Approximated stenosis was not different between treatment groups at randomization (Supporting Information, Table S1 ). Animals in acute heart failure after rapidly increased afterload can exhibit paradoxically low trans‐stenosis flow velocity. These animals were identified through dilated left atria and allocated in equal numbers to treatment groups. Animals were randomly assigned to treatment, with 26 animals to vehicle (veh), 29 to valsartan (val), and 28 to sacubitril/valsartan (sac/val). Sixteen animals were sham operated and subsequently received vehicle treatment.
Treatment and housing
All rats were housed in a controlled environment with 12 h light/dark cycle and controlled humidity and temperature, with free access to food and water. Animals were housed in pairs when possible. Treatment was delivered by oral gavage, with drugs dissolved in distilled water. Drugs were dosed according to manufacturer recommendations: sac/val 68 mg/kg/day and valsartan 31 mg/kg/day. All rats were fed 4 mL/kg of solution once daily, with dose adjusted once per week. Animals were sacrificed and tissues harvested after 8 weeks of treatment, as previous experience with the model indicates significant deleterious remodelling at this time point. Some sham animals were harvested at later time point.
Magnetic resonance imaging
Magnetic resonance imaging was performed in a subset of animals at endpoint, using a 9.4T MR system (Varian, USA) (see Supporting Information, Data S1 , for further description). Cine MRI images were acquired to calculate left ventricular volumes and EF, as previously described. 12 Cine segmentation was performed using Segment v3.0 R7820 (http://segment.heiberg.se). 13 Tissue phase mapping (TPM) slices were acquired, one mid‐ventricular short axis and one long axis, allowing calculation of systolic and diastolic strains and strain rates as previously described. 14 , 15 Combination of TPM with data from echocardiography allowed calculation of the ratio of early mitral inflow velocity to the global diastolic strain rate (E/e'SR). Data were analysed in a blinded fashion using MATLAB (The MathWorks Inc., USA).
Echocardiography
A more extensive description of the echocardiography protocol can be found in Supporting Information, Data S1 . Briefly, echocardiography was performed at randomization and after 8 weeks. Trans‐stenosis flow velocity was measured from long axis using pulsed‐wave Doppler. Pulsed‐wave Doppler measurements of mitral flow were performed from an apical view. M‐mode measurements of left ventricle, aorta, and left atrium were acquired from long axis. Analysis of echocardiographic data was performed by a single, blinded operator, using Vevo‐2100 software (Visualsonics, Canada).
Non‐invasive blood pressure readings
We measured arterial blood pressure and heart rate at endpoint using a CODA High‐Throughput non‐invasive tail cuff system with volume–pressure‐recording sensors (Kent Scientific Corporation, Torrington Connecticut). Measurements were performed in awake animals, placed on a heating platform, with a dark blanket covering animals to facilitate calm. Acclimatization cycles were performed to accustom animals to measurements.
Sacrifice
Deep anaesthesia was attained at 4% isoflurane mixed with O2, and the abdomen was opened. Blood was sampled from vena cava inferior into pre‐chilled EDTA and heparin tubes, underwent chilled centrifugation, and was put onto ice before freezing. Samples were stored at −80°C until further analysis. The diaphragm was opened and the heart, excised while still beating, put into cold sterile saline before being weighed. Atria were collected, and the free wall of the right ventricle dissected off and weighed. Left ventricle was weighed, and a mid‐ventricular cross‐sectional slice was taken for histology. Tissue for histological preparations was fixed in formalin, and the remaining left ventricular tissue was split into septum and free wall. All remaining harvested tissues were put into Eppendorf tubes, snap frozen using liquid nitrogen, and stored at −80°C.
Polymerase chain reaction
Quantitative real‐time polymerase chain reaction (qRT‐PCR) was run as previously described by Melleby et al. 16 A more extensive description can be found in Supporting Information, Data S1 . Briefly, RNA was isolated by RNeasy mini and concentration measured with Nanodrop ND‐1000 Spectrophotometer. cDNA was synthesized using iScript cDNA Synthesis. Gene expression levels were measured by qRT‐PCR using TaqMan assays or ddPCR.
Enzyme‐linked immunosorbent assays
Competitive enzyme‐linked immunosorbent assay (ELISA) kits were run on EDTA plasma samples, according to manufacturer's instructions, using commercially available pre‐coated plates. The following kits were used: B‐type NP (BNP) (Abnova, catalognr: KA1861) and atrial NP (ANP) (Abnova, catalognr: KA1680).
Histological sectioning and staining
A more extensive protocol can be found in Supporting Information, Data S1 . Briefly, sections were formalin fixed and paraffin embedded. Sections (4 μm) were stained using Masson's trichrome. High‐resolution images were acquired using an automated slide scanner system at ×20 bright field magnification. Fibrosis was quantified with ZEN blue software and ImageJ, using custom thresholds for labelling of myocardium and stained collagen fibres. Mask fitting was confirmed by visual inspection of every slide. All image manipulation and analyses were performed by a single operator blinded to treatment group.
Statistical analyses
Statistical analyses were performed in GraphPad Prism version 8, except survival analyses, which were completed in R (R Foundation for Statistical Computing, Vienna, Austria). Results are expressed as mean values with standard error of the mean, unless otherwise specified. Comparisons of three or more groups were performed using one‐way ANOVA, with Tukey's post‐hoc test, unless otherwise specified. Prior to ANOVA, prerequisite of normality was tested using D'Agostino–Pearson normality test and, in case of suspected violation of normality, inspection of Q–Q plots. Where normality presumption was violated, data were analysed by Kruskal–Wallis test, with subsequent Dunn's test.
Results
At 8 weeks, there was a non‐significant trend towards higher mortality in vehicle‐treated animals (data not shown). Bodyweight at sacrifice did not differ between groups (Supporting Information, Table S1 ).
Sacubitril/valsartan has superior anti‐hypertrophic effects compared with valsartan alone
Aortic banding increased left ventricular weight by 45% in vehicle compared with sham (Figure 1A ), whereas animals treated with sac/val had a 29% increase, significantly lower than vehicle (P = 0.01). Valsartan alone yielded a 42% increase compared with sham, significantly higher than animals treated with sac/val (P = 0.04), and not significantly different from vehicle. Left ventricular expression of Myh7 was only significantly increased in vehicle (Figure 1D ). Ventricular expression of ANP and BNP was increased in all groups compared with sham.
Figure 1.
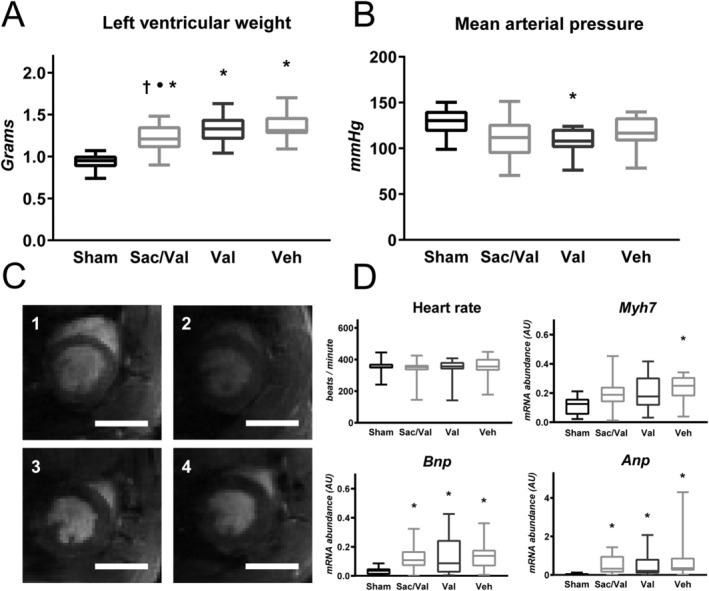
Sacubitril/valsartan (sac/val) superiorly prevents myocardial hypertrophy. (A) Sac/val reduces left ventricular hypertrophy compared with both valsartan and vehicle, as assessed by weighing left ventricle (P = 0.04 vs. val, P = 0.01 vs. veh). Valsartan alone confers no reduction compared with vehicle (P = 0.92) (n = sham: 16, sac/val: 23, val: 24, veh: 19). (B) Mean peripheral arterial pressure was measured through tail cuff. Only animals treated with valsartan showed significant reduction compared with sham (P = 0.02), with sac/val and vehicle showing non‐significant reduction (sac/val vs. sham: P = 0.06; veh vs. sham: P = 0.41) (n = sham: 10, sac/val: 21, val: 22, veh: 15). (C) Representative mid‐ventricular short axis images from Cine magnetic resonance imaging: 1. sham; 2, sac/val; 3, val; 4, veh. Scale bar represents 10 mm. (D) Heart rate, measured by tail cuff, was not different between groups. Myh7 is only significantly up‐regulated in vehicle (P = 0.004) (n = sham: 14, sac/val: 22, val: 24, veh: 18), whereas atrial natriuretic peptide (ANP) and B‐type natriuretic peptide (BNP) are up‐regulated in all groups (ANP: Kruskal–Wallis test, P < 0.0001; BNP: one‐way ANOVA, P = 0.01) (n = sham: 14, sac/val: 21, val: 23, veh: 18) (• denotes P < 0.05 vs. valsartan, † denotes P < 0.05 vs. vehicle, * denotes P < 0.05 vs. sham. Boxplots show median, with whiskers covering 2.5–97.5 percentile).
Blood pressure is not reduced through sacubitril/valsartan treatment
There was no difference in mean arterial pressure between treatment groups (Figure 1B ). Valsartan treatment was the only group with significant reduction compared with sham, with a lowering of 16% (P = 0.02), whereas sac/val did not show a significant reduction.
Diastolic function is preserved in sacubitril/valsartan
We investigated the rate of ventricular relaxation through TPM MRI and found longitudinal early diastolic strain rate to be significantly reduced in valsartan and vehicle compared with sham, but not reduced in sac/val (Figure 2 ). There were no differences in heart rate between groups, as measured by tail cuff (Figure 1 ). Both valsartan and vehicle showed significant elevations in E/e'SR compared with sham, whereas sac/val did not, suggesting preservation of diastolic function (Supporting Information, Table S1 ). Left ventricular expression of Serca2, as measured by qPCR, was not significantly altered in any group.
Figure 2.

Sacubitril/valsartan ameliorates diastolic dysfunction. Reduced early diastolic strain rate is a marker of diastolic dysfunction. Both animals treated with vehicle and valsartan showed significant decreases compared with sham (val vs. sham: P = 0.02; veh vs. sham: P = 0.03), indicating impaired relaxation. This decrease was not observed in animals treated with sacubitril/valsartan (sac/val vs. sham: P = 0.41), indicating a preservation of relaxation (n = sham: 9, sac/val: 17, val: 16, veh: 13) (* denotes P < 0.05 vs. sham).
Fibrosis is not affected by treatment
Left ventricular expression of collagens 1 and 3 showed significant up‐regulation in all banded groups compared with sham, with no difference between treatment groups (Figure 3 ). Mmp2 expression followed the same pattern, whereas TIMP4 was not significantly altered. Histological quantification after Masson's staining revealed that banded animals, as analysed together, had increased left ventricular collagen content compared with sham (Supporting Information, Figure S1 ), with no difference observed between treatment groups.
Figure 3.
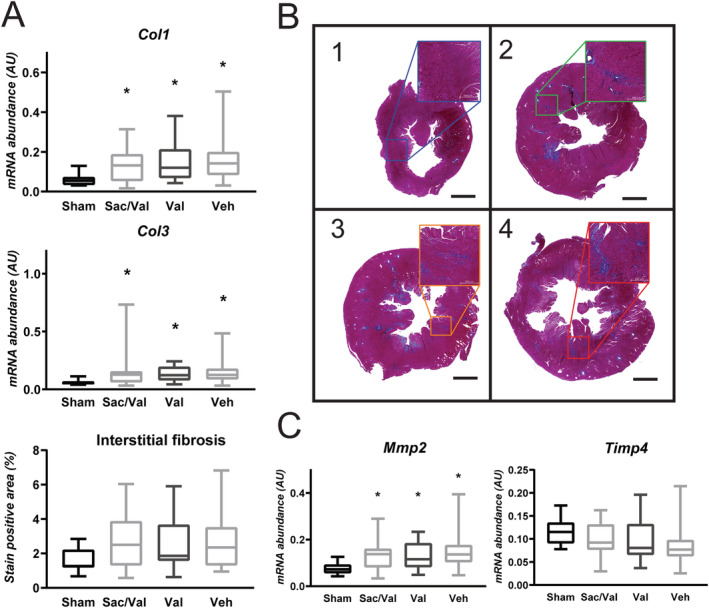
Fibrosis is increased in left ventricle of all banded groups. (A) Aortic banding (AB) induced fibrosis as measured by ventricular expression of Col1 and 3 (Col1: one‐way ANOVA of log‐transformed data, P = 0.002, n = sham: 14, sac/val: 21, val: 24, veh: 18; Col3: one‐way ANOVA of log‐transformed data, P = 0.002, n = sham: 14, sac/val: 22, val: 24, veh: 18). Interstitial fibrosis was significantly increased compared with sham when all banded groups were pooled (unpaired t‐test with Welch's correction, P < 0.0001, n = sham: 14, AB: 66), while mean interstitial fibrosis was similar in all treatment groups (Kruskal–Wallis test, P = 0.92, n = sac/val: 22, val: 25, veh: 19). (B) Representative photomicrographs from 1, Sham; 2, sac/val; 3, val; 4, veh. Scale bar represents 2000 μm. Adjustment of contrast to match reference image has been applied in all images. (C) Mmp2, a known regulator of collagen turnover, was increased in all banded groups (one‐way ANOVA of log‐transformed data, P = 0.002, n = sham: 14, sac/val: 21, val: 24, veh: 18), while Timp4 showed no significant difference (one‐way ANOVA of log‐transformed data, P = 0.12, n = sham: 14, sac/val: 22, val: 24, veh: 18) (* denotes P < 0.05 vs. sham). Boxplots show median, with whiskers covering 2.5–97.5 percentile.
Early systolic dysfunction is not ameliorated
Ejection fraction was not reduced by AB in our model (Supporting Information, Table S1 ). We obtained several additional indices of left ventricular function through TPM MRI. Circumferential strain was not reduced after banding, whereas longitudinal strain was reduced in all banded groups compared with sham, with no difference between treatment groups (Figure 4 ). All banded groups showed significant reductions in both circumferential and longitudinal systolic strain rate, with neither sac/val nor valsartan providing significant improvements compared with vehicle (Supporting Information, Table S1 ).
Figure 4.
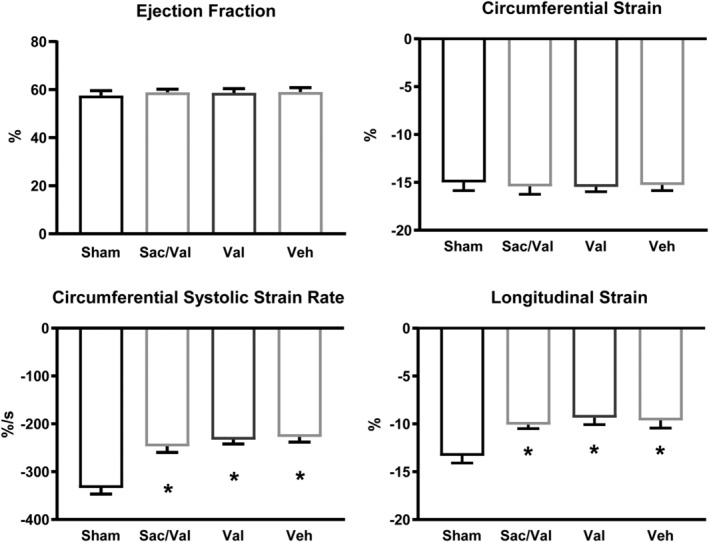
Left ventricular systolic function is depressed in all banded groups. Parameters of overt left ventricular systolic dysfunction showed no change in response to aortic banding (AB), including ejection fraction (EF) and circumferential strain. More sensitive markers of early left ventricular systolic dysfunction, such as longitudinal strain, and both longitudinal (not shown) and circumferential strain rate, demonstrated a depressed systolic function in all banded groups, with no difference between treatments. Cine (n = sham: 12, sac/val: 17, val: 17, veh: 16). Tissue phase mapping (TPM; circumferential: n = sham: 9, sac/val: 18, val: 17, veh: 16. Longitudinal: n = sham: 9, sac/val: 17, val: 16, veh: 13) (* denotes P < 0.05 vs. sham).
Pulmonary congestion is reduced by sacubitril/valsartan treatment
Lung weight was 41% higher in vehicle compared with sham (Kruskal–Wallis, P = 0.02), while it was not significantly different from sham in sac/val nor valsartan (Supporting Information, Table S1 ). Vehicle had a 36% higher right ventricular weight compared with sham (P < 0.001), whereas the ventricular weights of both sac/val and valsartan were similar to sham and significantly lower than vehicle (P = 0.005 and P = 0.01). Right ventricular hypertrophy can progress to dysfunction over time. We assessed right ventricular systolic strain and strain rate through TPM MRI, finding no significant difference between groups, indicating preserved right ventricular systolic function in this model.
Circulating natriuretic peptides
We measured circulating levels of both ANP and BNP in plasma through ELISA. No increase in either peptide was found in any treatment group compared with sham, with no differences between groups (Supporting Information, Figure S2 ).
Discussion
In the present study, we showed that treatment with sac/val has a protective effect on cardiac structure and function in a rat model of pressure overload and preserved EF. In particular, we found that sac/val leads to less hypertrophy and preserves diastolic function.
Aortic banding increased left ventricular weight, and treatment with sac/val reduced the hypertrophic response when compared with both vehicle treatment and valsartan, indicating an additional effect from NEPi compared with angiotensin receptor blocker alone. These findings align with previous studies by other groups, including Burke et al. who demonstrated the same effect in a mouse model of HFrEF. Several factors can drive hypertrophy, among them afterload on the heart, which is partly determined by arterial blood pressure. Pre‐stenotic pressure measurement is not feasible in AB without stabbing through the heart. Therefore, we measured peripheral blood pressure using a tail cuff system. Our study showed no decrease in mean arterial pressure with sac/val, indicating that observed superior anti‐hypertrophic effect, compared with angiotensin receptor blocker, is not blood pressure mediated but rather represents direct cardioprotective actions. The lack of blood pressure reduction in sac/val could relate to systemic hypotension induced by aortic constriction, resulting in activation of RAAS, tempering the natriuretic and diuretic effect of NEP inhibition. Furthermore, NEP is known to degrade pro‐hypertensive factors such as endothelin‐1 and angiotensin. 17 Blood pressure‐independent effects have previously been observed in a pre‐clinical HFrEF model by von Lueder et al. 9 and in sub‐analyses of patients included in both the PARADIGM (HFrEF) and PARAMOUNT (HFpEF) trials. 18 , 19 Our study indicates that these blood pressure‐independent effects extend to pressure overload‐induced remodelling. A possible explanation for these results could lie in the hypothesized main mechanism of action from NEPi—enhancement of NPs. Activation of NP receptor‐A in cardiomyocytes inherently prevents myocardial hypertrophy, 20 thereby leading to blood pressure‐independent cardioprotective effects.
Left ventricular hypertrophy represents part of the adverse cardiac remodelling during chronic pressure overload. Another important pathological change lies in alterations of diastolic function. We therefore measured relaxation of the heart through TPM MRI, finding that early diastolic strain rate was depressed in banded animals. E/e'SR is another indicator of diastolic function, which has been shown to correlate well with ventricular filling pressures 21 and prognosis. 22 E/e'SR was increased in banded animals, indicating diastolic dysfunction. While treatment with valsartan alone did not impact these changes, treatment with sac/val ameliorated changes in both early diastolic strain rate and E/e'SR, suggesting preservation of diastolic function with NEPi.
Several underlying factors influence diastolic function, among them the passive stiffness of the heart. Myocardial deposition of collagen is an important determinant of such stiffness. Our model was characterized by increased fibrosis, where neither treatment with sac/val nor valsartan attenuated these adverse changes. Anti‐fibrotic effect of sac/val has been shown in rats previously 9 , 11 , 23 ; however, several of these observations were made in animals with HFrEF, a condition where beneficial effects of ARNi have been established in patients. Our model is closer to an HFpEF state, and the lack of impact on fibrosis could reflect a differential treatment effect between disease models with reduced and preserved EF.
While degree of fibrosis influences cardiac relaxation, several other factors interplay, such as titin phosphorylation and active removal of calcium from the cytosol. 24 We found no change in the left ventricular expression of Serca2. Although protein turnover may influence actual tissue levels, these data suggest that the observed preservation of diastolic function is not due to higher levels of Serca2. While expression of Serca2 might not be the explanation, NPs are known to have positive effects on diastolic function independent of remodelling. 25 Therefore, experiments investigating the effects of sac/val on live cell calcium handling and titin function might further elucidate the underlying mechanism.
Aortic banding induced early features of systolic dysfunction, such as reductions in longitudinal strain and strain rate, while EF remained normal. Such subtle dysfunction is often seen in human HFpEF and is linked to poor prognosis. 26 , 27 These adverse changes were not impacted by treatment with sac/val. Systolic dysfunction in cardiac pressure overload is partly caused by remodelling of subendocardial regions, with deposition of collagen fibres. 28 Myocardial sheets in this region are predominately responsible for longitudinal strain, and as such, the lack of benefit from treatment is in line with lacking anti‐fibrotic effect. However, several previous studies have shown beneficial effects of sac/val on systolic function in post‐infarction heart failure, 9 , 29 volume overload, 23 and pressure overload in mice, 10 but not in rats. 11 These studies have been characterized by reduced EF, suggesting that effects of sac/val on systolic function may be lesser before EF falls, a finding that is in line with results from human trials. PARAGON‐HF, a large multicentre trial of ARNi in humans with HFpEF, was recently published and did not meet its primary endpoint of cardiovascular death and total hospitalizations. 4 While generating uncertainty about the role of ARNi in treatment of HFpEF, sub‐analyses of the material gave indication of treatment effect in lower ranges of normal EF. 30 In addition to EF being a determinant of treatment effect, disease aetiology may also play a role, and more granular subdivisions might facilitate targeted therapy. 31 Although benefit has been shown in a mouse model of pressure overload‐induced HFrEF, further studies are needed to investigate whether sac/val confers such benefits in other species and models of cardiac dysfunction with preserved EF.
Lung weight was increased in vehicle compared with sham, while both sac/val and valsartan alone showed no increase. Increase in lung weight can be caused by several factors, including pulmonary fibrosis, congestion, and remodelling. 32 The finding of increased right ventricular weight could reflect right ventricular hypertrophy as a compensatory change in response to increased afterload. This increased weight was ameliorated by both sac/val and valsartan alone, which could indicate that treatment had beneficial effects on both afterload of the right side of the heart and pulmonary remodelling.
We did not find an increase in circulating NPs. Reports from previous studies are conflicting, where some show increased circulating levels while others do not. 11 , 29 , 33 A previous human trial by Kobalava et al. did not find an increase in circulating ANP with administration of sac/val twice daily, 34 while Nougue et al. found an increase in circulating ANP as measured by MS/MS. 35 The lack of an increase in our data could indicate that circulating levels of ANP and BNP do not necessarily rise with NEPi in all disease states, depending on baseline production. Furthermore, neprilysin is expressed locally in tissues, and therefore, local inhibition of breakdown might contribute to potentiation of NP signalling without accompanying increases in circulating levels. Several other substrates of neprilysin also exist, and their correlation with hypertrophy and fibrosis represents an interesting avenue for future investigations.
One of the limitations of our study is how our findings cannot delineate the effects of sac/val as the disease phenotype transitions from preserved EF into overt HFrEF. Furthermore, while our estimations of diastolic function, utilizing both MRI and echocardiography, have the advantage of being close to the techniques used in clinical practice, the gold standard of intraventricular pressure readings was not attainable in this model without stabbing through the left ventricle. Future studies could utilize a debanding model to circumvent this limitation. Our measurements of NPs were performed with ELISA. Analysis with mass spectrometry would be needed for exact determination of circulating levels of peptide fragments, such as performed by Nougue et al. 35 Our model of cardiac pressure overload does not reflect the heterogeneity of human HFpEF but has the advantage of allowing investigation of sac/val effect in this specific aetiology and better separation from ARNi effects on systemic blood pressure.
In summary, our findings show that sac/val, compared with valsartan alone, better prevents left ventricular hypertrophy and ameliorates diastolic dysfunction in a pressure overload model of HFpEF. Previous studies have shown positive effects in HFrEF, while the early stages of systolic dysfunction in our model are more similar to the human condition of HFpEF. Held together with results from the PARAGON trial, these findings support the notion that sac/val most likely holds the largest therapeutic potential in HFrEF, while still having favourable effects in cardiovascular disease with a normal range EF. Observed anti‐hypertrophic effect in preserved EF furthermore highlights a potential for NEPi use in patient populations suffering from conditions characterized by extensive hypertrophic remodelling, such as hypertrophic cardiomyopathy or chronic hypertension.
Conflict of interest
Rizwan Hussain was an employee of Novartis AG at the time of study conduct. The other authors declare that they are free from conflict of interest.
Funding
This work was supported by K.G. Jebsen Center for Heart Failure Research and Bjørknes University College. Drugs utilized in the study were provided free of charge from Novartis AG.
Supporting information
Figure S1. Fibrosis is increased in aortic banding: Interstitial fibrosis, as measured by Masson's Trichrome, was increased after aortic banding, compared to sham (Welch's t test, P < 0.0001) (n = Sham: 14, AB: 66) (* denotes P < 0.05 vs Sham. Boxplots show median, with whiskers covering 2.5–97.5 percentile).
Figure S2. Circulating natriuretic peptides are not increased: Circulating ANP and BNP was not significantly increased in any treatment group compared to sham (n = Sham: 16, Sac/Val: 19, Val: 23, Veh: 19. (· denotes P < 0.05 vs Valsartan, † denotes P < 0.05 vs Vehicle, * denotes P < 0.05 vs Sham. Boxplots show median, with whiskers covering 2.5–97.5 percentile).
Data S1. Supporting Information
Table S1. Supporting Information
Acknowledgements
The authors would like to thank Hilde Dishington, Dina Behmen, Marianne Lunde, and Lili Zhang for their valuable contributions to this paper. Histological images were acquired at the Norbrain‐Slidescanning Facility at the Institute of Basic Medical Sciences, University of Oslo, a resource funded by the Research Council of Norway.
Nordén, E. S. , Bendiksen, B. A. , Andresen, H. , Bergo, K. K. , Espe, E. K. , Hasic, A. , Hauge‐Iversen, I. M. , Veras, I. , Hussain, R. I. , Sjaastad, I. , Christensen, G. , and Cataliotti, A. (2021) Sacubitril/valsartan ameliorates cardiac hypertrophy and preserves diastolic function in cardiac pressure overload. ESC Heart Failure, 8: 918–927. 10.1002/ehf2.13177.
This study was carried out in the Institute for Experimental Medical Research, Oslo, Norway.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Administration USFaD . FDA approves new drug to treat heart failure. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm2015 [cited 2016 02/27]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm Accessed 27 February 2016.
- 3. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 5. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Everett RJ, Tastet L, Clavel M‐A, Chin CWL, Capoulade R, Vassiliou VS, Kwiecinski J, Gomez M, Beek EJR, White AC, Prasad SK, Larose E, Tuck C, Semple S, Newby DE, Pibarot P, Dweck MR. Progression of hypertrophy and myocardial fibrosis in aortic stenosis. Circ Cardiovasc Imaging 2018; 11: e007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hyp 2007; 9: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarzani R, Spannella F, Giulietti F, Balietti P, Cocci G, Bordicchia M. Cardiac natriuretic peptides, hypertension and cardiovascular risk. High Blood Press Cardiovasc Prev 2017; 24: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015; 8: 71–78. [DOI] [PubMed] [Google Scholar]
- 10. Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail 2019; 12: e005565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maslov MY, Foianini S, Orlov MV, Januzzi JL, Lovich MA. A novel paradigm for sacubitril/valsartan: beta‐endorphin elevation as a contributor to exercise tolerance improvement in rats with preexisting heart failure induced by pressure overload. J Card Fail 2018; 24: 773–782. [DOI] [PubMed] [Google Scholar]
- 12. Schneider JE, Wiesmann F, Lygate CA, Neubauer S. How to perform an accurate assessment of cardiac function in mice using high‐resolution magnetic resonance imaging. J Cardiovasc Magn Reson 2006; 8: 693–701. [DOI] [PubMed] [Google Scholar]
- 13. Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med Imaging 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espe EKS, Aronsen JM, Skårdal K, Schneider JE, Zhang L, Sjaastad I. Novel insight into the detailed myocardial motion and deformation of the rodent heart using high‐resolution phase contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013; 15: 82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Espe EKS, Aronsen JM, Norden ES, Zhang L, Sjaastad I. Regional right ventricular function in rats: a novel magnetic resonance imaging method for measurement of right ventricular strain. Am J Physiol: Heart Circ Phys Ther 2020; 318: H143–h153. [DOI] [PubMed] [Google Scholar]
- 16. Melleby AO, Strand ME, Romaine A, Herum KM, Skrbic B, Dahl CP, Sjaastad I, Fiane AE, Filmus J, Christensen G, Lunde IG. The heparan sulfate proteoglycan glypican‐6 is upregulated in the failing heart, and regulates cardiomyocyte growth through ERK1/2 signaling. PLoS ONE 2016; 11: e0165079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail 2017; 19: 710–717. [DOI] [PubMed] [Google Scholar]
- 18. Jhund PS, Claggett B, Packer M, Zile MR, Voors AA, Pieske B, Lefkowitz M, Shi V, Bransford T, McMurray JJ, Solomon SD. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: an analysis of the PARAMOUNT trial. Eur J Heart Fail 2014; 16: 671–677. [DOI] [PubMed] [Google Scholar]
- 19. Bohm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJV. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J 2017; 38: 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase‐A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A 2001; 98: 2703–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, Schultheiss H‐P, Tschöpe C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure–volume loop analysis. Eur Heart J Cardiovasc Imaging 2010; 11: 743–751. [DOI] [PubMed] [Google Scholar]
- 22. Ersboll M, Andersen MJ, Valeur N, Mogensen UM, Fakhri Y, Thune JJ, Moller JE, Hassager C, Sogaard P, Kober L. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two‐dimensional speckle‐tracking study. Eur Heart J 2014; 35: 648–656. [DOI] [PubMed] [Google Scholar]
- 23. Maslov MY, Foianini S, Mayer D, Orlov MV, Lovich MA. Synergy between sacubitril and valsartan leads to hemodynamic, antifibrotic, and exercise tolerance benefits in rats with preexisting heart failure. Am J Physiol: Heart Circ Phys Ther 2019; 316: H289–h297. [DOI] [PubMed] [Google Scholar]
- 24. LeWinter MM, Meyer M. Mechanisms of diastolic dysfunction in heart failure with a preserved ejection fraction: if it's not one thing it's another. Circ Heart Fail 2013; 6: 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizuno O, Onishi K, Dohi K, Motoyasu M, Okinaka T, Ito M, Isaka N, Nakano T. Effects of therapeutic doses of human atrial natriuretic peptide on load and myocardial performance in patients with congestive heart failure. Am J Cardiol 2001; 88: 863–866. [DOI] [PubMed] [Google Scholar]
- 26. Kraigher‐Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014; 63: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 2015; 132: 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishizu T, Seo Y, Kameda Y, Kawamura R, Kimura T, Shimojo N, Xu D, Murakoshi N, Aonuma K. Left ventricular strain and transmural distribution of structural remodeling in hypertensive heart disease. Hypertension 2014; 63: 500–506. [DOI] [PubMed] [Google Scholar]
- 29. Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, Nestler JA, Devarakonda T, Ghosh S, Das A, Salloum FN. Sacubitril/valsartan averts adverse post‐infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol 2018; 72: 2342–2356. [DOI] [PubMed] [Google Scholar]
- 30. Solomon SD, Vaduganathan M, B LC, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer MA, Desai A, Lund LH, Kober L, Anand I, Sweitzer N, Linssen G, Merkely B, Luis Arango J, Vinereanu D, Chen CH, Senni M, Sibulo A, Boytsov S, Shi V, Rizkala A, Lefkowitz M, McMurray JJV. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020; 141: 352–361. [DOI] [PubMed] [Google Scholar]
- 31. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019; 40: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension 2012; 59: 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu J, Noe A, Chandra P, Al‐Fayoumi S, Ligueros‐Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual‐acting angiotensin receptor‐neprilysin inhibitor (ARNi). J Clin Pharmacol 2010; 50: 401–414. [DOI] [PubMed] [Google Scholar]
- 34. Kobalava Z, Kotovskaya Y, Averkov O, Pavlikova E, Moiseev V, Albrecht D, Chandra P, Ayalasomayajula S, Prescott MF, Pal P, Langenickel TH, Jordaan P, Rajman I. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther 2016; 34: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nougue H, Pezel T, Picard F, Sadoune M, Arrigo M, Beauvais F, Launay JM, Cohen‐Solal A, Vodovar N, Logeart D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail 2019; 21: 598–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fibrosis is increased in aortic banding: Interstitial fibrosis, as measured by Masson's Trichrome, was increased after aortic banding, compared to sham (Welch's t test, P < 0.0001) (n = Sham: 14, AB: 66) (* denotes P < 0.05 vs Sham. Boxplots show median, with whiskers covering 2.5–97.5 percentile).
Figure S2. Circulating natriuretic peptides are not increased: Circulating ANP and BNP was not significantly increased in any treatment group compared to sham (n = Sham: 16, Sac/Val: 19, Val: 23, Veh: 19. (· denotes P < 0.05 vs Valsartan, † denotes P < 0.05 vs Vehicle, * denotes P < 0.05 vs Sham. Boxplots show median, with whiskers covering 2.5–97.5 percentile).
Data S1. Supporting Information
Table S1. Supporting Information


