Abstract
Aims
Sarcopenia has been found to be frequently associated with co‐morbidity among patients with heart failure (HF). However, there remain insufficient data to accurately estimate the global prevalence of sarcopenia in HF. Therefore, the purpose of this research was to conduct a systematic review and meta‐analysis to estimate the current overall prevalence of sarcopenia in patients with HF.
Methods and results
We searched relevant databases for studies published up to 13 July 2020, assessing sarcopenia in vpatients with HF. After careful screening, data of included articles were extracted with a predesigned Excel form. Then the pooled prevalence of sarcopenia in patients with HF was calculated using the random‐effects model. The Q test was used to assess the heterogeneity, and I 2 statistic was calculated to quantify and evaluate the heterogeneity. Subgroup analyses were conducted to determine potential sources of heterogeneity. A total of 2852 articles were initially identified, and after removing duplicate publications and applying the selection criteria, we reviewed 79 full‐text articles. Finally, 11 articles (n = 1742 patients with HF) were included in this systematic review and meta‐analysis. The pooled prevalence of sarcopenia in patients with HF was 34% [95% confidence interval (CI): 22–47%, I 2 = 96.59%] and ranged from 10% to 69%. However, substantial heterogeneity between studies (I 2 = 96.59%, P < 0.001) was observed. There was no significant heterogeneity between subgroups by sex (P = 0.803) or the method used to define sarcopenia (P = 0.307). While the heterogeneity between subgroups by population setting was statistically significant (P < 0.001), the pooled prevalence of sarcopenia was 55% (95% CI: 43–66%) for hospitalized patients with HF and 26% (95% CI: 16–37%) for ambulatory patients.
Conclusions
Sarcopenia was a common condition in patients with HF, and the prevalence of hospitalized patients was higher than for ambulatory patients. Early detection of sarcopenia was therefore important in patients with HF, and it was important to implement interventions so that physical therapists or managerial dieticians can easily be introduced into clinical practice.
Keywords: Sarcopenia, Muscle wasting, Heart failure, Multimorbidity, Meta‐analysis
Introduction
Heart failure (HF) is a common chronic disease and represents the terminal stages of several types of heart disease, and its incidence is increasing annually. 1 As a syndrome with poor prognosis, HF has a significant influence on patient well‐being and quality of life. HF currently affects approximately 26 million patients worldwide, and its prevalence increases dramatically with age. 2 As a result, the elderly population represents over 80% of all patients with HF. The disproportionate prevalence of HF in later life is largely attributable to long‐term exposure to cardiovascular risk factors. 3 Indeed, HF negatively influences patient functional capacity and ability to fulfil daily activities. Previous studies have found that HF has a significant impact on patient muscle function and body composition, which has been clearly associated with considerable morbidity, institutionalization, and mortality. 4 , 5
Sarcopenia, a primarily age‐dependent syndrome, has been defined as the age‐related decline in skeletal muscle mass, strength, and physical performance and found to be frequently associated with co‐morbidity among patients with HF. 6 , 7 Changes in muscle function and composition are considered critical determinants in the progression of HF. 8 Indeed, an interplay may exist between the pathophysiological pathways involved in HF, age‐related changes in body composition, and sarcopenia. 9 The adaptive changes in the musculoskeletal system following the occurrence of HF play a key role in the development of many symptoms related to HF syndrome. 10 Thus, sarcopenia may contribute to the poor prognosis seen in patients presenting with HF. Sarcopenia affects approximately 20% of older adults with HF, which exceeds the prevalence observed in individuals of the same age with no HF. 11 Remarkably, older patients with HF with sarcopenia have been associated with poor physical performance and oxygen consumption, worsening left ventricular function, and higher hospitalization rates. 12 Therefore, the recognition of sarcopenia in the context of HF and the implementation of targeted therapeutic strategies may help ameliorate the poor patient functional capacity, before the wasting disorder progresses to a later stage. 3 , 13
The prevalence of low appendicular mass may vary substantially in different populations. 14 Currently, there remain insufficient data to accurately estimate the global prevalence of sarcopenia in HF. Therefore, we conducted a systematic review and meta‐analysis to appraise the best available evidence on the prevalence of sarcopenia in patients with HF. Estimating the overall prevalence of sarcopenia, according to the current operational definitions, recommended by a multidisciplinary consensus, will provide further insight into its burden during HF. Our secondary objectives were to verify possible factors affecting the prevalence of sarcopenia in HF and the effect of sarcopenia on the outcome of patients with HF.
Methods
Search strategy and data sources
We performed an electronic search of PubMed, Embase, Medline, Web of Science, and Scopus, to identify all relevant articles relating to sarcopenia and HF. We searched all records published from inception through to 13 July 2020, using the following terms: ‘sarcopenia’, ‘muscle atrophy’, ‘muscle wasting’, ‘HF’, ‘cardiac failure’, ‘heart decompensation’, and ‘myocardial failure’. We further hand searched bibliographies of relevant publications to identify potential articles missed by the principal search.
Selection criteria and screening
For this systematic review, we used the following inclusion criteria: (i) all studies had to be case controlled and of longitudinal or cross‐sectional design; (ii) research subjects: ≥18 years old, patients with HF diagnosed according to the clinical gold standard; (iii) assessment of sarcopenia had to be implemented according to the sarcopenia consensus criteria (comprehensive: muscle mass and muscle strength or physical performance are combined; non‐comprehensive: the aforementioned three aspects are only partially combined or applied separately); (iv) measurement of lean mass or muscle mass had to be assessed by at least one of four main methods: bioelectrical impedance analysis, dual‐energy X‐ray absorptiometry, magnetic resonance imaging, or computed tomography; and (v) the prevalence of sarcopenia (primary outcome) in patients with HF had to be reported.
To provide additional insight into the prevalence of sarcopenia in patients with HF, we also investigated possible factors affecting the prevalence of sarcopenia in HF and the effect of sarcopenia on the outcome of patients with HF.
We limited our inclusion to original publications, without language restriction, and excluded studies using the following criteria: (i) non‐original research such as reviews and meta‐analyses, comments, guidelines, editorials or letters, conference summaries, and case reports; (ii) repeated research (refers to the same article or the same data used in the article); (iii) studies that referred to other populations other than patients with HF; (iv) the number of cases or prevalence of sarcopenia in the study subjects is not reported; and (v) the sample size is less than 10.
The searched study citations were imported to EndNote for duplicate checking. After duplicate studies were deleted, two independent co‐authors screened all the articles by title and abstract. Disagreements were resolved by consensus between both co‐authors, together with a third co‐author. The citations were classified as eligible, uncertain about eligibility, or excluded. The full texts of articles classed as ‘eligible’ and ‘uncertain about eligibility’ were obtained for further review and evaluation.
Data extraction and quality assessment
We used a predefined data collection form (Data S1 ), in which we summarized relevant information on research characteristics (first author, publication year, and period of study), characteristics of the study population (the number, source, age, and sex), and outcome assessment (prevalence of sarcopenia and definition of sarcopenia). When relevant data were not available, the corresponding authors of the selected publications were contacted to complement the electronic searches.
The methodological quality of selected studies was assessed using an 11‐item checklist (Table S2 ), which was recommended by the Agency for Healthcare Research and Quality. An item would be scored ‘0’ if it was answered ‘NO’ or ‘UNCLEAR’; if it was answered ‘YES’, then the item scored ‘1’. Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; and high quality = 8–11. 15
Statistical analysis and graphing
We summarized the main characteristics of included studies in tables. Meta‐analyses on the prevalence of sarcopenia in patients with HF were undertaken using a random‐effects model. Forest plots were generated to describe the prevalence of sarcopenia and corresponding 95% confidence intervals (CIs), for each study and overall estimate. The Q test was used to test the heterogeneity, and I 2 statistic was calculated to quantify and evaluate the heterogeneity (low: 25–50%, moderate: 50–75%, and high: >75%). To discover potential sources of heterogeneity, subgroup analyses were conducted by sex, population setting, and evaluation method of sarcopenia. To assess the stability of the pooled results, sensitivity analysis was conducted by excluding each study at a time. A funnel plot was generated to visually assess the publication bias, and Egger's test and Begg's test were also conducted. All the aforementioned analyses and graphing were conducted in the STATA 15.0 software. A P value ≤ 0.05 was considered as statistically significant.
Results
Search results and characteristics of included studies
A total of 2852 articles were found from the aforementioned databases after the systematic search. After discarding duplicates, the titles and abstracts of 1870 articles were screened. Then, with one additional article identified from the reference list, the full texts of the remaining 79 articles were reviewed. Eventually, 11 articles were included in the final analysis (Figure 1 ).
Figure 1.
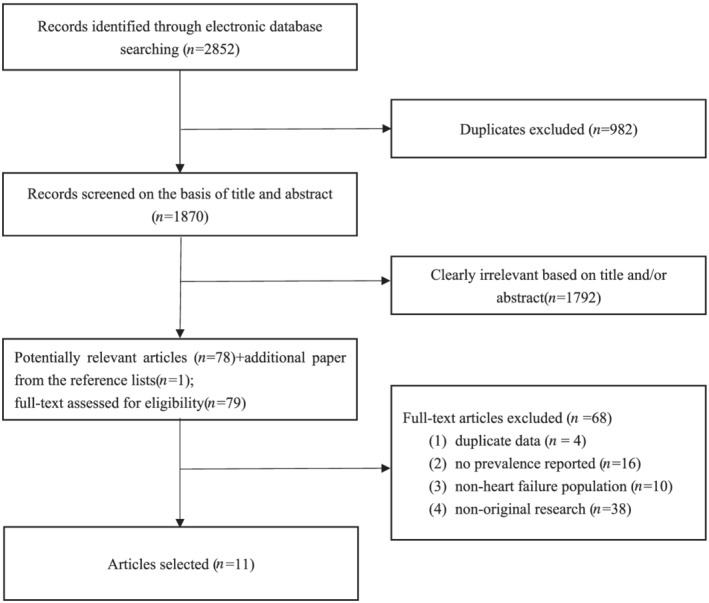
Study flow diagram demonstrating the search processes for identifying eligible articles.
The main characteristics and results of quality assessment of all included studies are listed in Table 1 . Among the 11 eligible studies published between 2016 and 2020, eight adopted cross‐sectional design and three adopted cohort design. A total of 1742 patients with HF from seven ambulatory studies and four hospitalized studies were involved, and most of them were male (n = 1272). Nine studies reported mean age of the subjects, ranging from 37.3 to 79.8 years at study entry. Seven of the selected studies used the non‐comprehensive definition of sarcopenia, and the remaining four used a comprehensive definition. Quality assessment scores of all included studies were between 7 and 10 points, nine studies were regarded as ‘high quality’, and the remaining two studies were regarded as ‘medium quality’ (Table S3 ).
Table 1.
Main characteristics of selected studies in the meta‐analysis
| Authors (publication year) | Study design | Population settings | Sample size | Male | Female | Age (χ ± s) | Participants with sarcopenia (n) | Assessment of sarcopenia | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Onoue et al. (2016) 16 | Cohort study | Hospitalized | 119 | 73 | 46 | 76.1 ± 6.2 | 82 | Non‐comprehensive | 7 |
| Emami et al. (2018) 17 | Cross‐sectional study | Ambulatory | 207 | 207 | — | 67.3 ± 10.1 | 44 | Non‐comprehensive | 9 |
| Fonseca et al. (2020) 6 | Cross‐sectional study | Ambulatory | 168 | 168 | — | — | 66 | Non‐comprehensive | 8 |
| Dos Santos et al. (2017) 18 | Cross‐sectional study | Ambulatory | 228 | 181 | 47 | 68.8 ± 9.6 | 37 | Non‐comprehensive | 8 |
| Canteri et al. (2019) 12 | Cross‐sectional study | — | 79 | 46 | 33 | 65.6 ± 13 | 8 | Comprehensive | 8 |
| Nozaki et al. (2019) 19 | Cohort study | Ambulatory | 191 | 136 | 55 | 73.3 ± 7.3 | 20 | Comprehensive | 10 |
| Tsuchida et al. (2018) 20 | Cohort study | Hospitalized | 38 | 25 | 13 | 75.0 ± 11.4 | 20 | Non‐comprehensive | 7 |
| Fonseca et al. (2019) 21 | Cross‐sectional study | Ambulatory | 116 | 116 | — | 55 ± 9.0 | 33 | Comprehensive | 8 |
| Kono et al. (2020) 22 | Cross‐sectional study | Hospitalized | 186 | 81 | 105 | 79.8 ± 9.64 | 77 | Non‐comprehensive | 8 |
| Zhao et al. (2020) 1 | Cross‐sectional study | Hospitalized | 355 | 207 | 148 | — | 198 | Comprehensive | 8 |
| Hajahmadi et al. (2017) 23 | Cross‐sectional study | Ambulatory | 55 | 32 | 23 | 37.3 ± 10.1 | 26 | Non‐comprehensive | 8 |
Prevalence of sarcopenia in patients with heart failure
Overall pooled prevalence of sarcopenia in patients with heart failure
The prevalence of sarcopenia in patients with HF ranged from 10% to 69%, and the overall pooled prevalence of sarcopenia was 34% (95% CI: 22–47%). There was substantial heterogeneity between studies (I 2 = 96.59%, P < 0.001, Figure 2 ).
Figure 2.
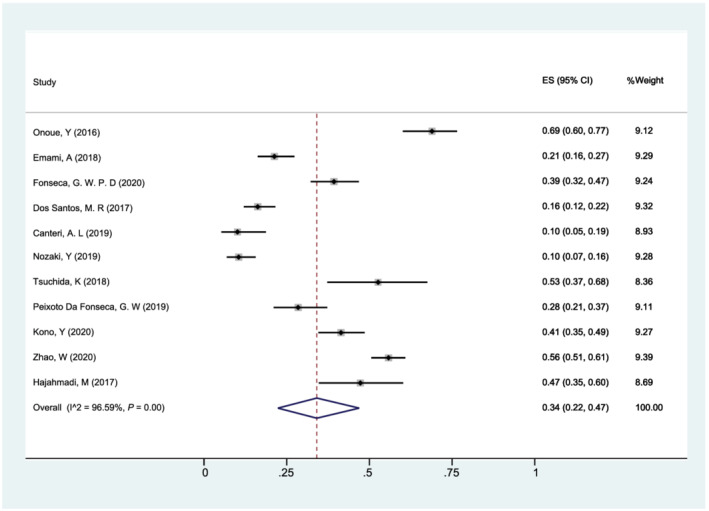
Forest plot of the overall pooled prevalence of sarcopenia in patients with heart failure using a random‐effects model; the Q test and I 2 statistic were used to test the heterogeneity. CI, confidence interval; ES, effect size (the propotion of sarcopenia).
The result of sensitivity analysis showed that the pooled prevalence of sarcopenia varied from 31% (95% CI: 20–43%) (when Onoue et al. was excluded) to 37% (95% CI: 24–50%) (when Canteri et al. was excluded) and indicated that the stability of overall prevalence of sarcopenia in patients with HF was not influenced by a single study (Table S1 , Figure 3 ).
Figure 3.
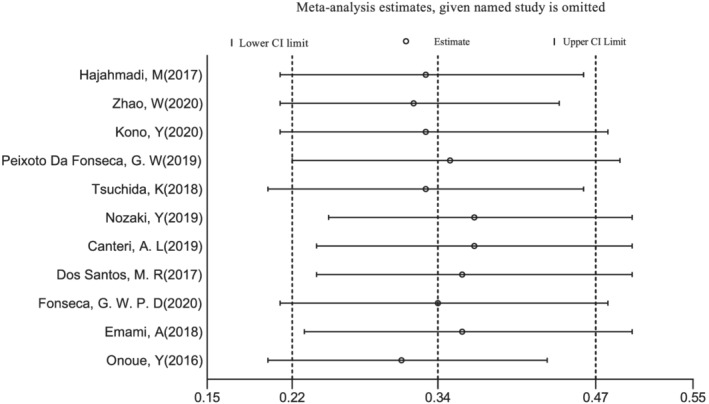
Sensitivity analysis for the effect of individual studies (given named study in the Y axis is omitted) on the pooled prevalence of sarcopenia. CI, confidence interval.
An Egger's publication bias plot was generated, and the visual symmetry of the funnel plot suggested that there was minimal publication bias (Figure 4 ). Moreover, both results of Egger's test (P = 0.943) and Begg's test (P = 0.533) also indicated that there was minimal potential risk of publication bias.
Figure 4.
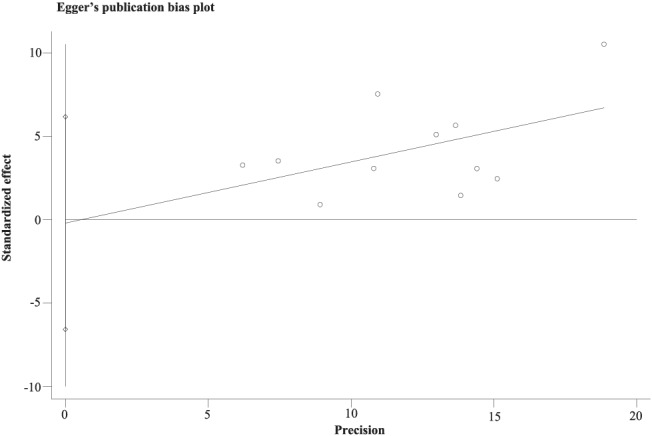
The Egger's funnel plot of the 11 included studies in the meta‐analysis using Egger's test.
Subgroup analysis
We included 10 studies and stratified our analysis according to sex (Figure 5 ). The pooled prevalence of sarcopenia in patients with HF was 37% (95% CI: 26–49%) for men and 33% (95% CI: 13–58%) for women. There was no significant heterogeneity between subgroups by sex (P = 0.803), while significant heterogeneity within subgroups was found (male: I 2 = 93.65%; female: I 2 = 95.45%).
Figure 5.
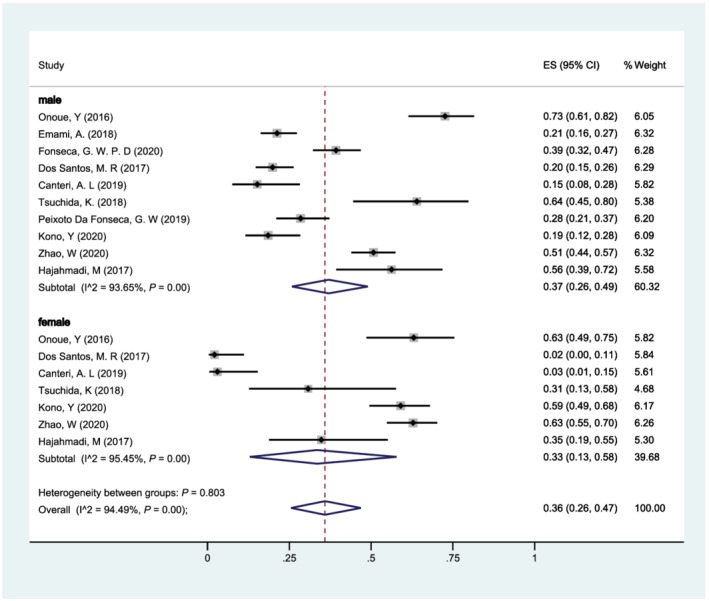
Forest plot of the prevalence of sarcopenia in patients with heart failure of different gender (male vs. female) using a random‐effects model; the Q test and I 2 statistic were used to test the heterogeneity. CI, confidence interval; ES, effect size.
According to the two main population settings, (i) hospitalized and (ii) ambulatory, a total of 10 studies were included to stratify our analysis (Figure 6 ). The pooled prevalence of sarcopenia was 55% (95% CI: 43–66%) for hospitalized patients with HF and 26% (95% CI: 16–37%) for ambulatory patients. The heterogeneity between subgroups was statistically significant (P < 0.001), while the heterogeneity within subgroups was also found (hospitalized: I 2 = 87.17%; ambulatory: I 2 = 92.45%).
Figure 6.
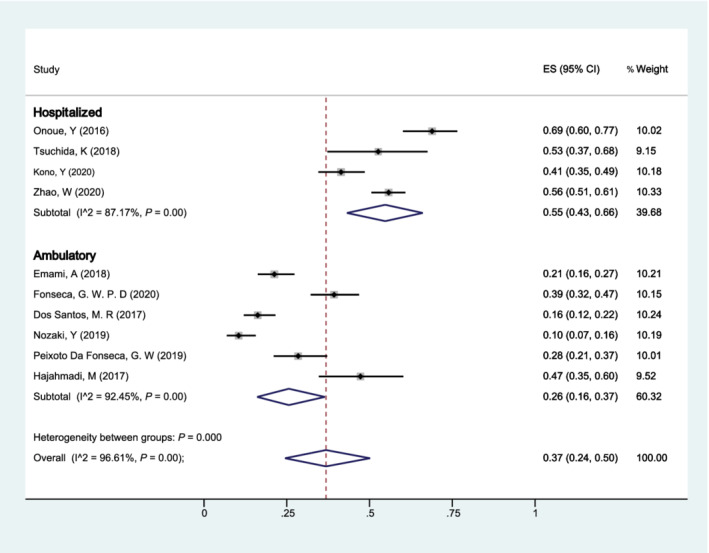
Forest plot of the prevalence of sarcopenia in patients with heart failure of different population settings (hospitalized vs. ambulatory) using a random‐effects model; the Q test and I 2 statistic were used to test the heterogeneity. CI, confidence interval; ES, effect size.
Additionally, we stratified our analysis according to the method used to define sarcopenia in all 11 studies: (i) comprehensive and (ii) non‐comprehensive (Figure 7 ). The pooled prevalence of sarcopenia in patients with HF was 24% (95% CI: 5–51%) and 40% (95% CI: 26–55%), respectively. No significant heterogeneity was found between subgroups (P = 0.307), while there was significant heterogeneity within subgroups (comprehensive: I 2 = 98.14%; non‐comprehensive: I 2 = 95.44%).
Figure 7.
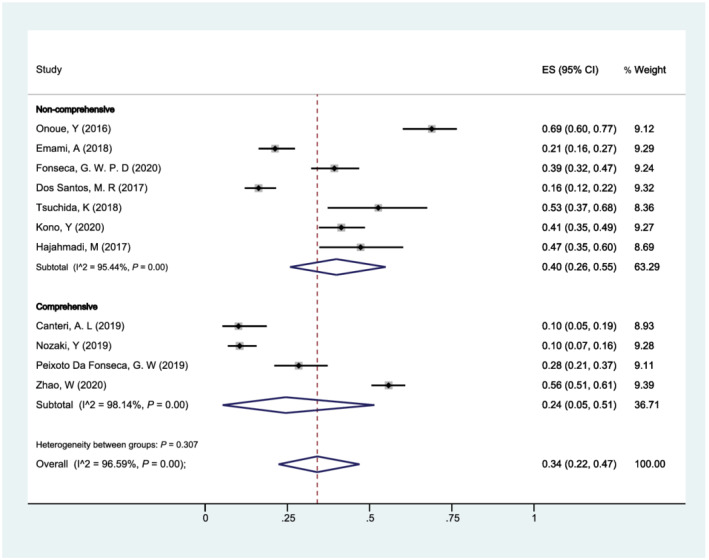
Forest plot of the prevalence of sarcopenia in patients with heart failure of different methods used to define sarcopenia (comprehensive vs. non‐comprehensive) using a random‐effects model; the Q test and I 2 statistic were used to test the heterogeneity. CI, confidence interval; ES, effect size.
Factors associated with sarcopenia in patients with heart failure
Regarding the risk factors for sarcopenia in patients with HF, age and body mass index (BMI) were frequently mentioned in studies. 1 , 6 , 12 , 16 , 18 , 20 , 21 , 22 , 23 When compared with the sarcopenia group, the non‐sarcopenia group was older 1 , 6 , 12 , 16 , 18 , 21 , 22 and had a lower BMI. 6 , 16 , 18 , 20 , 21 , 22 , 23
Seven studies compared the left ventricular ejection fraction (LVEF) between patients with HF with and without sarcopenia. Two studies 23 , 24 observed that LVEF was significantly lower in the sarcopenia group, when compared with the non‐sarcopenia group. However, one study 22 observed the opposite result. In addition, the remaining four studies 6 , 18 , 20 , 21 did not observe a statistical difference between the two groups.
Several studies have reported that patients from the sarcopenia group had significantly higher B‐type natriuretic peptide (BNP) levels, when compared with the non‐sarcopenia group, 16 , 20 , 21 whereas Fonseca et al. 6 did not observe a statistical difference in BNP levels between the two groups.
The effect of sarcopenia on heart failure
There were two prospective studies investigating the effect of sarcopenia on the outcome of patients with HF. Onoue et al. 24 divided the patients with HF into two groups based on the presence or absence of sarcopenia and defined using sarcopenia scores. After a median of 495 days of follow‐up, the Kaplan–Meier curve indicated that the HF event (included HF‐related hospitalization and deaths due to HF) free survival rates were significantly lower in the sarcopenia group. The stepwise multivariate Cox proportional hazard analysis identified the sarcopenia score (hazard ratio: 1.03; 95% CI: 1.01–1.05, P < 0.01) as independent and significant predictors of future HF events. Furthermore, multivariate Cox proportional hazard analysis using the forced entry method also indicated that the sarcopenia score was a significant predictor of future HF events, with adjusted factors suspected for associations with HF or nutrition. Nozaki et al. 19 also conducted a prospective cohort study to explore predictors of cardiac events in patients with HF, comprising cardiac death or HF‐related hospitalization. The multivariate Cox proportional hazard analysis revealed that sarcopenia was significantly associated with a cardiac event in the New York Heart Association Class II–IV group (hazard ratio: 4.44; 95% CI: 1.09–18.14; P = 0.04) after adjusting for sex, BNP, and estimated glomerular filtration rate.
Discussion
To the best our knowledge, this systematic review using meta‐analysis is the first to provide a comprehensive overview of the current overall prevalence of sarcopenia in patients with HF. Because both sarcopenia and HF have serious effects on the health of the population, our results may provide a preliminary basis for the prevention and treatment of HF‐related co‐morbid sarcopenia.
Overall, we found that 34% of individuals with HF had sarcopenia. The prevalence estimates of sarcopenia varied between 10% and 69%. Most of the included studies were of high quality, and the pooled rate was robust and not affected by publication bias. This rate was higher than the prevalence rate of sarcopenia in the community‐dwelling elderly population. 9 , 25 , 26 Among our included studies, only one 12 compared the prevalence of sarcopenia in HF group and control group, but no statistical difference was found (10.1% vs. 3.5%, P = 0.09), which is inconsistent with that mentioned in previous studies, where the prevalence of patients with HF with sarcopenia is higher than healthy subjects of the same age. 3 , 9 This may be limited by the number of studies and sample size. Therefore, more studies based on large samples are necessary to strengthen our understanding of the prevalence and importance of sarcopenia in patients with HF. In addition, we observed large heterogeneity across studies; therefore, further stratified analysis was carried out to explore its source.
The prevalence of sarcopenia in patients with HF largely varied by population settings. The rate of sarcopenia in hospitalized patients was significantly higher than that of outpatients. This finding can be explained by two main factors: first, HF cases from the ambulatory population are more often in an early stage of the disease or even asymptomatic, and second, according to the mean age, those hospitalized participants were older than the mean age range of the outpatient‐based studies. Therefore, differences between population settings should be considered when assessing the risk of sarcopenia.
There was no statistical difference in the pooled prevalence of sarcopenia in patients with HF between men and women. Of the six included studies that compared the sex composition of patients with or without sarcopenia, four reported that there was no statistical difference in sex composition. It is reasonable to conclude, therefore, that sex might not play a role on the prevalence of sarcopenia in patients with HF. However, previous studies reported that the association between sarcopenia and functional decline was more significant in men than in women, 27 , 28 which deserves further research into the influence of sarcopenia on sex‐related differences.
The significant between‐study heterogeneity was not explained by the method used to define sarcopenia. Although the prevalence of sarcopenia in studies using comprehensive methods seems to be lower than those using non‐comprehensive methods (24% vs. 40%), this difference was not statistically significant. A possible reason may be cursory grouping. Due to different methods used to define sarcopenia, included studies were simply divided into only two subgroups, according to whether the muscle mass, muscle strength, and physical performance were considered comprehensively or not. Four studies used a comprehensive approach, two 1 , 19 of which applied the standards suggested by the Asian Working Group, and the other two 12 , 21 applied the algorithm developed by the European Working Group. Similarly, they used muscle mass and muscle function (walking speed or handgrip strength) as necessary factors to define sarcopenia, although the cut‐off values were not completely consistent. Most of our selected studies (n = 7) 6 , 16 , 17 , 18 , 20 , 22 , 23 applied non‐comprehensive criteria to define sarcopenia in patients with HF. Heterogeneity within groups implied that there remained differences within estimates of sarcopenia between these methods. Currently, there is still a lack of uniformity in measurement standards, especially in cut‐off points across these criteria. 11 , 29 , 30 , 31 Additional studies are needed to establish which functional test and cut‐offs should be used across different stages of HF.
Multiple factors were considered associated with the presence of sarcopenia in patients with HF in the selected studies. These factors included age, BMI, LVEF, and BNP. It is well established that the prevalence of sarcopenia increases with aging; after all sarcopenia is widely defined as an age‐related condition. 26 , 32 BMI was lower in patients with sarcopenia, when compared with those without, and this is similar to other studies. 13 Our findings can be explained by the fact that patients with HF with higher BMIs had better outcomes. A patient with HF with higher BMI may have a better skeletal muscle composition, because of less metabolic and neurohormonal derangements. 33 Furthermore, our study found that reduced LVEF may be related to sarcopenia in patients with HF. This finding is similar to the conclusions of previous studies showing that the degree skeletal myofibril atrophy and its functional alteration are correlated with lower LVEF. 34 Historically, patients with HF have been categorized based on their LVEF, which is important in HF management because LVEF has been shown to be predictive of adverse outcomes, even in the absence of symptomatic HF. 35 , 36 Therefore, the grading of LVEF in the predictive role of sarcopenia in patients with HF may be a direction worthy of further research. In addition, BNP is a response to ventricular volume expansion and pressure overload. Some research reported that BNP levels were highly associated with a poor prognosis of patients with HF, 37 , 38 which may explain that our multiple studies found that the sarcopenia group had higher BNP levels when compared with the non‐sarcopenia group. More studies are needed to identify the main characteristics of patients with HF who present with sarcopenia.
There have only been two prospective studies that have found that sarcopenia was significantly associated with an HF event (included HF‐related hospitalization and death). Many studies, however, have found that the pathophysiology of HF and sarcopenia share common elements, such as oxidative stress, inadequate nutrition, infiltration of skeletal muscle by fat and connective tissue, and increased pro‐inflammatory cytokines. 39 , 40 , 41 , 42 Breathlessness, fatigue, and palpitations are common symptoms of HF, when patients perform physical activities and deterioration in exercise function further limit physical activity. It is likely that the reduced muscle function in sarcopenia, combined with HF symptoms, and the limited physical activity of patients with HF are associated with an increased risk of physical disability, which further increases the deterioration of patients with HF and leads to further HF events. 19 However, the mechanism behind the relationship between skeletal muscle loss and failing heart remains elusive. Further studies are needed to determine whether sarcopenia can be a potential therapeutic target and thereby improve outcomes in patients with HF.
We feel that the strengths of our systematic review included a comprehensive search strategy and the involvement of two independent co‐authors, at all stages of the process. Nevertheless, this work was not devoid of limitations, and several factors should be considered when interpreting our results. First, most of the articles included in the analysis were of cross‐sectional design, and the only three cohort studies used sarcopenia as a baseline assessment only. Therefore, the coexistence of sarcopenia and HF could not explain the occurrence of the two conditions. Second, the studies used different consensus criteria, and the disease may be affected by some unknown factors, resulting in different estimates of sarcopenia prevalence. Despite being in the subgroup analysis, we still observed the heterogeneity of the studies, which may affect the external generality of pooled prevalence. It should be emphasized that the main aim of this systematic review and meta‐analysis was to provide a comprehensive overview of the current overall prevalence of sarcopenia in patients with HF. The discovery of more influencing factors still requires further original research in the future. Finally, although we have explored several risk factors affecting sarcopenia in patients with HF, this was not the main purpose of the included studies. The comparison between the sarcopenia and non‐sarcopenia groups only represented the results of univariate analysis, and the influence of confounding factors was not considered. Therefore, in future research, multivariate analysis is needed to clarify the effects of these factors on sarcopenia in HF.
In conclusion, it is important to highlight our finding that sarcopenia was a frequent condition in patients with HF, and the prevalence of hospitalized patients was higher than that of ambulatory patients. Age, BMI, LVEF, and BNP may also be associated with sarcopenia in HF, and sarcopenia was significantly associated with future HF events, and therefore, early detection of sarcopenia is important for patients with HF. It is, therefore, necessary to devise interventions that a physical therapist or managerial dietician can easily introduce into clinical practice.
Conflict of interest
None declared.
Funding
This work was supported by the Science and Technology Planning Project of Shenzhen City, Guangdong Province, China (Grant No. SZGW2018002); the Science and Technology Planning Project of Shenzhen City, Guangdong Province, China (Grant No. JCYJ20180703145202065); Shenzhen Medical Key Discipline Construction Fund; and Sanming Project of Medicine in Shenzhen (Grant No. SZSM201811093).
Author contributions
J.X. and Z.‐G.Z. conceived and designed this research. X.‐L.Y., H.‐M.Z., and P.L. searched literatures, Y.Z. and J.Z. screened literature and extracted the data, and J.Z. and W.‐Q.N. performed statistical analyses and graphing. Y.Z. wrote the initial draft of the paper. J.X. and Z.‐G.Z. provided major suggestions for the revision of the paper. All authors edited and approved the final manuscript.
Supporting information
Table S1. Result of sensitivity analysis
Table S2. AHRQ checklist.docx
Table S3. Scores of all the items of AHRQ checklist for each study.docx
Data S1. Data collection form and the result of collection
Acknowledgement
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Zhang, Y. , Zhang, J. , Ni, W. , Yuan, X. , Zhang, H. , Li, P. , Xu, J. , and Zhao, Z. (2021) Sarcopenia in heart failure: a systematic review and meta‐analysis. ESC Heart Failure, 8: 1007–1017. 10.1002/ehf2.13255.
Contributor Information
Jian Xu, Email: anniexu73@126.com.
Zhiguang Zhao, Email: 1498384005@qq.com.
References
- 1. Zhao W, Lu M, Wang X, Guo Y. The role of sarcopenia questionnaires in hospitalized patients with chronic heart failure. Aging Clin Exp Res 2020: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seferović PM. Introduction to the special issue entitled ‘Heart failure management of the elderly patient: focus on frailty, sarcopenia, cachexia, and dementia’. Eur Heart J Suppl 2019; 21: L1–L3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collamati A, Marzetti E, Calvani R, Tosato M, D'Angelo E, Sisto AN, Landi F. Sarcopenia in heart failure: mechanisms and therapeutic strategies. J Geriatr Cardiol 2016; 13: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saitoh M, Dos Santos MR, Ebner N, Emami A, Konishi M, Ishida J, Valentova M, Sandek A, Doehner W, Anker SD. Nutritional status and its effects on muscle wasting in patients with chronic heart failure: insights from studies investigating co‐morbidities aggravating heart failure. Wien Klin Wochenschr 2016; 128: 1–8. [DOI] [PubMed] [Google Scholar]
- 5. Sandek A, Doehner W, Anker SD, Von Haehling S. Nutrition in heart failure: an update. Curr Opin Clin Nutr Metab Care 2009; 12: 384–391. [DOI] [PubMed] [Google Scholar]
- 6. Fonseca G, Dos Santos MR, de Souza FR, Takayama L, Rodrigues Pereira RM, Negrao CE, Alves MNN. Discriminating sarcopenia in overweight/obese male patients with heart failure: the influence of body mass index. ESC Heart Fail 2020; 7: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016; 222: 41–46. [DOI] [PubMed] [Google Scholar]
- 8. Curcio F, Testa G, Liguori I, Papillo M, Abete P. Sarcopenia and heart failure. Nutrients 2020; 12: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. Esc Heart Failure 2017; 4: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mauro Z, Andrea R, Francesca C, Clara B, Gloria M, Francesco F. Sarcopenia, cachexia and congestive heart failure in the elderly. Endocr Metab Immune Disord ‐ Drug Targets (Formerly Current Dru) 2013; 13: 58–67. [DOI] [PubMed] [Google Scholar]
- 11. Cruz‐Jentoft AJ, Glistan B, Jrgen B, Yves B, Olivier B, Tommy C, Cyrus C, Francesco L, Yves R, Aihie SA. Sarcopenia: revised European consensus on definition and diagnosis. Age and Agng 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canteri AL, Gusmon LB, Zanini AC, Nagano FE, Rabito EI, Petterle RR, Jonasson TH, Boguszewski CL, Borba VZC. Sarcopenia in heart failure with reduced ejection fraction. Am J Cardiovasc Dis 2019; 9: 116. [PMC free article] [PubMed] [Google Scholar]
- 13. Susann F, Matthias T, Anja S, Nicole E, Carsten T. Muscle wasting in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 14. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, Maggi S, Dennison E, Al‐Daghri NM, Allepaerts S. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018; 9: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, Zheng R, Mao X, Chen T, He W. Prevalence of suicide attempts among chinese adolescents: a meta‐analysis of cross‐sectional studies. Compr Psychiatry 2015; 61: 78–89. [DOI] [PubMed] [Google Scholar]
- 16. Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, Araki S, Sakamoto K, Tsujita K, Yamamoto E, Yamamuro M, Kojima S, Kaikita K, Hokimoto S. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol 2016; 215: 301–306. [DOI] [PubMed] [Google Scholar]
- 17. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, Hasenfuss G, Anker SD, von Haehling S. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur J Heart Fail 2018; 20: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 18. Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J, Emami A, Springer J, Sandek A, Doehner W, Anker SD, von Haehling S. Sarcopenia and endothelial function in patients with chronic heart failure: results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA‐HF). J Am Med Dir Assoc 2017; 18: 240–245. [DOI] [PubMed] [Google Scholar]
- 19. Nozaki Y, Yamaji M, Nishiguchi S, Fukutani N, Tashiro Y, Shirooka H, Hirata H, Yamaguchi M, Tasaka S, Matsubara K, Matsushita T, Hikita Y, Oya K, Aoyama T, Mabuchi H. Sarcopenia predicts adverse outcomes in an elderly outpatient population with New York Heart Association class II–IV heart failure: a prospective cohort study. Aging Med Healthcare 2019; 10: 53–61. [Google Scholar]
- 20. Tsuchida K, Fujihara Y, Hiroki J, Hakamata T, Sakai R, Nishida K, Sudo K, Tanaka K, Hosaka Y, Takahashi K, Oda H. Significance of sarcopenia evaluation in acute decompensated heart failure. Int Heart J 2018; 59: 143–148. [DOI] [PubMed] [Google Scholar]
- 21. Fonseca GWPD, Santos MRD, Souza FRD, Costa MJAD, Haehling SV, Takayama L, Pereira RMR, Negrão CE, Anker SD, Alves MJDNN. Sympatho‐vagal imbalance is associated with sarcopenia in male patients with heart failure. Arq Bras Cardiol 2019; 122: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kono Y, Izawa H, Aoyagi Y, Ishikawa A, Sugiura T, Mori E, Ueda S, Fujiwara W, Hayashi M, Saitoh E. The difference in determinant factor of six‐minute walking distance between sarcopenic and non‐sarcopenic elderly patients with heart failure. J Cardiol 2020; 75: 42–46. [DOI] [PubMed] [Google Scholar]
- 23. Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, Malek H, Naderi N. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle 2017; 8: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, Araki S, Sakamoto K, Tsujita K, Yamamoto E. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol 2016; 215: 301–306. [DOI] [PubMed] [Google Scholar]
- 25. Morley JE, Anker SD, Haehling SV. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology—update 2014. J Cachexia Sarcopenia Muscle 2014; 5: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, Aoyama T, Arai H. Prevalence of sarcopenia in community‐dwelling Japanese older adults. J Am Med Dir Assoc 2013; 14: 911–915. [DOI] [PubMed] [Google Scholar]
- 27. Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75‐year‐old men and women: a 5‐year prospective follow‐up study of the NORA cohort in Göteborg, Sweden. Nutrition 2009; 25: 613–619. [DOI] [PubMed] [Google Scholar]
- 28. Auyeung TW, Lee JSW, Leung J, Kwok T, Woo J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3,153 older adults—an alternative measurement of sarcopenia and sarcopenic obesity. Age 2013; 35: 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumgartner RN. Epidemiology of sarcopenia among the elderly in New Mexico (vol 147, pg 755, 1998). Am J Epidemiol 1999; 149: 1161–1161. [DOI] [PubMed] [Google Scholar]
- 30. Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean Sarcopenic Obesity Study. Int J Obes (Lond) 2009; 33: 885–892. [DOI] [PubMed] [Google Scholar]
- 31. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018; 14: 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, Rozentryt P, Rauchhaus M, Karpov R, Tkachuk V, Parfyonova Y, Zaritskey AY, Shlyakhto EV, Cleland JG, Anker SD. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). J Cachexia Sarcopenia Muscle 2010; 1: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delp MD, Duan C, Mattson JP, Musch TI. Changes in skeletal muscle biochemistry and histology relative to fiber type in rats with heart failure. J Appl Physiol 1997; 83: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 35. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: clinical implications and future directions. Jacc Heart Failure 2017: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003; 108: 977–982. [DOI] [PubMed] [Google Scholar]
- 37. Fonarow GC, Peacock WF, Phillips CO, Givertz MM, Lopatin M. Admission B‐type natriuretic peptide levels and in‐hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 38. Harrison A, Morrison LK, Krishnaswamy P, Kazanegra R, Clopton P, Dao Q, Hlavin P, Maisel AS. B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med 2002; 39: 131–138. [DOI] [PubMed] [Google Scholar]
- 39. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P . Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol 1983; 38: 673–677. [DOI] [PubMed] [Google Scholar]
- 41. Von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol 2013; 45: 2257–2265. [DOI] [PubMed] [Google Scholar]
- 42. Schaap LA, Pluijm SMF, Deeg DJH, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Marco P, Rubin SM, Tylavsky FA, Visser M, Health ABC Study. Higher inflammatory marker levels in older persons: associations with 5‐year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009; 64: 1183–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Result of sensitivity analysis
Table S2. AHRQ checklist.docx
Table S3. Scores of all the items of AHRQ checklist for each study.docx
Data S1. Data collection form and the result of collection


