Abstract
Aims
Assessing reversibility of pulmonary vascular changes through vasoreactivity testing (VRT) optimizes end‐stage heart failure patient selection for heart transplant. All efforts should be made to unload the left ventricle and reduce pulmonary vascular resistance to effectively exclude irreversible pulmonary hypertension.
Methods and results
We reviewed our centre's cardiac transplant registry database (2009–2017) for VRT and compared haemodynamic responses with 40 ppm inhaled NO (n = 14), 14–17 μg inhaled iloprost (n = 7), and 24 h 0.1 μg/kg/min intravenous levosimendan (n = 14). Response to levosimendan was assessed by repeat right heart catheterization within 72 h. Baseline clinical and haemodynamic features were similar between groups. VRT was well tolerated in all patients. All drugs effectively reduced pulmonary artery pressures and transpulmonary gradient while increasing cardiac index, although levosimendan had a greater impact on cardiac index increase (P = 0.036). Levosimendan was the only drug that reduced pulmonary artery wedge pressure (P = 0.004) and central venous pressures (P < 0.001) and increased both left and right ventricular stroke work indexes (P = 0.020 and P = 0.042, respectively) and cardiac power index (P < 0.001) compared with NO and iloprost. Right ventricular end‐diastolic pressures and central venous pressure were only decreased by levosimendan. The rate of positive responses (≥10 mmHg decrease or final mean pulmonary artery pressure ≤40 mmHg with increased/unaltered cardiac index) was lower with inhaled iloprost (14%) than with either levosimendan or NO (71% and 64%, respectively; P < 0.05).
Conclusions
Levosimendan may be a safe and effective alternative for pulmonary hypertension reversibility assessment or a valuable pre‐test medical optimization tool in end‐stage heart failure patient assessment for heart transplantation offering extended haemodynamic benefits. Whether it increases the rate of positive responses or allows a better selection of candidates to heart transplantation remains to be established.
Keywords: Vasoreactivity test, Heart failure, Heart transplantation, Levosimendan
Introduction
Heart transplantation remains the treatment of choice and gold standard therapy for advanced, refractory, or end‐stage heart failure patients without contraindications. 1 Although the frontier between reversible and irreversible changes in pulmonary vasculature remains ill defined, 2 irreversible pulmonary hypertension puts potential heart transplant candidates at high risk of right ventricular failure post‐transplantation. 3 Therefore, candidate selection warrants vasoreactivity testing whenever pulmonary artery systolic pressure is ≥50 mmHg and either the transpulmonary gradient is ≥15 mmHg or pulmonary vascular resistance is >3 Wood units with systolic arterial blood pressure >85 mmHg. 4 A positive response can be defined on the basis of reduction of pulmonary vascular resistance and pulmonary artery wedge pressure with concomitant increase in cardiac output, although cut‐offs remain uncertain and ultimately the complex decision to place patients on the waiting list is left to attending physicians. Most importantly, when the test is negative, intensification of medical therapy and/or left ventricular unloading with medical devices such as left ventricular assist devices are advised to definitely exclude reversibility of pulmonary vascular changes. 4 Inhaled NO has been the drug of choice for vasoreactivity testing due to its short half‐life, minimal systemic effects, and reduced costs. Nevertheless, there is no consensus on a specific agent or protocol 5 and inodilators such as milrinone 6 or levosimendan 7 that act both at the pulmonary vasculature and at the myocardium can be valuable alternatives.
Throughout the past years, inhaled NO, inhaled iloprost, and levosimendan have been used to assess pulmonary hypertension and pulmonary vascular resistance reversibility at our heart transplant centre during work up for heart transplantation. Our aim is to compare their haemodynamic effects in a retrospective observational study.
Methods
All adult patients with end‐stage heart failure that underwent right heart catheterization with concomitant vasoreactivity testing during workup for cardiac transplantation were retrieved from our centre's cardiac transplant registry database (2009–2017). Right heart catheterization data were recovered from our institution's haemodynamics lab. All data were processed upon anonymization. The study was approved by the institution's ethics committee and strictly complies with the ethical standards of the Declaration of Helsinki, the Geneva Declaration, the Belmont Report, and Good Clinical Practices from the Food and Drug Administration. Informed consent was waived due to the retrospective nature of the study.
Baseline clinical evaluation, biometric, blood test, ongoing therapies, and echocardiography data were retrieved from the exam that immediately preceded baseline right heart catheterization. Mean life expectancy according to the Seattle Heart Failure Model was calculated. Left ventricular ejection fraction was estimated by Simpson's biplane method; right ventricular dysfunction was defined as by fractional area change <35%, tricuspid annular plane systolic excursion < 17 mm, or pulsed tissue Doppler S′ wave peak velocity <9.5 cm/s. 8
Right heart catheterization and vasoreactivity testing were performed according to the institution's protocols. Briefly, after previous optimization of each patient's clinical condition, right heart catheterization was carried out after an 8 h fast in the supine position under resting condition. Supplemental oxygen was administered whenever peripheral capillary oxygen saturation was lower than 90% as assessed by pulse oximetry. Cardiac output was determined by indirect Fick method and indexed for body surface area according to DuBois and DuBois's formula. Arterial and mixed venous blood samples were obtained simultaneously for determination of oxygen saturation. Oxygen consumption was estimated from nomograms. Corrections for oxygen therapy were not performed because none of the patients was on oxygen therapy other than nasal cannula with 4 L/min maximum flow (28% inspired oxygen fraction). Central haemodynamics were assessed by a femoral artery and pulmonary artery catheter inserted either by the femoral, braquial or basilic veins. Pressure transducers were zeroed at mid‐thoracic position. Pulmonary artery wedge pressure was read at end‐expiration. Tracings were analysed, manually averaging data of at least three cycles (five in case of arrhythmia).
Transpulmonary gradient was estimated as the difference between mean and wedge pulmonary artery pressures while diastolic transpulmonary gradient as the difference between diastolic and wedge pressures. Indexed systemic and pulmonary vascular resistances were estimated as the ratio between the difference of mean arterial and central venous pressures (transsystemic gradient) or the transpulmonary gradient, respectively, and cardiac index while left and right ventricular stroke work indexes were obtained by multiplying stroke volume index by these gradients, respectively. Cardiac power index, a measure of effective hydraulic energy transmitted by the left ventricle to systemic arteries, was defined as the product between cardiac index and mean arterial pressure after conversion to Watts. 9 Pulmonary artery compliance was estimated as the ratio between stroke volume index and pulmonary artery pulse pressure.
Stable haemodynamic recordings before and after vasoreactivity testing with inhaled NO and iloprost were retrieved. The institution's inhaled NO administration protocol was 40 ppm by face mask during 10 min (MiniKINOX™, Air Liquide, France) whereas inhaled iloprost (Ventavis®, Bayer Schering Pharma, Germany) was administered at a concentration of 10 μg/mL for 15 min by nebulizer Prodose AAD System (Bayer Schering Pharma AG, Germany) to achieve and aerosolized dose of 14–17 μg.
As for levosimendan, the institution's protocol was repeat catheterization within 72 h of baseline right heart catheterization and after a 24 h perfusion of 0.1 μg/kg/min levosimendan without loading dose (Simdax®, Orion Pharma, Finland). Major changes to ongoing therapy during this 72 h period, namely in inotropes, diuretics and vasopressors, were excluded. Of note, although in strict sense this is not vasoreactivity testing and could rather be viewed as intensified heart failure therapy, for simplicity sake, we will apply the term vasoreactivity testing in broad sense to include levosimendan intervention throughout the rest of the manuscript.
As per institutional protocol, tests were interrupted whenever patients became symptomatic or intolerant. Positive response to acute vasodilator test is variably defined in the literature. Most widely accepted criteria derive mainly from cohorts of patients with idiopathic or hereditary pulmonary arterial hypertension and were adopted because they predict long‐term response to calcium channel blockers. 10 In this setting, patients are usually classified as responders when mean pulmonary artery pressure decrease is ≥10 mmHg or absolute value decreases to ≤40 mmHg with increased or unchanged cardiac output. Nevertheless, in pulmonary hypertension, due to left heart disease, the definition of a positive response is still ill defined. Based on Costard‐Jackle and Fowler's 1992 observation of improved outcomes in a cohort of 301 consecutive heart transplant patients that underwent vasoreactivity testing with sodium nitroprusside, 11 some reports suggest that using pulmonary vascular resistance decrease below 2.5 Wood units with preserved systolic blood pressure. 12 Nevertheless, findings on other, more recent, cohorts suggest other criteria. 13 Another simple and straightforward approach is to consider achieving pulmonary artery systolic pressure <50 mmHg and either transpulmonary gradient <15 mmHg or pulmonary vascular resistance ≤3 Wood units, which are the reverse criteria based on indications for vasoreactivity testing by the International Society for Heart Lung Transplantation. 4 Judgement is usually based on three classical favourable haemodynamic responses, decreased pulmonary vascular resistances, increased cardiac index, and decreased pulmonary artery wedge pressure. At our centre, prespecified criteria are a drop ≥25% in transpulmonary gradient and an increase ≥40% in cardiac index with any PAWP decrease. Patients are deemed positive responders when at least two of these criteria verify and fully responsive when all three criteria apply. Nevertheless, the ultimate decision to transplant a patient remains a heart team decision based on a comprehensive individual case evaluation.
Clinical outcomes on follow‐up were assessed as of December 2019 and cross checked with the national Portuguese Health Ministry clinical electronical repository—Registo de Saúde Eletrónico.
Statistical analysis
Statistical analysis was carried out with the aid of RStudio (Version 1.2.5033, RStudio, Inc.) and IBM SPSS Statistics V25 (IBM corporation). Data and residuals were checked for normality with Shapiro–Wilk's test. Homogeneity of variances was checked by Levene's test. Continuous data are presented as mean ± standard deviation or median (interquartile range) according to distribution. Categorical data are presented as count (percentage). Baseline continuous group features were compared with one‐way ANOVA or non‐parametric Kruskal–Wallis according to normality of residuals, respectively, whereas categorical data were compared with Fisher's test with computation of P values by Monte‐Carlo simulation and multigroup comparisons with z‐test with Bonferroni corrected P values. Haemodynamic outcomes of vasoreactivity testing were compared with two‐way repeated‐measures ANOVA with post‐hoc multigroup comparisons by Holm–Sidak's method or with the rank‐based non‐parametric R‐package nparLD (Nonparametric Analysis of Longitudinal Data in Factorial Experiments) in which case, P values are reported for the ANOVA‐type statistic, each group's evolution is visually represented by relative treatment effects, and multigroup comparisons were tested by subsampling with Bonferroni correction of P values. 14 Statistical significance was set at two‐tailed P < 0.05.
Results
All patients had severe reduced ejection fraction heart failure with elevated pulmonary vascular resistances. The aetiology was either ischemic or dilated non‐ischemic; none of the patients had significant valvular disease other than functional mitral regurgitation. No differences were observed between patients that underwent vasoreactivity testing with levosimendan, NO, or iloprost regarding demographic and biometric features, aetiology of heart failure, and disease severity as assessed by the New York Heart Association class, left ventricular ejection fraction, left ventricular and left atrial dilation, plasma B‐type natriuretic peptide levels, and rate of right ventricular dysfunction. Groups were also comparable regarding renal function as assessed by plasma creatinine levels. Likewise, no differences were observed in ongoing medication with either implanted cardiac defibrillators/cardiac resynchronization devices or drugs such as beta blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor antagonists, or mineralocorticoid receptor antagonists. Nevertheless, patients from the iloprost group had higher life expectancy according to the Seattle Heart Failure Model (Table 1 ). None of the patients was under mechanical circulatory support with either intra‐aortic balloon pump or left ventricular assist devices. All patients were classified under Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) Profile 4 except for one patient in the levosimendan group who was already on inotrope support with dobutamine.
Table 1.
Baseline features before vasoreactivity testing
| Levosimendan (n = 14) | NO (n = 14) | Iloprost (n = 7) | P value | |
| Age (years) | 54 (51–60) | 56 (51–63) | 53 (49–61) | 0.796 |
| Male gender (%) | 13 (93) | 12 (86) | 5 (71) | 0.398 |
| Body mass index (Kg/m2) | 23.1 (22.0–24.5) | 23.7 (22.3–26.0) | 27.6 (23.6–36.4) | 0.098 |
| Body surface area (m2) | 1.75 ± 0.15 | 1.79 ± 0.23 | 1.84 ± 0.22 | 0.621 |
| Ischemic aetiology (%) | 9 (64) | 8 (57) | 6 (86) | 0.580 |
| NYHA class IV (%) | 6 (43) | 4 (29) | 2 (29) | 0.731 |
| LVEF (%) | 21 (13–25) | 22 (18–26) | 25 (14–37) | 0.750 |
| LVED diameter (mm) | 70 ± 10 | 65 ± 14 | 72 ± 10 | 0.313 |
| LA diameter (mm) | 48 (44–54) | 52 (48–56) | 54 (52–61) | 0.112 |
| Atrial fibrillation (%) | 5 (36) | 8 (57) | 4 (57) | 0.516 |
| RV dysfunction (%) | 9 (64) | 11 (79) | 4 (57) | 0.579 |
| Plasma creatinine (mg/dL) | 1.3 (1.1–1.4) | 1.3 (1.0–1.5) | 0.9 (0.9–1.0) | 0.363 |
| ICD/CRT‐D (%) | 12 (86) | 11 (79) | 5 (71) | 0.862 |
| BB (%) | 14 (100) | 14 (100) | 7 (100) | 1.000 |
| ACEi/ARA (%) | 9 (64) | 7 (50) | 5 (71) | 0.737 |
| MRA (%) | 10 (71) | 9 (64) | 5 (71) | 1.000 |
| Furosemide (mg) | 85 (60–120) | 70 (40–90) | 60 (40–120) | 0.285 |
| SHFM mean life expectancy (years) | 3.1 (1.9–4.1) | 4.2 (3.0–5.6) | 5.0 (4.6–6.8)* | 0.026 |
| BNP (pg/mL) | 1,550 ± 1,210 | 1,460 ± 941 | 581 ± 561 | 0.258 |
Baseline features of end‐stage heart failure patients that underwent vasoreactivity testing during assessment for heart transplantation. ACEi, angiotensin‐converting enzyme inhibitors; ARA, angiotensin receptor antagonists; BB, β‐blockers; BNP, type‐B natriuretic peptide; CRT‐D, cardiac resynchronization therapy defibrillator device; ICD, implanted cardiac defibrillator; LA, left atrial; LVED, left ventricular end diastolic; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; RV, right ventricular; SHFM, Seattle Heart Failure Model. Values are mean ± standard deviation or median (interquartile range) for continuous variables according to normality of distribution and count (percentage) for categorical variables.
Vs. levosimendan and NO by Fisher's test.
On baseline, haemodynamic evaluation groups were also comparable overall except for arterial blood pressure, which was higher in the iloprost group compared with both levosimendan and NO, and transpulmonary gradient and indexed pulmonary vascular resistances, which were higher in iloprost compared with NO (Table 2 , Panel C of Figure 1 ). All except for one patient in the NO group were classifiable as combined post‐capillary and pre‐capillary pulmonary hypertensive according to haemodynamic definitions for pulmonary hypertension due to left heart disease. 2
Table 2.
Right heart catheterization and vasoreactivity test
| P value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | ME | I | Multigroup comparisons (ME) | Multigroup comparisons (I) | ||||||||||
| Lev | NO | Ilo | Lev | NO | Ilo | D | T | D*T | Lev vs. NO | Lev vs. Ilo | NO vs. Ilo | Lev*T | NO*T | Ilo*T | |
| SAP (mmHg) | 105 ± 15 | 106 ± 17 | 129 ± 24 | 109 ± 12 | 104 ± 17 | 131 ± 29 | 0.009 | 0.580 | 0.361 | 0.014 | 0.011 | ||||
| DAP (mmHg) | 67 ± 15 | 62 ± 8 | 76 ± 13 | 65 ± 7 | 60 ± 6 | 75 ± 13 | 0.007 | 0.333 | 0.973 | 0.005 | |||||
| MAP (mmHg) | 80 ± 13 | 77 ± 9 | 94 ± 18 | 79 ± 10 | 74 ± 7 | 92 ± 18 | 0.005 | 0.373 | 0.835 | 0.018 | 0.004 | ||||
| SaO2 (%) | 95 ± 2 | 95 ± 4 | 94 ± 3 | 96 ± 2 | 99 ± 2 | 94 ± 2 | 0.004 | 0.007 | 0.03 | <0.001 | |||||
| HR (/min) | 72(66–80) | 74(62–80) | 85(77–93) | 80(70–80) | 74(62–80) | 85(77–93) | 0.236 | 0.188 | 0.188 | ||||||
| CI (L/min/m2) | 1.4 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.4 | 2.0 ± 0.4 | 1.9 ± 0.6 | 1.9 ± 0.5 | 0.877 | <0.001 | 0.036 | <0.001 | |||||
| (mL/min/m2) | 70(67–75) | 67(66–73) | 67(62–71) | 74(72–77) | 67(66–73) | 67(62–71) | 0.056 | 0.036 | 0.036 | 0.036 | |||||
| CPI (W/m2) | 0.25(0.19–0.31) | 0.29(0.27–0.31) | 0.3(0.29–0.4) | 0.35(0.31–0.39) | 0.32(0.26–0.37) | 0.36(0.27–0.5) | 0.424 | <0.001 | <0.001 | <0.001 | |||||
| PVRI (WU/m2) | 13.8 ± 4.6 | 11.1 ± 4.0 | 15.6 ± 5.4 | 8.0 ± 3.0 | 6.1 ± 3.3 | 12.1 ± 7.9 | 0.027 | <0.001 | 0.440 | 0.028 | |||||
| SPAP (mmHg) | 71(64–78) | 59(51–70) | 75(70–89) | 56(50–64) | 54(43–69) | 62(62–77) | 0.052 | <0.001 | 0.061 | ||||||
| DPAP (mmHg) | 31(24–39) | 26(22–29) | 29(27–38) | 28(23–29) | 25(21–29) | 30(25–42) | 0.107 | 0.122 | 0.546 | ||||||
| MPAP (mmHg) | 43(40–51) | 38(33–44) | 51(42–55) | 38(35–43) | 36(29–44) | 46(38–60) | 0.051 | <0.001 | 0.114 | ||||||
| TPG (mmHg) | 19 ± 6 | 18 ± 7 | 24 ± 4 | 16 ± 6 | 10 ± 5 | 22 ± 12 | 0.008 | <0.001 | 0.094 | 0.006 | |||||
| DTPG (mmHg) | 4(0–9) | 6(3–9) | 10(8–12) | 4(1–7) | 2(0–4) | 10(−1–13) | 0.167 | 0.094 | 0.587 | ||||||
| PAWP (mmHg) | 26 ± 4 | 21 ± 4 | 26 ± 10 | 22 ± 8 | 26 ± 7 | 27 ± 9 | 0.561 | 0.493 | 0.004 | 0.005 | |||||
| CVP (mmHg) | 15(13–16) | 10(8–11) | 13(9–19) | 8(5–10) | 9(5–12) | 12(5–17) | 0.4901 | <0.001 | <0.001 | <0.001 | |||||
| SmvO2 (%) | 46 ± 11 | 55 ± 11 | 56 ± 11 | 57 ± 12 | 63 ± 14 | 61 ± 10 | 0.215 | <0.001 | 0.181 | ||||||
| PACi (mL/m2/mmHg) | 0.5(0.4–0.7) | 0.7(0.7–0.8) | 0.5(0.4–0.6) | 0.8(0.7–1.2) | 1.0(0.7–1.1) | 0.6(0.6–0.9) | 0.081 | <0.001 | 0.191 | ||||||
Baseline haemodynamic parameters and response to vasoreactivity test (before and after, respectively) in patients that underwent levosimendan (Lev, n = 14), NO (n = 14), and iloprost (Ilo, n = 7). P values for main effects (MEs) of drug (D) and time (T) and interaction (I, D*T) as well as multigroup comparisons for main effects and interaction are reported in the rightmost columns. SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; SaO2, peripheral oxygen saturation; HR, heart rate; CI, cardiac index; , oxygen consumption indexed for body surface area; CPI, cardiac power index; PVRI, pulmonary vascular resistance index; SPAP, systolic pulmonary artery pressure; DPAP, diastolic pulmonary artery pressure; MPAP, mean pulmonary artery pressure; TPG, transpulmonary gradient; DTPG, diastolic transpulmonary gradient; PAWP, pulmonary artery wedge pressure; CVP, central venous pressure; SmvO2, mixed venous oxygen saturation; PACi, pulmonary artery compliance for indexed volumes. Values are mean ± standard deviation when assumptions for parametric two‐way repeated measures ANOVA were met or median (interquartile range) when non‐parametric tests were applied.
Figure 1.
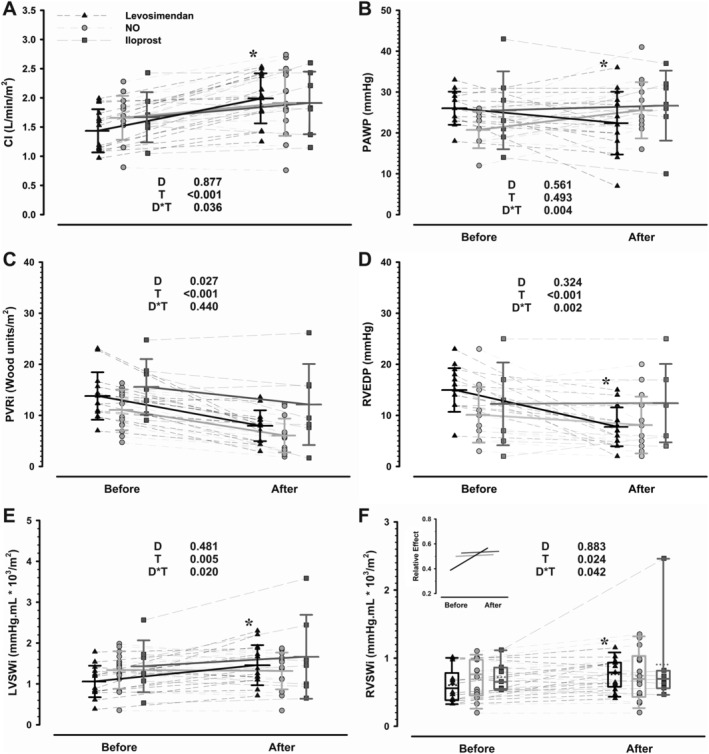
Response of cardiac index (CI, Panel A), pulmonary artery wedge pressure (PAWP, Panel B), indexed pulmonary vascular resistances (PVRi, Panel C), right ventricular end‐diastolic pressure (RVEDP, Panel D), left ventricular stroke work index (LVSWi, Panel E), and right ventricular stroke work index (RVSWi, Panel F) to vasodilator challenge with levosimendan (n = 14), NO (n = 14), and iloprost (n = 7) in end‐stage heart failure patients. Stable baseline and post‐drug infusion data are represented (before and after, respectively). Group means and standard deviations are represented in Panels A–E whereas box plots are represented in Panel F, according to test assumptions. Individual patient values are depicted as point plots with connecting thin lines while thick lines represent changes in means for Panels A–E and relative treatment effect in the insert to Panel F. P values for main effects of drug (D) and time (T) and interaction (D*T) are represented in each panel. *P < 0.05 levosimendan vs other groups' response over time.
During vasoreactivity test, none of the drugs elicited relevant hypotension or heart rate changes. None of the patients became symptomatic or discontinued the test. Peripheral oxygen saturation was improved only by NO (Table 2 ). All drugs increased cardiac index and mixed venous oxygen saturation but the magnitude of increase in cardiac index was higher with levosimendan (P = 0.036 for interaction, Panel A of Figure 1 ). Indeed, the increase in left ventricular stroke work index and cardiac power index was also significantly higher for levosimendan compared with both NO and iloprost (Panel E of Figure 1 and Table 2 , respectively). Moreover, levosimendan was the only drug that was able to reduce pulmonary artery wedge pressure during the test (P = 0.004 for interaction, Panel B of Figure 1 ). Regarding pulmonary vasculature, all drugs effectively reduced pulmonary artery pressures and transpulmonary gradient while increasing pulmonary artery compliance (Table 2 ). Nevertheless, the rise in right ventricular stroke index was higher with levosimendan infusion. Of note, right ventricular diastolic pressures and central venous pressure were only decreased by levosimendan (Panel D of Figure 1 and Table 2 , respectively). Raw haemodynamic data are provided in Table S1.
In Table 3 we present the main haemodynamic responses and positive responders applying qualitative criteria. According to our centre's practice 9 (64%) patients that underwent vasoreactivity testing with levosimendan were considered positive responders which was significantly higher than 2 (29%) and 1 (14%) responders in the NO and iloprost groups, respectively. Complete haemodynamic response was only observed in 2 patients from the levosimendan group (14%). Applying the criteria validated for pulmonary arterial hypertension, which predicts long‐term responsiveness to calcium channel blockers, the rate of positive responses was comparable between levosimendan and NO, 10 and 9 patients, respectively, and lower with iloprost, only one patient (P < 0.05). Likewise, response to iloprost was lower applying as criterium the reversion to no indication for vasoreactivity testing according to the International Society for Heart and Lung Transplantation criteria, none of the patients from the iloprost group would be deemed responders while five and six patients from the levosimendan and NO groups would, respectively.
Table 3.
Positive haemodynamic response to vasoreactivity testing
| Levosimendan | NO | Iloprost | |
| mPAP <40 mmHg/decrease ≥10 mmHg with increased/unchanged CI | 10 (71) | 9 (64) | 1 (14)* |
| PVR < 2.5 WU with systolic BP ≥ 85 mmHg | 1 (7) | 6 (43) | 1 (14) |
| PASP < 50 mmHg with either TPG < 15 mmHg or PVR ≤ 3WU | 5 (36) | 6 (43) | 0* |
| Institution's criteria | |||
| TPG drop ≥25% | 7 (50) | 12 (86) | 3 (43) |
| CI increase ≥40% | 8 (57)* | 0 | 2 (29) |
| PAWP decrease | 9 (64) | 3 (21) | 3 (43) |
| Two out of 3 criteria | 9 (64)* | 2 (14) | 1 (14) |
| Fully responsive | 2 (14) | 0 | 0 |
Qualitative assessment of reversibility of pulmonary hypertension using various criteria in patients that underwent levosimendan (Lev, n = 14), NO (n = 14), and iloprost (Ilo, n = 7). The criteria are presented in the following order: Sitbon's criteria used to establish responsiveness to calcium‐channel blockers in idiopathic pulmonary artery hypertension, 10 Costard‐Jackle's 1992 heart transplanted patient cohort derived criteria, 11 reversion to no indication for vasoreactivity testing according to the International Society for Heart and Lung Transplantation criteria, 4 and finally, our own centre's protocol (please refer to the main manuscript). BP, blood pressure; CI, cardiac index; mPAP, mean pulmonary artery pressure; PASP, pulmonary artery systolic pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; TPG, transpulmonary gradient; WU, Wood units. Values are count (percentage).
P < 0.05 vs. other groups by z‐test for proportions (Bonferroni corrected) when significant differences were found on Fisher's test.
Ten (71%), four (29%), and one patients (15%) from the levosimendan, NO and iloprost groups underwent heart transplantation, respectively. Amongst the patients that underwent transplantation, periprocedural acute right ventricular failure was correspondingly observed in four (40%), three (75%), and one patients (100%), warranting mechanical circulatory support with right ventricular assist device in two patients from the levosimendan group and in one patient from each of the other groups. One patient from each of the levosimendan and iloprost groups died from acute right ventricular failure, the patient from the levosimendan group, however, died in the operation theatre due to surgical intercurrences, which led to extensive bleeding culminating in acute right ventricular failure while the patient from the iloprost group died 2–3 weeks post‐operatively despite mechanical circulatory support. Acute right ventricular failure after transplantation was evenly observed amongst responders and non‐responders in the levosimendan group (two patients each) according to the International Society for Heart and Lung Transplantation criteria, while in the NO group, the right ventricle failed in one responder and two non‐responders and the single transplanted patient in the iloprost group was also a non‐responder that presented right ventricular failure after transplantation.
In the levosimendan group, the two other causes of death were haemorrhagic stroke and systemic fungemia with multiple organ dysfunction and the remaining cause of death in the NO group was stroke.
Discussion
This retrospective review of a single‐centre's case series of patients with end‐stage heart failure undergoing vasodilator challenge as part of the planned work up for heart transplant listing revealed that the inodilator levosimendan is a safe and feasible alternative to inhaled pulmonary vasodilators NO and iloprost with the potential to increase the rate of positive responses possibly due to its direct myocardial actions.
Over 50% of patients listed for heart transplantation have elevated pulmonary vascular resistances, which increase mortality risk following orthotopic heart transplantation. 3 Identifying patients in whom pulmonary vascular resistance elevation is reversible by vasoreactivity testing reduces but does not eliminate risk. 13 Current guidelines favour inhaled NO for vasoreactivity testing, and to our knowledge, this is the mainstay in most cardiac transplant centres. 2 Indeed, NO is a short‐acting drug devoid of systemic side effects, namely, systemic hypotension that frequently warrants discontinuation of systemic vasodilators such as nitroprusside. It acts by selectively vasodilating the pulmonary vasculature and decreases pulmonary shunt 15 explaining improved oxygenation in our group of NO patients. One drawback of NO administration is the increase in left ventricular filling pressure particularly in severe heart failure patients due to increased venous return to poorly compliant left ventricles. 16 Indeed, NO is ill‐advised by some experts that still prefer classic systemic vasodilators (nitroprusside) due to the potential of NO to induce flash pulmonary oedema. 17 A common alternative is inhaled iloprost, which according to some authors, has a larger pulmonary vasodilator effect than NO, 18 while others report comparable effects. 19 Inhaled iloprost is also of easy administration and short acting but has more systemic effects, namely, systemic vasodilation, due to slower inactivation. Inodilators such as milrinone, a bipyridine derivative type 3 phosphodiesterase inhibitor, have also been employed successfully for vasoreactivity testing with the advantage of convenient intravenous administration and lower incidence of systemic hypotension compared with simple vasodilators. 20 Using inodilators as an alternative to simple pulmonary vasodilators for vasoreactivity testing raises a key pathophysiological question, one could argue that selective pulmonary vasodilation without confoundment from cardiac performance would be desirable to ascertain whether pulmonary vascular resistances are fixed or irreversible, but it is highly questionable that this can be achieved in vivo even by the most selective pulmonary vasodilator due to concomitant load changes and reflex responses; thus, one may argue as well that the assessment of vascular resistance response should be carried out under optimized cardiac performance, 6 , 21 which means the highest achievable cardiac index at the lowest achievable left ventricular preload.
Levosimendan is a calcium‐sensitizer inodilator, which does not increase myocardial oxygen consumption and is devoid of potential arrhythmogenic effects, offering long‐lasting haemodynamic improvement owing to active metabolites with long half‐life. Contrarily to other inotropes, it has not been linked to increased mortality risk in clinical trials or wide clinical usage. 22 It is endorsed by the European Society of Cardiology to improve cardiac output and tissue perfusion in hypotensive acute heart failure patients providing that filling status is normal, 23 but several clinical trials have also suggested beneficial effects of repeated administration in end‐stage heart failure patients. 24 , 25 Indeed, although none of these trials individually showed a survival effect some meta‐analyses support increased survival in patients receiving levosimendan. 26 The outcomes of the Repetitive Levosimendan Infusion for Patients With Advanced Chronic Heart Failure (LeoDOR) trial (NCT03437226) are eagerly awaited in this regard.
Due to its unique pharmacology and the surmounting evidence suggesting beneficial effects in end‐stage heart failure patients, levosimendan has been used for vasoreactivity testing in our centre. To our knowledge, this is the first report of a case series where levosimendan is compared with inhaled pulmonary vasodilators for vasoreactivity testing, although a single case report has been published. 7 Overall, the safety profile and effects of levosimendan on the pulmonary vasculature and pulmonary vascular resistance were comparable with inhaled pulmonary vasodilators, but there was a significantly higher increase in cardiac index, left ventricular stroke work index, and cardiac power index, a known predictor of mortality in cardiogenic shock 27 and advanced heart failure. 9 Noticeably, levosimendan did not elicit systemic hypotension, and it was the only drug that reduced pulmonary artery wedge pressure as well as central venous pressure and right ventricular preload, an effect that we previously reported in an animal model. 28 Indeed, according to a mixed model meta‐analysis pooling data from previous studies comparing various drugs for vasoreactivity testing in advanced heart failure only nitroprusside, nitroglycerin, and milrinone decreased pulmonary artery wedge pressure. 29
Contrarily to inhaled pulmonary arteries vasodilators, assessing the effects of levosimendan in vasoreactivity testing may require repeat right heart catheterization, which could extend length of hospital stay. Nevertheless, most of end‐stage heart failure patients already undergo vasoreactivity testing to assess the potential for heart transplantation during hospital admissions for heart failure exacerbation. In this setting, levosimendan may be advantageous due to its sustained beneficial haemodynamic effects. Moreover, our protocol did not include a loading dose because of concerns for potential systemic hypotension, we cannot exclude that protocols with a loading dose may allow an assessment of levosimendan's effects in a single procedure. Also, levosimendan might alternatively be used for medical optimization of heart failure patients prior to vasoreactivity testing with pulmonary vasodilators.
In this case series, a positive haemodynamic response to vasoreactivity test was more common amongst patients that underwent levosimendan. Still, we must underscore that this is a single‐centre observational study with small sample size, precluding definitive conclusions. Although no major differences were observed between groups on baseline haemodynamics, there was a trend for higher central venous pressure in the levosimendan and iloprost groups, denoting congestion and the mean life expectancy predicted by the Seattle Heart Failure Model tended to be lower in the levosimendan group. Also, although no deviations from institution's protocols were observed there was a trend for increased use of levosimendan and decreased use of iloprost, during the timespan of the study. Cardiac index was derived by the indirect Fick method, which is a source of error. Additionally, definition of positive response to vasodilator challenge remains controversial. Whether or not to list patients for heart transplantation is a complex decision that is rather based on the clinical scenario, local policies, and donor availability. Furthermore, even if levosimendan could increase the rate of positive responders, the impact on prognosis after heart transplantation remains uncertain.
In conclusion, the inodilator levosimendan is a safe and effective alternative to standard inhaled pulmonary vasodilator challenge in the assessment of heart transplant candidates amongst patients with end‐stage reduced ejection fraction heart failure, which offers extended haemodynamic benefits. Whether it increases the rate of positive responses remains to be established.
Conflict of interest
None declared.
Funding
This study was supported by the projects: PTDC/BTM‐SAL/32616/2017 financed by FCT Fundação para a Ciência e Tecnologia and by the European Regional Development Fund (ERDF), through the Programa Operacional Regional NORTE2020 (POCI‐01‐0145‐FEDER‐032616); SAICT‐PAC/0047/2015 financed by FCT Fundação para a Ciência e Tecnologia and by the European Structural and Investment Funds (ESIF), through the Programa Operacional Regional Lisboa 2020 (POCI‐01‐0145‐FEDER‐016385); Cardiovascular R&D Center – UnIC (UIDB/00051/2020 and UIDP/00051/2020) funded by national funds through FCT ‐ Fundação para a Ciência e Tecnologia.
F. A. Saraiva is supported by Universidade do Porto/FMUP and FSE‐Fundo Social Europeu, NORTE 2020‐Programa Operacional Regional do Norte, NORTE‐08‐5369‐FSE‐000024‐Programas Doutorais.
Supporting information
Table S1 Right heart catheterization and vasoreactivity test (non‐indexed parameters).
Acknowledgements
The authors would like to thank to all the staff in the Interventional Cardiology Laboratory and also to all the medical team involved in the Heart Failure Consultation Group of the Cardiology Department of São João Hospital Center.
Tavares‐Silva, M. , Saraiva, F. , Pinto, R. , Amorim, S. , Silva, J. C. , Leite‐Moreira, A. F. , Maciel, M. J. , and Lourenço, A. P. (2021) Comparison of levosimendan, NO, and inhaled iloprost for pulmonary hypertension reversibility assessment in heart transplant candidates. ESC Heart Failure, 8: 908–917. 10.1002/ehf2.13168.
References
- 1. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hulsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 2. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Group ESCSD . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 3. Kirklin JK, Naftel DC, Kirklin JW, Blackstone EH, White‐Williams C, Bourge RC. Pulmonary vascular resistance and the risk of heart transplantation. J Heart Transplant 1988; 7: 331–336. [PubMed] [Google Scholar]
- 4. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A, International Society for Heart Lung Transplantation Infectious Diseases C, International Society for Heart Lung Transplantation Pediatric Transplantation C, International Society for Heart Lung Transplantation Heart F, Transplantation C . The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10‐year update. J Heart Lung Transplant 2016; 35: 1–23. [DOI] [PubMed] [Google Scholar]
- 5. Sharma A, Obiagwu C, Mezue K, Garg A, Mukherjee D, Haythe J, Shetty V, Einstein AJ. Role of vasodilator testing in pulmonary hypertension. Prog Cardiovasc Dis 2016. ‐Feb; 58: 425–433. [DOI] [PubMed] [Google Scholar]
- 6. Abramov D, Haglund NA, Di Salvo TG. Effect of milrinone infusion on pulmonary vasculature and stroke work indices: a single‐center retrospective analysis in 69 patients awaiting cardiac transplantation. Am J Cardiovasc Drugs 2017; 17: 335–342. [DOI] [PubMed] [Google Scholar]
- 7. Ammirati E, Musca F, Oliva F, Garascia A, Pacher V, Verde A, Cipriani M, Moreo A, Martinelli L, Frigerio M. Levosimendan reverted severe pulmonary hypertension in one patient on waiting list for heart transplantation. Int J Cardiol 2013; 168: 4518–4519. [DOI] [PubMed] [Google Scholar]
- 8. Alsoos F, Khaddam A. Echocardiographic evaluation methods for right ventricular function. J Echocardiogr 2015; 13: 43–51. [DOI] [PubMed] [Google Scholar]
- 9. Yildiz O, Aslan G, Demirozu ZT, Yenigun CD, Yazicioglu N. Evaluation of resting cardiac power output as a prognostic factor in patients with advanced heart failure. Am J Cardiol 2017; 120: 973–979. [DOI] [PubMed] [Google Scholar]
- 10. Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long‐term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 11. Costard‐Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol 1992; 19: 48–54. [DOI] [PubMed] [Google Scholar]
- 12. Ghio S, Crimi G, Temporelli PL, Traversi E, La Rovere MT, Cannito A, Vizza D, Scelsi L, Raineri C, Guazzi M, Oltrona Visconti L. Haemodynamic effects of an acute vasodilator challenge in heart failure patients with reduced ejection fraction and different forms of post‐capillary pulmonary hypertension. Eur J Heart Fail 2018; 20: 725–734. [DOI] [PubMed] [Google Scholar]
- 13. Butler J, Stankewicz MA, Wu J, Chomsky DB, Howser RL, Khadim G, Davis SF, Pierson RN 3rd, Wilson JR. Pre‐transplant reversible pulmonary hypertension predicts higher risk for mortality after cardiac transplantation. J Heart Lung Transplant 2005; 24: 170–177. [DOI] [PubMed] [Google Scholar]
- 14. Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 2012; 50: 23. [Google Scholar]
- 15. Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991; 83: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 16. Loh E, Stamler JS, Hare JM, Loscalzo J, Colucci WS. Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction. Circulation 1994; 90: 2780–2785. [DOI] [PubMed] [Google Scholar]
- 17. Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, Gomberg‐Maitland M, Murali S, Frantz RP, McGlothlin D, Horn EM, Benza RL. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012; 31: 913–933. [DOI] [PubMed] [Google Scholar]
- 18. Sablotzki A, Hentschel T, Gruenig E, Schubert S, Friedrich I, Muhling J, Dehne MG, Czeslick E. Hemodynamic effects of inhaled aerosolized iloprost and inhaled nitric oxide in heart transplant candidates with elevated pulmonary vascular resistance. Eur J Cardiothorac Surg 2002; 22: 746–752. [DOI] [PubMed] [Google Scholar]
- 19. Haraldsson A, Kieler‐Jensen N, Nathorst‐Westfelt U, Bergh CH, Ricksten SE. Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest 1998; 114: 780–786. [DOI] [PubMed] [Google Scholar]
- 20. Givertz MM, Hare JM, Loh E, Gauthier DF, Colucci WS. Effect of bolus milrinone on hemodynamic variables and pulmonary vascular resistance in patients with severe left ventricular dysfunction: a rapid test for reversibility of pulmonary hypertension. J Am Coll Cardiol 1996; 28: 1775–1780. [DOI] [PubMed] [Google Scholar]
- 21. Strong C, Raposo L, Castro M, Madeira S, Tralhão A, Ventosa A, Rebocho MJ, Almeida M, Aguiar C, Neves JP, Mendes M. Haemodynamic effects and potential clinical implications of inhaled nitric oxide during right heart catheterization in heart transplant candidates. ESC Heart Fail 2020; 7: 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agostoni P, Farmakis DT, Garcia‐Pinilla JM, Harjola VP, Karason K, von Lewinski D, Parissis J, Pollesello P, Polzl G, Recio‐Mayoral A, Reinecke A, Yerly P, Zima E. Haemodynamic balance in acute and advanced heart failure: an expert perspective on the role of levosimendan. Card Fail Rev 2019; 5: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 24. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 25. Comin‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, Garcia Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodriguez JL, Grau M, Bruguera J, Investigators L‐HS . Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 20: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 26. Silvetti S, Nieminen MS. Repeated or intermittent levosimendan treatment in advanced heart failure: an updated meta‐analysis. Int J Cardiol 2016; 202: 138–143. [DOI] [PubMed] [Google Scholar]
- 27. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G, Investigators S . Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44: 340–348. [DOI] [PubMed] [Google Scholar]
- 28. Tavares‐Silva M, Alaa M, Leite S, Oliveira‐Pinto J, Lopes L, Leite‐Moreira AF, Lourenco AP. Dose‐response head‐to‐head comparison of inodilators dobutamine, milrinone, and levosimendan in chronic experimental pulmonary hypertension. J Cardiovasc Pharmacol Ther 2017; 22: 485–495. [DOI] [PubMed] [Google Scholar]
- 29. Guglin M, Mehra S, Mason TJ. Comparison of drugs for pulmonary hypertension reversibility testing: a meta‐analysis. Pulm Circ 2013; 3: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Right heart catheterization and vasoreactivity test (non‐indexed parameters).


