Abstract
Aims
There is currently no gold standard in evaluating frailty in patients with heart failure (HF), and the prognostic value of frailty according to the Canadian Study of Health and Aging Clinical Frailty Scale (CFS) on mortality in patients with HF is still unknown.
Methods and results
Among consecutive 596 patients after their discharge from HF in Kokura Memorial Hospital (Kitakyushu, Japan) during 2015, their frailty at discharge was assessed according to CFS. Patients were classified into three groups: low (N = 232, 38.9%), intermediate (N = 230, 38.6%), and high (N = 134, 22.5%). The primary endpoint was defined as 2 year all‐cause death. The mean age was 76.6 ± 10.1 years, and 55.3% were men in entire cohort. There were significant differences in age, living environment, and dementia among low, intermediate, and high CFS groups. Left ventricular ejection fraction (LVEF) and co‐morbidities such as severe renal failure and severe anaemia tended to increase with frailty severity, while body mass index (BMI) and albumin level tended to decrease with frailty severity. Two year cumulative incidences of all‐cause death for the three groups were 12.8%, 25.4%, and 52.7% (P < 0.001), respectively. This significant difference in the risk for all‐cause death among the CFS groups was driven by the risk for cardiac (8.6%, 14.2%, and 31.0%, respectively, P < 0.001) and non‐cardiac death (4.6%, 13.0%, and 31.4%, respectively, P < 0.001). The multivariate analysis showed that high frailty was independently associated with all‐cause death (intermediate CFS group: adjusted hazard ratio, 1.43, 95% confidence interval, 0.86–2.36, P = 0.16; high CFS group: adjusted hazard ratio, 3.90, 95% confidence interval, 2.32–6.55, P < 0.001), and this result was consistent, irrespective of stratification based on age, sex, BMI, and LVEF without significant interaction.
Conclusions
The simple CFS tool was successful in predicting the risk for all‐cause death in patients with HF, and frailty according to CFS was independently associated with all‐cause death irrespective of stratification based on age, sex, BMI, and LVEF without significant interaction. The CFS is a valuable prognostic tool in clinical settings.
Keywords: Frailty, Clinical Frailty Scale, Heart failure, Death
Introduction
Frailty is a geriatric syndrome that is defined as a state of reduced physiological reserve against pathological or iatrogenic stressors due to age‐related impairments. 1 An ageing population leads to an increase in not only heart failure (HF) patients but also frail patients. 2 , 3 While previous large‐scale trials have enabled the development of effective treatments for HF, especially for reduced ejection fraction, the findings of these trials were less applicable to frail patients. These trials tend to exclude frail patients because of their various co‐morbidities, unwillingness to participate, or physicians discouraging participation. 4 There are emerging data that suggest that frailty is an independent prognostic factor for HF patients, which formed the basis for assessing frailty in HF patients. Moreover, frailty is an important factor from the view of quality of life enhancement and appropriate end‐of‐life care.
However, the best assessment instrument for frailty is still not known. 5 While most instruments are time consuming and require physical measurements, 6 the Canadian Study of Health and Aging Clinical Frailty Scale (CFS) is a very simple frailty assessment tool that is practical in busy clinical settings. 7 Although CFS has been shown to correlate with mortality in patients with cardiovascular disease, 8 , 9 data on the association between CFS and HF are limited. 10 , 11 We sought to evaluate the impact of frailty according to the CFS on 2 year all‐cause death after hospitalization for HF in an all‐comer registry from a single centre.
Methods
Study population
Among consecutive 641 patients in an all‐comer, single‐centre registry who were hospitalized because of HF at Kokura Memorial Hospital (Kitakyushu, Japan) during 2015, 45 patients were excluded because of in‐hospital death. The remaining 596 patients were discharged and enrolled in the present study. Written informed consent of the patients was waived because the patients were retrospectively enrolled. None of the patients refused participation in the study when contacted for follow‐up. This opt‐out consent strategy was in accordance with the guidelines of the Japanese Ministry of Health, Labour and Welfare. The institutional review board of Kokura Memorial Hospital approved the protocol of this observational study. Moreover, the study was conducted following the Declaration of Helsinki. The objectives and detailed design are available on the University Hospital Medical Information Network (UMIN000041519).
Frailty was assessed at discharge by healthcare professionals in the cardiovascular centre using Canadian Study of Health and Aging CFS (Supporting Information, Table S1 ). 7 , 12 Patients were divided into three groups according to the CFS level: low (CFS Levels 1–3), intermediate (CFS Levels 4–6), and high (CFS Levels 7–9).
Clinical outcomes
We obtained follow‐up clinical information from medical records, patients' referral documents, and telephone interviews with the patients or their families. The primary outcome measure was defined as all‐cause death at 2 years. We also assessed cardiac death and non‐cardiac death according to the Academic Research Consortium criteria. 13 We assessed rehospitalization for HF as a secondary outcome measure. HF decompensation was diagnosed using the Framingham criteria. 14 All baseline variables and clinical outcomes were adjudicated by 10 assessors who reviewed the source documents.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median (inter‐quartile ranges) and were compared using a Kruskal–Wallis test. Categorical variables were presented as values and percentages and were compared using a χ 2 test. Kaplan–Meier curves were used to estimate the cumulative incidence of clinical events, and the differences of cumulative incidence among CFS groups were estimated by log‐rank test. Cumulative incidence of cardiac death, non‐cardiac death, and HF rehospitalization were also estimated by Grey's test, which account for the competing risk for all‐cause death. In comparison of baseline characteristics, Bonferroni‐adjusted P values of 0.0167 were used as the criterion of statistical significance. To test the predictive ability of the CFS, multivariate Cox proportional hazard models were constructed, which comprised variables identified from previous studies 15 , 16 , 17 as potential risk factors for all‐cause mortality and rehospitalization for HF. For the post hoc analyses, we dichotomized patients based on (i) median age of 80 years, (ii) sex, (iii) median body mass index (BMI) level of 22.5, and (iv) left ventricular ejection fraction (LVEF) values of 50%, to explore interactions between the presence and absence of these factors that correlate with frailty and effects of frailty on the risk of 2 year all‐cause death and rehospitalization for HF. LVEF cut‐off point was decided according to the definition of HF with preserved ejection fraction (HFpEF) in the 2016 European Society of Cardiology guideline. 18 A two‐tailed P < 0.05 was considered statistically significant for all tests. All analyses were performed using JMP Version 14.3.0 (SAS Institute Incorporated, Cary, NC, USA).
Results
Baseline characteristics
The CFS level was low (1–3) in 232 (38.9%) patients, intermediate 4 , 5 , 6 in 230 (38.6%) patients, and high 7 , 8 , 9 in 134 (22.5%) patients (Figure 1 ). None of the patients with HF were comparable with CFS1. The baseline clinical characteristics of the CFS groups are summarized in Table 1 . There were significant differences in age, living environment, and dementia among low, intermediate, and high CFS groups. Co‐morbidities such as severe renal failure, severe anaemia, and severe tricuspid regurgitation and LVEF tended to increase with frailty severity, while BMI and albumin level tended to decrease with frailty severity. The result of post hoc test regarding the baseline characteristics was shown Supporting Information, Table S2 .
Figure 1.
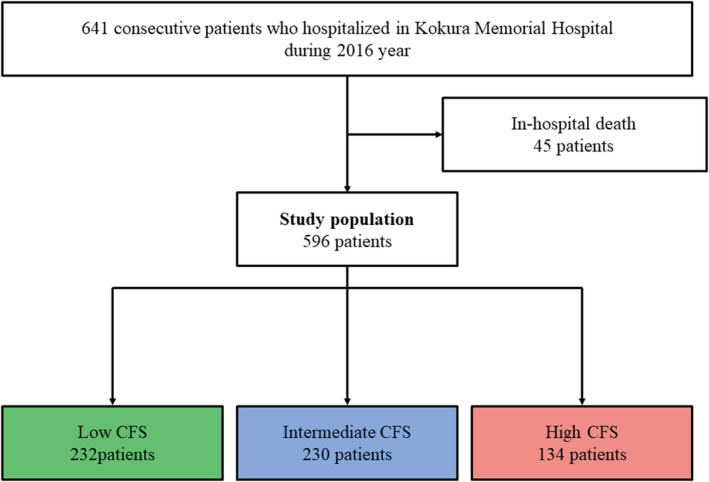
Study patient flow. Clinical Frailty Scale (CFS) was derived from Canadian Study of Health and Aging.
Table 1.
Baseline characteristics according to the Clinical Frailty Scale groups
| Overall (N = 596) | Clinical Frailty Scale groups | P values | |||
|---|---|---|---|---|---|
| Low (1–3) | Intermediate (4–6) | High (7–9) | |||
| N = 232 | N = 230 | N = 134 | |||
| Patient characteristics | |||||
| Age (years) | 76.6 ± 10.1 | 69.5 ± 11.6 | 80.2 ± 9.0 | 83.4 ± 9.1 | <0.001 |
| Age ≥75 years | 380 (63.9%) | 87 (36.7%) | 180 (80.0%) | 113 (85.0%) | <0.001 |
| Men | 329 (55.3%) | 161 (67.9%) | 103 (45.8%) | 65 (48.9%) | <0.001 |
| Body mass index | 23.0 ± 4.4 | 24.1 ± 4.1 | 22.6 ± 4.7 | 22.1 ± 3.8 | 0.004 |
| Living environment | <0.001 | ||||
| Living alone | 161 (26.1%) | 71 (30.0%) | 64 (28.4%) | 26 (19.5%) | |
| Living with one or more people | 399 (67.1%) | 164 (69.2%) | 152 (67.6%) | 83 (62.4%) | |
| Admission to a nursing facility | 35 (5.9%) | 2 (0.8%) | 9 (4.0%) | 24 (18.1%) | |
| Receiving welfare | 82 (13.8%) | 40 (16.9%) | 27 (12.0%) | 15 (11.3%) | 0.21 |
| Smoking status | <0.001 | ||||
| Current smoker | 88 (14.8%) | 55 (23.2%) | 24 (10.7%) | 9 (6.8%) | |
| Past smoker | 193 (32.4%) | 90 (38.0%) | 64 (28.4%) | 39 (29.3%) | |
| Hypertension | 595 (64.7%) | 146 (61.6%) | 150 (66.8%) | 89 (66.9%) | 0.44 |
| Diabetes mellitus | 192 (32.3%) | 88 (37.1%) | 56 (24.9%) | 48 (36.1%) | 0.01 |
| Dyslipidaemia | 201 (33.8%) | 08 (38.1%) | 72 (32.0%) | 39 (29.3%) | 0.17 |
| Prior myocardial infarction | 120 (20.2%) | 50 (21.1%) | 35 (15.6%) | 35 (26.3%) | 0.045 |
| Prior stroke | 72 (12.1%) | 19 (8.0%) | 39 (17.3%) | 14 (10.5%) | 0.008 |
| Prior heart failure | 216 (36.4%) | 70 (29.5%) | 94 (42.2%) | 52 (39.1%) | 0.01 |
| Prior peripheral artery disease | 58 (9.8%) | 23 (9.7%) | 19 (8.4%) | 16 (12.0%) | 0.55 |
| Atrial fibrillation | 239 (40.2%) | 88 (37.1%) | 98 (43.6%) | 53 (39.9%) | 0.37 |
| Prior ventricular tachycardia or fibrillation | 29 (4.9%) | 13 (5.5%) | 10 (4.4%) | 6 (4.5%) | 0.85 |
| Liver cirrhosis | 8 (1.3%) | 7 (3.0%) | 1 (0.4%) | 0 (0%) | 0.01 |
| Chronic obstructive pulmonary disease | 44 (7.4%) | 15 (6.3%) | 22 (9.8%) | 7 (5.3%) | 0.21 |
| Prior malignancy | 71 (11.9%) | 23 (9.7%) | 28 (12.4%) | 20 (15.0%) | 0.30 |
| Dementia | 59 (9.9%) | 9 (3.8%) | 23 (10.2%) | 27 (20.3%) | <0.001 |
| Renal failure | |||||
| eGFR < 30 mL/min/1.73 m2 or dialysis | 177 (29.6%) | 50 (21.1%) | 76 (33.8%) | 51 (38.4%) | <0.001 |
| eGFR 30–59 mL/min/1.73 m2 | 280 (47.1%) | 114 (48.1%) | 106 (47.1%) | 60 (45.1%) | 0.86 |
| Prior open‐heart surgery | 78 (13.1%) | 26 (11.0%) | 35 (15.6%) | 17 (12.8%) | 0.35 |
| Prior revascularization | 208 (35.0%) | 82 (34.6%) | 72 (32.0%) | 54 (40.6%) | 0.26 |
| Prior coronary artery graft bypass surgery | 51 (8.6%) | 23 (9.7%) | 18 (8.0%) | 10 (7.5%) | 0.71 |
| Prior percutaneous coronary intervention | 179 (30.1%) | 72 (30.4%) | 62 (27.6%) | 45 (33.8%) | 0.45 |
| Prior myocardial ablation | 40 (6.7%) | 20 (8.4%) | 14 (6.2%) | 6 (4.5%) | 0.32 |
| Albumin | 3.7 (3.4–4.0) | 3.8 (3.5–4.1) | 3.7 (3.4–4.0) | 3.5 (3.2–3.8) | <0.001 |
| Anaemia (haemoglobin <11 g/dL) | 249 (41.9%) | 74 (31.2%) | 103 (45.8%) | 72 (54.1%) | <0.001 |
| Thrombocytopenia (platelet <100 × 109/L) | 52 (8.7%) | 19 (8.0%) | 20 (8.9%) | 13 (9.8%) | 0.84 |
| Brain natriuretic peptide | 997 ± 944 | 918 ± 816 | 995 ± 907 | 1141 ± 1185 | 0.28 |
| Left ventricular ejection fraction | 46.2 ± 15.1 | 42.6 ± 15.4 | 48.1 ± 15.3 | 49.7 ± 14.4 | <0.001 |
| Left ventricular ejection fraction ≤35% | 143 (26.0%) | 77 (34.7%) | 43 (21.4%) | 23 (18.0%) | <0.001 |
| Severe aortic regurgitation | 5 (0.8%) | 1 (0.4%) | 3 (1.3%) | 1 (0.8%) | 0.55 |
| Severe aortic stenosis | 35 (5.9%) | 10 (4.2%) | 17 (7.6%) | 8 (6.0%) | 0.30 |
| Severe mitral regurgitation | 19 (3.2%) | 6 (2.5%) | 11 (4.9%) | 2 (1.5%) | 0.16 |
| Severe mitral stenosis | 4 (0.7%) | 1 (0.4%) | 1 (0.4%) | 2 (1.5%) | 0.48 |
| Severe tricuspid regurgitation | 22 (3.7%) | 2 (0.8%) | 11 (4.9%) | 9 (6.8%) | 0.003 |
| Medication at discharge | |||||
| Beta‐blocker | 448 (75.3%) | 192 (81.0%) | 163 (72.4%) | 93 (69.9%) | 0.03 |
| Angiotensin‐converting enzyme inhibitor | 219 (36.8%) | 97 (40.9%) | 71 (31.6%) | 51 (38.4%) | 0.10 |
| Angiotensin II receptor blocker | 209 (35.1%) | 80 (33.8%) | 82 (36.4%) | 47 (35.3%) | 0.83 |
| Mineralocorticoid receptor antagonist | 298 (50.1%) | 127 (53.6%) | 108 (48.0%) | 63 (47.4%) | 0.38 |
eGFR, estimated glomerular filtration rate.
Continuous variables are given as mean ± standard deviation or median (inter‐quartile range). Categorical variables are given as number (%).
Clinical outcomes
The 2 year follow‐up rate for clinical information was 93.5%. Of the entire cohort, 150 (25.2%) patients suffered from all‐cause death and 211 (35.4%) patients suffered from rehospitalization due to HF during the 2 year follow‐up period. Among the low, intermediate, and high CFS groups, the cumulative 2 year incidence of all‐cause death (12.8%, 25.4%, and 52.7%, respectively, P < 0.001) and rehospitalization for HF (29.6%, 41.2%, and 59.7%, respectively, P < 0.001) was significantly higher in frailer patients (Figure 2 A and 2 B ). The significant difference in the risk for all‐cause death among the CFS groups was driven by the risk for cardiac (8.6%, 14.2%, and 31.0%, respectively, P < 0.001) and non‐cardiac death (4.6%, 13.0%, and 31.4%, respectively, P < 0.001) (Figure 2 C and 2 D ). When competing risks were taken into account, group differences in cardiac deaths, non‐cardiac deaths, and HF rehospitalizations were in line with the main analyses (Supporting Information, Table S3 ). Compared with CFS Level 2, the risk for all‐cause death was incrementally higher in patients with CFS Levels 3–9 (Figure 3 A and Supporting Information, Table S4 ). After dividing the patients into two groups according to the median age of 80 years, the difference in the cumulative incidence of 2 year mortality per CFS level was slight between younger strata (<80 years) and older strata (≥80 years) (Figure 3 B ).
Figure 2.
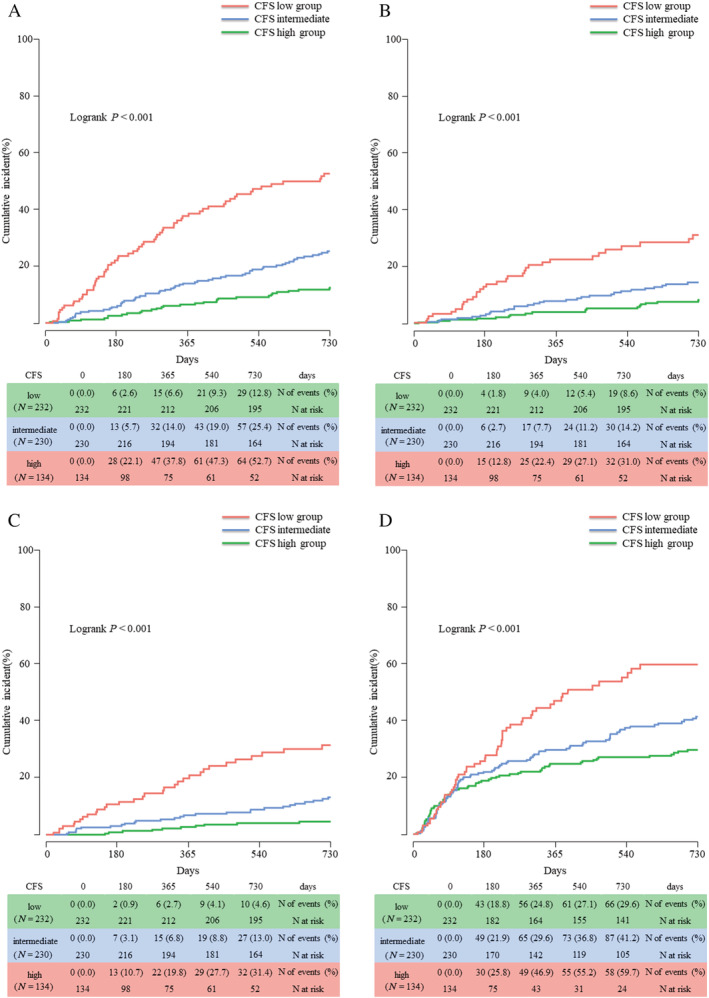
Kaplan–Meier curves for (A) all‐cause death, (B) cardiac death, (C) non‐cardiac death, and (D) rehospitalization for heart failure according to the Clinical Frailty Scale (CFS) groups.
Figure 3.
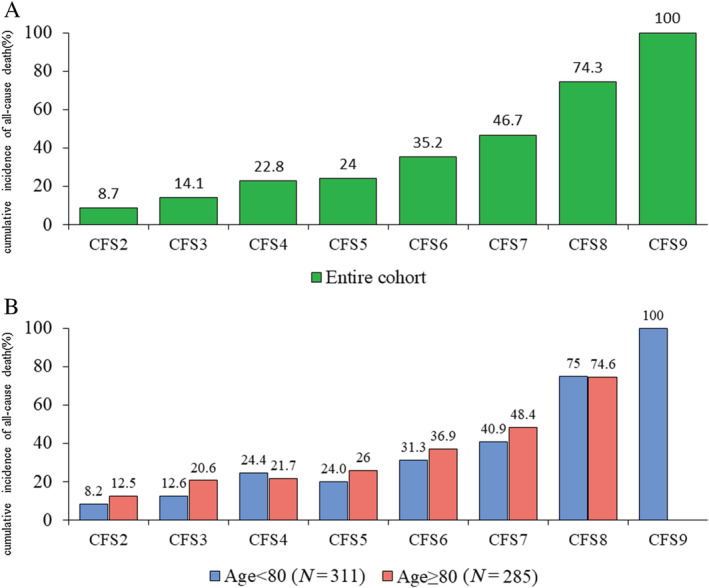
Two year cumulative incidence of all‐cause death per Clinical Frailty Scale (CFS) level (A) in entire cohort (B) in younger strata (<80 years) and older strata (≥80 years).
In the multivariate analysis, CFS group was independently associated with the risk for all‐cause death [intermediate CFS group: adjusted hazard ratio (HR), 1.43, 95% confidence interval (CI), 0.86–2.36, P = 0.16; high CFS group: adjusted HR, 3.90, 95% CI, 2.32–6.55, P < 0.001] and rehospitalization due to HF (intermediate CFS group: adjusted HR, 1.52, 95% CI, 1.04–2.44, P = 0.03; high CFS group: HR, 2.44, 95% CI, 1.60–3.74, P < 0.001; reference: low CFS group; Table 2 ).
Table 2.
Univariate and multivariable Cox proportional hazard models for all‐cause mortality and rehospitalization for heart failure at 2 years
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P values | Hazard ratio (95% confidence interval) | P values | |
| All‐cause death | ||||
| Low CFS group | Reference | Reference | ||
| Intermediate CFS group | 2.12 (1.35–3.31) | 0.001 | 1.43 (0.86–2.36) | 0.16 |
| High CFS group | 5.73 (3.69–8.89) | <0.001 | 3.90 (2.32–6.55) | <0.001 |
| Rehospitalization for heart failure | ||||
| Low CFS group | Reference | Reference | ||
| Intermediate CFS group | 1.53 (1.11–2.10) | 0.01 | 1.52 (1.04–2.21) | 0.03 |
| High CFS group | 2.96 (2.08–4.22) | <0.001 | 2.44 (1.60–3.74) | <0.001 |
CFS, Clinical Frailty Scale.
Adjusted variables were as follows: age; sex; body mass index; left ventricular ejection fraction; current smoking status; a history of heart failure (whether or not a de novo case), diabetes, and chronic obstructive pulmonary disease; systolic blood pressure; haemoglobin; estimated glomerular filtration rate; prescription of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, beta‐blocker, and mineralocorticoid receptor antagonist; and New York Heart Association Class III/IV at discharge.
We stratified the patients into two groups according to the median age of 80 years, the median BMI of 25.5, the LVEF values of 50%, and sex. The multivariate analysis showed that CFS was independently associated with the risk for all‐cause death and HF rehospitalization at 2 years, regardless of stratification based on age, BMI, LVEF, and sex, without significant interaction (Figure 4 ). While only the male patients with intermediate CFS have the significantly higher risk for all‐cause death than those with low CFS, there was no significant interaction (interaction P = 0.24).
Figure 4.
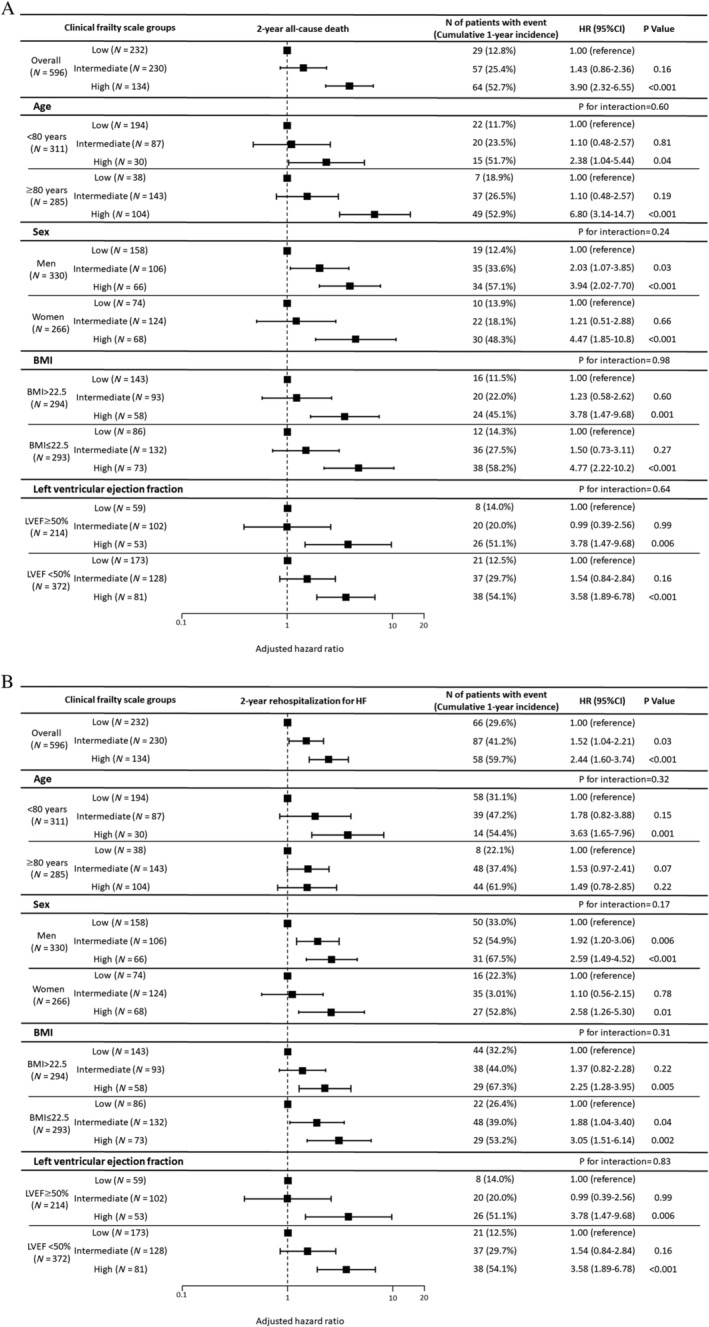
Forest plot for the adjusted hazard ratios (HRs) of (A) 2 year all‐cause mortality and (B) 2 year rehospitalization rate for heart failure (HF) according to the Clinical Frailty Scale groups. BMI, body mass index; CI, confidence interval; LVEF, left ventricular ejection fraction.
Discussion
The primary findings of this study are as follows:
In an all‐comer, single‐centre HF registry with a mean age of 76.6 ± 10.1 years, 61.1% of the patients were regarded as frail according to the CFS (high: 22.5% and intermediate: 38.6%). Frailer patients more often suffered all‐cause death, which was driven by risk for both cardiac and non‐cardiac death and rehospitalization due to HF during the 2 year follow‐up period after discharge from HF.
Compared with CFS Level 2, the risk for all‐cause death was incrementally higher in patients with CFS Levels 3–9. After dividing the patients into two groups according to the median age of 80 years, the difference in the cumulative incidence of 2 year mortality per CFS level was slight between younger strata (<80 years) and older strata (≥80 years).
Clinical Frailty Scale group was independently associated with 2 year all‐cause death and rehospitalization due to HF.
The association between CFS group and 2 year all‐cause death and rehospitalization due to HF remained significant even after the stratification based on age, sex, BMI, and LVEF in multivariate analysis.
The first demonstration of the association between frailty and HF was conducted by Cacciatore et al. in 2005. 19 Since then, frailty is an increasingly recognized metric that has been reported to be associated with adverse events. These studies have shown that the association between frailty and HF is close and frailty has a greater impact on mortality, disability, and hospitalization especially in patients with HF than in those without HF. 10 , 20 , 21 , 22 The prevalence of frailty varies widely depending on the definition of frailty used and the study cohorts but is thought to range from 15% to 77%. 23 Of the many assessment tools for frailty, the CFS requires little time to administer and is easy to use in clinical settings because it does not involve physical measurements, which makes it particularly suitable for frail patients. A previous study showed that the CFS had high sensitivity, specificity, and misclassification out of six assessment instruments for frailty. Moreover, the study also showed that despite the subjective component of the CFS, the inter‐operator agreement was good. 6 The assessment of frailty using the CFS is not the gold standard; nevertheless, it can be considered that the CFS is sufficiently practical in clinical settings.
The Heart Failure Association of the European Society of Cardiology recommends the assessment of four domains: psycho‐cognitive, functional, clinical, and social, 5 while the CFS includes only cognitive and functional elements in multiple frailty domains. The previous studies showed that a greater number of frailty domains are associated with a worse prognosis. 24 , 25 The modified frailty index, which is a multidimensional frailty assessment tool that included four domains of frailty: physical, mental, nutritional, and socio‐economic, was superior to Fried's frailty index 26 in terms of the predictive ability of mortality. 27 These results indicate that multidimensional frailty evaluation is valid and required. However, the relationship between predictive power and the administration time would be a trade‐off. The difference in administration time between CFS and modified frailty index would be ~5–10 min. Physicians should select the evaluation method, which is appropriate for each clinical setting. To explore the best assessment tool of frailty, which combines simplicity and predictive power, further study is needed.
In our study, we found that frail patients were older and more likely to have HFpEF and many co‐morbidities. The patients in higher CFS level group were more likely to have suffered 2 year all‐cause death and HF rehospitalization, which indicated that the CFS is effective in predicting the risk for all‐cause death and HF rehospitalization. It is notable that the difference between younger strata (<80 years) and older strata (≥80 years) in the cumulative incidence of 2 year mortality per CFS level was very slight, while the risk for all‐cause death was incrementally higher in patients with CFS Levels 3–9. This result could be interpreted that frailty is more important factor than chronological age in prognostic values. Moreover, the 2 year cumulative incidence of all‐cause death was >50% in patients in high CFS group. The discussion about treatment policy such as surgery or chemotherapy is needed according to not only patient's chronological age but also systemic state including frailty.
The CFS group was independently associated with all‐cause death and HF rehospitalization in the multivariate analysis. However, because chronological age is a well‐known predictor of death in most cardiology studies 28 and closely correlated with frailty, we considered that its impact could not be ignored even in the multivariable models adjusted for age. Therefore, we conducted a post hoc analysis by classifying patients into two age groups using a median age of 80 years. We detected no significant interactions between age and CFS group regarding risk for death or HF rehospitalization. The CFS consistently identified high‐risk patients, irrespective of the stratification based on age, sex, BMI, and LVEF in multivariate analysis. The CFS level, irrespective of these factors, can provide important information about prognoses of HF patients, which can enhance quality of life and enable appropriate end‐of‐life care that involves the patients and their families. While only the male patients with intermediate CFS have the significantly higher risk for all‐cause death than those with low CFS, there was no significant interaction (interaction P = 0.24). This result might be driven by the small sample size. To clarify the impact of sex on frail patients or to identify patients who can benefit from rehabilitation, which prevent the progress of their frailty, further research is needed.
Limitations
There are several limitations to this study. First, it is uncertain whether the results are generalizable because we used a single‐centre design. Second, CFS is a semiquantitative scale of frailty. Although medical professionals have assessed the CFS, its reproducibility has not been evaluated. Third, we did not compare the CFS with other frailty assessment tools.
Conclusions
The simple CFS tool was successful in predicting the risk for all‐cause death in patients with HF, and frailty according to CFS was independently associated with all‐cause death irrespective of stratification based on age, sex, BMI, and LVEF without significant interaction. The CFS is a valuable prognostic tool in clinical settings.
Conflict of interest
None declared.
Funding
None.
Supporting information
Table S1. Clinical Frailty Scale.
Table S2. Post‐hoc test in baseline characteristics.
Table S3. Clinical Frailty Scale and 1‐year cumulative incidence of cardiac death, non‐cardiac death, and HF rehospitalization.
Table S4. Univariate and Multivariable Cox Proportional Hazard Models for All‐Cause Mortality and rehospitalization for heart failure at 2 years per CFS level.
Kanenawa, K. , Isotani, A. , Yamaji, K. , Nakamura, M. , Tanaka, Y. , Hirose‐Inui, K. , Fujioka, S. , Mori, S. , Yano, M. , Ito, S. , Morinaga, T. , Fukunaga, M. , Hyodo, M. , and Ando, K. (2021) The impact of frailty according to Clinical Frailty Scale on clinical outcome in patients with heart failure. ESC Heart Failure, 8: 1552–1561. 10.1002/ehf2.13254.
Contributor Information
Kenji Kanenawa, Email: kanesannsann@yahoo.co.jp.
Masato Fukunaga, Email: masato_f0728@yahoo.co.jp.
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vitale C, Uchmanowicz I. Frailty in patients with heart failure. Eur Heart J 2019; 21: L12–L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 4. Coats AJS. Heart failure management of the elderly patient: focus on frailty, sarcopaenia, cachexia, and dementia: conclusions. Eur Heart J Suppl 2019; 21: L36–L38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, Seferovic P, Coats AJ. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 6. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. JACC Heart Fail 2019; 7: 291–302. [DOI] [PubMed] [Google Scholar]
- 7. Rockwood K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Tsuchikane E, Suzuki T, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Watanabe Y, Hayashida K, Yashima F, Inohara T, Kakefuda Y, Arai T, Yanagisawa R, Tanaka M, Kawakami T, Maekawa Y, Takashi K, Yoshitake A, Iida Y, Yamazaki M, Shimizu H, Yamada Y, Jinzaki M, Tsuruta H, Itabashi Y, Murata M, Kawakami M, Fukui S, Sano M, Fukuda K, Hosoba S, Sato H, Teramoto T, Kimura M, Sago M, Tsunaki T, Watarai S, Tsuzuki M, Irokawa K, Shimizu K, Kobayashi T, Okawa Y, Miyasaka M, Enta Y, Shishido K, Ochiai T, Yamabe T, Noguchi K, Saito S, Kawamoto H, Onishi H, Yabushita H, Mitomo S, Nakamura S, Yamawaki M, Akatsu Y, Honda Y, Takama T, Isotani A, Hayashi M, Kamioka N, Miura M, Morinaga T, Kawaguchi T, Yano M, Hanyu M, Arai Y, Tsubota H, Kudo M, Kuroda Y, Kataoka A, Hioki H, Nara Y, Kawashima H, Nagura F, Nakashima M, Sasaki K, Nishikawa J, Shimokawa T, Harada T, Kozuma K. Impact of the Clinical Frailty Scale on outcomes after transcatheter aortic valve replacement. Circulation 2017; 135: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 9. Takeji Y, Yamaji K, Tomoi Y, Okazaki J, Tanaka K, Nagae A, Jinnouchi H, Hiramori S, Soga Y, Ando K. Impact of frailty on clinical outcomes in patients with critical limb ischemia. Circ Cardiovasc Interv 2018; 11: e006778. [DOI] [PubMed] [Google Scholar]
- 10. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 2017; 106: 533–541. [DOI] [PubMed] [Google Scholar]
- 11. Costa D, Aladio M, Girado CA, Pérez de la Hoz R, Berensztein CS. Frailty is independently associated with 1‐year mortality after hospitalization for acute heart failure. Int J Cardiol Heart Vasc 2018; 21: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morley JE, Vellas B, Abellan Van Kan G, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW, Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115: 2344–2351. [DOI] [PubMed] [Google Scholar]
- 14. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993; 88: 107–115. [DOI] [PubMed] [Google Scholar]
- 15. Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51 043 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2014; 16: 173–179. [DOI] [PubMed] [Google Scholar]
- 16. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K, Yoshikawa T. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC heart Fail 2018; 5: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 19. Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long‐term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005; 35: 723–730. [DOI] [PubMed] [Google Scholar]
- 20. Yang X, Lupón J, Vidán MT, Ferguson C, Gastelurrutia P, Newton PJ, Macdonald PS, Bueno H, Bayés‐Genís A, Woo J, Fung E. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta‐analysis. J Am Heart Assoc 2018; 7: e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol 2010; 55: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gianluca Testa IL, Curcio F, Russo G, Bulli G, Galizia G, Della‐Morte D, Gargiulo G, Basile C, Cacciatore F, Bonaduce D, Abete P. Multidimensional frailty evaluation in elderly outpatients with chronic heart failure: a prospective study. Eur J Prev Cardiol 2019; 26: 1115–1117. [DOI] [PubMed] [Google Scholar]
- 23. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2017; 236: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleipool EEF, Wiersinga JHI, Trappenburg MC, Rossum AC, Dam CS, Liem SS, Peters MJL, Handoko ML, Muller M. The relevance of a multidomain geriatric assessment in older patients with heart failure. ESC Heart Fail 2020; 7: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H, Kasai T, Nagamatsu H, Ozawa T, Izawa K, Yamamoto S, Aizawa N, Yonezawa R, Oka K, Momomura SI, Kagiyama N. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail 2020; 22: 2112–2119. [DOI] [PubMed] [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M157. [DOI] [PubMed] [Google Scholar]
- 27. Abete P, Basile C, Bulli G, Curcio F, Liguori I, Della‐Morte D, Gargiulo G, Langellotto A, Testa G, Galizia G, Bonaduce D, Cacciatore F. The Italian version of the “frailty index” based on deficits in health: a validation study. Aging Clin Exp Res 2017; 29: 913–926. [DOI] [PubMed] [Google Scholar]
- 28. Lloyd‐Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. The Lancet 1999; 353: 89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Frailty Scale.
Table S2. Post‐hoc test in baseline characteristics.
Table S3. Clinical Frailty Scale and 1‐year cumulative incidence of cardiac death, non‐cardiac death, and HF rehospitalization.
Table S4. Univariate and Multivariable Cox Proportional Hazard Models for All‐Cause Mortality and rehospitalization for heart failure at 2 years per CFS level.


