Abstract
Aims
The implantable cardiac defibrillator/cardiac resynchronization therapy with defibrillator‐based HeartLogic™ algorithm has recently been developed for early detection of impending decompensation in heart failure (HF) patients; but whether this novel algorithm can reduce HF hospitalizations has not been evaluated. We investigated if activation of the HeartLogic algorithm reduces the number of hospital admissions for decompensated HF in a 1 year post‐activation period as compared with a 1 year pre‐activation period.
Methods and results
Heart failure patients with an implantable cardiac defibrillator/cardiac resynchronization therapy with defibrillator with the ability to activate HeartLogic and willingness to have remote device monitoring were included in this multicentre non‐blinded single‐arm trial with historical comparison. After a HeartLogic alert, the presence of HF symptoms and signs was evaluated. If there were two or more symptoms and signs apart from the HeartLogic alert, lifestyle advices were given and/or medication was adjusted. After activation of the algorithm, patients were followed for 1 year. HF events occurring in the 1 year prior to activation and in the 1 year after activation were compared. Of the 74 eligible patients (67.2 ± 10.3 years, 84% male), 68 patients completed the 1 year follow‐up period. The total number of HF hospitalizations reduced from 27 in the pre‐activation period to 7 in the post‐activation period (P = 0.003). The number of patients hospitalized for HF declined from 21 to 7 (P = 0.005), and the hospitalization length of stay diminished from average 16 to 7 days (P = 0.079). Subgroup analysis showed similar results (P = 0.888) for patients receiving cardiac resynchronization therapy during the pre‐activation period or not receiving cardiac resynchronization therapy, meaning that the effect of hospitalizations cannot solely be attributed to reverse remodelling. Subanalysis of a single‐centre Belgian subpopulation showed important reductions in overall health economic costs (P = 0.025).
Conclusion
Activation of the HeartLogic algorithm enables remote monitoring of HF patients, coincides with a significant reduction in hospitalizations for decompensated HF, and results in health economic benefits.
Keywords: Chronic heart failure, ICD, CRT, Remote monitoring, Heart failure hospitalization
Introduction
Heart failure (HF) is a major and still growing medical and economic problem with significant morbidity and mortality. Industrialized countries spend 1–2% of their healthcare budgets on HF care, and most costs are attributable to HF hospitalizations. 1 HF hospitalizations are associated with high mortality and reduce quality of life. 2 , 3 Accordingly, preventing HF hospitalizations is of utmost and equal importance for patients, doctors, and third payers.
Home (remote) monitoring to prevent HF hospitalizations has gained interest over the last decade. Earlier detection of fluid retention may facilitate ambulatory treatment before patients severely decompensate. 4 In theory, this could result in better care at lower costs. Clinical trials so far have particularly focused on measuring blood pressure and body weight at home, with mixed results. 4 , 5 , 6 An alternative approach is telemonitoring through implantable cardiac defibrillators (ICDs). 7 Parameters that are sensed by and stored on the ICD include—among others—heart rhythm, pacing percentages, thoracic impedance, and physical activity. 8 An advantage of telemonitoring of ICDs is that it requires almost no effort from the patient, because data are continuously gathered automatically and transferred to a cloud‐based server. 7 So far, trials on telemonitoring of ICDs have mainly focused on the early detection of arrhythmias and device complications such as lead fractures. 7 , 9 The results of studies evaluating the clinical benefit of remote monitoring of ICDs are mixed. A systematic review of 11 randomized controlled trials showed that remote monitoring of ICDs reduced planned hospital visits and lowered monetary costs, while it did not affect mortality. 9 On the other hand, a systematic review of nine randomized controlled trials demonstrated a survival benefit in hospitals that used daily transmission of data. 10 Furthermore, a major randomized controlled trial of remote monitoring of intrathoracic impedance found that hospital admissions even increased with no survival benefit. 11
The MultiSENSE trial 8 investigated a novel algorithm (HeartLogic™, Boston Scientific, Marlborough, MA, USA) that incorporated multiple parameters recorded by the ICD. It combines heart sounds (S1 and S3), respiration rate, thoracic impedance, nightly heart rate, and physical activity. All parameters are automatically collected and analysed by the ICD and incorporated in a single numeric value that can trigger an alert. In the validation step of the MultiSENSE trial, 8 more than 400 patients had the algorithm installed on their ICD. The sensors were combined into a single score (the HeartLogic index). When a patient was hospitalized, the algorithm was read out. It was shown that an increase in the HeartLogic index could predict an HF episode with a sensitivity of 70%. HF episodes could be predicted up to a median of 34 days in advance. 8 The MultiSENSE trial 8 was an observational study in which the algorithm was reviewed after an HF hospitalization had occurred. The design, therefore, did not allow for proof of lower HF hospitalization rates. Until this day, it remains unclear whether HeartLogic can help to reduce hospitalizations for decompensated HF in a real‐world setting.
Methods
Patient population
Patients were recruited from the HF outpatient clinic of the Departments of Cardiology from the Onze Lieve Vrouw Hospital Aalst (Belgium), the Leiden University Medical Centre (the Netherlands), and the Fondazione Cardiocentro Ticino (Switzerland) between 3 January 2018 and 21 December 2019. Patients with HF [according to the European Society of Cardiology (ESC) guidelines 12 ] and an ICD featuring the HeartLogic multisensor algorithm were eligible for inclusion, provided that sufficient follow‐up of pre‐activation and post‐activation time was available. Patients were excluded if they were <18 years old or pregnant or when historical comparison (pre‐activation vs. post‐activation) was not possible. The study was conducted in accordance with the Declaration of Helsinki, applicable local law, and the European directive for data protection (General Data Protection Regulation). The local three institutional ethical committees approved the study.
Study design
This was a multicentre non‐blinded single‐arm study with pre‐activation and post‐activation comparison within each patient. All patients were provided remote monitoring of their ICD via LATITUDE (Boston Scientific) and signed an informed consent agreeing to undergo remote device monitoring. Furthermore, a technical service, organized by the company providing the remote monitoring system, was available in case of technical questions. Patients were followed via two ways. First, regularly scheduled outpatient clinic visits (standard care). Patients were treated and followed‐up in accordance with ESC guidelines. 12 Second, via HeartLogic, patients were continuously followed‐up according to a standardized protocol, which is described in the succeeding text. HeartLogic was therefore an additional service for patients that did not replace (parts of) regular care.
HeartLogic
All patients had an ICD implanted (RESONATE or MOMENTUM, Boston Scientific) that supports the HeartLogic algorithm. As previously described, 8 data are continuously collected from five sensors, embedded in the ICD: first heart sound (S1), third heart sound (S3) (and the ratio of the two), intrathoracic impedance, nightly heart rate, and physical activity. The HeartLogic index is the result of a complex algorithm of which the formula is unknown to its users. Briefly, the HeartLogic index is a single number score based on alterations in the five previously mentioned sensors. The number of sensors in which an alteration is measured and/or the rapidity of the change measured determines the level of the HeartLogic index. A high HeartLogic index (standard set at >16) indicates an impending decompensation. A low HeartLogic index (<16) indicates a stable clinical status. Previous research reported that this algorithm has a sensitivity of 70%, an unexplained alert rate of 1.47 per patient year, and a negative predictive value of 99.8%. 8 The HeartLogic index is transmitted to the hospital via the telemonitoring system (LATITUDE, Boston Scientific), which sends the HeartLogic index to the LATITUDE platform. If the threshold of the HeartLogic index is surpassed, an alert is given by the system. A report, showing the trend in the HeartLogic index, as well as the relative contributions of the five sensors, is provided. Figure 1 displays a representative example of a HeartLogic alert report.
Figure 1.
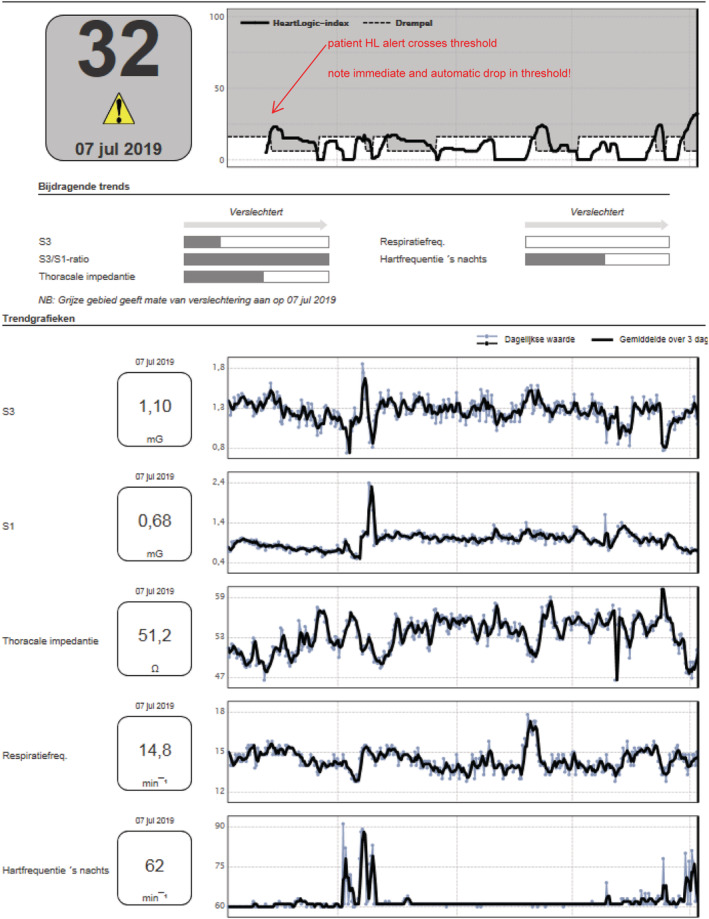
Representative example of a HeartLogic™ (HL) report.
Protocol for follow‐up
Remote monitoring data were reviewed on a daily basis by a trained ICD technician and/or HF nurse. In case of an alert, the HeartLogic report was transferred to the HF caregiver. Within 72 h, the patient was contacted by phone, and the following parameters were structurally evaluated: worsening HF symptoms or signs, weight, blood pressure, and heart rhythm. In case there were two or more other symptoms or signs of HF (besides the HeartLogic index) based on the ESC guidelines, lifestyle advices were given and/or medical treatment was adjusted in line with the ESC HF guidelines. 12 If there were less than two symptoms or signs of HF, a new remote evaluation in 2 weeks was planned. This remote evaluation again consisted of a medical history, rhythm assessment, and evaluation of weight, blood pressure, and the level of the HeartLogic index. Again, if there were two or more symptoms or signs (apart from the HeartLogic index), the treating cardiologist provided lifestyle advices or adjusted medical treatment in line with the ESC HF guidelines. 12 After three remote evaluations with no or less than two symptoms or signs of HF per evaluation, an alert was disregarded and classified as false positive.
Data collection and endpoints
Clinical data were collected from the electronic medical record of all three participating centres. HeartLogic data were collected from the LATITUDE website from the moment that calibration was completed. Baseline was defined as the first day on which the calibration was completed, and the HeartLogic index was transferred to the LATITUDE website. The pre‐activation period was the 1 year before activation of HeartLogic; the post‐activation period was the period from baseline to the end of follow‐up. Of note, the elective hospitalization for implantation and the calibration period (generally 45 days, specific for the HeartLogic feature) were not taken into account because no HeartLogic alerts can be generated at this time point. This methodology is equal to the MultiSENSE data analysis. The primary endpoint of this study was the total number of hospital admissions for decompensated HF in the pre‐activation period as compared with the post‐activation period. An HF admission was defined in accordance with the ‘2017 Cardiovascular and stroke endpoints definitions for clinical trials’ 13 as an ‘unscheduled hospital admission for a primary diagnosis of HF with a length of stay that either exceeds 24 h or crosses a calendar day’. At presentation, at least two typical signs and two typical symptoms of HF needed to be present. 12 Patients had to receive intravenous diuretics as part of their treatment during hospitalization. 13
Secondary outcomes were the number of patients hospitalized for decompensated HF, the mean number of HF hospital admissions per patient, and mean hospital length of stay in days. In addition, the total number of 1 day clinic visits, mean number of 1 day clinic visits per patient, and the number of patients with a 1 day clinic visit was evaluated. Furthermore, the total number of ambulatory outpatient clinic visits and the mean number of ambulatory visits per patient were investigated. In order to distinguish whether a potential reduction in HF events was (partly) driven by cardiac resynchronization therapy (CRT) implantation and reverse remodelling in the pre‐activation or post‐activation period, a pre‐specified subgroup analysis was planned in patients with a CRT implantation in the pre‐activation period vs. patients with an ICD or a CRT that was in place for at least 1 year. Causes of mortality were noted by the responsible researcher of the centre of inclusion.
Performance of the HeartLogic index was determined by calculating sensitivity, specificity, and unexplained alert rate per patient year. The same definitions were applied as in the MultiSENSE study. 8 A true positive alert could be explained by a hospitalization for HF, early signs or symptoms, which resulted in the administration of IV diuretics or in the increase of peroral diuretics. A non‐HF, yet clinically meaningful alert was considered an alert that did not correlate with an impending HF episode but that resulted in a meaningful clinical action. In order to analyse the association between alerts and clinical events, as in the MultiSENSE study, we linked that alert to the clinical event if the alert began before the occurrence of the clinical event and did not return to baseline earlier than 30 days before the event.
Financial analysis
In the subpopulation of Onze Lieve Vrouw Hospital Aalst (Belgium), a thorough health economic analysis was performed to study the effect of the use of HeartLogic. In contrast to the comparable level of HF care, the financing systems and practical organization of the healthcare systems in Belgium, the Netherlands, and Switzerland are largely different. Therefore, a pooled health economic analysis of all the three centres could not be performed.
The retrospective financial analysis consists of a comparison between the resource use (hospital admissions for HF event and ambulatory visits 12 months before the activation of HeartLogic) (pre‐activation period) and 12 months after the activation of HeartLogic (post‐activation period) within the same patient population. The post‐activation period started 45 days after the activation of HeartLogic to allow for its calibration.
Total costs per patient were estimated by following the Belgian Health Care Knowledge Centre guideline for economic evaluation 14 and by using extrapolation factors to deal with the mixed system of hospital financing. The complex methodology is based on the Belgian Health Care Knowledge Centre reference guideline to analyse health economic costs in Belgium. 14 Cost data were extracted from the financial department of the Onze Lieve Vrouw Hospital Aalst and include hospital and ambulatory costs (i.e. laboratory tests, medical imaging, and consultations) only for Aalst patients. Medication costs are not included in this analysis because it was impossible to retrieve all out‐of‐hospital pharmacy costs. Costs are reported in euro taking into account inflation rates (https://ec.europa.eu/eurostat/web/hicp/data/database).
Statistical analysis
Descriptive data are reported as mean ± standard deviation for normally distributed continuous variables, or median with inter‐quartile range (IQR) in the case of non‐normally distributed variables, unless specifically mentioned otherwise. Normality testing was performed by the Kolmogorov–Smirnov and the Shapiro–Wilk test. Differences between mean data were compared by Student's t‐test for Gaussian variables and the Mann–Whitney U (Wilcoxon rank‐sum) test for non‐normally distributed variables. The F test was used to check the hypothesis of equality of variance. Proportional differences were compared by applying χ 2 analysis or Fisher's exact test, as appropriate. A P‐value lower than 0.05 was considered statistically significant. Statistical analysis was performed by means of GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient population
In total, 74 patients were included in the study. As shown in Table 1 , age was 67.2 ± 10.3 years, 62 (84%) were male, 27 (36%) had an ischaemic aetiology of HF, and left ventricular ejection fraction was 31 ± 11%. At the moment of HeartLogic activation, 64 (86%) had a CRT with defibrillator (CRT‐D) device, and 10 (14%) had an ICD device. Of interest, 34 patients (46%) were included from the Onze Lieve Vrouw Hospital Aalst, 30 patients (41%) from the Leiden University Medical Centre, and 10 patients (14%) from the Lugano Fondazione Cardiocentro Ticino.
Table 1.
Patient characteristics (N = 74)
| N | % | |
|---|---|---|
| Age (mean ± SD) | 67.2 ± 10.3 | n/a |
| Male | 62 | 84% |
| Ischaemic aetiology HF | 27 | 36% |
| NYHA Class I/II/III | 15/35/24 | 20%/47%/32% |
| CRT‐D device | 64 | 86% |
| ICD device | 10 | 14% |
| Diabetes mellitus | 15 | 20% |
| Serum creatinine in mg/dL (mean ± SD) | 1.29 ± 0.55 | n/a |
| eGFR in mL/min/BSA (mean ± SD) | 60 ± 23 | n/a |
| Medical therapy (N = 68) | ||
| Beta‐blocker | ||
| Pre‐activation | 56 | 82% |
| Post‐activation | 61 | 89% |
| ACE‐I/ARB/ARNI | ||
| Pre‐activation | 56 | 82% |
| Post‐activation | 56 | 82% |
| MRA | ||
| Pre‐activation | 36 | 53% |
| Post‐activation | 45 | 66% |
| Diuretics (loop + thiazide) | ||
| Pre‐activation | 47 | 69% |
| Post‐activation | 48 | 70% |
| Ivabradine | ||
| Pre‐activation | 3 | 4% |
| Post‐activation | 3 | 4% |
| Echocardiographic parameters | ||
| LVEF (mean ± SD in %) | 31 ± 11 | n/a |
| LVEDD (mean ± SD in mm) | 61 ± 9 | n/a |
| MR mild/moderate/severe | 52/11/10 | 70%/15%/14% |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BSA, body surface area; CRT‐D, cardiac resynchronization therapy with defibrillator; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardiac defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SD, standard deviation.
Follow‐up period
During a median follow‐up of 365 days (IQR 0) after HeartLogic activation, five patients died. The cause of death was non‐HF related in all patients: one patient died of urosepsis, one of saddle pulmonary embolism, one of lung cancer, and one of endocarditis. One patient committed suicide. These patients were excluded from the analysis of primary and secondary endpoints and from the subanalyses. One patient was lost to follow‐up after device implantation. According to the municipality register, he was still alive when follow‐up was completed. This patient was also excluded from the analysis. Subsequently, 68 patients were included in the analyses of primary and secondary endpoints.
HeartLogic alerts in the post‐activation period
In the 68 patients included in the analysis, during a median post‐activation period of 365 days (IQR 0), in total, 51 HeartLogic alerts were objectified. These alerts occurred in only 24 patients (36%), meaning that 44 patients (64%) had no single alert during the follow‐up period. Six patients (9%) had only one HeartLogic alert during their follow‐up, while 11 patients had two alerts (16%) and 7 patients had three or more HeartLogic alerts (10%). The average number of alerts per patient was 0.75 ± 1.16 with a mean duration of alert of 33.7 ± 14.1 days. The number of false positive alerts was 8 (16%), the number of true positive alerts was 37 (72%), and the number of non‐HF‐related yet clinically meaningful alerts (e.g. leading to the diagnosis of pneumonia) was 3 (6%). Three alerts were not classified because worsening HF went along with a concomitant non‐HF diagnosis (e.g. pneumonia). Two events of worsening HF were not detected by HeartLogic. The unexplained alert rate per patient year in our population was 0.16, the sensitivity of HeartLogic was approximately 90% (confidence interval 77–97%), and the specificity was approximately 89% (confidence interval 79–95%).
Heart failure events in pre‐activation vs. post‐activation period
In the 68 patients included in the analysis of primary and secondary endpoints, the median duration of the pre‐activation period was 365 days (IQR 0), and the median duration of the post‐activation period was 365 days (IQR 0). The total number of hospitalizations for decompensated HF declined from 27 in the pre‐activation period to 7 in the post‐activation period (Table 2 ). This corresponds to a mean number of hospitalizations per patient year of 0.39 ± 0.08 in the pre‐activation period and 0.11 ± 0.04 in the post‐activation period (P = 0.003) (Figure 2 ). These HF hospitalizations occurred in 21 (31%) patients in the pre‐activation period vs. in 7 (10%) patients in the post‐activation period (P = 0.005). The duration of hospitalizations was longer in the pre‐activation period than in the post‐activation period (16 ± 14 vs. 7 ± 5 days; P = 0.079). A patient had on average 0.5 ± 0.7 one day clinic visits and 1.9 ± 2.5 ambulatory visits in the pre‐activation period compared with 0.6 ± 1.2 and 1.7 ± 2.5, respectively, in the post‐activation period. Both differences were not statistically significant with P‐values of P = 0.732 (1 day clinic visits) and P = 0.757 (outpatient clinic visits), respectively. The proportion of patients with 1 day clinic visit remained relatively stable (24 patients pre‐activation vs. 19 patients post‐activation, P = 0.461).
Table 2.
HF events in the pre‐activation vs. post‐activation period (N = 68)
| Pre‐activation | Post‐activation | |
|---|---|---|
| Total number of HF admissions | 27 | 7 |
| Total number of 1 day clinic visits | 32 | 42 |
| Total number of ambulatory visits | 132 | 117 |
HF, heart failure.
Figure 2.
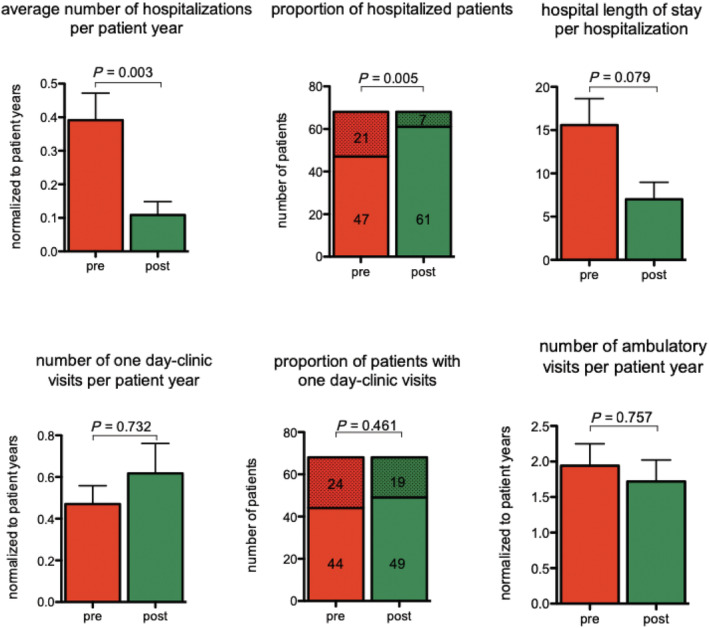
Number of hospitalizations, average duration of hospitalization, and number of patients hospitalized. Analysis of hospitalizations for heart failure before and after activation of HeartLogic™.
Subgroup analysis in de novo cardiac resynchronization therapy implantations
In order to exclude that the reduction of hospitalizations could be solely attributed to reverse remodelling by effective CRT, a subanalysis was made by defining a subpopulation of patients with a de novo CRT in the pre‐activation period vs. patients with a single‐chamber or two‐chamber ICD or a CRT that was present for at least 1 year prior to HeartLogic activation. In total, 44 (64%) patients had a de novo CRT implantation during the pre‐implantation period. The other 24 (36%) patients had an ICD or a CRT device that was implanted more than 1 year before HeartLogic activation. Between both groups, age (68 ± 11 vs. 65 ± 9 years) and male gender (81% vs. 89%) were similar. Patients with de novo CRT implantation during the pre‐implantation period had a lower left ventricular ejection fraction (27.4 ± 10.2% vs. 38.4 ± 9.9%; P < 0.001) and a larger left ventricular end‐diastolic diameter (63 ± 9 vs. 58 ± 9 mm; P = 0.031) and tended to have a higher New York Heart Association class (P = 0.045). The number of HeartLogic alerts per patient in the post‐activation period was similar in both groups (0.83 vs. 0.7; P = 0.640). As shown in Table 3 , for both groups, the mean number of hospital admissions for decompensated HF was similar in the pre‐activation period. In both groups, there was a significant decline in the post‐activation period. Of interest, the size of the reduction was similar in both groups, indicating that the decline in hospitalizations was not primarily driven by CRT implantation in the pre‐activation period.
Table 3.
Subgroup analysis: CRT vs. no CRT
| De novo CRT subgroup (n = 44) | ICD or CRT > 1 year (n = 24) | ||||
|---|---|---|---|---|---|
| Pre‐activation period | Post‐activation period | Pre‐activation period | Post‐activation period | P for interaction | |
| Total number of HF hospital admissions | 20 | 5** | 7 | 2** | 0.888 |
| Number of patients hospitalized for HF (%) | 16 (36%) | 5 (11%)** | 5 (21%) | 2 (8%)** | 0.787 |
| Mean number HF hospital admissions per patient | 0.45 | 0.11* | 0.29 | 0.08* | 0.481 |
| Mean HF hospital length of stay in days | 17.9 | 7.8 | 8.2 | 5.0 | 0.576 |
| Total number of 1 day clinic visits | 16 | 32 | 16 | 10 | 0.673 |
| Mean number of 1 day clinic visits per patient | 0.36 | 0.73 | 0.67 | 0.42 | 0.673 |
| Number of patients with 1 day clinic visits (%) | 13 (29%) | 14 (32%) | 11 (46%) | 5 (21)* | 0.305 |
| Total number of ambulatory visits | 93 | 89 | 39 | 28 | n/a |
| Mean number of ambulatory visits per patient | 2.11 | 1.62 | 2.02 | 1.17 | 0.082 |
CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardiac defibrillator.
Data are presented as mean (±standard deviation) or numbers (%).
P < 0.05.
P < 0.01 for pre‐activation and post‐activation difference within the same subgroup.
Health economic analysis: resource use before and after activation of HeartLogic in the Onze Lieve Vrouw Aalst (Belgian) subgroup
Results of the financial analysis are shown in Tables 4 and 5 . Resource use analysis revealed a substantial drop in overall health economic costs. Including the patients that deceased during follow‐up, a dramatic reduction in total cost, average total cost per patient (P = 0.025), and average hospitalization cost per patient (P = 0.028) after activation of HeartLogic was noted (Table 4 ). Furthermore, there was no significant increase in total ambulatory cost per patient (P = 0.968). The differences in before activation vs. after activation of HeartLogic were even more pronounced if the deceased patients (non‐cardiac deaths) were excluded from the pre‐activation vs. post‐activation analysis (Table 5 ). All subtypes of costs decreased in absolute numbers (hospital per diem cost −€3685, laboratory test −€736 and medical imaging costs −€3362, and other costs −€2175 per patient, respectively).
Table 4.
Overview of the cost per patient (30 patients, including deceased patients)
| Before activation of HL (a) | After activation of HL (b) | Difference (a − b) | P‐values for comparison between groups | |
|---|---|---|---|---|
| Total costs (hospitalization and ambulatory) | €425 057 | €126 311 | €298 746 | — |
| Average total costs per patient | €14 169 | €4210 | €9958 | 0.025 |
| Average hospitalization costs per patient | €12 799 | €2827 | €9972 | 0.028 |
| Average ambulatory costs per patient | €1370 | €1383 | −€14 | 0.968 |
| Hospital per diem costs per patient | €5399 | €1713 | €3685 | 0.120 |
| Laboratory tests costs per patient | €1596 | €860 | €736 | 0.365 |
| Medical imaging costs per patient | €3733 | €372 | €3362 | 0.0001 |
| Other costs per patient | €3441 | €1265 | €2175 | 0.037 |
HL, HeartLogic™.
Table 5.
Overview of the cost per patient (25 patients, excluding deceased patients)
| Before activation of HL (a) | After activation of HL (b) | Difference (a – b) | P‐values for comparison between groups | |
|---|---|---|---|---|
| Total costs (hospitalization and ambulatory) | €254 783 | €47 633 | €207 150 | — |
| Average total costs per patient | €10 191 | €1905 | €8286 | 0.003 |
| Average hospitalization costs per patient | €8902 | €378 | €8523 | 0.003 |
| Average ambulatory costs per patient | €1290 | €1527 | −€237 | 0.535 |
| Hospital per diem costs per patient | €3453 | €233 | €3220 | 0.006 |
| Laboratory tests costs per patient | €610 | €385 | €225 | 0.252 |
| Medical imaging costs per patient | €3655 | €206 | €3449 | 0.0001 |
| Other costs per patient | €2474 | €1081 | €1392 | 0.118 |
HL, HeartLogic™.
Discussion
The main finding of the current study is that activation of the ICD/CRT‐D‐based multisensor algorithm HeartLogic enables effective remote monitoring of HF patients and that after activation a significant reduction in hospitalizations for decompensated HF was observed. In particular, there was a decline in the total number of hospitalizations, a decrease in the number of patients admitted for decompensated HF, and a near‐significant reduction in the length of hospitalization. The beneficial effect on hospitalizations was similar in patients with a recent CRT‐D implantation (potentially with reverse remodelling) and in patients without a recent CRT‐D implantation (i.e. without reverse remodelling), evidencing that HeartLogic substantially contributed to the decline in frequency and duration of HF hospitalizations.
Telemonitoring for HF has been investigated as a tool to reduce mortality and morbidity of HF. Previous studies substantially differed in patient selection, monitoring strategy, and effect of the intervention on outcome. Past studies have evaluated the effect of remote monitoring of intrathoracic impedance, remote monitoring of arrhythmias and device complications, and remote monitoring of signs and symptoms, but the studies showed mixed results. 4 , 5 , 6 , 7 Physiologically, fluid retention reduces intrathoracic impedance and results in increased sympathetic tone (and thus affects heart rate variability). Thus, fluid retention can be detected early by monitoring these markers. 8 In general, however, monitoring of intrathoracic impedance only is insufficient to predict impending decompensation. The distinguishing feature of HeartLogic is that multiple sensors are recorded simultaneously, weighted, and combined into a single alert number. An additional benefit of monitoring device markers is that they are automatically collected; the patient does not need to perform the measurements herself or himself. This likely improves compliance.
The current ESC guidelines on HF state that diuretics are recommended to reduce the signs and symptoms of fluid retention and that the dosage must be adjusted in case of progressive symptoms. 12 However, research has indicated that symptoms are a relatively late indicator of worsening HF. 15 Therefore, increasing the dosage of diuretics after a patient itself reports worsening symptoms and signs of fluid retention is often too late to prevent hospitalization. The MultiSENSE study showed that the HeartLogic algorithm could detect an HF event with a median 34 days in advance. Of note, 2 weeks before an HF event, already 89% of study patients had an alert. Symptoms are thought to occur 2–3 days before a HeartLogic alert, although it is often reported as a dichotomous variable. Given the previously reported false alert rate of 1.5 per patient year of the algorithm, 8 adjusting diuretic treatment purely based on the alert was not preferred, as it may impair kidney function in patients with a false positive alert. However, with the prospect of increasing evidence for the clinical usefulness of HeartLogic, this might be a strategy in the future.
Of note, in our study, the sensitivity of HeartLogic, its negative predictive value, and the unexplained alert rate were in line with the results of the MultiSENSE study. The unexplained alert rate was fairly low, having a positive impact on the practical workload when using HeartLogic in daily clinical practice.
The reduction in hospitalizations for decompensated HF found in this study is most probably—yet not exclusively—related to early detection of fluid retention. Subsequent adjustment of therapeutic regimen (either lifestyle advices or increasing diuretic dose) has likely prevented worsening of fluid accumulation and thus prevented a hospital admission. The shorter duration of the hospitalization probably indicates that fluid overload is diagnosed at an earlier stage.
In line with the clinical results, a concomitant significant reduction in hospitalization costs was noted after activation of HeartLogic. This reduction in cost did not go along with an increase in ambulatory cost. The ambulatory cost after activation of HeartLogic was unaltered. Although these health economic results cannot be interpreted without taking into account the specific Belgian healthcare financing system, the principal message is that in our study, the use of HeartLogic resulted in a significant drop in hospitalizations, which is of benefit for the patient, the healthcare system, and third payer parties.
Despite the currently reported promising results, there remain knowledge gaps regarding the use of HeartLogic in daily practice. The MANAGE‐HF (NCT03237858) trial investigates if diuretic titration can be based purely on the HeartLogic index. Until these results are available, we propose that HeartLogic should be used as an additional diagnostic and follow‐up tool in managing HF patients. In addition, the clinical relevance of the height of the HeartLogic index, as well as the rate of increase of the HeartLogic index, remains to be investigated.
Strengths, limitations, and future research
To the best of our knowledge, this was one of the first studies to structurally evaluate the ability of the HeartLogic algorithm to reduce HF hospitalizations in a real‐world HF population. Some limitations have to be noted when interpreting the results. First, this was not a blinded study and some data were collected retrospectively. As such, researchers and clinicians were aware of a patient's study participation. Second, the protocol was implemented in a continuously evolving healthcare practice. Because of our before and after design, the reduction in HF events might have been augmented or might have been influenced by other variables. The subgroup in patients receiving a CRT‐D device during the pre‐activation period evidenced, however, that the results are not only attributable to CRT. Lastly, as far as the health economic data are concerned, some parameters that might influence the results were not quantified because of practical limitations (e.g. out‐of‐hospital medication use). Yet the pre‐activation and post‐activation differences in resource use were so substantial and across different costing items that it is highly unlikely for these additional factors to influence the results in a meaningful way.
Conclusion
The current study demonstrates that activation of the ICD‐based multisensor algorithm HeartLogic enables remote monitoring of HF patients and that after activation a significant reduction in hospitalizations for decompensated HF was observed going along with substantial reduction in health economic resource use.
Clinical perspectives
Standard ambulatory follow‐up of HF patients has currently failed to risk stratify patients and to avoid decompensation, resulting in considerable morbidity, mortality, and socio‐economic costs. The integration of available device‐based parameters in an alert‐based system allows for an earlier intervention hereby preventing impending HF decompensation, hospitalization, and hospitalization‐related costs.
Translational outlook
Chronic HF continues to increase as a clinical entity, especially in the elderly. Novel methods to follow large cohorts of patients are needed, to take care of these patients in an effective and efficient way. Our paper adds knowledge to the emerging option of remote monitoring of HF patients.
Conflict of interest
R.W.T. reports receiving speaker fees from Boston Scientific. W.A.H. reports that Cardiac Research Centre (CRI) Aalst receives consultancy fees on his behalf from all major device companies (Boston Scientific, Abbott St Jude, MicroPort, Medtronic, and Biotronik).
Funding
W.A.H. reports that Cardiac Research Centre (CRI) Aalst receives an educational and research grant from Boston Scientific Corporation with Reference Number ISRRM11793. The Department of Cardiology of OLV Aalst reports receiving research and educational grants from Boston Scientific Corporation, Medtronic, Biotronik, Abbott Laboratories, and MicroPort. The Department of Cardiology of the LUMC reports receiving unrestricted research and educational grants from Boston Scientific Corporation, Medtronic, and Biotronik.
Treskes, R. W. , Beles, M. , Caputo, M.‐L. , Cordon, A. , Biundo, E. , Maes, E. , Egorova, A. D. , Schalij, M. J. , Van Bockstal, K. , Grazioli‐Gauthier, L. , Vanderheyden, M. , Bartunek, J. , Auricchio, A. , Beeres, S. L. M. A. , and Heggermont, W. A. (2021) Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Failure, 8: 1541–1551. 10.1002/ehf2.13252.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133: 447–454. [DOI] [PubMed] [Google Scholar]
- 2. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, Fonarow GC. Association of the Hospital Readmissions Reduction Program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol 2018; 3: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis EF, Li Y, Pfeffer MA, Solomon SD, Weinfurt KP, Velazquez EJ, Califf RM, Rouleau JL, Kober L, White HD, Schulman KA, Reed SD. Impact of cardiovascular events on change in quality of life and utilities in patients after myocardial infarction: a VALIANT study (valsartan in acute myocardial infarction). JACC Heart failure. 2014; 2: 159–165. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, de Marco T, Escarce JJ, Evangelista LS, Hanna B, Ganiats TG, Greenberg BH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M, Tong K, Fonarow GC, for the Better Effectiveness After Transition—Heart Failure (BEAT‐HF) Research Group . Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the Better Effectiveness After Transition—Heart Failure (BEAT‐HF) randomized clinical trial. JAMA Intern Med 2016; 176: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011; 123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 7. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Søgaard P. Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet 2014; 384: 583–590. [DOI] [PubMed] [Google Scholar]
- 8. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA, Wariar R, Zhang Y, Singh JP. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart failure 2017; 5: 216–225. [DOI] [PubMed] [Google Scholar]
- 9. Klersy C, Boriani G, De Silvestri A, Mairesse GH, Braunschweig F, Scotti V, Balduini A, Cowie MR, Leyva F. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta‐analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail 2016; 18: 195–204. [DOI] [PubMed] [Google Scholar]
- 10. Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, Roberts‐Thomson KC, Young GD, Sanders P, Ganesan AN. Remote monitoring of implantable cardioverter‐defibrillators: a systematic review and meta‐analysis of clinical outcomes. J Am Coll Cardiol 2015; 65: 2591–2600. [DOI] [PubMed] [Google Scholar]
- 11. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu CM, Gerritse B, Borggrefe M, DOT‐HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, Morrow DA, Targum SL, Sila CA, Hai MTT, Jaff MR, Joffe HV, Cutlip DE, Desai AS, Lewis EF, Gibson CM, Landray MJ, Lincoff AM, White CJ, Brooks SS, Rosenfield K, Domanski MJ, Lansky AJ, McMurray J, Tcheng JE, Steinhubl SR, Burton P, Mauri L, O'Connor CM, Pfeffer MA, Hung HMJ, Stockbridge NL, Chaitman BR, Temple RJ. Standardized Data Collection for Cardiovascular Trials Initiative (SCTI). 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018; 137: 961–972. [DOI] [PubMed] [Google Scholar]
- 14. Cleemput I NM, Van de Sande S, Thiry N. Belgian guidelines for economic evaluations and budget impact analyses: second edition. Health Technology Assessment (HTA). KCE Report 183C. 2012;D/2012/10.273/54.
- 15. Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005; 112: 841–848. [DOI] [PubMed] [Google Scholar]


