Abstract
Aims
We sought to clarify the role of ventriculo–arterial (V–A) coupling in the treatment of nonischemic dilated cardiomyopathy (NIDCM) by adding a mineralocorticoid receptor antagonist (MRA) to conventional anti‐failure therapy.
Methods and results
We employed cardiac magnetic resonance imaging to quantify left ventricular (LV) contractility and V–A coupling in normal subjects at rest (n = 11) and in patients with NIDCM (n = 12) before and after long term anti‐failure therapy, in which MRA was added to conventional anti‐failure therapy. After ≥6 months' treatment in NIDCM patients, LV volumes and mass decreased, and the LV ejection fraction increased from a median of 24% (17, 27) (interquartile range IQR) to 47 (42, 52) (P < 0.002), with a marked reduction in arterial elastance (Ea) from 2.89 mmHg/mL (2.34, 4.0) to 1.50 (1.29, 1.95) (P < 0.002), similar to Ea of normal subjects, 1.53 (1.34, 1.67) (P > 0.05). The V–A coupling ratio, Ea/end‐systolic elastance (single‐beat method), decreased by −1.08 (−1.96, −0.55), (P = 0.003), as did Ea/end‐systolic pressure/end‐systolic pressure ratio, −0.54 (0.35, 0.87), (P = 0.002). The preload recruitable stroke work (PRSW) increased as did PRSW indexed for Ea (both P = 0.002), which reflected ‘total circulatory performance’.
Conclusions
In NIDCM, adding MRA to conventional anti‐failure therapy markedly improved LV ejection fraction and reduced peripheral vascular resistance, due to both improved LV contractility and especially to enhanced V–A coupling, as Ea decreased to normal. Total circulatory performance was a sensitive indicator of both LV pump performance and the arterial loading conditions.
Keywords: Nonischemic cardiomyopathy, Ventricular/vascular coupling haemodynamics, Magnetic resonance imaging, Spironolactone
Introduction
Nonischemic dilated cardiomyopathy (NIDCM) is characterized by depressed left ventricular (LV) contractility with adverse LV remodelling, myocardial fibrosis and heart failure (HF) with reduced ejection fraction (HFrEF). 1 This constellation activates compensatory neurohormonal mechanisms that increase systemic vascular resistance (SVR). 2
Standard therapy for HFrEF affects both the LV myocardium and the peripheral circulation. Beta‐adrenergic blocking drugs (BBs), such as carvedilol, improve myocardial structure and function 3 and reduce SVR. 4 Angiotensin‐converting enzyme inhibitors (ACE‐I) reduce perivascular collagen 5 and reduce SVR, 2 reducing unfavourable LV remodelling. 6
Randomized clinical trials in HFrEF patients reduced mortality by treatment with mineralocorticoid receptor antagonist(s) (MRA). 7 Aldosterone causes excess vascular stiffness 8 and stimulates LV interstitial fibrosis, 9 which can be blocked and partly reversed by MRA. 10 , 11 Relatively little attention has been paid to the role of the peripheral vasculature in improving LV performance in HFrEF. 12 , 13 However, MRA up‐regulate endothelial nitric oxide, 14 causing peripheral vascular dilatation and lower SVR, 15 which may contribute to beneficial LV remodelling. 16 , 17
The coupling of LV contractile properties with the peripheral circulation [ventriculo–arterial (V–A) coupling] can be expressed in the pressure–volume plane as the interaction of the LV end‐systolic pressure (ESP) volume relationship (ESPVR) and the systemic arterial elastance (Ea). 18 Other methods for estimating LV performance include the ratio of LV end‐systolic pressure/end‐systolic volume, 19 the single‐beat calculation of LV elastance, 20 preload recruitable stroke work (PRSW), 21 and its single‐beat calculation. 22
We sought to clarify the influence of V–A coupling on changes in LV ejection fraction (LVEF) and indices of LV contractility during the treatment of NIDCM. For this, we used serial cardiac magnetic resonance imaging (CMR) and the above‐mentioned methods in patients with NIDCM before and after adding MRA to conventional anti‐failure therapy with BB and ACE‐I and angiotensin receptor blocking (ARB) drugs. 23 , 24 The CMR method was employed based on its accuracy for quantitating LV mass and volume. 25 , 26
Methods
Study design
We enrolled patients with newly diagnosed NIDCM who were recruited from the Vanderbilt University Medical Center and the Veterans Affairs Tennessee Valley Medical Center. Eligible participants were 18–80 years old, of any ethnic background and either sex, New York Heart Association Functional Class II–IV, with an echocardiographic LVEF of ≤35% and serum potassium level less than 5.0 mmol/L (5 mEq/dL) while on medical therapy for HF (including stable BB and ACE‐I/ARB for ≥3 months). Exclusion criteria were the need for an implantable cardioverter‐defibrillator, evidence of prior myocardial infarction on electrocardiogram, a positive stress test or coronary artery disease with ≥50% stenosis in a major epicardial artery at angiography, severe chronic obstructive airway disease precluding adenosine use, creatinine >220 μmol/L (2.5 mg/dL), glomerular filtration rate <30 mL/min/1.73m2, uncontrolled atrial fibrillation, current spironolactone therapy, and physician preference. Of 590 screened clinic visits, 16 patients who met the inclusion and exclusion criteria were recruited. Among these, four did not complete the study due to erroneous inclusion of one patient, bronchoconstriction from adenosine used for CMR (n = 1), implantation of implantable cardioverter‐defibrillator (n = 1), and patient withdrawal (n = 1), leaving a total of 12 patients who completed the study protocol. Significant coronary atherosclerosis was excluded by coronary angiography (n = 10) or the absence of ischemia or infarction on nuclear stress perfusion imaging (n = 2).
The NIDCM patients underwent a 5 day trial of spironolactone to assess tolerability. After this drug was discontinued, the patients underwent CMR at baseline and after ≥6 months of spironolactone therapy added to ACE‐I/ARB and BB anti‐failure treatment. The CMR protocol is described later. The patients took spironolactone 25 mg daily for ≥6 months, up‐titrated to 50 mg daily if possible. The doses of ACE‐I/ARB and BB drugs remained constant. Eleven normal subjects were recruited by advertisement and had no history of hypertension, diabetes mellitus, or heart disease. They were studied at rest at a single time point using similar CMR technique.
This investigation conforms with the principles outlined in the Declaration of Helsinki. The NIDCM study was approved by both the Vanderbilt and Nashville Veterans Affairs Institutional Review Boards. The normal subject study was approved by the Vanderbilt Institutional Review Board. All patients provided written informed consent.
Cardiac magnetic resonance imaging acquisition and analysis
Cardiac magnetic resonance imaging was performed using a commercially available 1.5‐T Siemens Magnetom Avanto scanner (Erlangen, Germany) using methods previously published. 23 During CMR, the blood pressure was measured using an magnetic resonance imaging compatible automated cuff sphygmomanometer, and heart rate was recorded from telemetry.
Data analysis
Cardiac magnetic resonance imaging
The end‐diastolic and end‐systolic endocardial borders on CMR were outlined using system software (Argus software Version B17, Siemens, Erlangen, Germany) (NIDCM patients) or Medis software (normal subjects) and adjusted manually as needed to calculate LVEF. The end‐diastolic volume (EDV), end‐systolic volume (ESV) and stroke volume (SV), were divided by body surface area, yielding these indices (mL/m2). Simplified pressure–volume diagrams were constructed from CMR data and the noninvasive blood pressure values for each patient.
Echocardiography acquisition and analysis
All subjects underwent standard two‐dimensional, M‐mode, and Doppler transthoracic echocardiography according to American Society of Echocardiography guidelines. Imaging was performed using Phillips IE33 echocardiographs using standard transducers. For calculation of ENd(est), the ratio of the pre‐ejection period (PEP) to the total systolic period (Q‐S2 interval) was determined using Doppler tracings of the LV outflow tract. Among normal subjects, SV was calculated using the LV outflow tract diameter and velocity time integral. 27
Indices of contractility
End‐systolic pressure
The ESP (Pes) was calculated as 0.9 × systolic blood pressure (SBP). 28
End‐systolic elastance (single‐beat method)
A single‐point estimate of the LV contractile state was calculated as
Ees (sb) = [Pd − (ENd (est) × Ps × 0.9)]/[SV × ENd (est)], 20
in which Ees(sb) = end‐systolic elastance using a single‐beat method (SBM) (mmHg/mL), Pd = systemic arterial diastolic pressure (mmHg), Ps = systolic arterial pressure (mmHg), SV = stroke volume (mL), and ENd(est) = group averaged LV elastance at the onset of ejection. To calculate ENd(est), the ratio of the PEP to the total systolic period (Q‐S2 interval) was determined using Doppler tracings of the LV outflow tract on echocardiograms obtained as baseline or follow‐up studies. These data were available in 10 of 12 HF patients at baseline and for five patients who had echocardiograms within 1.5 years after completing the study. The mean values for PEP and Q‐S2 were applied to the remaining HF patients. All normal subjects had echocardiograms within 6 hours of their CMR study.
Magnetic resonance imaging was employed to calculate SV instead of echocardiography as performed previously. 20 To evaluate our CMR adaptation, in 10 normal subjects, echocardiography was employed for estimating PEP, Q‐S2, and SV, and results were compared with end‐systolic elastance (single‐beat method) [Ees (sb)] using CMR SV.
End‐systolic pressure/end‐systolic volume ratio
The ESP/end‐systolic volume (P/V) ratio was calculated as Pes/Ves. 4
End‐systolic volume pressure of 100 mmHg
The ESV at a pressure of 100 mmHg (End‐systolic volume at pressure 100 mmHg) was calculated as an expression of contractility, needing minimal extrapolation, if any, from measured values, similar to the work of others. 29
Stroke work
This was calculated as stroke work (SW) = SV × MAP, 21 where MAP = mean systemic arterial pressure (mmHg).
Preload recruitable stroke work
This was expressed as Mw = SW/EDV − Vw, where Vw is the X‐axis intercept of this slope and Mw is the slope of the relation, 21 employing a single beat method (SBM), SBMw. 22
Systemic arterial properties
Total systemic vascular resistance
This was calculated as total systemic vascular resistance (TVR) = 80 × MAP/CO (dyn s cm−5), where CO = cardiac output (L/min), converted to Wood units (mmHg‐min/L).
Arterial elastance
The Ea was expressed as Pes/SV. 18
Ventriculo–arterial coupling
Arterial elastance/end‐systolic elastance (single‐beat method)
The V–A coupling ratio expressed as the relation of Ea and LV contractility expressed as Ees (sb), 18 Ea/Ees(sb).
Arterial elastance/end‐systolic pressure/end‐systolic volume ratio
The V–A coupling ratio expressed as the relation of Ea and LV contractility using the end‐systolic Pes/Ves ratio 4 Ea/[P/V].
Total circulatory performance
To evaluate the hypothesis that Ea is incorporated in PRSW, 30 , 31 we compared its main component, SW/EDV, with SBMw and then indexed the result for Ea by comparing the relation of [(SW/EDV)/Ea] to SBMw. Incorporating Ea was considered an index of total circulatory performance (TCP) that reflected both LV contractility and arterial loading conditions.
Statistical analysis
Descriptive statistics are presented as median (25th to 75th percentile) or counts (percentages). Either the Wilcoxon rank‐sum or Fisher's exact test was used for comparisons between normal subjects and patients with NIDCM. For paired data examining changes in parameters of cardiac structure and function before and after spironolactone, the Wilcoxon signed‐rank test was used. Comparisons of the slopes of the relationships between SW/EDV (or [SW/EDV]/Ea) and SBMw before and after MRA therapy were performed using linear regression with an interaction term with robust adjustment. Differences between the slopes of SW/EDV to SBMw and [SW/EDV]/Ea to SBMw were assessed using the ‘suest’ package in Stata. Analyses were performed using Stata v15.0 (Stata Corp., Austin, TX, USA).
Results
Baseline characteristics
Selected baseline data of each patient group are listed in Tables 1 and 2 . Other details of the NIDCM patients were published previously. 23 The NIDCM and normal subjects were of similar ages. Before study entry, the median duration of taking a BB and either an ACE‐I or ARB drug was 21 weeks, and the median duration on stable doses of BB was 16 weeks. The baseline CMR study was at a median of 10 weeks (IQR 6–16) after the screening echocardiogram that met inclusion criteria. There was a strong correlation between Ees (sb) determined by echocardiography alone compared with Ees (sb) using CMR for SV (R 2 = 0.79, P = 0.0006).
Table 1.
Characteristics at time of baseline CMR
| NIDCM N = 12 | Normal N = 11 | P | |
|---|---|---|---|
| Age (years) | 52 (45, 55) | 55 (52, 58) | 0.039 |
| Male | 8 (67%) | 3 (27%) | 0.10 |
| Medications | |||
| Beta blocker | 12 (100%) | 0 (0%) | |
| ACE‐I or ARB | 12 (100%) | 0 (0%) | |
| Diuretic | 7 (58%) | 0 (0%) | |
| BMI (kg/m2) | 30.0 (25.7, 34.3) | 24.7 (21.0, 27.8) | 0.023 |
| BSA (m2) | 2.07 (1.85, 2.33) | 1.63 (1.56, 2.01) | 0.019 |
| NYHA class | |||
| 1 | 0 (0%) | 7 (100%) | |
| 2 | 5 (42%) | 0 (0%) | |
| 3 | 7 (58%) | 0 (0%) | |
| Heart rate (bpm) | 67 (58, 71) | 62 (57, 68) | 0.48 |
| Systolic BP (mmHg) | 121 (115, 125) | 120 (113, 125) | 0.62 |
| Diastolic BP (mmHg) | 67 (63, 73) | 72 (67, 82) | 0.25 |
| MAP (mmHg) | 84 (80, 90) | 87 (82, 95) | 0.39 |
Median (interquartile range). ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocking drug; BMI, body mass index; BP, blood pressure; bpm, beats per minute; BSA, body surface area; CMR, cardiac magnetic resonance imaging; MAP, mean arterial pressure; mmHg, millimetres Mercury; NIDCM, nonischemic dilated cardiomyopathy; NYHA, New York Heart Association.
Table 2.
Baseline parameters of LV performance and ventriculo–arterial coupling
| NIDCM N = 12 | Normal N = 11 | P | |
|---|---|---|---|
| LVEF (%) | 24 (17, 27) | 66 (62, 67) | <0.001 |
| LVEDVI (mL/m2) | 82 (74, 91) | 63 (55, 75) | 0.002 |
| LVESVI (mL/m2) | 63 (56, 78) | 19 (17, 28) | <0.001 |
| LVSVI (mL/m2) | 19 (14, 21) | 41 (37, 47) | <0.001 |
| LV mass (g) | 169 (154, 203) | 69 (49, 77) | <0.001 |
| TVR (Wood units) | 2.69 (2.23, 3.85) | 1.48 (1.38, 1.75) | <0.001 |
| Ea | 2.89 (2.34, 4.00) | 1.53 (1.34, 1.67) | <0.001 |
| Ees (sb) | 0.96 (0.75, 1.43) | 1.44 (1.26, 1.64) | 0.016 |
| Ea/Ees (sb) | 3.08 (2.54, 3.78) | 0.95 (0.91, 1.11) | <0.001 |
| Pes/Ves | 0.83 (0.65, 0.95) | 3.04 (2.63, 3.49) | <0.001 |
| V0 | 16 (−14, 38) | −38 (−39, −24) | 0.001 |
| ESV‐100 | 121 (104, 148) | 30 (27, 37) | <0.001 |
| SBMw | 35 (24, 45) | 113 (101, 119) | <0.001 |
| TCP | 7.4 (3.4, 11.4) | 40.2 (35.2, 43.8) | <0.001 |
| Ea/[Pes/Ves] | 3.24 (2.65, 4.83) | 0.51 (0.49, 0.60) | <0.001 |
| Vw | 71.3 (60.2, 90.2) | 51.1 (49.2, 56.4) | 0.012 |
Median (interquartile range). Pairwise comparisons between groups using Wilcoxon rank sum test, with Bonferroni corrected threshold P < 0.0166 for significance. Ea, arterial elastance (mmHg/mL); Ea/[Pes/Ves], arterial elastance/end‐systolic pressure/end‐systolic volume ratio; Ees (sb), end‐systolic elastance (single‐beat method) (mmHg/mL); ESV‐100, end‐systolic volume at 100 mmHg (mL); LV, left ventricular; LVEDVI, LV end‐diastolic volume index; LVEF, LV ejection fraction; LVESVI, LV end‐systolic volume index; LVSVI, LV stroke volume index; Pes/Ves, end‐systolic pressure/end‐systolic volume ratio; SBMw, slope of preload recruitable stroke work relation (single‐beat method) (erg.cm−3 103); TCP, total circulatory performance (stroke work/end‐diastolic volume/Ea); TVR, total vascular resistance; V0, end‐systolic volume at SBP 0 mmHg (mL); Vw, LV volume at Stroke Work 0.
Left ventricular remodelling in nonischemic dilated cardiomyopathy
Following ≥6 months of spironolactone therapy (median 45 weeks, IQR 27–74) CMR showed evidence for beneficial remodelling, with significant reductions in LV EDVI and ESVI, associated with an increase in SVI and LVEF (Figure 1 , Table 3 ).
Figure 1.
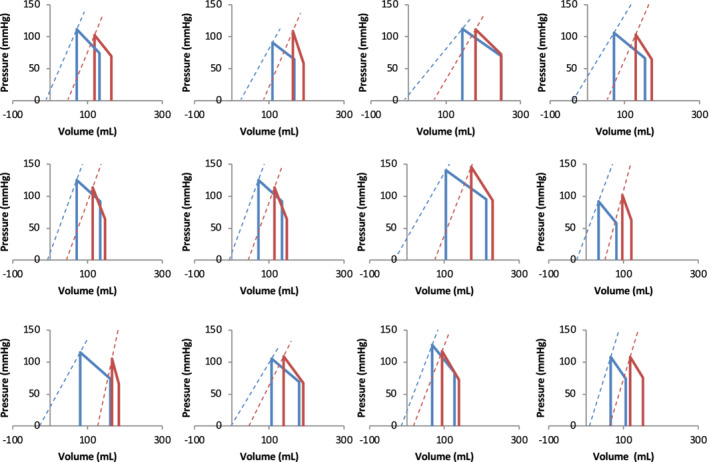
Pressure–volume relations of patients with nonischemic dilated cardiomyopathy before (red) and after anti‐failure therapy (blue). Dashed lines show Ees (sb) and calculated V0. ESV‐100, end‐systolic volume pressure of 100 mmHg; LVEF, left ventricular ejection fraction; P/V, end‐systolic pressure/end‐systolic volume; SBM, single‐beat method.
Table 3.
LV performance and ventriculo–arterial coupling at baseline and after adding mineralocorticoid receptor antagonist in NIDCM patients
| Baseline | Follow‐up | Change | P | |
|---|---|---|---|---|
| Heart rate (bpm) | 67 (58, 71) | 72 (65, 76) | 5 (3, 12) | 0.021 |
| Systolic BP (mmHg) | 121 (115, 125) | 122 (112, 134) | 2 (−5, 11) | 0.46 |
| Diastolic BP (mmHg) | 67 (63, 73) | 72 (65, 79) | 4 (−1, 10) | 0.059 |
| MAP (mmHg) | 84 (80, 90) | 89 (81, 97) | 1 (−1, 10) | 0.21 |
| LVEF (%) | 24 (17, 27) | 47 (42, 52) | 22 (15, 34) | 0.002 |
| LVEDVI (mL/m2) | 82 (74, 91) | 74 (59, 82) | −8 (−14, −5) | 0.003 |
| LVESVI (mL/m2) | 63 (56, 78) | 36 (31, 46) | −24 (−32, −19) | 0.002 |
| LVSVI (mL/m2) | 19 (14, 21) | 36 (28, 40) | 15 (9, 19) | 0.002 |
| LV mass (g) | 169 (154, 203) | 151 (138, 190) | −12 (−21, −6) | 0.008 |
| TVR (Wood units) | 2.69 (2.23, 3.85) | 1.34 (1.15, 1.87) | −1.17 (−2.06, −0.73) | 0.002 |
| Ea | 2.89 (2.34, 4.00) | 1.50 (1.29, 1.95) | −1.19 (−2.26, 0.53) | 0.002 |
| Ees (sb) | 0.96 (0.75, 1.43) | 0.79 (0.69, 1.11) | −0.10 (−0.35, 0.02) | 0.12 |
| Vo ml | 16 (−14, 38) | −56 (−73, −37) | −70 (−102, −34) | 0.003 |
| Vw | 71.3 (60.2, 90.2) | 61.7 (47.0, 79.0) | −11.9 (−18.1, −4.4) | 0.004 |
| ESV‐100 | 121 (104, 148) | 60 (52, 82) | −54 (−63, −39) | 0.002 |
| Ea/Ees (sb) | 3.08 (2.55, 3.78) | 1.89 (1.75, 2.07) | −1.08 (−1.96, −0.55) | 0.003 |
| Pes/Ves | 0.83 (0.65, 0.95) | 1.52 (1.17, 1.70) | 0.70 (0.35, 0.79) | 0.002 |
| SBMw | 35.1 (24.0, 44.7) | 74.1 (61.5, 77.5) | 31.0 (25.8, 54.2) | 0.002 |
| TCP | 7.4 (3.4, 11.4) | 25.0 (22.7, 33.9) | 19.3 (13.0, 28.9) | 0.002 |
| Ea/[Pes/Ves] | 3.24 (2.65, 4.83) | 1.12 (0.91, 1.4) | 0.54 (0.35, 0.87) | 0.002 |
Median (interquartile range). Ea, arterial elastance (mmHg/mL); Ea/[Pes/Ves], arterial elastance/end‐systolic pressure/end‐systolic volume ratio; Ees (sb), end‐systolic elastance (single‐beat method) (mmHg/mL); ESV‐100, end‐systolic volume at 100 mmHg (mL); LV, left ventricular; LVEDVI, LV end‐diastolic volume index; LVEF, LV ejection fraction; LVESVI, LV end‐systolic volume index; LVSVI, LV stroke volume index; Pes/Ves, end‐systolic pressure/end‐systolic volume ratio; SBMw, slope of preload recruitable stroke work relation (single‐beat method) (erg.cm−3 103); TCP, total circulatory performance (stroke work/end‐diastolic volume/Ea); TVR, total vascular resistance; V0, end‐systolic volume at SBP 0 mmHg (mL); Vw, LV volume at Stroke Work 0.
Indices of contractility
There were significant changes in the P/V ratio, ESV‐100, SW/EDV, and SBMw, all consistent with improved contractility, except for Ees (sb) (Figure 2 , Table 3 ). At baseline, Ees (sb) was 0.96 (0.75, 1.43) mmHg/mL, which was less than in our normal subjects and normal values in the literature, 20 , 32 and was 0.79 (0.69, 1.11) at follow‐up (P = 0.12). With a decrease in ESV, the calculated V0 shifted leftward.
Figure 2.
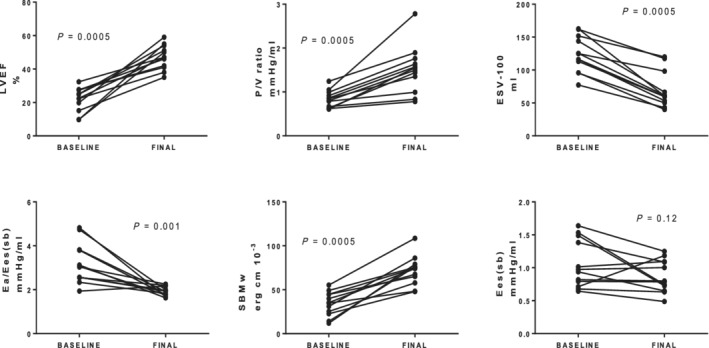
Estimates of left ventricular contractility before and after anti‐failure therapy. Please see text for abbreviations.
Peripheral circulation
There was a strong correlation between Ea and TVR in NIDCM patients (R 2 = 0.85, P < 0.0001). The Ea of NIDCM patients was 2.89 (2.34, 4.00) mmHg/mL at baseline, and as SV increased, Ea declined sharply to 1.50 (1.29, 1.95) at follow‐up (P = 0.002).
Ventriculo–arterial coupling
Arterial elastance/end‐systolic elastance (single‐beat method)
At baseline, patients with NIDCM, compared with normal subjects, had a greater V–A coupling ratio, Ea/Ees (sb), due to greater Ea and lower Ees (sb). At follow‐up, Ea declined sharply to a value similar to normal subjects (P > 0.05) (Tables 2 and 3 ) because of the increase in SV. However, the V–A coupling ratio, Ea/Ees (sb), was still greater than in normal subjects (P < 0.001) (Tables 2 and 3 ).
Arterial elastance/end‐systolic pressure/end‐systolic volume ratio
At baseline, patients with NIDCM had a similarly greater V–A coupling ratio compared with normal subjects (Table 2 ). There was a marked decrease in in this value at follow‐up (Table 3 , Figure 3 ), although this measure of V–A coupling remained greater than normal (P < 0.001).
Figure 3.
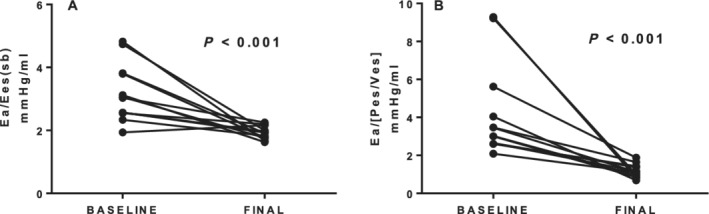
Changes in ventriculo–arterial coupling using two definitions of left ventricular contractility. (A). Arterial elastance/end‐systolic elastance (single‐beat method) [Ea/Ees (sb)]. (B). Arterial elastance/end‐systolic pressure/end‐systolic volume ratio (Ea/[Pes/Ves]). ESV, end‐systolic volume; SBM, single‐beat method.
Total circulatory performance
At baseline, TCP was markedly different between NIDCM and normal subjects, but lay along the same regression line (Figure 4 ). At baseline, in the NIDCM patients, the slope of the relation between [SW/EDV] and Mw was similar to the slope of the relation when indexed for Ea (Figure 5 , Panel A vs. C) (P = 0.61). After MRA, the values of [SW/EDV] increased markedly, but the slope of the [SW/EDV] relation to Mw remained similar to baseline (Panel A vs. B) (P = 0.254). In contrast, however, after MRA, the slope of the relation of [SW/EDV]/Ea to Mw was significantly greater than without this adjustment (Panel B vs. D) (P = 0.003) and significantly greater than its baseline value (Panel D vs. C) (P = 0.033).
Figure 4.
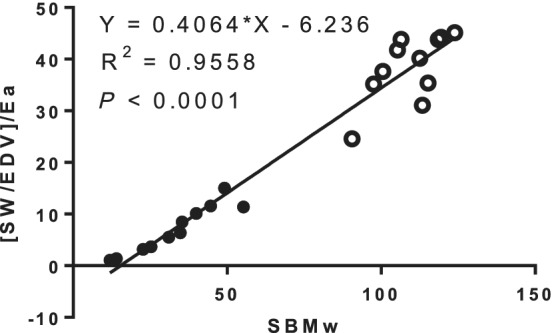
Relation between preload recruitable stroke work as SBMw vs. [SW/EDV]/Ea in patients with nonischemic dilated cardiomyopathy and normal subjects. The values are markedly different between the two groups but the relations are similar. Closed circles: nonischemic dilated cardiomyopathy. Open circles: normal. ESV, end‐systolic volume; SBM, single‐beat method.
Figure 5.
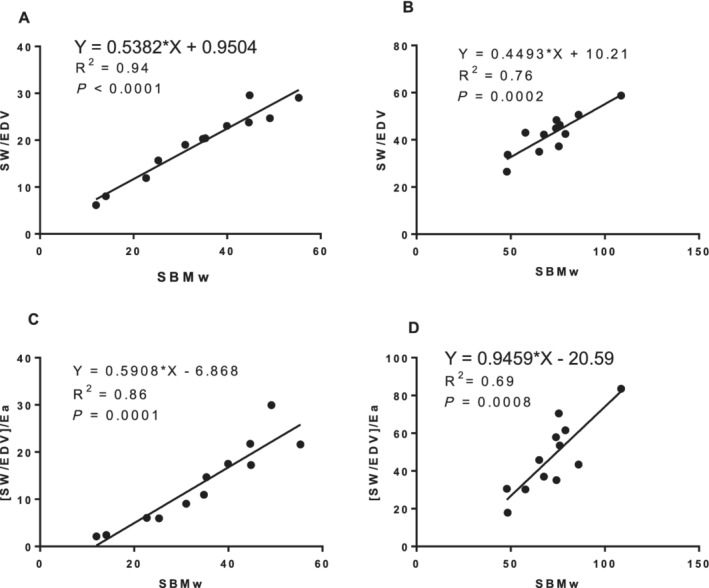
Preload recruitable stroke work (SW) (PRSW) as SBMw in patients with nonischemic dilated cardiomyopathy. Comparison of SBMw and SW/EDV before (A) and after adding mineralocorticoid receptor antagonist (MRA) (B). Comparison of SBMw and SW/EDV adjusted for peripheral vascular elastance (Ea) (TCP) before (C) and after adding MRA (D). PRSW increased markedly after adding MRA (Panel A vs. B). Increased slope of the [(SW/EDV)/Ea]‐SBMw relation after MRA demonstrated both increase in PRSW and sensitivity to Ea (Panel B vs. D). Please refer to the text for details. EDV, end diastolic volume; SBM, single‐beat method.
Discussion
Among patients with NIDCM, the addition of MRA to BB and ACE‐I/ARB produced beneficial LV remodelling, 23 greater LV contractility, and normalization of Ea, indicating strongly enhanced V–A coupling, all leading to a marked improvement in LVEF.
Aldosterone and ventriculo–arterial coupling in heart failure
Randomized clinical trials in HFrEF patients have demonstrated reductions in mortality rates with MRA, 7 likely due to reducing the effects of circulating aldosterone. The serum aldosterone and the aldosterone/renin ratio are significant factors in peripheral vascular stiffness, 8 and spironolactone can improve this by up‐regulating arterial nitric oxide synthase 33 and reducing endothelial dysfunction. 14 , 34
The V–A coupling has been studied in untreated patients with NIDCM and normal subjects, with greater Ea/Ees in NIDCM (2.02 vs. 0.46). 32 At baseline, our results were similar. Three prior studies examined V–A coupling after long‐term anti‐failure therapy including MRA. These found 18–42% decreases in the V–A coupling ratio, but with variability in the estimates of LV contractility. 4 , 12 , 13 In the present study, we found a sharp decrease in Ea, but no change in Ees (sb), producing an average 32% decrease in Ea/Ees (sb).
Beneficial left ventricular remodelling compared with peripheral vascular effects
Anti‐failure therapy has direct myocardial effects. BB drugs benefit myocardial metabolism. 3 ACE‐I reduce myocardial inflammation, vascular remodelling, 5 and the rate of adverse LV remodelling. 6 Spironolactone inhibits myocardial collagen synthesis. 11
However, even lacking myocardial effects, drugs such as nitroprusside can reduce SVR, 35 increase SV, and decrease ESV, thus increasing LVEF. 21 Similarly, spironolactone enhances peripheral vascular dilatation both in animal models and in patients. 14 , 34 Thus, LVEF could, in theory, improve by reducing SVR, as seen here, without improving LV contractility. In fact, however, there was both a decrease in SVR and an increase in contractility.
Classic experiments in the isolated supported heart show that ESPVR is affected little by the arterial characteristics 18 but LV performance, as judged by SV and SW, increases with lower SVR, 18 which is also supported by an analytical model. 36 ‘Optimal’ LV performance depends on the clinical context. Thus, greater SV might be the goal in treating HFrEF, rather than SW, although greater SW is associated with greater LV mechanical efficiency. 36 , 37
Parameters of contractility in NIDCM
Several parameters of contractility (P/V, ESV‐100, V0, and PRSW), suggested an increase in contractility, except for Ees (sb). All these indices depend on SV, which increased greatly as the SVR decreased. The equation for Ees (sb) (refer to Methods) shows the marked influence of SV on this, because SBP did not change significantly (median difference 1 mmHg) but SVI increased markedly, yielding no increase in Ees (sb). Thus, it is likely that Ees (sb) is inadequate to estimate LV contractility in this situation. Similar to the present study, Maurer et al. 4 analysed the effects of carvedilol on patients with HFrEF and found an increase in Pes/Ves, an increase in SV, and a significant decrease in Ea/[Pes/Ves] in responders to carvedilol. Dekleva et al. analysed elderly patients in the CIBIS study and found similar results to ours, including no change in Ees (sb), which they termed Elv. 38
There may be other influences on the Ees (sb) result. Possibly, the myocardial mechanism of ‘shortening deactivation’ is responsible for the Ees (sb) result, based on experiments finding lower ESPVR with reduced afterload 29 and lower ESP as SV increased. 39 Others found lower ESP on ejecting beats than isovolumic beats, 40 , 41 and that LV pressure is reduced at greater flow rates, termed ‘flow‐induced deactivation’. 42 The Ees (sb) might be affected by these LV mechanical properties or also by reflex feedback. 43 Consistent with our results using Ees (sb), afterload reduction with nitroprusside had no effect on another single‐beat estimate of contractility. 44
Incorporation of peripheral vascular resistance into analysis of left ventricular contractility
Preload recruitable stroke work
The PRSW, expressed as SBMw, increased sharply with MRA, consistent with an increase in LV contractility (Table 3 , Figure 4A,B ) but with no significant difference in the slopes of the relations between SW/EDV and SBMw after treatment. PRSW is a highly linear index of LV contractility in the intact dog model with autonomic blockade, 21 not affected by phenylephrine or nitroprusside infusions. Its linearity is thought due to incorporating Ea into PRSW, 30 , 31 where, mathematically, a linear SW–EDV relation could only exist if there is autoregulation in which Ea changes with ED volume. 30 McClain et al. concluded that PRSW was a descriptor of TCP, rather than only intrinsic myocardial performance. 31 The mechanism of such autoregulation is unclear because beta‐adrenergic blockade and atropine were employed in these studies. 21 , 31
Total circulatory performance
In this study, we intentionally incorporated Ea into the analysis of SW/EDV vs. SBMw, and there was a much steeper slope of the [SW/EDV]/Ea relation to SBMw following anti‐failure therapy (which we termed total circulatory performance, TCP) (Figure 4C vs. Figure 4D ). This is likely due to both greater SV and reduced Ea and contrasts with the lack of change in the slope of SBMw (Figure 5A vs. Figure 5B ), in spite of the marked decrease in Ea. This direct incorporation of Ea into the [SW/EDV] relation demonstrated both increased contractility and the importance of Ea in TCP. The results of PRSW and TCP in the treatment of actual patients with HFrEF are a step beyond prior analyses in animal models.
Possible effects of fibrosis on left ventricular performance
The large increase in LVEF in our patients with NIDCM was greater than in other studies of HFrEF using MRA. 12 , 13 This may be a specific attribute of our study population, compared with chronic NIDCM. 1 Our patients had a relatively short duration of HF symptoms, no replacement fibrosis, and little interstitial fibrosis. 45 Other studies of MRA in HFrEF have not documented the duration of HF symptoms or the amount of interstitial fibrosis. 45
Limitations
The fewer echocardiograms carried out after the follow‐up CMR study were a potential limitation for not having paired PEP and the total duration of systole (QS2 interval) when calculating Ees (sb) for all patients. However, this was of minimal importance, because the main determinants of Ees (sb) are SBP, SV, and the complex regression equation that specifies End, as shown in the formula (Methods). The total duration of systole is similar in normal and HF patients, while PEP is longer and LVET is shorter (the latter consistent with reduced SV in HF). 46 The mean difference between PEP in HF patients and normal subjects is 42 ms, which is 30% greater than normal. 46 We calculated the effect of PEP on Ees (sb), using our results for SBP and SV at follow‐up. Even including a very significant 10% decrease in PEP, concordant with much improved contractility, would change Ees (sb) by only 0.1 mmHg/mL, a minimal difference. These factors support our Ees (sb) results.
The calculation of Ees (sb) led to a decrease in V0 to negative values in most patients. While this shift is consistent with improved contractility, we did not emphasize this because the LV ESPVR is likely curvilinear at non‐physiological low pressures. 29
Others have studied V–A coupling in HF by measuring pulsatile arterial loading. 47 But nevertheless, even with simpler methods, the present study showed highly significant changes in V–A coupling. We studied a relatively small group of patients, but CMR is very accurate for defining LV volume 25 and shows significant changes using fewer subjects. 48
Conclusions
Adding MRA to anti‐failure therapy with BB and ACE‐I/ARB drugs, produced a marked improvement in LVEF due primarily to reduced peripheral vascular resistance, reflected by Ea, with contributions from beneficial LV remodelling and improved LV contractility. It is important to judge the effects treating HFrEF in patients with NIDCM by using indices such as Ea/Ees (sb), PRSW and TCP that reflect V–A coupling. The parameter, TCP, best expressed both the improvement in LV pump performance and the reduction in arterial load. Future studies of the treatment of NIDCM prior to replacement fibrosis may allow similar results.
Clinical trials registration
https://register.clinicaltrials.gov/Clinical Trials. Gov ID NCT00574119.
Conflict of interest
None declared.
Funding
This work was supported in part by a Discovery Grant from the Vanderbilt University Medical Center grant UL1RR024975 NCRR/NIH award UL1TR000445) from the National Center for Advancing Translational Sciences; (U01HL100398) (Dr Sawyer); Astellas Pharmaceuticals (grant REGA‐14E03); National Institutes Health (grant K23 HL128928) (Dr Gupta); (T32 HL 07411‐31); National of Health/National of Child Health and Human Development (K12 HD 043483‐11); National Institute on Aging) (Paul B. Beeson K23AG048347 award), and Eisenstein Women's Heart Fund (Dr Bell).
Acknowledgements
The authors thank Adam Stein, AS, RT; Francesca Sabo, BS, RT; Donald CiFelli, BS, RT; Janine E. Belote, Sr CNMT, RT (CT); Joseph Forrester, Sr CNMT, RT (CT); Barbara Konz, RN; Amber Brock, RN; Debra Rassel, RN; Linda Howerton, RN; and Brenda White, RN for their contributions to this study.
Lawson, M. A. , Hansen, D. E. , Gupta, D. K. , Bell, S. P. , Adkisson, D. W. , Mallugari, R. R. , Sawyer, D. B. , Ooi, H. , and Kronenberg, M. W. (2021) Modification of ventriculo–arterial coupling by spironolactone in nonischemic dilated cardiomyopathy. ESC Heart Failure, 8: 1156–1166. 10.1002/ehf2.13161.
From the Division of Cardiovascular Medicine, Vanderbilt University School of Medicine and the Cardiology Section, VA Tennessee Valley Healthcare System, Nashville, TN. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1. Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, Todiere G, Srebot V, Passino C, Aquaro GD, Emdin M, Lombardi M. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast‐enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging 2013; 6: 790–799. [DOI] [PubMed] [Google Scholar]
- 2. Faxon DP, Halperin JL, Creager MA, Gavras H, Schick EC, Ryan TJ. Angiotensin inhibition in severe heart failure: acute central and limb hemodynamic effects of captopril with observations on sustained oral therapy. Am Heart J 1981; 101: 548–556. [DOI] [PubMed] [Google Scholar]
- 3. Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta‐blocking agents. N Engl J Med 2002; 346: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 4. Maurer MS, Sackner‐Bernstein JD, El‐Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail 2009; 2: 189–196. [DOI] [PubMed] [Google Scholar]
- 5. Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, Diez J, Drexler H, Ferrari R, van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Luscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. The relevance of tissue angiotensin‐converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol 2001; 88: 1l–20l. [DOI] [PubMed] [Google Scholar]
- 6. Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long‐term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation 1992; 86: 431–438. [DOI] [PubMed] [Google Scholar]
- 7. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 8. Lieb W, Larson MG, Benjamin EJ, Yin X, Tofler GH, Selhub J, Jacques PF, Wang TJ, Vita JA, Levy D, Vasan RS, Mitchell GF. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation 2009; 119: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber KT. Aldosterone in congestive heart failure. N Engl J Med 2001; 345: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 10. Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation 2005; 112: 2940–2945. [DOI] [PubMed] [Google Scholar]
- 11. Brilla CG, Zhou G, Matsubara L, Weber KT. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 1994; 26: 809–820. [DOI] [PubMed] [Google Scholar]
- 12. Bozkurt B, Bolos M, Deswal A, Ather S, Chan W, Mann DL, Carabello B. New insights into mechanisms of action of carvedilol treatment in chronic heart failure patients—a matter of time for contractility. J Card Fail 2012; 18: 183–193. [DOI] [PubMed] [Google Scholar]
- 13. Vizzardi E, Sciatti E, Bonadei I, D'Aloia A, Tartiere‐Kesri L, Tartiere JM, Cohen‐Solal A, Metra M. Effects of spironolactone on ventricular–arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clinical res cardiology: official j German Cardiac Soc 2015; 104: 1078–1087. [DOI] [PubMed] [Google Scholar]
- 14. Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000; 101: 594–597. [DOI] [PubMed] [Google Scholar]
- 15. Abiose AK, Mansoor GA, Barry M, Soucier R, Nair CK, Hager D. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol 2004; 93: 1564–1566. [DOI] [PubMed] [Google Scholar]
- 16. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Matsui T, Kinoshita M. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001; 37: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 17. Chan AK, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WW, Wong JT, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol 2007; 50: 591–596. [DOI] [PubMed] [Google Scholar]
- 18. Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983; 245: H773–H780. [DOI] [PubMed] [Google Scholar]
- 19. Kronenberg MW, Uetrecht JP, Dupont WD, Davis MH, Phelan BK, Friesinger GC. Intrinsic left ventricular contractility in normal subjects. Am J Cardiol 1988; 61: 621–627. [DOI] [PubMed] [Google Scholar]
- 20. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol 2001; 38: 2028–2034. [DOI] [PubMed] [Google Scholar]
- 21. Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC Jr, Rankin JS. Linearity of the Frank–Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 1985; 71: 994–1009. [DOI] [PubMed] [Google Scholar]
- 22. Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single‐beat technique in humans. Am J Physiol Heart Circ Physiol 2003; 284: H744–H750. [DOI] [PubMed] [Google Scholar]
- 23. Bell SP, Adkisson DW, Lawson MA, Wang L, Ooi H, Sawyer DB, Kronenberg MW. Antifailure therapy including spironolactone improves left ventricular energy supply‐demand relations in nonischemic dilated cardiomyopathy. J Am Heart Assoc 2014; 3: pii: e000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradham WS, Bell SP, Adkisson DW, Smith HM, Harrell FE Jr, Lawson MA, Ooi H, Sawyer DB, Kronenberg MW. Myocardial T1 measurement predicts beneficial LV remodeling after long‐term heart failure therapy. J Card Fail 2017; 23: 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation 1990; 82: 154–163. [DOI] [PubMed] [Google Scholar]
- 26. Allison JD, Flickinger FW, Wright JC, Falls DG 3rd, Prisant LM, VonDohlen TW, Frank MJ. Measurement of left ventricular mass in hypertrophic cardiomyopathy using MRI: comparison with echocardiography. Magn Reson Imaging 1993; 11: 329–334. [DOI] [PubMed] [Google Scholar]
- 27. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 28. Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992; 86: 513–521. [DOI] [PubMed] [Google Scholar]
- 29. van der Velde ET, Burkhoff D, Steendijk P, Karsdon J, Sagawa K, Baan J. Nonlinearity and load sensitivity of end‐systolic pressure–volume relation of canine left ventricle in vivo. Circulation 1991; 83: 315–327. [DOI] [PubMed] [Google Scholar]
- 30. Takaoka H, Suga H, Goto Y, Hata K, Takeuchi M. Cardiodynamic conditions for the linearity of preload recruitable stroke work. Heart Vessels 1995; 10: 57–68. [DOI] [PubMed] [Google Scholar]
- 31. McClain LC, Wright LD, Bose RK, Spratt JA, Maier GW. Afterload sensitivity of nonlinear end‐systolic pressure–volume relation vs preload recruitable stroke work in conscious dogs. J Surg Res 1998; 75: 6–17. [DOI] [PubMed] [Google Scholar]
- 32. Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 1989; 65: 483–493. [DOI] [PubMed] [Google Scholar]
- 33. Thai HM, Do BQ, Tran TD, Gaballa MA, Goldman S. Aldosterone antagonism improves endothelial‐dependent vasorelaxation in heart failure via upregulation of endothelial nitric oxide synthase production. J Card Fail 2006; 12: 240–245. [DOI] [PubMed] [Google Scholar]
- 34. Bauersachs J, Heck M, Fraccarollo D, Hildemann SK, Ertl G, Wehling M, Christ M. Addition of spironolactone to angiotensin‐converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J Am Coll Cardiol 2002; 39: 351–358. [DOI] [PubMed] [Google Scholar]
- 35. Guiha NH, Cohn JN, Mikulic E, Franciosa JA, Limas CJ. Treatment of refractory heart failure with infusion of nitroprusside. N Engl J Med 1974; 291: 587–592. [DOI] [PubMed] [Google Scholar]
- 36. Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol 1986; 250: R1021–R1027. [DOI] [PubMed] [Google Scholar]
- 37. Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985; 56: 586–595. [DOI] [PubMed] [Google Scholar]
- 38. Dekleva M, Lazic JS, Soldatovic I, Inkrot S, Arandjelovic A, Waagstein F, Gelbrich G, Cvijanovic D, Dungen HD. Improvement of ventricular–arterial coupling in elderly patients with heart failure after beta blocker therapy: results from the CIBIS‐ELD Trial. Cardiovasc Drugs Ther 2015; 29: 287–294. [DOI] [PubMed] [Google Scholar]
- 39. Suga H, Kitabatake A, Sagawa K. End‐systolic pressure determines stroke volume from fixed end‐diastolic volume in the isolated canine left ventricle under a constant contractile state. Circ Res 1979; 44: 238–249. [DOI] [PubMed] [Google Scholar]
- 40. Hunter WC. End‐systolic pressure as a balance between opposing effects of ejection. Circ Res 1989; 64: 265–275. [DOI] [PubMed] [Google Scholar]
- 41. Igarashi Y, Goto Y, Yamada O, Ishii T, Suga H. Transient vs. steady end‐systolic pressure–volume relation in dog left ventricle. Am J Physiol 1987; 252: H998–H1004. [DOI] [PubMed] [Google Scholar]
- 42. Vaartjes SR, Boom HB. Left ventricular internal resistance and unloaded ejection flow assessed from pressure‐flow relations: a flow‐clamp study on isolated rabbit hearts. Circ Res 1987; 60: 727–737. [DOI] [PubMed] [Google Scholar]
- 43. Baan J, Van der Velde ET. Sensitivity of left ventricular end‐systolic pressure–volume relation to type of loading intervention in dogs. Circ Res 1988; 62: 1247–1258. [DOI] [PubMed] [Google Scholar]
- 44. Shishido T, Hayashi K, Shigemi K, Sato T, Sugimachi M, Sunagawa K. Single‐beat estimation of end‐systolic elastance using bilinearly approximated time‐varying elastance curve. Circulation 2000; 102: 1983–1989. [DOI] [PubMed] [Google Scholar]
- 45. Bradham WS, Bell SP, Huang S, Harrell FE Jr, Adkisson DW, Lawson MA, Sawyer DB, Ooi H, Kronenberg MW. Timing of left ventricular remodeling in nonischemic dilated cardiomyopathy. Am J Med Sci 2018; 356: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968; 37: 149–159. [DOI] [PubMed] [Google Scholar]
- 47. Chirinos JA, Sweitzer N. Ventricular–arterial coupling in chronic heart failure. Card Fail Rev 2017; 3: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34. [DOI] [PubMed] [Google Scholar]


