Abstract
Cardiogenic shock (CS) portends high morbidity and mortality in the contemporary era. Despite advances in temporary mechanical circulatory supports (MCS), their routine use in CS to improve outcomes has not been established. Delays in diagnosis and timely delivery of care, disparities in accessing adjunct therapies such revascularization or MCS, and lack of a systematic approach to care of CS contribute to the poor outcomes observed in CS patients. There is growing interest for developing a standardized multidisciplinary team‐based approach in the management of CS. Recent prospective studies have shown feasibility of CS teams in improving survival across a spectrum of CS presentations. Herein, we will review the rationale for CS teams focusing on evidence supporting its use in streamlining care, optimizing revascularization strategies, and patient identification and MCS selection. The proposed structure and flow of CS teams will be outlined. An in‐depth analysis of four recent studies demonstrating improved outcomes with CS teams is presented. Finally, we will explore potential implementation hurdles and future directions in refining and widespread implementation of dedicated cross‐specialty CS teams.
Keywords: Cardiogenic shock, Cardiogenic shock teams, Cardiogenic shock centres
Introduction
Cardiogenic shock (CS) is characterized by reduced cardiac output along with abnormal multi‐organ blood flow and oxygen delivery to meet metabolic demands. CS is the most common cause of death in patients with acute myocardial infarction (AMI) with mortality rates as high as 50% 1 , 2 ; however, CS‐AMI constitutes only 30% of all patients presenting with CS. The CS outcomes in non‐AMI patients are less established but remains similarly disappointing. 3 In CS‐AMI, a survival advantage has been demonstrated for patients who undergo successful reperfusion with primary coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery. 4 Notably, other modern cardiac intensive care unit (CICU) interventions such as vasopressor and inotropic drug infusions, haemodynamic monitoring, and intra‐aortic balloon pump (IABP) counterpulsation have shown no improvement in CS outcomes. 5 , 6 There has been growing interest for use of mechanical circulatory support (MCS) devices, such as ventricular assist devices (VADs) and microaxial flow pump catheter (Impella), offering greater haemodynamic support than IABP. 7 , 8 , 9 Despite the superior haemodynamic profile of newer MCS devices, they have not been shown to improve clinical outcomes. 10 , 11 , 12 Therefore, new approaches to care of CS patients have focused on mechanisms beyond MCS and revascularization. A recent promising initiative has focused on establishing CS teams to provide rapid identification, early resuscitation, and multidisciplinary management of this population. A multidisciplinary approach that encompasses all aspects of CS care is sensible given the dynamic course of disease with rapidly changing treatment targets. Preliminary studies have generated optimism that a team‐based approach to CS can optimize treatment, from medical to invasive management, and improve clinical outcomes. In this article, we will review the rationale and current literature for developing a multidisciplinary care for this critically ill population.
Cardiogenic shock teams to streamline care
Team‐based care for critical illness has been a fundamental tenet in many areas of medicine. The implementation of code teams, in‐hospital rapid response teams for decompensating patients, stroke, and trauma teams have been paramount in improving outcomes in patients with acute and time‐sensitive conditions. 13 , 14 , 15 Although having a multidisciplinary CS team has only recently garnered interest, a team‐based ‘heart team’ approach has been commonplace in care of patients in other areas of cardiology. Multidisciplinary ‘heart teams’ have been adopted in the management of complicated cardiac arrhythmia, 16 heart transplant, complex coronary revascularization, 17 and structural heart interventions. 18 , 19 More recently, there has been a trend towards specialized cardiac arrest centres in patients with out‐of‐hospital cardiac arrest to provide an all‐encompassing and contemporaneous evidence‐based resuscitation and post arrest care. 20 , 21 Bundled care at a cardiac arrest centre has been suggested to improve survival to hospital discharge with good neurological outcome and increased 30 day survival compared with admission at non‐specialized centres. 22 , 23 The 2019 American Heart Association focused update on cardiac‐arrest systems of care now provides a Class IIa recommendation for transfer of patients to cardiac arrest centres. 24 Given that cardiac arrest is commonly complicated by shock and the added hazard of cardiac arrest in CS patients, 25 , 26 it is conceivable that implementing specialized CS centres could confer the same benefits.
Despite major breakthroughs in the fields of percutaneous and surgical revascularization and MCS devices, the outcomes of CS have remained unacceptably poor, with a mortality range of 31% to 39% across a wide spectrum of CS in contemporary CICUs. 27 The disappointing outcomes in patients with CS, despite these advancements, may be partly attributed to delays in recognition and subsequent timely deployment of appropriate resources for management of CS. Significant delays in offering guideline‐directed interventions can occur due to additional time needed for initial stabilization of the critically ill CS patients. In the AHA Mission Lifeline System Accelerator project, fewer than 40% of ST‐Elevation Myocardial Infarction (STEMI) patients complicated with CS achieved the first medical contact‐to‐device time targets. 28 The multicentre Feedback Intervention and Treatment Times in ST‐Elevation Myocardial Infarction (FITT‐STEMI) trial showed that every 10 min treatment delay in first medical contact‐to‐balloon time resulted in 3.3 additional deaths in 100 PCI‐treated patients, with a 10‐fold rise in mortality rate within the early hours of infarction in CS patients as compared to haemodynamically stable patients. 29 Similarly, by decreasing the contact‐to‐balloon time to less than 90 min, one out of five CS patients could be saved. Therefore, mobilizing the proper units and earlier transfer of CS patients to specialized centres can improve the outcomes of this highly morbid population. However, development of shock teams should not replace the traditional network of STEMI activation, but instead complement existing STEMI systems to address the needs of the sickest CS‐AMI patient.
A key reason for developing dedicated CS centres and streamlining systems of care is the considerable variation seen in practice patterns of CS patients with worse outcomes in hospitals with lower CS volumes. The results from the National Cardiovascular Data Registry CathPCI of CS patients showed that IABP or MCS were more commonly employed in large hospitals (>600 beds) and university or teaching hospitals (as compared with private or community hospitals). 30 Among over half a million patients admitted with CS in the Nationwide Inpatient Sample database in the United States, a 5% reduction in mortality was observed in the hospitals with the lowest volume quartile as compared with the highest volume quartile of CS cases (odds ratio for inpatient mortality 1.27 vs. 1.12, respectively). 31 An important factor that may have contributed to the observed disparity was the significantly greater use of early revascularization (36.4% vs. 20.6%) and MCS (33.5% vs. 16.9%) in higher compared with lower CS volume centres, respectively. In a more recent analysis of 362 065 patients with AMI‐CS, there was a serial decrease in mortality with increasing hospital size (42.4% and 39% between small and large hospitals, respectively). 32 Compared with smaller hospitals, larger centres had increased use of early coronary angiography (41.8% vs. 30.3%), PCI (49.9% vs. 36.6%), and MCS (46.3% vs. 32.9%). Furthermore, appropriate patient selection for advanced therapies may be a determinant of outcomes. Patient selection for MCS also differs in hospitals with different rates of MCS utilization, which could affect variation in clinical outcomes across hospitals. 33
Overall, given the complexity of CS, dedicated training and experience is needed to maintain competency in delivering safe and effective non‐invasive and invasive interventions. It is possible to build upon the volume–outcome relationship by establishing multidisciplinary CS teams within specialized referral centres adept at providing comprehensive care for CS patients. 34 , 35 , 36
Structure of cardiogenic shock teams
The earliest efforts to centralize CS care were focused on a ‘travelling shock team’ concept where a group of physicians with expertise at managing CS was deployed to a spoke centre. The Mayo Clinic Arizona team included a cardiothoracic surgeon or heart failure/transplant cardiologist, perfusionist, and ICU nurses. 37 The travelling team would focus on stabilizing the patient and deciding on initiation of MCS prior to transfer. In their pilot study of 27 patients, 15 underwent extracorporeal membrane oxygenation (ECMO) placement prior to transfer, 25 patients survived transfer to the tertiary CS centre, and 14 patients survived to discharge. In a similar study of the cardiac‐RESCUE programme, a mobile team consisting of cardiac surgeon, intensivist, perfusionist, and a nurse provided MCS with ECMO to non‐tertiary centres in Paris. 38 Their programme resulted in long‐term survival in approximately one‐third of refractory CS patients (32 out of 75 patients).
Contemporary CS teams generally comprise an advanced heart failure cardiologist, a cardiothoracic surgeon, an interventional cardiologist, and intensivist (including cardiac intensivist). Other members of the team include a critical care nurse, perfusionist, and a respiratory therapist. Cardiac catheterization laboratory staff, critical care nurses, and perfusionists should be available as appropriate. Once activated, all team members are expected to participate in the decisions surrounding patient management and therapeutic options. Commonly, the CICU attending or heart failure specialist would activate the CS team after initial assessment for appropriate criteria for team activation. 39 The term ‘shock doc’ has been proposed for the physician in charge of coordinating with the other parties the critical team‐based decisions and interventions, such as urgent MCS placement, streamlining care in CICU, and day‐to‐day management of the patient. 40
A categorized level of care for CS has been suggested based on the capability of the hospital. 41 This is similar to the three‐tiered CICU classification system in which a Level I CICU is assigned the ‘regional hub’, Level II the ‘secondary referral centre’, and the Level III the ‘community CICU’. 42 A Level I CS centre implies a tertiary hospital with full‐time PCI and advanced MCS capabilities. After appropriate assessment by the advance heart failure specialist, refractory CS should trigger activation of the ‘shock team’, which includes transferring from the spokes hospitals to the Level I hub centre with concurrent consultation with the cardiac intensivist. 43 The cardiac catheterization laboratory staff including the interventionist and cardiac surgeon should also be simultaneously notified for immediate angiography and possible need for MCS support. 41 , 43 , 44 In the INOVA Heart and Vascular Institute (IHVI) cardiogenic shock pathway, the shock team was activated via a ‘shock line’ prompting a multidisciplinary discussion with the four specialists involved. 45 , 46 In the University of Ottawa Heart Institute (UOHI) code shock protocol, a smartphone application was employed for code shock activation and subsequent online virtual discussion among the CS team members. 47 Irrespective of the initial management plan, there should be ongoing daily communication between all team members to discuss management strategies and timing for escalation or de‐escalation of care. 39
Role of cardiogenic shock team in early revascularization
A key objective of centralizing CS care is to obtain expedient access to a cardiac catheterization laboratory with expertise in managing haemodynamically unstable patients. The role of early revascularization in CS complicating AMI is well established. In the landmark SHOCK trial, which included 302 patients with CS‐AMI, although there was no 30 day survival benefit with early invasive approach, the mortality rates were significantly lower in the revascularization cohort in comparison with the medical therapy group at 6 months and 1 year (50.3% vs. 63.1% and 46.7% vs. 33.6%, respectively). 4 , 48 A prospective observational study of the National Registry of Myocardial Infarction supported these findings with decreased in in‐hospital mortality from 60.3% to 47.9% with early revascularization in patients with CS following AMI. 49
The proficiency of medical staff, including the interventionists and cardiac surgeons, to provide acute PCI or CABG is independently associated with successful revascularization. 50 , 51 There appears to be a positive correlation between the volume of procedures and the outcomes of CABG or PCI. Multiple studies have reported improved survival after primary PCI for STEMIs in high‐volume centres and by high‐volume operators. A 5 year analysis of over 2 million PCIs in United States showed a decrease in mortality and complication rates with increasing quartiles of operator volume with mortality rates of 1.68%, 1.15%, 0.87%, and 0.59% in first (≤15 PCIs yearly), second (16 to 44 PCIs yearly), third (45 to 100 PCIs yearly), and fourth (>100 PCIs yearly) quartiles of operator volume, respectively. 50 A meta‐analysis of 15 studies (10 PCI and 7 CABG studies) revealed lower in‐hospital mortality in large‐volume (more than 600 cases annually) as compared with lower volume (less than 600 cases annually) PCI [odds ratio (OR) 0.89, confidence interval (CI) 0.83–0.91] and CABG (OR 0.85; CI 0.79–0.92) centres. 51 The importance of procedural competence is crucial in the management of CS patients who present with tenuous haemodynamics. Accordingly, establishing systems of care with dedicated CS centres identified as hubs with proficiency in performing high‐risk interventions has the potential to improve outcomes in CS patients and limit procedural complications.
Role of cardiogenic shock team in early mechanical circulatory support
Despite haemodynamic advantages of MCS, large trials powered for efficacy and safety are lacking in CS. 4 , 11 , 52 , 53 A potential limitation of these trials was a selection bias in choosing patients who were either extremely sick with irreversible neurological injury (i.e. post‐arrest CS patients in IMPRESS trial) or insufficiently sick to derive meaningful benefits from MCS (i.e. mild to moderately severe CS patients in IABP‐SHOCK II trial). Timing of MCS initiation is also thought to contribute to success of therapy. CS teams can play a crucial role to (1) evaluate those that may derive benefit from MCS, (2) to provide more timely access for these patients, and (3) to prevent individual biases and identify those that may be too sick to derive any benefits. It is hoped that previous efforts failing to show consistent clinical improvements with MCS can be overcome by optimization of MCS care with specialized CS teams.
One potential role of having CS teams is earlier recognition of refractory shock in order to expedite the initiation of MCS. For CS, a similar metric as STEMI's door‐to‐balloon time, or ‘door‐to‐support’ time could be developed to reflect the time between onset of CS and initiation of MCS. 54 It has been proposed that early identification of CS to rapidly implement MCS can improve clinical outcomes. 55 , 56 , 57 In a study of 287 patients with AMI‐CS receiving an Impella less than 1.25 h from shock onset, a 66% in‐hospital survival was achieved compared with 37% and 26% survival in patients who received MCS within 1.25 to 4.25 h or exceeding 4.25 h of CS onset, respectively. 55 In a non‐randomized cohort from the USpella registry, among patients with CS following AMI, Impella insertion prior to PCI increased survival by 24.4% (number needed to treat of five) compared with insertion after PCI. 56 A meta‐analysis of three studies comprising a total of 370 patients indicated that early initiation of Impella in CS following AMI leads to a 48% reduction in 30 day mortality compared with late MCS initiation. 58 Despite the strong physiological bases, these associations require confirmation in randomized control studies.
The Detroit Cardiogenic Shock Initiative enacted a regional protocol for management of CS in AMI patients focusing around rapid insertion of MCS and use of pulmonary artery catheter haemodynamic monitoring to guide subsequent therapy. 59 In this pilot protocol of 41 patients, a rapid door‐to‐support time averaging 83 min was obtained, with 85% survival rate until device explant and 76% survival rate to discharge; this was a marked improvement compared with conventional expected outcomes. This study cultivated the National Cardiogenic Shock Initiative where multiple US institutions adopted the same CS protocol‐based approach emphasizing on early initiation of MCS. 60 About 98.9% of enrolled CS patients underwent Impella placement with 74% of patients receiving it pre‐PCI. Also, a striking rapid door‐to‐MCS time of 85 ± 63 min was achieved. With an early MCS approach, the number of inotrope infusions was reduced in 51% of patients, a potential marker of improved outcomes. In the UOHI code shock protocol, there was a trend towards increased MCS utilization among patients treated with adoption of a CS team approach (45%) as compared with a conventional treated group (28%). 47 In the IHVI shock team study, 44% of patients presented to the hub CS centre had escalation of MCS. 46 Every hour of delay in intensification of therapy was associated with 10% increased mortality risk, with improved outcomes when MCS was initiated within 5 h of patient presentation.
Although more robust data are required before routine adoption of MCS in CS, a strategy adopting rapid assessment for MCS can be facilitated by establishing dedicated CS protocols. A concern raised with CS teams is the potential delay in care and execution of critical decisions with increasing numbers of providers involved. However, in the Utah Cardiac Recovery (UCAR) shock team experience, a multidisciplinary approach did not delay care with a shock‐to‐support time of 19 ± 5 h in shock team vs. 25 ± 8 h in control (P = 0.52) with an increased survival after establishing a dedicated multidisciplinary CS team. 61
Patient selection and choice of mechanical circulatory support device
Patient selection for MCS is critical in obtaining a successful outcome. Given the high morbidity and substantial costs associated with MCS, its judicious use to carefully selected CS patients is critical. 62 Given the lack of clear guidelines for appropriate patient selection for MCS application, development of CS teams can help in selecting the right patient for the right device. In the IHVI and National Cardiogenic Shock Initiative shock team protocols, presumed more robust haemodynamic markers such as cardiac power output 63 and pulmonary artery pulsatility index (PAPi) 64 were used as haemodynamic criteria for MCS patient selection, assessing response to therapy and whether escalation/de‐escalation of MCS was needed. It should be kept in mind that the use of these metrics is mostly based on observational data rather than randomized control trial evidence.
The appropriate MCS device for a given CS stage is of utmost importance to maximize the survival benefit while minimizing the risks. MCS devices should be tailored to patient profile to offer the highest chance of haemodynamic augmentation, and this process can be facilitated by the emergence of CS teams. Different types of MCS devices have different efficacy in the management of CS, with the newer percutaneous VAD and venoarterial (VA)‐ECMO providing greater haemodynamic support that IABP. 65 , 66 , 67 Also, MCS devices with dedicated right ventricular support may be used in patients with refractory right heart failure. 68 In the UCAR shock team study, there was a significant variation in MCS‐device type with increased use of Impella and VA‐ECMO in the shock team cohort as compared with controls, although no survival advantage was observed in relation to device type (Table 1 ). 61 In the UOHI shock experience, Impella—particularly the 5.0 L device—was used more commonly in the CS team managed group in relation to the standard care cohort. 47 In the INOVA‐SHOCK registry, percutaneous VADs (especially Impella CP) either alone or in combination to VA‐ECMO were used more commonly than IABP (45% vs. 27%), with frequent escalation of IABP to VAD after activating the CS team. 46 Until there are convincing data from randomized studies demonstrating a survival benefit based on MCS type, a CS team‐based approach can streamline eligibility for different types of MCS to choose accordingly. Lastly, development of multidisciplinary CS teams can help in excluding patients from invasive therapies with advanced comorbidities or impending futility. 69
Table 1.
Studies to date using dedicated cardiogenic shock teams and protocols
| Study | Number of patients | Quality Measures/Goals | Intervention(s) | Outcome(s) |
|---|---|---|---|---|
|
National Cardiogenic Shock Initiative 60 Basir et al. 2019 Prospective single‐arm study |
Total: 171 All patients with AMI‐CS *No control group |
1. MCS use pre‐PCI 2. Shock onset to device <90 min 3. Establish TIMI 3 Flow 4. Complete revascularization 5. Maintain CPO > 0.6 W 6. Maintain PAPi >0.9 7. Routine RHC use |
PCI: 171 of 171 patients MCS [Impella 2.5, CP, or RP]: 169 of 171 pts • 74% pre‐PCI • 7.1% during PCI • 18.9% post‐PCI RHC: 154 of 171 pts |
MCS pre‐PCI: 74% RHC usage: 92% Maintain CPO > 0.6 W: 62% Door to support time: 85 ± 63 min Survival to discharge: 72% |
|
Inova Heart and Vascular Institute Cardiogenic Shock Initiative 46 Tehrani et al. 2019 Prospective, pre‐intervention and post‐intervention study |
Total: 204 AMI‐CS: 81 ADHF‐CS: 122 *Control group not presented |
1. Rapid CS identification 2. Early MCS (LV and RV) 3. RHC: Thresholds at 24 h: i. Lactate <3 ii. CPO >0.6 W iii. PAPi >1.0 4. Minimize inotropes/vasopressors 5. Cardiac recovery |
PCI: 82 of 204 patients MCS: 135 of 204 pts • 35.3% IABP • 44.9% Impella only • 6.4% VA‐ECMO only • 13.5% Impella + VA‐ECMO RHC: 167 of 204 patients |
30 day survival: • Pre‐shock team implementation: 47% • After 1 year of shock team implementation: 58% • After 2 years of shock team implementation: 77% (P < 0.01) |
|
Utah Cardiac Recovery shock team 61 Taleb et al. 2019 Prospective, pre‐intervention and post‐intervention study |
Total: 244 N = 123 treatment; N = 121 control AMI‐CS: N treatment = 75 N control = 85 Non‐AMI CS: N treatment = 48 N control = 36 |
If STEMI: 1. Central arterial access for LVEDP measurement 2. Consideration for MCS and simultaneous angiogram‐PCI 3. Urgent RHC If not STEMI: 1. Urgent RHC 2. Consideration for MCS 3. Possible LHC as needed |
MCS: 123 of 123 in shock team (vs. control) • 30.2% IABP (vs. 62.8%) • 33.3% Impella (vs. 9.9%) • 8.9% VA‐ECMO (vs. 5%) • 27.6% combination of devices (vs.. 22.3%) P value for MCS type <0.001 |
Shock to support time: 19 ± 5 (vs. 25 ± 8 h, P = NS) Mean length of MCS support: 121 ± 13 (vs. 104 ± 16 h, P = NS) In‐hospital survival: 61% (vs. 47.9%; P = 0.04) 30 day all‐cause mortality HR 0.61 [95% CI, 0.41–0.93] |
|
University of Ottawa Heart Institute code shock team 47 Lee et al. 2020 Retrospective, CPO, MCS, PAPi, RHC pre‐intervention and post‐intervention study |
Total: 100 N treatment = 64 N control = 36 AMI‐CS: N treatment = 7 N control = 6 Non‐AMI CS: N treatment = 57 N control = 30 |
1. Confirmation of CS 2. Resuscitation 3. Medical optimization 4. Temporary MCS evaluation 5. Heart transplant, LVAD evaluation |
Revascularization: 12 of 100 patients—all AMI‐CS (75% PCI, 8% CABG, 17% both) MCS: 29 of 64 in shock team (vs. 10 of 36 in control) • 34% IABP (vs. 40%) • 28% Impella (vs. 10%) • 7% VA‐ECMO (vs. 10%) • 14% combination (vs. 11%) P value for MCS type: 0.08 RHC: 50 of 100 patients |
Temporary MCS use: 45% (vs. 28%, P = 0.08) In‐hospital survival: 69% (vs. 61%; P = NS) 30 day survival: 72% (vs. 69%; P = NS) Long‐term survival: 67% (vs. 42%; P = 0.04) Cumulative survival: HR 0.53 [95% CI 0.28–0.99] |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CI, confidence interval; CPO, cardiac power output; CS, cardiogenic shock; HR, hazard ratio; IABP, intra‐aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support; NS, not significant; PAPi, pulmonary artery pulsatility index; PCI, primary coronary intervention; RHC, right heart catheterization; VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
Association of cardiogenic shock teams with outcomes
Four published observational studies from North American centres have evaluated the role of multidisciplinary CS teams, using protocol‐based approaches (Table 1 and Figure 1 ). Basir et al., based on their experience with the Detroit Cardiogenic Shock Initiative, established the National Cardiogenic Shock Initiative, a regional protocol for CS in patient with AMI. 59 , 60 The investigators developed a CS protocol focused on best practices including early activation of catheterization laboratory, early use of MCS preferably within 90 min of presentation, and routine use of invasive haemodynamic monitoring. A total of 171 patients from 35 US centres were enrolled in which 167 (97.7%) survived the index procedure and 123 (71.9%) survived to discharge.
Figure 1.
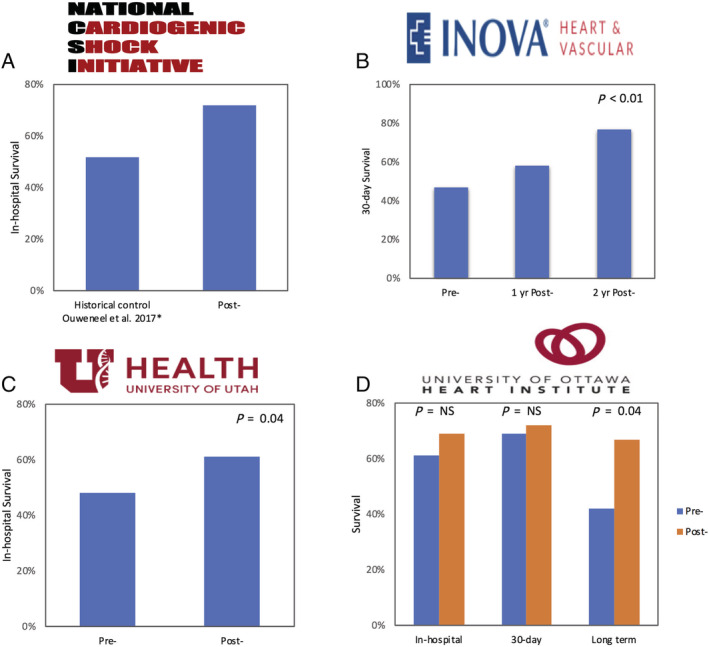
Survival outcomes pre‐shock and post‐shock team/protocol implementation in the (A) National Cardiogenic Shock Initiative, (B) INOVA Heart and Vascular Institute Shock Team Protocol, (C) Utah Cardiac Recovery shock team, and (D) University of Ottawa Heart Institute Code shock team. *Data from the IMPRESS in Severe Shock Trial. 12 **No baseline institutional survival outcomes or controls reported in the National Cardiogenic Shock Initiative.
The IHVI investigators developed a shock team protocol for management of patients with CS. 45 , 46 The team‐based approach demonstrated a 30 day survival among patients with CS at 57.9%, which was a marked improvement from 47% the year prior to the shock team implementation. One year after implementation of the programme, about 76.6% of patients with shock treated in the IHVI pathway survived to 30 days (P < 0.01). The survival benefit of this team‐based shock care was more pronounced in CS following AMI (44% in 2016, 63% in 2017, and 82% in 2018) as compared with CS following acute decompensate heart failure (60% in 2016, 63% in 2017, and 72% in 2018). In the latest proof of concept study, the UCAR shock team compared 123 consecutive patients with refractory CS between 2015 and 2018 managed through a ‘shock team’ approach with 121 control patients treated with traditional algorithms. 61 After institution of a shock team, there was 13.1% absolute risk reduction in in‐hospital death and a reduction in 30 day all‐cause mortality with an adjusted HR of 0.61 (95% CI, 0.41–0.93). More recently, the UOHI code shock protocol displayed an improved long‐term survival with establishing an interdisciplinary CS team compared with standard care (HR 0.50; 95% CI 0.28–0.99) over a median follow up of 240 days. 47 Notably, there were no in‐hospital or 30 day survival benefit. This may in part be accounted by the lower incidence of AMI‐CS and ischemic cardiomyopathy, two CS phenotypes with higher inherent mortality. Although the outcomes in CS is still discouraging despite the use of various interventions, emergence of CS centres with staffed multidisciplinary ‘shock teams’ offers an encouraging vision in CS care with potential to improve survival.
Cardiogenic shock teams in managing ‘beyond shock’
The understanding of CS has shifted away from a ‘haemodynamic’ aberration towards a ‘haemometabolic’ insult that might not respond to treatment of the underlying haemodynamic problem alone. 54 , 70 This requires a multifaceted approach to management of the multi‐organ dysfunction that ensues CS. The role of CS teams thereby extends beyond optimizing revascularization and advanced MCS therapies. CS teams can help in consolidating cardiac‐related medical therapy from choice and titration of vasoactive agents to the need for non‐cardiac interventions including renal replacement therapy and neurological prognostication. Timely initiation of mechanical ventilation and mode of ventilation could attenuate multi‐organ dysfunction in CS patients. 71 A multidisciplinary shock team approach can take advantage of subspecialists with expertise in intensive care and ventilation strategies. 72
Lastly, a multidisciplinary approach to CS can integrate advanced diagnostics and therapeutics with non‐medical and palliative aspect of care. A retrospective analysis of national inpatient sample database from almost half a million patients with CS following AMI showed significant underutilization of palliative care services despite the high mortality associated with CS. 73 Therefore, CS team can help with treatment de‐escalation, earlier incorporation of palliative care measures, and facilitate transition to comfort care if necessary.
Challenges in implementing cardiogenic shock teams
A number of hurdles exist in establishing CS teams and centres. To implements successful CS protocols, multiple cross‐specialists, and personnel with adequate expertise and competence in managing the most critically ill patients are required. Frequent training and quality improvement should be incorporated for the members of the CS teams to sustain adequate clinical and procedural proficiency. This is specifically important for centres where CS cases are sporadic. It is also quite burdensome at an institutional and individual level to maintain a 24 h/7 day CS programme. In addition to the funding required to run CS teams, the costs associated with temporary MCS 74 can be prohibitive for single payer health care systems.
Expediency is also imperative in establishing CS teams where critical decisions and interventions are needed but might be delayed with the increasing number of stakeholders involved. These challenges could be mitigated by designating a ‘shock doc’ who could triage the initial calls and ensures appropriate activation of the rest of the team and also coordinate the care to avoid delays. The extensive resources needed to maintain CS teams require constant institutional administration support, sufficient funding and staffing, and ongoing cost‐effective analysis. It also needs to be a regional buy in from health authorities into a hub‐and‐spoke model for CS management similar to STEMI systems of care. Design of CS teams requires consideration of local infrastructure, including involvement of referral centres and EMS for expedient triage and transport of patients. In addition, designing a system to address all stages of shock, rather than just those at more advance stages continues to be a challenge. Lastly, consistent data collection is needed to follow outcomes and provide a framework for quality improvement feedback for CS teams.
Future directions
The increasing treatment complexity of CS creates new opportunities for dedicated multidisciplinary CS teams to gain traction. Since the initial inception of the formalized team‐based CS protocols however, our knowledge about CS and different advanced therapies has grown. There is significant heterogeneity in the CS definition, patient selection, treatment protocols, and outcome measures in these studies. The new SCAI CS definition has added more granularity in the severity of shock and allows for better multidisciplinary communication. 25 It also helps to refine our MCS selection based on the CS stage, as each MCS device can have a variable outcome at different acuity stages. 26 , 75 Given the time‐sensitive nature of managing patients with CS and dynamic need for escalation or de‐escalation of treatment, dedicated CS teams can help in efficiently navigating different therapies and optimizing outcomes. There needs to be further randomized evidence—perhaps generated by pragmatic, multi‐centred, stepped wedged strategy trials—evaluating the clinical and cost effectiveness of the CS teams in relation to standard care before routine adoption in the critical care cardiology landscape.
Conclusions
Despite revascularization and MCS advancements, the outcomes of CS have remained poor largely due to fragmentation of care and disparity of practice patterns. There is now increasing evidence from observational registries supporting the establishment of a hub‐and‐spoke care system for CS. Multidisciplinary shock teams utilizing protocol‐driven care is feasible and can improve survival in patients with CS. The role of CS teams is to facilitate timely diagnosis, appropriate use of invasive haemodynamics, revascularization strategies, and implementation of MCS. CS teams should systematically include cardiovascular specialists with dedicated expertise in different arrays of CS care and respond to the evolving advancements in this field. Given the observational nature of the current studies, further prospective large‐scale randomized trials are required to determine the clinical efficacy and cost effectiveness of standardized team‐based approach to CS.
Moghaddam, N. , van Diepen, S. , So, D. , Lawler, P. R. , and Fordyce, C. B. (2021) Cardiogenic shock teams and centres: a contemporary review of multidisciplinary care for cardiogenic shock. ESC Heart Failure, 8: 988–998. 10.1002/ehf2.13180.
References
- 1. Anderson ML, Peterson ED, Peng SA, Wang TY, Ohman EM, Bhatt DL, Saucedo JF, Roe MT. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: a report from NCDR. Circ Cardiovasc Qual Outcomes 2013; 6: 708–715. [DOI] [PubMed] [Google Scholar]
- 2. Miller L. Cardiogenic shock in acute myocardial infarction: the era of mechanical support. J Am Coll Cardiol 2016; 67:1881‐1884: 1881–1884. [DOI] [PubMed] [Google Scholar]
- 3. Shah M, Patel B, Tripathi B, Agarwal M, Patnaik S, Ram P, Patil S, Shin J, Jorde UP. Hospital mortality and thirty day readmission among patients with non‐acute myocardial infarction related cardiogenic shock. Int J Cardiol 2018; 270: 60–67. [DOI] [PubMed] [Google Scholar]
- 4. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne‐Nickens P, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 6. Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R. Intra‐aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev 2015: CD007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Neill WW, Kleiman NS, Moses J, Henriques JPS, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Džavík V, Popma J, Douglas PS, Ohman M. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra‐aortic balloon pump in patients undergoing high‐risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012; 126: 1717–1727. [DOI] [PubMed] [Google Scholar]
- 8. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short‐term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014; 64: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 9. Lauten A, Engström AE, Jung C, Empen K, Erne P, Cook S, Windecker S, Bergmann MW, Klingenberg R, Lüscher TF, Haude M, Rulands D, Butter C, Ullman B, Hellgren L, Modena MG, Pedrazzini G, Henriques JPS, Figulla HR, Ferrari M. Percutaneous left‐ventricular support with the Impella‐2.5‐assist device in acute cardiogenic shock: results of the Impella‐EUROSHOCK‐registry. Circ Heart Fail 2013; 6: 23–30. [DOI] [PubMed] [Google Scholar]
- 10. Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv 2017; 10: e004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, De Waha S. Percutaneous short‐term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta‐analysis of randomized trials. Eur Heart J 2017; 38: 3523–3531. [DOI] [PubMed] [Google Scholar]
- 12. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BAJM, Tijssen JGP, Henriques JPS. Percutaneous mechanical circulatory support versus intra‐aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287. [DOI] [PubMed] [Google Scholar]
- 13. Morrison Laurie J, Neumar Robert W, Zimmerman Janice L, Link MS, Newby LK, McMullan PW Jr, Hoek TV, Halverson CC, Doering L, Peberdy MA, Edelson DP. Strategies for improving survival after in‐hospital cardiac arrest in the United States: 2013 consensus recommendations. Circulation 2013; 127: 1538–1563. [DOI] [PubMed] [Google Scholar]
- 14. Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med 2010; 170: 18–26. [DOI] [PubMed] [Google Scholar]
- 15. Celso B, Tepas J, Langland‐Orban B, Pracht E, Papa L, Lottenberg L, Flint L. A systematic review and meta‐analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. J Trauma 2006; 60: 371–378. [DOI] [PubMed] [Google Scholar]
- 16. Fumagalli S, Chen J, Dobreanu D, Madrid AH, Tilz R, Dagres N. The role of the arrhythmia team, an integrated, multidisciplinary approach to treatment of patients with cardiac arrhythmias: results of the European Heart Rhythm Association survey. Europace 2016; 18: 623–627. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez CE, Badhwar V, Dota A, Schindler J, Chu D, Smith AJC, Lee JS, Khandhar S, Toma C, Marroquin OC, Schmidhofer M, Bhama J, Wei L, Scolieri S, Esper S, Lee A, Mulukutla SR. Practical implementation of the coronary revascularization heart team. Circ Cardiovasc Qual Outcomes 2013; 6: 598–603. [DOI] [PubMed] [Google Scholar]
- 18. Chambers JB, Lloyd G, Rimington HM, Parkin D, Hayes AM, Baldrock‐Apps G, Topham A. The case for a specialist multidisciplinary valve clinic. J Heart Valve Dis 2012; 21: 1–4. [PubMed] [Google Scholar]
- 19. Holmes DR Jr, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol 2013; 61: 903–907. [DOI] [PubMed] [Google Scholar]
- 20. Yeung J, Matsuyama T, Bray J, Reynolds J, Skrifvars MB. Does care at a cardiac arrest centre improve outcome after out‐of‐hospital cardiac arrest? A syst rev Resus 2019; 137: 102–115. [DOI] [PubMed] [Google Scholar]
- 21. Callaway CW, Soar J, Aibiki M, Böttiger BW, Brooks SC, Deakin CD, Donnino MW, Drajer S, Kloeck W, Morley PT, Morrison LJ, Neumar RW, Nicholson TC, Nolan JP, Okada K, O'Neil BJ, Paiva EF, Parr MJ, Wang T‐L, Witt J. on behalf of the Advanced Life Support Chapter CollaboratorsInternational consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015; 132: S84–S145. [DOI] [PubMed] [Google Scholar]
- 22. Kragholm K, Malta Hansen C, Dupre ME, Xian Y, Strauss B, Tyson C, Monk L, Corbett C, Fordyce CB, Pearson DA, Fosbøl EL, Jollis JG, Abella BS, McNally B, Granger CB. Direct transport to a percutaneous cardiac intervention center and outcomes in patients with out‐of‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes 2017; 10: e003414. [DOI] [PubMed] [Google Scholar]
- 23. Elmer J, Rittenberger JC, Coppler PJ, Guyette FX, Doshi AA, Callaway CW, Pittsburgh Post‐Cardiac Arrest Service . Long‐term survival benefit from treatment at a specialty center after cardiac arrest. Resuscitation 2016; 108: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panchal AR, Berg KM, Cabañas JG, Kurz MC, Link MS, del Rios M, Hirsch KG, Chan PS, Hazinski MF, Morley PT, Donnino MW. American Heart Association focused update on systems of care: dispatcher‐assisted cardiopulmonary resuscitation and cardiac arrest centers: an update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation n.d 2019; 0 CIR.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 25. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, Stelling K, Thiele H, Diepen S, Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019; 94: 29–37. [DOI] [PubMed] [Google Scholar]
- 26. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 2019; 74: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 27. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis AP, Haleem A, Hollenberg SM, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Park JG, Phreaner N, Roswell RO, Schulman SP, Jeffrey Snell R, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes 2019; 12: e005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kochar A, Al‐Khalidi HR, Hansen SM, Shavadia JS, Roettig ML, Fordyce CB, Doerfler S, Gersh BJ, Henry TD, Berger PB, Jollis JG. Delays in primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction patients presenting with cardiogenic shock. JACC Cardiovasc Interv 2018; 11: 1824–1833. [DOI] [PubMed] [Google Scholar]
- 29. Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ott R, Mudra H, Seidl K, Schulze PC, Weiss C, Haimerl J, Friede T, Meyer T. Impact of treatment delay on mortality in ST‐segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT‐STEMI trial. Eur Heart J 2018; 39: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandhu A, McCoy LA, Negi SI, Hameed I, Atri P, Al'Aref SJ, Curtis J, McNulty E, Anderson HV, Shroff A, Menegus M. Use of mechanical circulatory support in patients undergoing percutaneous coronary intervention. Circulation 2015; 132: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 31. Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc 2015; 4: e001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR, Prasad A. Hospital‐level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 2019; 124: 491–498. [DOI] [PubMed] [Google Scholar]
- 33. Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ, Cohen DJ, Yeh RW. Hospital variation in the utilization of short‐term nondurable mechanical circulatory support in myocardial infarction complicated by cardiogenic shock. Circ Cardiovasc Interv 2019; 12: e007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dehmer GJ, Blankenship JC, Cilingiroglu M, Dwyer JG, Feldman DN, Gardner TJ, Grines CL, Singh M, Society for Cardiovascular Angiography and Interventions, American College of Cardiology, American Heart Association . SCAI/ACC/AHA expert consensus document: 2014 update on percutaneous coronary intervention without on‐site surgical backup. Catheter Cardiovasc Interv 2014; 84: 169–187. [DOI] [PubMed] [Google Scholar]
- 35. Harold JG, Bass TA, Bashore TM, Brindis RG, Brush JE Jr, Burke JA, Dehmer GJ, Deychak YA, Jneid H, Jollis JG, Landzberg JS, Levine GN, McClurken JB, Messenger JC, Moussa ID, Muhlestein JB, Pomerantz RM, Sanborn TA, Sivaram CA, White CJ, Williams ES, Halperin JL, Beckman JA, Bolger A, Byrne JG, Lester SJ, Merli GJ, Muhlestein JB, Pina IL, Wang A, Weitz HH. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures. J Am Coll Cardiol 2013; 62: 357–396. [DOI] [PubMed] [Google Scholar]
- 36. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 37. Jaroszewski DE, Kleisli T, Staley L, Pierce C, Scott R, Steidley DE, DeValeria P, Arabia FA. A traveling team concept to expedite the transfer and management of unstable patients in cardiopulmonary shock. J Heart Lung Transplant 2011; 30: 618–623. [DOI] [PubMed] [Google Scholar]
- 38. Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac‐RESCUE program). Eur Heart J 2013; 34: 112–120. [DOI] [PubMed] [Google Scholar]
- 39. Doll JA, Ohman EM, Patel MR, Milano CA, Rogers JG, Wohns DH, Kapur NK, Rao SV. A team‐based approach to patients in cardiogenic shock. Catheter Cardiovasc Interv 2016; 88: 424–433. [DOI] [PubMed] [Google Scholar]
- 40. Rab T. “Shock teams” and “Shock docs”. J Am Coll Cardiol 2019; 73: 1670–1672. [DOI] [PubMed] [Google Scholar]
- 41. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB, O'Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol 2018; 72: 1972–1980. [DOI] [PubMed] [Google Scholar]
- 42. le May M, van Diepen S, Liszkowski M, Schnell G, Tanguay JF, Granger CB, Ainsworth C, Diodati JG, Fam N, Haichin R, Jassal D. From coronary care units to cardiac intensive care units: recommendations for organizational, staffing, and educational transformation. Can J Cardiol 2016; 32: 1204–1213. [DOI] [PubMed] [Google Scholar]
- 43. Tchantchaleishvili V, Hallinan W, Massey HT. Call for organized statewide networks for management of acute myocardial infarction‐related cardiogenic shock. JAMA Surg 2015; 150: 1025–1026. [DOI] [PubMed] [Google Scholar]
- 44. Garan AR, Kirtane A, Takayama H. Redesigning care for patients with acute myocardial infarction complicated by cardiogenic shock: the “shock team”. JAMA Surg 2016; 151: 684–685. [DOI] [PubMed] [Google Scholar]
- 45. Truesdell AG, Tehrani B, Singh R, Desai S, Saulino P, Barnett S, Lavanier S, Murphy C. ‘Combat’ approach to cardiogenic shock. Interv Cardiol 2018; 13: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, Rosner C, Raja A, Barnett SD, Saulino P, deFilippi CR, Gurbel PA, Murphy CE, O'Connor CM. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol 2019; 73: 1659–1669. [DOI] [PubMed] [Google Scholar]
- 47. Lee F, Hutson JH, Boodhwani M, McDonald B, So D, de Roock S, Rubens F, Stadnick E, Ruel M, le May M, Labinaz M. Multidisciplinary code shock team in cardiogenic shock: a Canadian‐center experience. CJC Open 2020: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J, Sanborn T, Buller CE, Modur S, Forman R, Desvigne‐Nickens P, Jacobs AK, Slater JN, LeJemtel T, SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock . One‐year survival following early revascularization for cardiogenic shock. JAMA 2001; 285: 190–192. [DOI] [PubMed] [Google Scholar]
- 49. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005; 294: 448–454. [DOI] [PubMed] [Google Scholar]
- 50. Badheka AO, Patel NJ, Grover P, Singh V, Patel N, Arora S, Chothani A, Mehta K, Deshmukh A, Savani GT, Patel A, Panaich SS, Shah N, Rathod A, Brown M, Mohamad T, Tamburrino FV, Kar S, Makkar R, O'Neill WW, de Marchena E, Schreiber T, Grines CL, Rihal CS, Cohen MG. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5‐year United States experience (2005–2009). Circulation 2014; 130: 1392–1406. [DOI] [PubMed] [Google Scholar]
- 51. Post PN, Kuijpers M, Ebels T, Zijlstra F. The relation between volume and outcome of coronary interventions: a systematic review and meta‐analysis. Eur Heart J 2010; 31: 1985–1992. [DOI] [PubMed] [Google Scholar]
- 52. Hsu S, Kambhampati S, Sciortino CM, Russell SD, Schulman SP. Predictors of intra‐aortic balloon pump hemodynamic failure in non‐acute myocardial infarction cardiogenic shock. Am Heart J 2018; 199: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius‐Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schäfer A, Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019; 139: 1249–1258. [DOI] [PubMed] [Google Scholar]
- 54. Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Research 2017; 6: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 2017; 119: 845–851. [DOI] [PubMed] [Google Scholar]
- 56. O'Neill WW, Schreiber T, Wohns DHW, Rihal C, Naidu SS, Civitello AB, Dixon SR, Massaro JM, Maini B, Ohman EM. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella registry. J Interv Cardiol 2014; 27: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meraj PM, Doshi R, Schreiber T, Maini B, O'Neill WW. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol 2017; 30: 256–263. [DOI] [PubMed] [Google Scholar]
- 58. Flaherty MP, Khan AR, O'Neill WW. Early initiation of Impella in acute myocardial infarction complicated by cardiogenic shock improves survival: a meta‐analysis. JACC Cardiovasc Interv 2017; 10: 1805–1806. [DOI] [PubMed] [Google Scholar]
- 59. Basir MB, Schreiber T, Dixon S, Alaswad K, Patel K, Almany S, Khandelwal A, Hanson I, George A, Ashbrook M, Blank N. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: the Detroit Cardiogenic Shock Initiative. Catheter Cardiovas Intervs 2018; 91: 454–461. [DOI] [PubMed] [Google Scholar]
- 60. Basir MB., Kapur NK., Patel K., Salam M.A., Schreiber T., Kaki A., Hanson I., Almany S., Timmis S., Dixon S., Kolski B., Todd J., Senter S., Marso S., Lasorda D., Wilkins C., Lalonde T., Attallah A., Larkin T., Dupont A., Marshall J., Patel N., Overly T., Green M., Tehrani B., Truesdell A.G., Sharma R., Akhtar Y., McRae T. III, O'Neill B., Finley J., Rahman A., Foster M., Askari R., Goldsweig A., Martin S., Bharadwaj A., Khuddus M., Caputo C., Korpas D., Cawich I., McAllister D., Blank N., Alraies M.C., Fisher R., Khandelwal A., Alaswad K., Lemor A., Johnson T., Hacala M., O'Neill W.W., on behalf of the National Cardiogenic Shock Initiative Investigators Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv 2019;93:1173–1183. [DOI] [PubMed] [Google Scholar]
- 61. Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi‐Nicolau J, Alvarez Villela M, Welt F, Stehlik J, Gilbert EM, Wever‐Pinzon O, Morshedzadeh JH, Dranow E, Selzman CH, Fang JC, Drakos SG. Shock team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support. Circulation 2019; 140: 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol 2013; 61: 1209–1221. [DOI] [PubMed] [Google Scholar]
- 63. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel T, Cotter G, SHOCK Investigators . Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004; 44: 340–348. [DOI] [PubMed] [Google Scholar]
- 64. Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation [published correction appears in J Heart Lung Transplant. 2017 Nov;36(11):1272]. J Heart Lung Transplant 2016; 35: 67–73. [DOI] [PubMed] [Google Scholar]
- 65. Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW, TandemHeart Investigators Group . A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006; 152: 469.e1–469.e4698. [DOI] [PubMed] [Google Scholar]
- 66. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott‐Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra‐aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008; 52: 1584–1588. [DOI] [PubMed] [Google Scholar]
- 67. Garan AR, Takeda K, Salna M, Vandenberge J, Doshi D, Karmpaliotis D, Kirtane AJ, Takayama H, Kurlansky P. Prospective comparison of a percutaneous ventricular assist device and venoarterial extracorporeal membrane oxygenation for patients with cardiogenic shock following acute myocardial infarction. J Am Heart Assoc 2019; 8: e012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, Burkhoff D. Mechanical circulatory support devices for acute right ventricular failure. Circulation 2017; 136: 314–326. [DOI] [PubMed] [Google Scholar]
- 69. Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail 2012; 5: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lawler PR, Mehra MR. Advancing from a “hemodynamic model” to a “mechanistic disease‐modifying model” of cardiogenic shock. J Heart Lung Transplant 2018; 37: 1285–1288. [DOI] [PubMed] [Google Scholar]
- 71. van Diepen S, Hochman JS, Stebbins A, Alviar CL, Alexander JH, Lopes RD. Association between delays in mechanical ventilation initiation and mortality in patients with refractory cardiogenic shock [published online ahead of print, 2020 May 20]. JAMA Cardiol 2020; 5: 965–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FG, Kuvin JT, Lopez‐Sendon J, McAreavey D, Nallamothu B, Page RL 2nd, Parrillo JE, Peterson PN, Winkelman C, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Council on Quality of Care and Outcomes Research . Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation 2012; 126: 1408–1428. [DOI] [PubMed] [Google Scholar]
- 73. Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr, Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr, Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15‐year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc 2019; 8: e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A, Deshmukh AJ. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc 2018; 7: e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Diepen S, Baran DA, Mebazaa A. What is the role of medical therapy in cardiogenic shock in the era of mechanical circulatory support? Can J Cardiol 2020; 36: 151–153. [DOI] [PubMed] [Google Scholar]


