Abstract
Aims
Iron deficiency (ID) occurs in about 50% of patients with heart failure (HF). The European Society of Cardiology (ESC) recommends ID diagnostic testing in newly diagnosed patients with HF and during follow‐up, with intravenous iron supplementation (IS) only recommended in patients with HF with reduced ejection fraction (HFrEF). This study aimed to assess prevalence, clinical characteristics, and application of ESC guidelines for ID and IS in patients with HF in the real‐life clinical setting.
Methods and results
The French transversal multicentre OFICSel registry (300 cardiologists) conducted in 2017 included patients hospitalized for HF at least once in the previous 5 years. Diverse adult patients were eligible including inpatients and outpatients and those with acute and chronic HF. Data were collected from cardiologists and patients using study‐specific surveys. Data included demographic and clinical data, as well as HF and ID management data. Overall, 2822 patients, mainly male (69.3%) with a median age of 69 years (interquartile range 58–78), were included. A total of 1075 patients (38.1%) were tested for ID, with 364 (33.9%) diagnosed. Of these, 168 (46.2%) received IS: 128 (76.2%) intravenous IS and 40 (23.8%) oral. Among the 201 patients with HFrEF diagnosed with ID, 99 (49.3%) received IS: 79 (79.8%) intravenous IS and 20 (20.2%) oral.
Conclusions
In clinical practice, only one‐third of patients with HF had a diagnostic test for ID. In patients with ID with HFrEF, only 39.3% received intravenous IS as recommended. Thus, in general, cardiologists should be encouraged to follow the ESC guidelines to ensure optimal treatment for patients with HF.
Keywords: Heart failure, Iron deficiency, Iron supplementation, Diagnosis, Guidelines
Introduction
Chronic heart failure (HF) is a main public health issue with a substantial economic burden. Annually in France, there are more than a million patients with HF with 165 000 hospitalizations, with the cost of hospitalization estimated to be 2.5 billion euros. 1
Iron deficiency (ID) is described in approximately half of patients with HF. 2 ID, according to the European and American guidelines, is defined as having a ferritin level <100 μg/L, or a level between 100 and 299 μg/L with a transferrin saturation < 20%. 3 , 4 ID, in patients with HF, may be associated with anaemia. Several studies have demonstrated that ID is associated with HF severity in terms of New York Heart Association (NYHA) classification, exercise performance, 5 , 6 health‐related quality of life, 7 and higher hospitalization and death rates, irrespective of the presence of anaemia. 2 , 6 , 8 Iron supplementation (IS) for patients with HF with ID clinically improves exercise capacity and quality of life and reduces hospitalizations. 9 , 10 , 11 A very recent French analysis even suggests that IS could reduce hospitalization costs for worsening HF (by 35.8 million euros) and HF follow‐up costs (by 2.9 million euros) for the French national health insurance. 12 Consequently, current guidelines published by the European Society of Cardiology (ESC) suggest systematic ID diagnostic testing in newly diagnosed patients with HF and intravenous IS only in patients with heart failure with reduced ejection fraction (HFrEF). 3 Currently, there is a lack of real‐life data on how these guidelines are applied in terms of ID diagnosis and IS.
As part of the multicentre ‘Observatoire Français de l'Insuffisance Cardiaque et du Sel’ (OFICSel) registry, we aimed to describe ID prevalence in patients with HF; to assess the clinical application of the ESC guidelines, specifically concerning ID diagnosis and IS; and to investigate the epidemiological characteristics of patients with HF tested for ID and those subsequently treated.
Methods
Study design
OFICSel is a non‐interventional, observational, transversal, multicentre registry performed by the French Heart Failure Group [‘Groupe Insuffisance Cardiaque et Cardiomyopathies de la Société Française de Cardiologie’ (GICC)] of the French Society of Cardiology. Overall, about 300 cardiologists from private and hospital practices participated in the registry present in each of the 13 regions of France. Patients older than 18 years, both inpatients and outpatients, with acute or chronic HF hospitalized for acute HF at least once in the previous 5 years were eligible. Patients who did not understand French were not eligible. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (ethics committee number, CNIL‐916224; clinical trial number, CCTIRS No. 16‐109). This study followed the STROBE reporting guideline for cross‐sectional studies.
Data collection
Data were collected from patients and their cardiologists using study‐specific surveys. The patient's survey collected data on socio‐demographic, medical history, and quality of life (Minnesota Living with Heart Failure Questionnaire). The cardiologist's survey collected data on HF type (right, left, or global); the cause of HF; date of HF diagnosis; echocardiographic characteristics [left ventricular ejection fraction (LVEF)]; electrocardiogram data (sinus rhythm or atrial fibrillation); biological data [haemoglobin, N‐terminal prohormone of B‐type natriuretic peptide (NT‐proBNP), and serum creatinine concentrations]; medical treatments [including the type of anticoagulant with vitamin K antagonist (VKA) or novel oral anticoagulants (NOAC) and antiplatelet therapy]; and whether a multisite and/or implantable cardioverter defibrillator had been implanted.
Iron deficiency assessment
The cardiologist provided information on whether or not an ID diagnostic test had been performed. In patients who had ID diagnostic testing, the cardiologist specified whether or not the patient had ID: defined as ferritinaemia <100 μg/L or ferritinaemia between 100 and 299 μg/L and a transferrin saturation <20%. 3 The first assay performed was ferritinaemia; then only if the ferritinaemia value was between 100 and 299 μg/L was a transferrin saturation assay carried out. For patients diagnosed with ID, the cardiologist noted if IS was implemented. Data concerning anaemia were collected. Anaemia, using the laboratory test closest to registration, was defined as a haemoglobin level <12.0 g/dL in women and <13.0 g/dL in men, according to the World Health Organization recommendations. 13
Statistical analysis
Continuous variables are expressed as medians [interquartile ranges (IQRs)], and categorical variables as numbers or frequencies (%). Patient characteristics were compared using the χ 2 test for categorical data or using the Student's unpaired t‐test for continuous data. Multivariable logistic regression modelling was used to identify variables independently associated with ID diagnosis and with IS and to compute adjusted odds ratios (ORs) with 95% confidence intervals (CIs). The multivariable models were built using backward stepwise variable selection, with exit criteria set at P ≤ 0.1 to limit model overfitting. A two‐sided P‐value <0.05 was considered statistically significant. All statistical analyses were performed using STATA software, Version 15.1 (StataCorp, College Station, TX, USA).
Results
Participants of OFICSel study
From March to June 2017, 2822 patients were included. The study participant flow chart is shown in Figure 1 . The clinical and biological characteristics of the patients with HF in the study are provided in Table 1 . Overall, patients with HF were mostly male (69.1%), the median age was 69 years (IQR 58–78), and the median LVEF was 36% (IQR 29–50). Of the 2680 patients with data, 53.8% had HFrEF, 21.0% heart failure with mid‐range ejection fraction (HFmEF), and 25.2% heart failure with preserved ejection fraction (HFpEF). Moreover, 427 (16.4%) patients were diagnosed with HF within the previous 3 months. Also, HF was acute in 783 (27.7%) patients and chronic stable in 2039 (72.3%).
Figure 1.
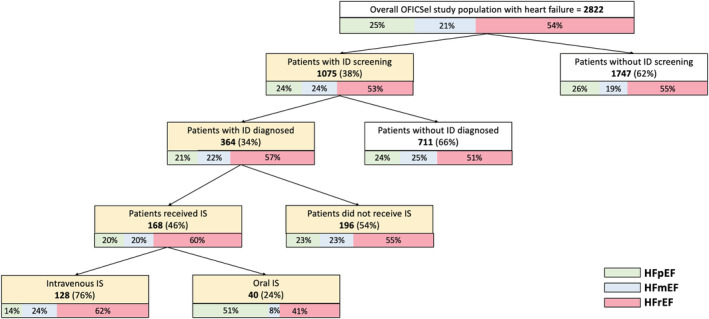
Flow diagram of patients included in the OFICSel study (n = 2822). HFmEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; IS, iron supplementation.
Table 1.
Clinical and biological characteristics of the study population depending on whether iron deficiency diagnostic testing was performed or not (n = 2822)
| All | ID diagnostic test performed | P‐value | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| n a (%) | 2822 | 1075 (38.1) | 1747(61.9) | ||
| Baseline characteristics | |||||
| Age, years (IQR) | 2729 | 69 (58–78) | 67 (56–77) | 69 (60–79) | 0.0002 |
| Male sex, n (%) | 2788 | 1956 (70.2) | 725 (68.0) | 1231 (71.5) | 0.051 |
| BMI, kg/m2 (IQR) | 2668 | 26.1 (23.2–30.1) | 26.1 (23.1–30.1) | 26.2 (23.2–30.1) | 0.577 |
| NYHA III–IV vs. I–II, n (%) | 2530 | 971 (38.4) | 350 (36.0) | 621 (39.9) | 0.049 |
| Current decompensation, n (%) | 2577 | 789 (30.6) | 313 (31.6) | 476 (30.0) | 0.507 |
| MLWHF total score (IQR) | 1090 | 48 (31–62) | 50 (31–65) | 46 (31–59) | 0.035 |
| Serum creatinine, μmol/L (IQR) | 2459 | 99.0 (76.0–128.0) | 99.0 (77.0–128.0) | 99.0 (76.0–127.0) | 0.830 |
| Haemoglobin, g/dL (IQR) | 2581 | 12.9 (11.6–14.2) | 12.9 (11.5–14.3) | 12.9 (11.7–14.1) | 0.434 |
| Anaemia, n (%) | 2581 | 1087 (42.1) | 439 (42.2) | 648 (42.1) | 0.935 |
| Baseline medical history, n (%) | |||||
| Diabetes | 2822 | 816 (28.9) | 297 (27.6) | 519 (29.7) | 0.237 |
| Hypertension | 2822 | 1578 (55.9) | 611 (56.8) | 967 (55.4) | 0.440 |
| Dyslipidaemia | 2822 | 1072 (38.0) | 416 (38.7) | 656 (37.6) | 0.542 |
| Smoking | 2822 | 321 (11.4) | 105 (9.8) | 216 (12.4) | 0.035 |
| AF | 2822 | 767 (27.2) | 271 (25.2) | 496 (28.4) | 0.065 |
| SAS | 2822 | 231 (8.2) | 88 (8.2) | 143 (8.2) | 1.000 |
| COPD | 2822 | 199 (7.1) | 70 (6.5) | 129 (7.4) | 0.379 |
| Renal dialysis | 2822 | 17 (0.6) | 10 (0.9) | 7 (0.4) | 0.077 |
| Clinical features of HF | |||||
| ICM | 2639 | 1162 (44.0) | 416 (40.9) | 746 (46.0) | 0.010 |
| LVEF, % (IQR) | 2680 | 36 (29–50) | 37 (28–48) | 35 (30–50) | 0.723 |
| HFrEF, n (%) | 1442 (53.8) | 554 (52.7) | 888 (54.5) |
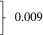
|
|
| HFmEF, n (%) | 563 (21.0) | 251 (23.9) | 312 (19.2) | ||
| HFpEF, n (%) | 675 (25.2) | 246 (23.4) | 429 (26.3) | ||
| Newly diagnosed HF < 3 months, n (%) | 2680 | 427 (16.4) | 173 (17.4) | 254 (15.8) | 0.283 |
| Last decompensation, n (%) | 2424 | ||||
| <3 months | 1068 (44.1) | 412 (43.6) | 656 (44.4) |
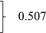
|
|
| 3 months to 1 year | 535 (22.1) | 220 (23.3) | 315 (21.3) | ||
| >1 year | 821 (33.9) | 313 (33.1) | 508 (34.3) | ||
| NT‐proBNP, pg/mL (IQR) | 1739 | 1811 (703–4384) | 1783 (663–4342) | 1855 (734–4405) | 0.332 |
| BNP, pg/mL (IQR) | 828 | 438 (177–855) | 351 (140–732) | 517 (214–948) | 0.020 |
| NT‐proBNP or BNP quartiles, n (%) | 2448 | ||||
| 1st | 615 (25.1) | 277 (27.4) | 338 (23.5) | ||
| 2nd to 3rd | 1214 (49.6) | 493 (48.8) | 721 (50.2) | 0.074 | |
| 4th | 619 (25.3) | 241 (23.8) | 378 (26.3) | ||
| Treatments, n (%) | |||||
| Diuretic | 2822 | 2144 (76.0) | 834 (77.6) | 1310 (75.0) | 0.117 |
| ACE inhibitor or ARB | 2822 | 1855 (65.7) | 694 (64.6) | 1161 (66.5) | 0.302 |
| ARNi | 2822 | 427 (15.1) | 179 (16.7) | 248 (14.2) | 0.077 |
| Beta‐blocker | 2822 | 2311 (81.9) | 925 (86.0) | 1386 (79.3) | <0.0001 |
| MRA | 2822 | 1269 (45.0) | 521 (48.5) | 748 (42.8) | 0.003 |
| VKA | 2822 | 986 (34.9) | 361 (33.6) | 625 (35.8) | 0.235 |
| NOAC | 2822 | 413 (14.6) | 160 (14.9) | 253 (14.5) | 0.769 |
| Antiplatelet therapy b | 2822 | 1330 (47.1) | 489 (45.5) | 841 (48.1) | 0.171 |
| ICD | 2822 | 725 (25.7) | 275 (25.6) | 450 (25.8) | 0.917 |
| CRT | 411 (14.6) | 158 (14.7) | 253 (14.5) | 0.875 | |
| Recruitment site | |||||
| Hospitalization | 2822 | 1350 (47.8) | 544 (56.7) | 806 (51.0) | |
| Consultation | 908 (32.2) | 300 (31.3) | 608 (38.5) | 0.001 | |
| Cardiac rehabilitation | 283 (10.0) | 116 (12.0) | 167 (10.5) | ||
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation/flutter; ARB, angiotensin receptor blocker; ARNi, inhibitor of angiotensin and neprilysin; BMI, body mass index; BNP, B‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; HFmEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; ICM, ischaemic cardiomyopathy; ID, iron deficiency; IQR, interquartile range; LVEF, left ventricular ejection fraction; MLWHF, Minnesota Living with Heart Failure Questionnaire with ≥18 items completed; MRA, mineralocorticoid antagonist; NOAC, novel oral anticoagulants; NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide; NYHA, New York Heart Association; SAS, sleep apnoea syndrome; VKA, vitamin K antagonist.
Number of data available.
Corresponded to aspirin, clopidogrel, ticagrelor, or prasugrel.
Regarding HF treatment, 2440 patients (86.5%) were treated with either an angiotensin‐converting enzyme inhibitor, an angiotensin receptor blocker, or an inhibitor of angiotensin and neprilysin; 2311 (81.9%) were treated with a beta‐blocker; 1269 (45.0%) received a mineralocorticoid antagonist; 2144 (76.0%) received a diuretic; 725 (25.7%) had an implantable cardioverter defibrillator; and 411 (14.6%) had received cardiac resynchronization therapy. Concerning anticoagulant and antiplatelet therapy, of the 2822 patients, 986 (34.9%) received VKA, 413 (14.6%) NOAC, and 1330 (47.1%) antiplatelet therapy.
Prevalence of iron deficiency and clinical characteristic of the patients according to iron deficiency status
Among the 2822 patients, 1075 (38.1%) patients had ID diagnostic testing within the previous year. Among those, 364 (33.9%) were diagnosed with ID.
Compared with patients without ID diagnostic test, patients with ID diagnostic test were younger (median age 67 vs. 69 years; P = 0.0002) and were less frequently smokers (P = 0.035). Regarding the type of HF, patients with ID diagnostic testing had ischaemic heart disease more frequently (P = 0.027). There were no differences between groups (patients with diagnostic testing vs. those without testing) regarding the median LVEF (P = 0.723), time from last decompensation (P = 0.507), and diagnosis of HF within the previous 3 months (P = 0.283). Similarly, concerning disease severity, there were no differences between groups for the rates of NYHA class III or IV (P = 0.231), median NT‐proBNP plasma concentration (P = 0.332), nor diuretic use (P = 0.117). Patients with ID diagnostic testing had beta‐blocker (P < 0.0001) and mineralocorticoid antagonist treatment (P = 0.003) more frequently than those without ID diagnostic testing (Table 1 ).
In multivariable analysis, ID diagnostic testing was more frequently performed in younger patients (OR 0.991, 95% CI 0.985–0.998; P = 0.013), non‐smokers (OR 0.65, 95% CI 0.48–0.87; P = 0.004), patients with lower body mass index (OR 0.80, 95% CI 0.67–0.96; P = 0.015), and those with beta‐blocker (OR 1.44, 95% CI 1.13–1.83; P = 0.003). Moreover, ID diagnostic testing occurred more frequent during hospitalizations than during consultations (Table 2 ).
Table 2.
Multivariable logistic regression analysis for iron deficiency performed, iron deficiency diagnosed, and iron supplementation performed
| ID performed | ID diagnosed | IS performed | ||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
| Gender, male | — | — | 0.55 (0.75–1.03) | 0.075 | — | — |
| Age | 0.991 (0.985–0.998) | 0.013 | — | — | — | — |
| BMI | 0.80 (0.67–0.96) | 0.015 | — | — | — | — |
| Smoking | 0.65 (0.48–0.87) | 0.004 | — | — | — | — |
| Beta‐blocker | 1.44 (1.13–1.83) | 0.003 | — | — | — | — |
| VKA | — | — | — | — | 2.40 (1.48–3.88) | 0.0004 |
| Recruitment site | ||||||
| Hospitalization | 1 (ref) | — | — | 1 (ref) | ||
| Consultation | 0.68 (0.56–0.82) | <0.0001 | — | — | 1.89 (1.06–3.39) | 0.032 |
| Cardiac rehabilitation | 0.94 (0.71–1.25) | 0.673 | — | — | 2.95 (1.34–6.49) | 0.007 |
| Current decompensation | — | — | 2.83 (2.09–3.83) | <0.0001 | — | — |
| LVEF, % | — | — | 0.98 (0.96–0.99) | 0.0006 | — | — |
| Haemoglobin | — | — | 0.76 (0.71–0.83) | <0.0001 | 0.88 (0.78–0.99) | 0.031 |
| NT‐proBNP or BNP quartiles | ||||||
| 1st | — | — | — | — | 1 (ref) | |
| 2nd to 3rd | — | — | — | — | 1.23 (0.60–2.51) | 0.574 |
| 4th | — | — | — | — | 2.39 (1.09–5.21) | 0.029 |
BMI, body mass index; BNP, B‐type natriuretic peptide; CI, confidence interval; ID, iron deficiency; IS, iron supplementation; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide; VKA, vitamin K antagonist.
Patients with iron deficiency
The clinical and biological characteristics of the 1075 patients tested for ID according to ID are shown in Supporting Information, Table S1 . Patients with ID were older (median age 69 vs. 66 years; P = 0.028), were more frequently women (36.0% vs. 29.9%; P = 0.044), had a higher median LVEF (P = 0.039), and had ischaemic heart disease (P = 0.0004) compared with those without ID. Patients with ID had more severe HF disease: higher rates of NYHA class III or IV and acute HF, a higher median NT‐proBNP plasma concentration, increased diuretic use, and a shorter time interval from the last decompensation (all P < 0.0001). Figure 2 shows the prevalence of ID according to NYHA and LVEF classes. The prevalence of ID increases with NYHA class severity. HF times of onset were similar in patients with or without ID (P = 0.44). Patients with diagnosed ID had more anaemia and kidney dysfunction (both P < 0.0001). Also, patients with ID were more frequently treated with beta‐blockers (P = 0.003) and VKA (P = 0.001). There was no difference between groups regarding the prescription of NOAC (P = 0.565) or antiplatelet therapy (P = 0.567). The ID prevalence was higher in patients with anaemia than patients without anaemia (P < 0.001; Figure 3 ).
Figure 2.
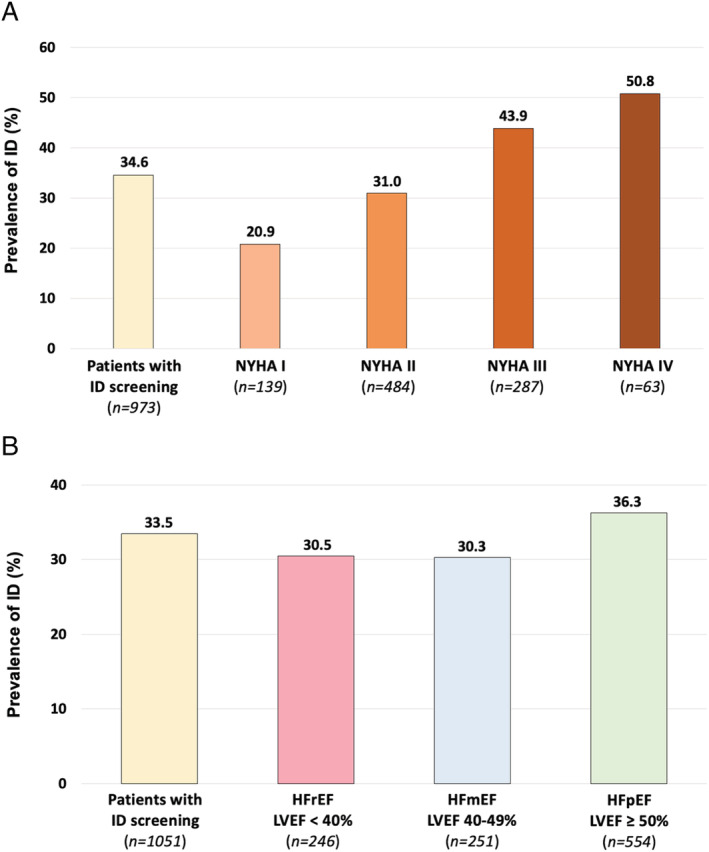
Prevalence of ID in patients stratified by NYHA class (A) and LVEF value (B). Bar chart of the ID prevalence in the 973 patients received ID diagnostic test and with NYHA status assessed (n = 973 of 1075), stratified by NYHA class (A), and the 1051 patients received ID diagnostic test and with LVEF value assessed, stratified by LVEF value (B). HFmEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Figure 3.
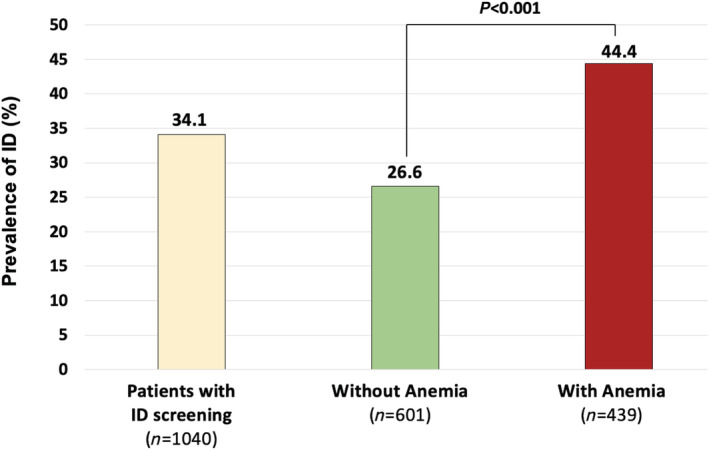
Prevalence of ID in patients stratified by the presence of anaemia. Bar chart of the ID prevalence in the 1040 patients received ID diagnostic test and with anaemia status assessed, stratified by the presence of anaemia. ID, iron deficiency.
In multivariable analysis, the presence of ID was independently associated with a higher rate of current acute HF (OR 2.83, 95% CI 2.09–3.83; P < 0.0001), with lower haemoglobin levels (OR 0.76, 95% CI 0.71–0.83; P < 0.0001), and lower LVEF (OR 0.98, 95% CI 0.96–0.98; P = 0.0006) (Table 2 ).
Patients receiving iron supplements
Clinical and biological characteristics of 364 patients diagnosed with ID according to IS are shown in Table 3 . Among those, 168 (46.2%) received IS of whom 128 (76.2%) were treated with intravenous IS (Table S2) and 40 (23.8%) with oral iron. Among the patients diagnosed with ID, there were 91/213 patients (42.7%) treated with IS during hospitalizations, 41/82 patients (50.0%) during consultations, and 22/36 patients (61.1%) during cardiac rehabilitations. Regarding the LVEF value, of those patients diagnosed with ID, there were 33/75 patients with HFpEF (44.0%), 34/76 patients with HFmEF (44.7%), and 99/201 patients with HFrEF (49.3%) treated with IS. Compared with patients with no IS, patients with IS more frequently had anaemia (P = 0.040), treatment with VKA (P < 0.0001), higher serum creatinine levels (P = 0.011), and higher rates of renal dialysis (P = 0.030). There was no difference between groups regarding the median LVEF (P = 0.423), recruitment site (P = 0.095), or number of patients recently diagnosed with HF within 3 months of registration (P = 0.992). Among the 201 patients with HFrEF with diagnosed ID, only 99 (49.3%) received IS: 79 (79.8%) intravenously and 20 (20.2%) orally.
Table 3.
Clinical and biological characteristics of patients diagnosed with iron deficiency (n = 364) according to whether iron supplementation was performed
| ID diagnosed | P‐value | ||
|---|---|---|---|
| With IS | Without IS | ||
| n (%) | 168 (46.2) | 196 (53.8) | |
| Baseline characteristics | |||
| Age, years (IQR) | 69 (58–78) | 69 (58–78) | 0.920 |
| Male sex, n (%) | 101 (60.5) | 128 (67.0) | 0.199 |
| BMI, kg/m2 (IQR) | 26.1 (23.3–30.8) | 25.4 (22.6–30.0) | 0.167 |
| NYHA III–IV vs. I–II, n (%) | 70 (47.0) | 85 (45.2) | 0.747 |
| Current decompensation, n (%) | 75 (48.3) | 90 (48.6) | 0.962 |
| MLWHF total score (IQR) | 52 (40–69) | 51 (35–65) | 0.416 |
| Serum creatinine, mg/dL (IQR) | 108 (80–150) | 105 (77–136) | 0.011 |
| Haemoglobin, g/dL (IQR) | 11.9 (10.3–13.1) | 12.4 (11.0–13.9) | 0.005 |
| Anaemia, n (%) | 98 (60.9) | 97 (50.0) | 0.040 |
| Baseline medical history, n (%) | |||
| Diabetes | 65 (38.7) | 62 (31.6) | 0.159 |
| Hypertension | 107 (63.7) | 117 (59.7) | 0.435 |
| Dyslipidaemia | 81 (48.2) | 81 (41.3) | 0.187 |
| Smoking | 13 (7.7) | 28 (14.3) | 0.049 |
| AF | 51 (30.4) | 49 (25.0) | 0.254 |
| SAS | 12 (7.1) | 21 (10.7) | 0.237 |
| COPD | 14 (8.3) | 21 (10.7) | 0.442 |
| Renal dialysis | 4 (2.4) | 0 (0.0) | 0.030 |
| Clinical features of HF | |||
| ICM, n (%) | 83 (51.2) | 84 (45.4) | 0.278 |
| LVEF % (IQR) | 35 (25–45) | 35 (27–46) | 0.423 |
| HFrEF, n (%) | 99 (59.6) | 102 (54.8) |
|
| HFmEF, n (%) | 34 (20.5) | 42 (22.6) | |
| HFpEF, n (%) | 33 (19.9) | 42 (22.6) | |
| Newly diagnosed HF < 3 months, n (%) | 26 (16.0) | 28 (16.1) | 0.992 |
| Last decompensation, n (%) | |||
| <3 months | 84 (54.2) | 96 (54.2) |

|
| 3 months to 1 year | 42 (27.1) | 30 (16.8) | |
| >1 year | 29 (18.7) | 51 (28.8) | |
| NT‐proBNP, pg/mL (IQR) | 3169 (1250–9000) | 2059 (850–4353) | 0.003 |
| BNP, pg/mL (IQR) | 361 (193–1011) | 468 (233–748) | 0.130 |
| NT‐proBNP or BNP quartiles, n (IQR) | |||
| 1st | 23 (14.0) | 36 (18.9) | |
| 2nd to 3rd | 80 (48.8) | 112 (58.9) | 0.007 |
| 4th | 61 (37.2) | 42 (22.1) | |
| Treatments, n (%) | |||
| Diuretic | 145 (86.3) | 166 (84.7) | 0.063 |
| ACE inhibitor or ARB | 105 (62.5) | 130 (66.3) | 0.447 |
| ARNi | 27 (16.1) | 24 (12.2) | 0.294 |
| Beta‐blocker | 142 (84.5) | 155 (79.1) | 0.182 |
| MRA | 91 (54.2) | 92 (46.9) | 0.169 |
| VKA | 104 (61.9) | 42 (21.4) | <0.0001 |
| NOAC | 22 (13.1) | 29 (14.8) | 0.641 |
| Antiplatelet therapy a | 73 (43.5) | 97 (49.5) | 0.250 |
| ICD | 54 (32.1) | 43 (21.9) | 0.028 |
| CRT | 28 (16.7) | 28 (14.3) | 0.530 |
| Recruitment site, n (%) | |||
| Hospitalization | 91 (59.1) | 122 (68.9) | |
| Consultation | 41 (26.6) | 41 (23.2) | 0.095 |
| Cardiac rehabilitation | 22 (14.3) | 14 (7.9) | |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation/flutter; ARB, angiotensin receptor blocker; ARNi, inhibitor of angiotensin and neprilysin; BMI, body mass index; BNP, B‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; HFmEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; ICM, ischaemic cardiomyopathy; ID, iron deficiency; IQR, interquartile range; IS, iron supplementation; LVEF, left ventricular ejection fraction; MLWHF, Minnesota Living with Heart Failure Questionnaire with ≥18 items completed; MRA, mineralocorticoid antagonist; NOAC, novel oral anticoagulants; NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide; NYHA, New York Heart Association; SAS, sleep apnoea syndrome; VKA, vitamin K antagonist.
Corresponded to aspirin, clopidogrel, ticagrelor, or prasugrel.
In multivariable analysis, IS treatment was independently associated with a higher prescription rate of VKA or NOAC (OR 2.05, 95% CI 1.26–3.32; P = 0.004), a higher NT‐proBNP plasma concentration (OR 2.26, 95% CI 1.02–4.99; P = 0.044), a lower haemoglobin level (OR 0.86, 95% CI 0.76–0.97; P = 0.012), and a lower LVEF (OR 0.98, 95% CI 0.96–0.99; P = 0.030) than those without IS (Table 2 ).
Discussion
The OFICSel registry obtained data that are highly representative of the French HF population. Patients were included from various healthcare structures throughout France. Our results show that ESC recommendations that ID diagnostic testing be performed in all patients with HF and intravenous IS be administered in those patients with HFrEF diagnosed with ID are not being followed. 3 Furthermore, the prevalence of ID in patients tested was 34%. Multivariate analyses found that patients' age, non‐ischaemic cardiomyopathy, non‐smoking, beta‐blocker treatment, and the recruiting healthcare centre were associated with increased ID diagnostic testing, while LVEF, haemoglobin levels, anticoagulant treatment, and NT‐proBNP levels were associated with prescription of IS (intravenous or oral).
The population included in this study was approximately a decade younger than the European HF population in the community 14 but similar to the large French ODIN cohort study. 15 Indeed, in this type of study using a self‐administered questionnaire, patients are often younger than in registry studies. In our study, the subgroup of patients with reduced LVEF were generally well treated, medically, with results similar to data from European registers for patients receiving an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (90.1% and 93.2%, respectively), a beta‐blocker (92.3% and 92.2%, respectively), or a mineralocorticoid antagonist (61.0% and 67.8%, respectively). 14 Moreover, the distribution of LVEF was fairly similar in our study and the European register [median 36% (29–50%) and median 39% (30–52%), respectively]. 14
In our study population, only 38.1% of patients with HF underwent ID diagnostic testing as recommended by the current guidelines. 3 , 16 Although the ESC guidelines recommend ID diagnosis in stable patients, 31.6% of patients underwent ID diagnostic testing during a hospitalization for HF decompensation. These findings are surprising because cardiologists participating in the OFICSel registry routinely treat patients with HF. It is unlikely that ID test effectiveness nor regulatory hurdles hamper prescription. Several reasons may explain the non‐systematic ID diagnostic testing. Firstly, the study was performed only 1 year after the guidelines were published: perhaps more time is required for these to be applied in real life. However, a more recent study published in 2018 reported similar results. 17 Secondly, HF is a complex disorder with various treatment strategies available. Although several treatments are known to decrease mortality, there is no evidence that IS reduces mortality. Consequently, ID and IS may not be of primary concern for HF treatment. However, IS may be beneficial and may result in sustainable improvement in functional capacity, symptoms and quality of life, and a significant reduction in hospitalizations for worsening HF. 10 Very recently, the AFFIRM‐AHF trial, in patients with HFrEF with ID who were stabilized after an episode of acute HF, showed that IS was safe and reduced the risk of HF hospitalizations, with no apparent impact on the risk of cardiovascular death. 18 For these reasons, expert working groups have published position papers to increase awareness among cardiologists concerning ID and interpretation of the ID test results. 16 , 17
In our study, the prevalence of ID, in patients tested, was 33.9%, lower than the 40–70% previously reported. 2 This is probably due to the non‐systematic ID diagnostic testing in our study. Our results are similar to those previously reported 19 , 20 and highlight the importance of ID, not only a predisposing factor for HF but also a consequence of HF severity. 21 , 22
There was no difference in ID diagnostic rates in patients newly diagnosed with HF, that is, within the last 3 months. However, current acute HF status was independently associated with ID diagnosis, as previously reported. 23 , 24 This suggests that ID diagnostic test during decompensation or worsening of the disease may be valuable, even in patients without ID at HF diagnosis. It is unlikely that the high incidence of ID in patients with acute HF in our study is related to initial haemodilution of the iron parameters, as reported for plasma haemoglobin. 25 Indeed, Androne et al. reported that in patients with anaemic chronic HF, 46% had haemodilution and 54% true anaemia. 25 Inflammation and ID may be associated, given that HF is an inflammatory condition, especially when decompensated. Iron levels are known to fluctuate as HF evolves: patients may become ID even if they had normal levels at HF diagnosis, 26 , 27 and ID prevalence for acute HF decreases from hospital admission to 30 days after discharge. 28 HF evolution is a complex process with several co‐morbidities, 21 especially in patients with HFpEF. 29 Current guidelines recommend ID diagnostic test at HF diagnosis. 3 However, patients with HF may benefit from more regular follow‐up and monitoring of ID, and when required IS, particularly during HF evolution, for example, the onset of AF or anaemia. The guidelines need to be updated to include continuous ID monitoring. 3 Practical recommendations that complement the guidelines have been published. They suggest that iron levels be evaluated in patients with existing chronic HF, particularly when HF is severe and symptomatic despite optimal HF treatment. 17 They also suggest that iron levels be assessed once or twice a year, as well as after hospitalization for patients with decompensated HF. 17 Our results show that there is a need for implementing these practical recommendations. We also suggest that studies be developed to access evidence‐based strategies for clinicians to support recommendations. Furthermore, specifically in patients with HFpEF, large morbimortality trials adapted to the evolution of co‐morbidities and specific risk factors should be proposed.
Regarding the cause of ID, several studies have reported that iron in patients with HF may be depleted by gastrointestinal bleeding from or exacerbated by anticoagulants. 21 , 30 Indeed, in our study, patients receiving VKA, as anticoagulants, had ID diagnosed more frequently. Interestingly, there was no difference in ID diagnostic rates in patients prescribed NOAC, compared with those prescribed VKA, even though lower rates of gastrointestinal bleeding are reported with NOAC. 31 As expected, patients diagnosed with ID more often had anaemia and kidney dysfunction. 27 In patients with ID and anaemia, the guidelines suggest investigating the underlying causes of anaemia and excluding other pathologies. 3 , 17
The current ESC guidelines recommend intravenous IS for treating ID in patients with symptomatic HFrEF. 3 Indeed, several studies have shown that oral IS is not as effective as intravenous IS in these patients. 32 Oral IS does not replenish iron stores nor improve the clinical status of patients with HF. 32 Moreover, oral IS is poorly tolerated in patients with HF, with more than 50% experiencing gastrointestinal side effects. 33
In our study, 1442 patients (53.8%) had HFrEF. Of these, 554 (38.4%) had a diagnostic test for ID. ID was diagnosed in 201 patients, with 36.3% of patients with HFrEF tested. Finally, of the 201 patients with HFrEF diagnosed with ID, 99 patients (49.3%) received IS: 39.3% received the recommended intravenous IS, and 10.0% oral IS. Overall, despite the potential benefit of intravenous IS, only a small proportion of patients with HFrEF benefited from optimal treatment with intravenous IS.
Perhaps clinicians are unaware of the superiority of intravenous IS. These findings suggest that guidelines are not consistently been applied in the real‐life clinical setting, as previously reported. 34 In the clinical setting, there are not only ethical, societal, and organizational constraints but also logistic issues. Indeed, as previously mentioned, intravenous IS, in France, requires a short‐stay hospitalization to manage possible hypersensitivity. 9 Consequently, intravenous IS is more expensive than oral IS. Interestingly, rules and regulations concerning the administration of intravenous IS vary among countries. 17
What about IS in patients other than those with HFrEF? The ESC guidelines recommend IS only in patients with HFrEF. 3 However, in patients with HFpEF, ID is associated with worse functional capacity compared with patients with HFrEF. 35 , 36 Interestingly, of the 76 patients with HFmEF diagnosed with ID, 34 (44.7%) received IS. Similarly, of the 75 patients with HFpEF diagnosed with ID, 33 (44.0%) received IS. Currently, in France, the use of IS for ID for patients with HFpEF is not established. In contrast, the American guidelines suggest that IS may be reasonable in all patients with symptomatic HF. 4 Hopefully, the ongoing Phase II FAIR‐HFpEF trial (NCT03074591) assessing intravenous IS in patients with HFpEF will determine the usefulness of IS in this population.
Study limitations
Our study has several limitations. Firstly, there is a possible selection bias. Furthermore, patients with consecutive HF were prospectively enrolled at each centre. However, for logistical reasons, each centre or cardiologist was initially only provided with 10 study‐specific surveys, with further surveys supplied on request. Secondly, our study population was on average younger (by about 10 years) and with more optimal treatment compared with most published HF registers. 3 Enrolled patients had to complete a lengthy study‐specific survey, which may have deterred older patients from participating. Thirdly, data concerning ID diagnostic testing and IS were based on declarative data from the patient's referring cardiologist. We assume that the cardiologist participating in the study would be aware of ID diagnostic testing or IS previously performed. The definition of ID was reported in the questionnaire to avoid any bias of interpretation of the presence or not of ID, and this definition was in accordance with the guidelines. 3 Moreover, the presence of a history of inflammatory disease that could influence the prevalence of ID was not collected in this study. Fourthly, we are unable to distinguish between absolute and functional ID in our data. 3 However, according to the guidelines, patients with HFrEF, with either absolute or functional ID, should be treated with intravenous IS. 37 Fifthly, the recommended intravenous IS is not easily accessible. In France, intravenous IS requires hospitalizations. Thus, cardiologists may be reluctant to test for ID because a diagnosis would imply that intravenous IS is not easily accessible. Sixthly, the result suggesting a significant association between VKA uptake and ID is limited by the lack of data on the international normalized ratio (INR) value in the study. Moreover, we did not collected details concerning the specific intravenous IS administered. Indeed, both ferric carboxymaltose and iron sucrose complex are available, but only ferric carboxymaltose is recommended by the ESC. Finally, as OFICSel is a large registry including physicians from different practices and patients with different type of HF, it might be representative of HF population in France. However, the extrapolation of these findings to other countries or specialists could be limited.
Conclusions
Of the 2822 patients with HF included in the multicentre OFICSel registry, only 38% of patients had an ID diagnostic test. This is despite the current ESC guidelines that recommend ID diagnostic testing in all patients with HF. Among the patients tested, more than a third were diagnosed with ID. Regarding IS, fewer than half of patients with HFrEF with diagnosed ID received IS. Our results suggest that cardiologists should be encouraged to follow the ESC guidelines, concerning ID testing and IS, to ensure optimal treatment for patients with HF.
Conflict of interest
T.P. received research grants from Servier. T.L. received consultant fees from Novartis. L.K.T. received consultant fees from Vifor. F.R. received consultant fees from Servier, Medtronic, Astra‐Zeneca, Novartis, MSD, Amgen, Sanofi, Pfizer, Mylan, and Boehringer. T.D. received consultant fees and research support from Novartis, Vifor, Pfizer, Alnylam, Akcea, and Resmed. A.C.S. received consultant fees and grants from Vifor, AstraZeneca, Bayer, Boehringer Ingelheim, WeHealth, MSD, and Novartis. The other authors declare that they have no conflicts of interest concerning this article.
Funding
This work was supported by Novartis, Vifor Pharma, and Daiichi Sankyo Company. None of the funding organizations played a role in the design and conduct of the study (including the collection, management, analysis, and interpretation of the data) or in the preparation, review, and approval of the manuscript.
Supporting information
Table S1. Clinical and biological characteristics of patients with iron deficiency in patients with ID diagnostic testing (n = 1075).
Table S2. Clinical and biological characteristics of patients diagnosed with iron deficiency according to whether intravenous iron supplementation was performed. (n = 364).
Acknowledgements
The OFICSel team would like to thank the French Heart Failure Group [Groupe Insuffisance Cardiaque et Cardiomyopathies (GICC)] of the French Society of Cardiology.
The OFICSel team would like to thank all the investigators (list not exhaustive) in private practice and their patients: Dr Kessler (Abreschviller); Dr Pollet (Aix‐les‐bains); Drs Luc Boulain and Jérôme Taieb (Aix‐en‐Provence); Dr Jean‐Jacques Leandri (Ajaccio); Drs François Pernin and Agnès Zumer (Alfortville); Drs J.M. Dupuis, Alain Furber, and Dr F. Rouleau (Angers); Dr Roland Carlioz (Avignon); Drs Emmanuel Roux and Stéphane Arques (Aubagne); Dr Nguyen Van Hung (Aurillac); Dr C. Monpere (Ballan‐Miré); Dr Benoit Cypriani (Besançon); Dr Laurent Sebbag (Bron); Dr Jean‐Paul Faure (Brive); Dr Lech Leszczynski (Brive La Gaillarde); Dr Gilles Sitruk (Brunoy); Dr Marc Essayagh (Cagnes‐sur‐mer); Dr Jacques Gauthier (Cannes); Dr Alain Ettner (Champigny‐sur‐Marne); Dr Laurence Moulinier‐Paquet (Charenton‐le‐pont); Dr Olivier Stora (Châteaubriant); Drs Michèle Lehuhant and Claire Supper (Charleville‐Mézieres); Drs Stéphane Messager, Nathalie Guigui, and Bernard Ledouarin (Créteil); Drs Thierry Domniez and Pierre Dugrand (Carcassonne); Dr Luong Minh Vu (Châtillon); Dr J.M. Casillas (Dijon); Drs R. Brion and S. Devaud (Dieulefit); Dr B. Talamat (Dourdan); Dr Alain Sqheir (Esbly); Dr Jean‐François Rousseau (Falaise); Dr Philippe Kramarz (Franois); Dr Laure Chaufourier (Honfleur); Dr Dominique Rakotoarimanana (Jonzac); Dr Philippe Bruneau (La Ciotat); Drs Marie Bourraindeloup, Jean‐Yves Poindron, Linda Larmé, Philippe Derkx, Christian Berges, and Céline Vaucelle (La Varenne Saint‐Hilaire); Dr Frédéric Mouquet (Lille); Dr Carole Abello (Macon); Dr Olivier Gartenlaub (Maisons‐Alfort); Drs Philippe Chazouillères and Philippe Verschueren (Montereau‐Fault‐Yonne); Drs Vladimir Ciobotaru and Monique Loscos (Nîmes); Dr Edouard Colcher (Nieuil‐l'espoir); Dr Frédéric Feldman (Nogent‐sur‐Marne); Drs Carpentier and Pierre Lemaire (Oignies); Dr Benoît Moquet (Paimpol); Drs Elsa Abitbol, Noël Allegrini, David Bacquet, Philippe Duc, Fatima Gueffaf, Dominique Guedj, Olivier Hoffman, Olivier Jobard, Maud Laporte, Anouk Meizel, Léon Ouazanna, and Pierre Sabouret (Paris); Dr Guy Jean‐Michel (Saint‐Étienne); Drs Mouloud Abès and Abderrahim Harouri (Saint‐Jean‐d'Angély); Dr Carole Dossetto (Saint‐Jean‐de‐Luz); Drs Frédéric Hurson, Nathalie Renaud, and Juliette Badie (Saint‐Maur‐des‐Fossés); Dr Tisseau (Saint‐Herblain); Dr Jean‐Maurice (Saint‐Pierre); Dr J.M. Guy (Saint‐Priest‐en‐Jarez); Dr Dionyssios Pongas (Saran); Dr Chalude Richard (Saumur); Dr Daul (Schirmeck); Dr Florian Zorès (Strasbourg); Drs Anne Leroux and Philippe Verdier (Sucy‐en‐Brie); Dr Khaled Lanouar (Thiais); Drs Amer Al Homsi and Abdel El Kenz (Vaux‐sur‐Mer); Dr Philippe Jauffrion (Villejuif); and Drs Nicolas Ivanoff and Thierry Morell (Vitry‐sur‐Seine).
The OFICSel team would also like to thank the following investigators in hospitals and rehabilitation centres and their patients: Prof Christophe Tribouilloy and Dr Catherine Szymanski (CHRU, Cardiology Department, Amiens); Dr Sebastien Terrazzoni (Private Hospital, Cardiology Department, Antony); Dr Anthony Chauvat (CHG, Cardiology Department, Argenteuil); Dr Cécile Lacote‐Roiron (Clinique, Department of Cardiology, Avignon); Drs Corone, T. Farrokhi, and A. Brucker (Rehabilitation Centre, Briis‐sous‐Forges); Prof Marie‐France Seronde (CHRU, Department of Cardiology, Besancon); Prof Christophe Meune (CHU Avicenne, Department of Cardiology, Bobigny); Prof Nicolas Mansencal (CHU Ambroise‐Paré, Boulogne‐Billancourt); Prof Jacques Mansourati and Mrs Marie Augagneur (CHRU, Brest); Prof Rémi Sabatier and Dr Damien Legallois (CHRU, Caen); Drs Stéphane Cosson and Xavier Rovanni (Private Hospital, Champigny‐sur‐Marne); Prof Michel Slama and Dr Bussière (Rehabilitation Centre, Châtillon); Drs Vincent Algalarrondon and Ludivine Elhiahou (CHU Antoine‐Béclère, Clamart); Prof Romain Eschaffier and Dr Claire Boiteux (CHRU, Department of Cardiology, Clermont‐Ferrand); Dr Arnaud Dellinger (CHG, Department of Cardiology, Chalon‐sur‐Saône); Drs Fathia Ait Yahia and François Koukoui (CHU Sud Francilien, Corbeille‐Essonnes); Profs Thibaud Damy, Luc Hittinger, Jean‐Luc Dubois‐Randé, Nicolas Lellouche, and Geneviève Derumeaux; and Drs Soulef Guendouz, Arnault Galat, Diane Bodez, Julien Ternacle, Nathalie Elbaz, Laura Ernande, Caroline Touboul, and Ségolène Rouffiac, as well as Mrs Mounira Kharoubi and Mélanie Bézard (CHU Henri Mondor, Department of Cardiology, Créteil); Kevin Richard and Aicha Barigou (CHU Henri Mondor, Rehabilitation Centre, Créteil); Drs Jean‐Christophe Eicher and Laurent Gabriek (CHRU, Department of Cardiology, Dijon); Drs Nachwan Ghanem and Caroline Chong‐Nguyen (CHG, Department of Cardiology, Eaubonne); Dr Lacroix (Rehabilitation Centre, Évecquemont); Profs J. Machecourt and Gérald Vanzetto and Dr Muriel Salvat (CHRU, Department of Cardiology, Grenoble); Prof Marie‐Christine Iliou (APHP‐Rehabilitation Centre, Issy‐les‐Moulineaux); Dr Rémi Cohen (CHG Lagny, Lagny‐sur‐Marne); Prof Patrick Assayag and Dr Emannuelle Berthelot (APHP‐CHU Bicêtre, Le Kremlin‐Bicêtre); Dr Martin Kloeckner (Centre Chirurgical Marie Lannelongue, Department of Cardiology, Le Plessis‐Robinson); Drs Philippe Garriges, Mathieu, and Agnès Oblak (CHG, Levallois Perret); Prof Nicolas Lamblin; Drs Pascal de Groote, Eléonore Hebbar, Marie Fertin, Anne Laure Madika, and Hélène Ridon (CHRU, Department of Cardiology, Lille); Profs Victor Aboyans, Dania Mohty, and Patrice Virot (CHRU, Department of Cardiology, Limoges); Dr Florence Durup (CHG, Department of Cardiology, Longjumeau); Dr Jean‐François Aupetit (CH Saint Joseph Saint Luc, Department of Cardiology, Lyon); Dr Laurent Sebbagh (CHU, Department of Cardiology, Lyon); Prof Gilbert Habib and Dr Renard Sébastien (CHRU La Timone, Marseille); Dr Erik Bouvier (Institut Jacques Cartier, Department of Cardiology, Massy); Dr Hervé Perchet (CHG, Department of Cardiology, Meaux); Dr Ugo Vergueylen (CHG, Department of Cardiology, Montfermeil); Prof François Roubille (CHRU, Department of Cardiology, Montpellier); Drs Albert Boccara, Aurès Chaib, Arnaud Koubbi, and Claire‐Marie Tissot (CHG, Department of Cardiology, Montreuil); Profs Faiez Zannad and Yves Juillière and Drs Nicolas Girerd, Oliver Huttin, and Christine Selton Sutty (CRHU, CIC and Department of Cardiology, Nancy); Prof Jean‐Noël Trochu (CHRU, Department of Cardiology, Nantes); Dr Jean‐Pierre Gueffet (Clinique, Department of Cardiology, Nantes); Prof Pierre Gibelin (CHRU, Department of Cardiology, Nice); Dr Jean‐Etienne Ricci (CHRU, Nimes); Drs P. Maribas, P. Hourdebaigt Larruse, and M.M. Lecardonnel Dessaint (CHG, Department of Cardiology, Orsay); Prof Guillaume Jondeau, Drs Guillaume Baudry, Théo Pezel, and Claire Bouleti (CHU Bichat, Department of Cardiology, Paris); Prof Michel Komajda; Drs Romain Cador, P. Abassade, and Garcon Philippe (Saint Joseph Hospital, Department of Cardiology, Paris); Profs Alain Cohen‐Solal and Damien Logeart; Dr Florence Beauvais (CHU Lariboisière, Department of Cardiology, Paris); Dr Laurent Sabbah (CHU Necker, Department of Cardiology, Paris); Prof Albert Hagège and Dr Mariana Mirabel (CHU HEGP, Department of Cardiology, Paris); Profs Richard Isnard and Philippe Charron; Dr Françoise Pousset (CHU Pitié Salpétrière, Department of Cardiology and Genetic, Paris); Prof Ariel Cohen and Dr Frank Boccara (CHU Saint Antoine, Department of Cardiology, Paris); Dr François Picard (CHRU, Department of Cardiology, Pessac); Dr Benoit Lequeux (CHRU, Department of Cardiology, Poitiers); Dr Barnabas Gellen (Polyclinique de Poitiers, Department of Cardiology, Poitiers); Drs Patrick Jourdain, Joel Dagorn, and Viviana Henegariu (CHG, Department of Cardiology, Pontoise); Dr Martin Fabrice (Gallien Private Hospital, Department of Cardiology, Quincy‐sous‐Sénart); Prof Erwan Donal and Dr François Le Helloco (CHRU, Department of Cardiology, Rennes); Dr Nicolas Coquerel (Polyclinique Saint Laurent, Department of Cardiology, Rennes); Drs S. Wasmer, Anouar Badani, Salim Chaouche, and Jean‐Pascal Chassard (CHG, Department of Cardiology, Romilly‐sur‐Seine); Prof Fabrice Bauer and Dr Frederic Anselme (CHRU, Department of Cardiology, Rouen); Drs Thierry Laperche, David Attias, Thierry Badoual, and Juliette Rousseau (Centre Cardiologique du Nord, Department of Cardiology and Rehabilitation Centre, Saint‐Denis); Dr Frédéric Roche (CHRU, Department of Physiology, Saint‐Étienne); Dr Christian Godreuil (Military Hospital, Department of Cardiology, Saint‐Mandé); Dr Eric Colpart (CHG, Saint‐Quentin); Drs Jospeh Abrial, Marie Pascale Bienvenu, and Jérôme Peyrou (CHG, Department of Cardiology, Saintes); Prof Gérald Roul (CHRU, Department of Cardiology, Strasbourg); Drs G. Chalhoub and Noura Zannad (CHG, Department of Cardiology, Thionville); Dr Jean‐Michel Tartière (CHG, Department of Cardiology, Toulon); Dr Lamia Tartière (Rehablitation Centre, Toulon); Profs Michel Galinier and Olivier Lairez (CHRU, Department of Cardiology, Toulouse); Profs Dominique Babuty and Laurent Fauchier (CHRU, Department of Cardiology, Tours); Drs Raphael Pierre and Aurélien Seeman (Clinique Saint Gatien, Tours); Dr Julien Jeanneteau (Private Hospital, Department of Cardiology, Trélazé); Drs Mohamed Mahrousseh, Bruno Maillier, and Philippe Thilleul (CHG, Department of Cardiology, Troyes); Drs Ahmed Bendriss, Raphael Dumaine, Philippe Meurin, Nathalie Renaud, Jean‐Yves Tabet, Hélène Weber, and Philippe Tournadre (Rehabilitation Centre, Villeneuve‐Saint‐Denis); and Drs Emmanuel Salengro, Edouard Fonseca, and Fawaz Nassar (Hôpital Intercommunal, Department of Cardiology, Villeneuve‐Saint‐Georges).
The authors would also like to thank Amy Whereart (Speak the Speech) and Trevor Stanbury (Pro‐Pens) for medical writing assistance.
Pezel, T. , Audureau, E. , Mansourati, J. , Baudry, G. , Ben Driss, A. , Durup, F. , Fertin, M. , Godreuil, C. , Jeanneteau, J. , Kloeckner, M. , Koukoui, F. , Kesri‐Tartière, L. , Laperche, T. , Roubille, F. , Cohen‐Solal, A. , and Damy, T. (2021) Diagnosis and Treatment of Iron Deficiency in Heart Failure: OFICSel study by the French Heart Failure Working Group. ESC Heart Failure, 8: 1509–1521. 10.1002/ehf2.13245.
References
- 1. Tuppin P, Riviere S, Rigault A, Tala S, Drouin J, Pestel L, Denis P, Gastaldi‐Menager C, Gissot C, Juilliere Y, Fagot‐Campagna A. Prevalence and economic burden of cardiovascular diseases in France in 2013 according to the national health insurance scheme database. Arch Cardiovasc Dis 2016; 109: 399–411. [DOI] [PubMed] [Google Scholar]
- 2. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582 e3. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F, American Heart Association Task Force on Practice G . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 5. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- 6. Okonko DO, Mandal AK, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011; 58: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 7. Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comin‐Colet J. Iron deficiency and health‐related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014; 174: 268–275. [DOI] [PubMed] [Google Scholar]
- 8. Cohen‐Solal A, Leclercq C, Deray G, Lasocki S, Zambrowski JJ, Mebazaa A, de Groote P, Damy T, Galinier M. Iron deficiency: an emerging therapeutic target in heart failure. Heart 2014; 100: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole‐Wilson PA, Ponikowski P, Investigators F‐HT. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, Investigators C‐H. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency dagger. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Luscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 12. Bourguignon S, Faller M, Champs FO, Moutier H, Levesque K, Caranhac G, Cohen‐Solal A. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail 2019; 6: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nutritional anaemias . Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405: 5–37. [PubMed] [Google Scholar]
- 14. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L, EuroHeart Survey I. Heart Failure Association ESoC. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006; 27: 2725–2736. [DOI] [PubMed] [Google Scholar]
- 15. Juilliere Y, Suty‐Selton C, Riant E, Darracq JP, Dellinger A, Labarre JP, Druelle J, Mulak G, Danchin N, Jourdain P, participants Oc . Prescription of cardiovascular drugs in the French ODIN cohort of heart failure patients according to age and type of chronic heart failure. Arch Cardiovasc Dis 2014; 107: 21–32. [DOI] [PubMed] [Google Scholar]
- 16. Cohen‐Solal A, Leclercq C, Mebazaa A, De Groote P, Damy T, Isnard R, Galinier M. Diagnosis and treatment of iron deficiency in patients with heart failure: expert position paper from French cardiologists. Arch Cardiovasc Dis 2014; 107: 563–571. [DOI] [PubMed] [Google Scholar]
- 17. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantahti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Gohring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA, investigators A‐A . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020; 396: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 19. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin‐Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010; 31: 1872–1880. [DOI] [PubMed] [Google Scholar]
- 20. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation 2003; 107: 223–225. [DOI] [PubMed] [Google Scholar]
- 21. Cohen‐Solal A. Iron deficiency in heart failure: why so frequent and which mechanisms? Eur Heart J 2019; ehz874. [DOI] [PubMed] [Google Scholar]
- 22. Ghafourian K, Shapiro JS, Goodman L, Ardehali H. Iron and heart failure: diagnosis, therapies, and future directions. JACC Basic Transl Sci 2020; 5: 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen‐Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillie L, Darne B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014; 16: 984–991. [DOI] [PubMed] [Google Scholar]
- 24. Nunez J, Comin‐Colet J, Minana G, Nunez E, Santas E, Mollar A, Valero E, Garcia‐Blas S, Cardells I, Bodi V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail 2016; 18: 798–802. [DOI] [PubMed] [Google Scholar]
- 25. Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation 2003; 107: 226–229. [DOI] [PubMed] [Google Scholar]
- 26. Sportouch L, Cautela J, Resseguier N, Pinto J, Ammar C, Gaubert M, Barraud J, Peyrol M, Laine M, Bonello L, Yvorra S, Paganelli F, Thuny F. Dynamic iron status after acute heart failure. Arch Cardiovasc Dis 2019; 112: 410–419. [DOI] [PubMed] [Google Scholar]
- 27. Rocha BML, Cunha GJL, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol 2018; 71: 782–793. [DOI] [PubMed] [Google Scholar]
- 28. van der Wal HH, Grote Beverborg N, Dickstein K, Anker SD, Lang CC, Ng LL, van Veldhuisen DJ, Voors AA, van der Meer P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J 2019; 40: 3616–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juilliere Y, Venner C, Filippetti L, Popovic B, Huttin O, Selton‐Suty C. Heart failure with preserved ejection fraction: a systemic disease linked to multiple comorbidities, targeting new therapeutic options. Arch Cardiovasc Dis 2018; 111: 766–781. [DOI] [PubMed] [Google Scholar]
- 30. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34: 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lega JC, Bertoletti L, Gremillet C, Chapelle C, Mismetti P, Cucherat M, Vital‐Durand D, Laporte S, Meta‐Embol G. Consistency of safety and efficacy of new oral anticoagulants across subgroups of patients with atrial fibrillation. PLoS One 2014; 9: e91398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E, Network NHFCR. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail 2015; 17: 248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feldman SF, Lesuffleur T, Olie V, Gastaldi‐Menager C, Juilliere Y, Tuppin P. Outpatient healthcare utilization 30 days before and after hospitalization for heart failure in France: contribution of the national healthcare database (Systeme national des donnees de sante). Arch Cardiovasc Dis 2020; 113: 401–419. [DOI] [PubMed] [Google Scholar]
- 35. Alcaide‐Aldeano A, Garay A, Alcoberro L, Jimenez‐Marrero S, Yun S, Tajes M, Garcia‐Romero E, Diez‐Lopez C, Gonzalez‐Costello J, Mateus‐Porta G, Cainzos‐Achirica M, Enjuanes C, Comin‐Colet J, Moliner P. Iron deficiency: impact on functional capacity and quality of life in heart failure with preserved ejection fraction. J Clin Med 2020; 9: 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Open Heart 2019; 6: e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pozzo J, Fournier P, Delmas C, Vervueren PL, Roncalli J, Elbaz M, Galinier M, Lairez O. Absolute iron deficiency without anaemia in patients with chronic systolic heart failure is associated with poorer functional capacity. Arch Cardiovasc Dis 2017; 110: 99–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical and biological characteristics of patients with iron deficiency in patients with ID diagnostic testing (n = 1075).
Table S2. Clinical and biological characteristics of patients diagnosed with iron deficiency according to whether intravenous iron supplementation was performed. (n = 364).


