Abstract
Aims
Congestive heart failure (CHF) and impaired renal function are two often co‐existing medical conditions and associated with adverse cardiovascular outcome. The aim of the current study was to assess renal and intraglomerular haemodynamics by constant infusion input clearance technique in subjects with CHF.
Methods and results
The group of subjects with CHF consisted of 27 individuals with HFpEF and 27 individuals with HFrEF and were compared with 31 healthy controls. Subjects underwent renal clearance examination to measure glomerular filtration rate (GFR) and renal blood and plasma flow (RBF and RPF) and to calculate intraglomerular haemodynamics such as resistances of the afferent (RA) and efferent arterioles (RE) as well as intraglomerular pressure (P glom). Measured GFR was lower in CHF subjects (68.1 ± 10.1 mL/min/1.73 m2) compared with controls (83.6 ± 13.4 mL/min/1.73 m2, P adj < 0.001) as was P glom (P adj < 0.001). Total renal vascular resistance (RVR) was higher in CHF subjects (87.3 ± 20.1 vs. 73.8 ± 17.1 dyn × s/cm5, P adj < 0.001) mediated by an increased resistance at the afferent site (3201 ± 1084 vs. 2181 ± 796 dyn × s/cm5, P adj < 0.001). Comparing HFpEF and HFrEF subjects, RA was higher in HFrEF subjects. The severity of CHF assessed by NT‐proBNP revealed an inverse association with renal perfusion (RPF r = −0.421, P = 0.002, RBF r = −0.414, P = 0.002) and a positive relation with RVR (r = 0.346, P = 0.012) at the post‐glomerular site (RE: r = 0.318, P = 0.022).
Conclusions
Renal function assessed by measured GFR is reduced and renal vascular resistance at the preglomerular, afferent site is increased in HFpEF and, to greater extent, in HFrEF. Our data indicate a close cardiorenal interaction in CHF.
Keywords: Heart failure, Preserved ejection fraction, Reduced ejection fraction, Renal haemodynamics, Cardiorenal interaction
Introduction
With life expectancy increasing, the prevalence and incidence of chronic heart failure (CHF) and chronic kidney disease (CKD) have been growing in parallel. 1 , 2 CHF and impaired renal function are two often co‐existing medical conditions, and the combination is known to be associated with adverse cardiovascular outcome and increased mortality. 3 , 4 , 5 Therefore, detailed analysis of the pathophysiological mechanisms that connect CHF and renal function represents a matter of major research activities.
The relationship between CHF and impaired renal function is bidirectional. Renal dysfunction has been shown to be an independent risk factor for the development of CHF, 6 , 7 and conversely, an increase in central venous pressure leads to renal dysfunction by reducing renal blood flow (RBF) and perfusion pressure in patients with CHF, which is related to the activation of the renin‐angiotensin‐aldosterone system. 8 , 9 , 10 This cardiorenal relationship has been demonstrated for subjects with CHF and both preserved (HFpEF) and reduced ejection fraction (HFrEF). 8 , 11 , 12 , 13 , 14 , 15
Hillege et al. found a close relation between renal function and mortality in a total of 1906 subjects with HFrEF. 16 In a retrospective analysis of the Studies of LV Dysfunction (SOLVD) cohort, renal dysfunction could be identified as an independent risk factor for mortality in subjects with left ventricular dysfunction. 17 Similarly, estimated creatinine clearance was shown to be an important predictor for all‐cause mortality in subjects with CHF. 18 Unger et al. retrospectively examined the relationship between renal function and echocardiographic parameters in 299 patients with HFpEF, revealing that CKD was independently associated with worse cardiac mechanics and outcomes. 13 Studying 217 participants from the PARAMOUNT trial with HFpEF, Gori et al. demonstrated that renal dysfunction was associated with abnormal left ventricular geometry, lower midwall fractional shortening, and higher NT‐proBNP. 19
In the majority of the studies published so far, renal function has been assessed with readily available tests such as determination of serum creatinine, estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio. However, these parameters, while established in clinical practice, only allow an approximate estimation of renal function. Constant infusion input clearance technique offers a more precise and detailed approach of evaluating renal function and perfusion. 20 With this technique, a reliable and valid measurement of GFR and renal plasma flow (RPF) is available for clinical studies, thus allowing us to further assess renal haemodynamic parameters such as RBF, renal vascular resistance (RVR), and intraglomerular haemodynamic parameters. 20 To the best of our knowledge, such a detailed analysis of renal and also intraglomerular haemodynamics has not yet been performed in subjects with CHF.
Therefore, the aim of the present study was to precisely assess renal function and intraglomerular haemodynamics by means of constant infusion input clearance technique in subjects with CHF—with both HFpEF and HFrEF—compared with healthy controls.
Methods
Study design
This was a cross‐sectional, observational single centre study performed at the Clinical Research Center of the Department of Nephrology and Hypertension, University Hospital Erlangen‐Nuremberg, Germany (www.crc‐erlangen.de). Between March and July 2019, subjects with HFrEF, HFpEF and healthy controls were recruited from the University outpatient clinics, by means of local newspaper advertisement and referring physicians.
Written informed consent was obtained from each subject before study inclusion. The study was conducted according to the tenets of the Declaration of Helsinki and the principles of good clinical practice guidelines. The study protocol has been approved by the local Ethics Committee of the University of Erlangen‐Nuremberg. The study was registered at http://www.clinicaltrials.gov (NCT03672591).
Study population
A total of 85 subjects were included into this study. The group of subjects with CHF and clinical stable conditions consisted of 27 individuals suffering from HFpEF and 27 individuals suffering from HFrEF according to 2016 European Guidelines for the diagnosis and treatment of acute and chronic heart failure. 21 Thereby, subjects were defined as suffering from HFpEF if they had a left ventricular ejection fraction (LVEF) of at least 50% and symptoms and/or signs of HF, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) values above 125 pg/mL, and evidence of relevant structural heart disease such as left ventricular hypertrophy or atrial enlargement and/or diastolic dysfunction. Subjects were defined as suffering from HFrEF if they had a left ventricular ejection fraction below 40% and had symptoms and/or signs of CHF. The group of healthy controls consisted of 31 subjects who had to be in good and stable health condition without any relevant preexisting medical condition or long‐term medication. Key exclusion criteria for all groups were acute cardiac decompensation, dyspnoea at rest, uncontrolled diabetes [fasting plasma glucose ≥240 mg/dL, glycated haemoglobin (HbA1c) ≥ 10%], uncontrolled arterial hypertension (≥180/110 mmHg), any history of stroke, transient ischaemic attack, instable angina pectoris or myocardial infarction within the last 6 months prior to study inclusion, significant valvular heart disease, known hypertrophic obstructive cardiomyopathy or known pericardial constriction as well as atrial fibrillation with a resting heart rate above 90 bpm. Subjects were also excluded if they suffered from subclinical or clinical hyperthyroidism, known allergic reaction to iodine, or medication with amiodarone. Other exclusion criteria were an eGFR below 30 mL/min/1.73 m2 and a body mass index higher than 40 kg/m2.
Clinical parameters
At Visit 1, demographic data of all participants including medical history and concomitant medication were obtained. In addition, fasting blood samples were drawn to measure NT‐proBNP, creatinine (with eGFR calculated), fasting plasma glucose, HbA1c, lipid levels, and other biochemical parameters such as liver enzymes and lipid levels. Assessment of office blood pressure and heart rate was carried out in standard fashion by validated devices (DINAMAP® PRO 100 V2, GE Critikon) in a seated position after 5 min of rest according to European Society of Cardiology/European Society of Hypertension guideline recommendations. 22
Renal clearance examination
Figure 1 shows an example of a renal clearance examination protocol. Renal haemodynamic parameters were determined by constant infusion input clearance technique with iohexol and p‐aminohippuric acid for measuring GFR and RPF, respectively. 23 , 24 , 25 , 26 Clearance examinations were performed at the same time in the morning in a quiet and temperature‐controlled room. After bolus infusion of iohexol and p‐aminohippuric acid over 15 min and a subsequent constant infusion over 90 min, a steady state between input and renal excretion of the tracer substances was reached. With respect to the application of iohexol, we followed the methodological approach of Dixon et al. 27 , 28 , 29 , 30 Target steady state concentration of iohexol was 100 μmol/L. This required a loading dose of 16.5 × body weight (kg). Thereafter, the constant infusion started with 0.5 mL/h of ACCUPAQUE 300™ (containing 647 mg iohexol/mL or 300 mg iod/mL), corresponding to 323 mg iohexol/h or 150 mg iodine/h. Renal haemodynamic parameters such as RBF and RVR were derived in standard fashion. 20 Total serum concentration and mean arterial pressure at the end of the infusion period were measured. Subsequently, intraglomerular pressure (P glom) as well as resistances of the afferent (RA) and efferent arterioles (RE) were calculated according to previously described formulas. 20 , 23 , 31 , 32
Figure 1.
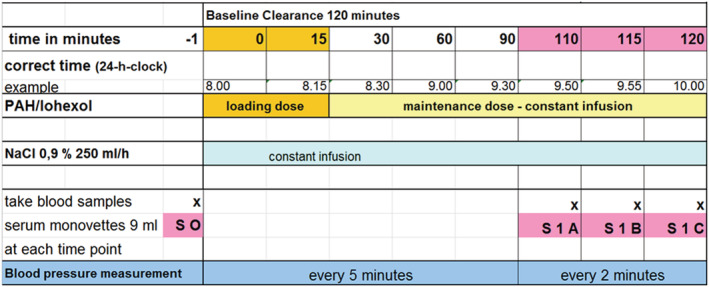
Renal clearance examination protocol.
Statistical analysis
Data are expressed as mean ± standard deviation or median and 95% confidence interval depending on data distribution. A two‐sided P value <0.05 was considered statistically significant. According to our statistical analysis plan we compared first all subjects with CHF (both HFpEF and HFrEF taken together) to healthy controls and then as a second step subjects with HFpEF versus those with HFrEF by Student's t‐test. Due to significant differences in age and systolic blood pressure between the three groups, we adjusted our results for these 2 cofactors by applying analysis of covariance. As a third step, bivariate correlation analyses were performed to assess the relation between NT‐proBNP and renal haemodynamic and intraglomerular parameters in subjects with CHF. All analyses were performed using SPSS software, version 21.0 (IBM Corporation, Chicago, IL, USA).
Results
Clinical characteristics of the study population
Table 1 provides the clinical characteristics of the study groups. Comparing the total group of subjects with CHF to healthy controls, CHF subjects were older (74.1 ± 7.5 vs. 43.0 ± 14.8 years, P < 0.001) and had a higher body mass index (28.7 ± 4.5 vs. 25.9 ± 5.1 kg/m2, P = 0.006) than healthy controls. There was no significant difference in gender distribution between the two groups (P = 0.392). Similarly, office systolic blood pressure was higher in the group of subjects with CHF compared with healthy controls (130.9 ± 18.0 vs. 123.2 ± 13.4 mmHg, P = 0.006). In CHF subjects, mean LVEF was 48.1 ± 11.4% and mean NT‐proBNP levels were 990.0 ± 1492.7 ng/L. Serum creatinine concentrations were higher (1.1 ± 0.3 vs. 0.8 ± 0.2 mg/dL, P < 0.001) and the derived eGFR (according to CKD‐EPI formula) lower (68.2 ± 17.3 vs. 102.3 ± 18.0 mL/min/1.73 m2, P < 0.001) in CHF subjects compared with healthy controls.
Table 1.
Clinical characteristics of the study population
| Parameter | CHF (n = 54) | Healthy controls (n = 31) | P value | HFpEF (n = 27) | HFrEF (n = 27) | P value |
|---|---|---|---|---|---|---|
| Age (years) | 74.1 ± 7.5 | 43.0 ± 14.8 | <0.001 | 76.7 ± 7.0 | 71.2 ± 7.2 | 0.003 |
| Gender (m/f) | 46/17 | 26/12 | 0.392 | 24/10 | 22/7 | 0.428 |
| Body mass index (kg/m2) | 28.7 ± 4.5 | 25.9 ± 5.1 | 0.006 | 28.7 ± 4.7 | 28.6 ± 4.4 | 0.933 |
| Office systolic blood pressure (mmHg) | 130.9 ± 18.0 | 123.2 ± 13.4 | 0.023 | 136.7 ± 15.8 | 125.3 ± 18.5 | 0.018 |
| Office diastolic blood pressure (mmHg) | 72.1 ± 8.5 | 74.5 ± 8.7 | 0.199 | 72.9 ± 8.2 | 71.4 ± 9.0 | 0.531 |
| LVEF (%) | 48.1 ± 11.4 | ‐ | ‐ | 56.3 ± 3.7 | 36.7 ± 3.2 | <0.001 |
| NT‐proBNP (ng/L) | 990.0 ± 1492.7 | 54.8 ± 11.3 | <0.001 | 786.1 ± 927.7 | 1214.9 ± 1929.9 | 0.266 |
| Creatinine (mg/dL) | 1.1 ± 0.3 | 0.8 ± 0.2 | <0.001 | 1.0 ± 0.4 | 1.1 ± 0.3 | 0.348 |
| eGFR a (mL/min/1.73 m2) | 68.2 ± 17.3 | 102.3 ± 18.0 | <0.001 | 69.6 ± 18.6 | 66.6 ± 15.8 | 0.494 |
| UACR (mg/g creatinine) | 70.8 ± 150.9 | 12.0 ± 14.1 | 0.034 | 90.0 ± 184.4 | 52.7 ± 113.7 | 0.487 |
Data are given as mean ± standard deviation (SD); CHF, chronic heart failure; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; UACR, urine albumin to creatinine ratio.
According to CKD‐EPI formula.
With respect to the cause of heart failure, n = 36 individuals (66.7%) suffered from ischaemic cardiomyopathy and n = 18 (33.3%) from non‐ischaemic (e.g. dilated or hypertensive) cardiomyopathy.
With respect to concomitant medication in the group of subjects with CHF, n = 21 (38.9%) were treated with angiotensin‐converting enzyme (ACE) inhibitors, n = 20 (37.0%) with angiotensin type 1 (AT1) receptor antagonists, n = 5 subjects (9.3%) with sacubitril/valsartan, n = 34 (63.0%) with beta‐blockers, n = 16 (29.6%) with spironolactone and n = 32 (59.3%) with thiazide or loop diuretics.
Comparing the two entities of CHF, subjects with HFpEF were older (76.7 ± 7.0 years) than subjects with HFrEF (71.2 ± 7.2 years, P = 0.003) and had a higher office systolic blood pressure (136.7 ± 15.8 vs. 125.3 ± 18.5, P = 0.018). Mean LVEF was 56.3 ± 3.7% in HFpEF and 36.7 ± 3.2% in HFrEF subjects (P < 0.001), respectively. Of interest, in our study, NT‐proBNP levels and serum creatinine as well as derived eGFR did not differ significantly between the 2 groups, although NT‐proBNP levels tended to be higher in HFrEF subjects (1214.9 ± 1929.9 ng/L) compared with HFpEF subjects (786.1 ± 927.7 ng/L, P = 0.266).
Renal function, perfusion, and intraglomerular haemodynamics
Table 2 shows a comparison of renal haemodynamic parameters between subjects with CHF and healthy controls. Measured GFR was lower in subjects with CHF (68.1 ± 10.1 mL/min/1.73 m2) compared with the control group (83.6 ± 13.4 mL/min/1.73 m2) before (P < 0.001) and after adjustment for age and systolic blood pressure (P adj < 0.001). RVR was significantly higher in subjects with CHF (87.3 ± 20.1 dyn × s/cm5) compared with healthy controls (73.8 ± 17.1 dyn × s/cm5, P adj < 0.001), as was RA (3201 ± 1084 vs. 2181 ± 796 dyn × s/cm5, P adj < 0.001). P glom was lower in subjects with CHF (49.7 ± 2.6 mmHg) compared with healthy controls (54.7 ± 3.8 mmHg, P adj < 0.001). RPF and RBF as well as RE did not differ significantly between subjects with CHF and control subjects after adjustment for age and office systolic blood pressure.
Table 2.
Comparison of renal haemodynamic parameters between subjects with HF and healthy controls
| Parameter | CHF (n = 54) | Healthy controls (n = 31) | P value | P adj value a |
|---|---|---|---|---|
| GFR (mL/min/1.73 m2) | 68.1 ± 10.1 | 83.6 ± 13.4 | <0.001 | <0.001 |
| RPF (mL/min/1.73 m2) | 565.0 ± 143.3 | 627.1 ± 161.8 | 0.070 | 0.245 |
| RBF (mL/min/1.73 m2) | 959.1 ± 244.8 | 1105.7 ± 258.6 | 0.012 | 0.067 |
| MAP (mmHg) | 91.7 ± 10.3 | 90.7 ± 9.7 | 0.675 | ‐ |
| RVR (dyn × s/cm5) | 87.3 ± 20.1 | 73.8 ± 17.1 | 0.003 | <0.001 |
| P glom (mmHg) | 49.7 ± 2.6 | 54.7 ± 3.8 | <0.001 | <0.001 |
| RA (dyn × s/cm5) | 3201 ± 1084 | 2181 ± 796 | <0.001 | <0.001 |
| RE (dyn × s/cm5) | 1307 ± 298 | 1414 ± 344 | 0.143 | 0.147 |
Data are given as mean ± standard deviation (SD). CHF, heart failure; GFR, glomerular filtration rate; MAP, mean arterial pressure; P glom, intraglomerular pressure; RA, resistance of the afferent arteriole; RBF, renal blood flow; RE, resistance of the efferent arteriole; RPF, renal plasma flow; RVR, renal vascular resistance.
Adjusted for age and systolic blood pressure.
Comparing subjects with HFpEF to those with HFrEF, there were no significant differences in measured GFR, RPF, RBF, P glom, or RE (Table 3 ). However, after adjustment for age and office systolic blood pressure, RA was higher in subjects with HFrEF compared with those with HFpEF (P adj = 0.001).
Table 3.
Comparison of renal haemodynamic parameters between subjects with HFpEF and HFrEF
| Parameter | HFpEF (n = 27) | HFrEF (n = 27) | P value | P adj value a |
|---|---|---|---|---|
| GFR (mL/min/1.73 m2) | 69.9 ± 11.2 | 66.3 ± 8.8 | 0.200 | 0.376 |
| RPF (mL/min/1.73 m2) | 571.7 ± 138.5 | 558.3 ± 150.3 | 0.736 | 0.674 |
| RBF (mL/min/1.73 m2) | 956.0 ± 257.6 | 962.2 ± 236.2 | 0.927 | 0.524 |
| MAP (mmHg) | 94.1 ± 9.5 | 89.4 ± 10.7 | 0.086 | ‐ |
| RVR (dyn × s/cm5) | 87.8 ± 18.7 | 86.8 ± 21.8 | 0.861 | 0.011 |
| P glom (mmHg) | 49.9 ± 2.7 | 49.5 ± 2.5 | 0.653 | 0.225 |
| RA (dyn × s/cm5) | 3192 ± 1065 | 3209 ± 1123 | 0.954 | 0.001 |
| RE (dyn × s/cm5) | 1357 ± 342 | 1258 ± 242 | 0.228 | 0.443 |
Data are given as mean ± standard deviation (SD). GFR, glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MAP, mean arterial pressure; P glom, intraglomerular pressure; RA, resistance of the afferent arteriole; RBF, renal blood flow; RE, resistance of the efferent arteriole; RPF, renal plasma flow; RVR, renal vascular resistance.
Adjusted for age and systolic blood pressure.
Bivariate correlation analysis in the group of subjects with CHF revealed a significant inverse correlation between NT‐proBNP levels and RPF (R = −0.421, P = 0.002) as well as RBF (r = −0.414, P = 0.002), whereas there was no significant association between NT‐proBNP and measured GFR (r = −0.071, P = 0.619). Of interest, there was a positive correlation between NT‐proBNP levels and RVR (r = 0.346, P = 0.012) as well as RE (r = 0.318, P = 0.022), but not with RA (r = 0.191, P = 0.176) and P glom (r = 0.048, P = 0.733) (Figure 2 ).
Figure 2.
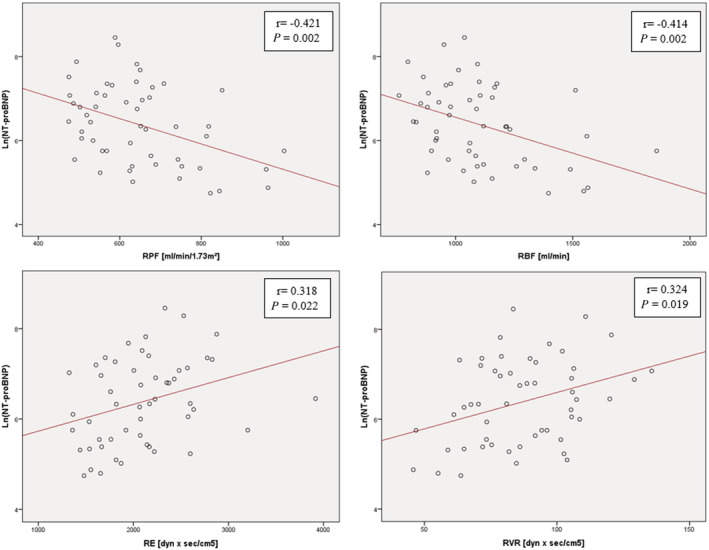
Correlation between NT‐proBNP and renal parameters. CHF, chronic heart failure; RBF, renal blood flow; RE, resistance of the efferent arteriole; RPF, renal plasma flow; RVR, renal vascular resistance.
Discussion
Detailed exploration of the pathophysiological mechanisms behind the relationship of CHF and renal function represents a matter of major research interest. Several studies have attempted to investigate the association between renal (dys‐)function and CHF. In all of these studies, renal function was assessed by estimated GFR (eGFR), determined by serum creatinine concentration. 13 , 16 , 17 , 18 , 19 However, eGFR provides only an approximate estimation of renal function. In order to understand cardiorenal interaction, complete assessment of renal haemodynamics is required.
In subjects with CHF, we precisely investigated renal haemodynamics (measured GFR, RPF, RBF, and RVR) by means of constant infusion input clearance technique, which is considered to be the gold standard technique for the measurement of renal function and perfusion. 33 We observed that measured GFR was significantly lower in subjects with CHF compared with healthy controls. After adjustment for age and systolic blood pressure, renal perfusion as assessed by RPF and RBF did not differ significantly between the groups, but total renal vascular resistance was higher in CHF subjects, which was mediated by an increase at the preglomerular, afferent site, suggesting preserved tubuloglomerular feedback. There was no significant difference in vascular resistance at the post‐glomerular, efferent site between patients with CHF and healthy controls, which can be attributed to the fact that the majority of subjects with CHF in this group were under medication with renin angiotensin system inhibitors known to decrease vascular resistance at the post‐glomerular, efferent site. Diuretics also affect renal haemodynamics and 59.3% of our population were on diuretics. Comparing CHF subjects with and without diuretic treatment, there were no significant differences (all P > 0.20) with respect to renal and intraglomerular haemodynamics (data not shown), which may be related to the fact that we excluded patients with decompensated CHF.
The findings of the current study should be interpreted in the context of neuroendocrine activation known to occur with progression of CHF. 34 , 35 In order to maintain the function of vital organs such as heart, kidney, and brain in case of hypoperfusion, neuroendocrine activation—in particular of the sympathetic nervous system and renin angiotensin aldosterone system—initially leads to increased chronotropia, inotropia, and vasoconstriction in the systemic circulation. We now observed that with increasing severity of CHF, as assessed by NT‐pro BNP, vasoconstriction increases in parallel in the renal vasculature. Further analysis of total RVR disclosed that NT‐pro BNP was closely related to the post‐glomerular, efferent resistance which is known to be the target of angiotensin II.
The role of NT‐proBNP in CHF and cardiorenal syndrome has been previously described. NT‐proBNP has been found to be among the best indicators for severity of CHF and a predictor for short‐term mortality. 36 The observed associations between NT‐proBNP and renal haemodynamics confirm the concept of a cardiorenal interaction. In the current study, we observed an association between reduced renal perfusion and renal vasoconstriction with progressive severity of CHF. The haemodynamic relation of NT‐proBNP with an increased resistance of the post‐glomerular efferent arterioles, which are the primary target of renin and angiotensin II, suggests that the neuroendocrine activation helps to preserve renal function. 37 A decrease in renal perfusion might cause tubular hypoxic injury and thereby progressive renal dysfunction as a long‐term consequence. 38
In patients with HFpEF, renal dysfunction is highly prevalent (30–60%) and associated with cardiac remodelling. 12 , 13 , 39 Our data do not indicate a substantial difference in renal and intraglomerular haemodynamics between patients with HFrEF and HFpEF. Only the resistance at the preglomerular afferent site was found to be increased in patients with HFrEF, pointing to either increased efferent sympathetic activity to the kidney or more advanced vascular remodelling in these patients.
However, renal and intraglomerular function can be affected by renal congestion in subjects with CHF. Due to increasing cardiac preload and therefore central venous pressure, renal venous pressure increases in parallel, subsequently leading to a reduction of RBF and GFR, as effective glomerular pressure may decrease. However, decompensated CHF and NYHA IV were exclusion criteria in this study. All subjects participating in this project were clinically recompensated and euvolaemic under diuretic treatment. No measurement of central venous pressure or other parameters of left ventricular preload was performed, which may be considered as a limitation of this study.
Treatment with the dual‐acting angiotensin receptor neprilysin inhibitor LCZ696 was associated with lower levels of creatinine and higher eGFR indicating a better preservation of renal function in comparison to treatment with valsartan only. 14 , 15 In our study, only five patients were treated with sacubitril/valsartan, and we therefore did not conduct any analysis of intraglomerular haemodynamics to further elucidate the renoprotective effects of sacubitril/valsartan. Another limitation of the current study is its cross‐sectional design without follow‐up examination, but in this study, we addressed the open question of altered renal and in particular glomerular haemodynamics in HFrEF and HFpEF by measuring (and estimating) glomerular filtration rate in parallel to renal perfusion. Structural and functional alterations of the kidney with increasing age have been described previously, including a decrease in GFR and RBF as well as alterations in intraglomerular haemodynamics such as a reduction in RA. 40 , 41 , 42 , 43 To compensate for the significant difference in age between CHF subjects and healthy controls, adjustment for age was performed in the current study by statistical means. CHF is a predominantly a disease of the elderly, with increased prevalence, incidence and severity with progressive aging. In contrast, recruitment of elderly healthy control subjects is challenging, some may consider it nearly impossible in the Western world, and we therefore relied on statistical adjustments to take age into account as a confounder.
Conclusions
In conclusion, the findings of the current study should be interpreted in the context of neuroendocrine activation in subjects suffering from CHF. In compensated CHF, both HFpEF and HFrEF, renal perfusion is preserved, renal function (assessed by measured GFR) is reduced and renal vascular resistance at the preglomerular, afferent site is increased in HFpEF and, to greater extent, in HFrEF. With progressive severity of CHF—as indicated by increasing NT‐proBNP—renal vascular resistance at the post‐glomerular site increases in parallel, potentially reflecting the predominantly intraglomerular action of angiotensin II at the glomerular vessels. The association between NT‐proBNP and renal haemodynamic parameters documents a close cardiorenal interaction by neuroendocrine activation due to CHF. Insofar, the results of the current study can help us to better understand the pathophysiological background of future results of larger studies in subjects with both HFpEF and HFrEF.
Conflict of interest
R. E. S. has received speaker fees and advisory board fees from Novartis Pharma GmbH, Germany, during the conduct of the study. The other authors declare that they have no competing interests.
Acknowledgements
We gratefully acknowledge the expert technical assistance of Dorothea Bader‐Schmieder, Ingrid Fleischmann, Kerstin Fröhlich‐Endreß, Ulrike Heinritz, Wiebke Maurer, Simone Pejkovic, and Sabine Thümmler (Clinical Research Center, Department of Nephrology and Hypertension, University Hospital Erlangen). The present work was performed in fulfilment of the requirements for obtaining the degree, Dr. Med. for T. S. This investigator initiated clinical trial and was supported by a grant provided by Novartis Pharma GmbH, Germany. No other funding source was involved in any part of the study or the decision to submit the manuscript for publication. Open access funding enabled and organized by Projekt DEAL.
Jung, S. , Bosch, A. , Kolwelter, J. , Striepe, K. , Kannenkeril, D. , Schuster, T. , Ott, C. , Achenbach, S. , and Schmieder, R. E. (2021) Renal and intraglomerular haemodynamics in chronic heart failure with preserved and reduced ejection fraction. ESC Heart Failure, 8: 1562–1570. 10.1002/ehf2.13257.
References
- 1. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 2. Tonelli M, Riella MC. Chronic kidney disease and the aging population. Kidney Int 2014; 85: 487–491. [DOI] [PubMed] [Google Scholar]
- 3. Filippatos G, Farmakis D, Parissis J. Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J 2014; 35: 416–418. [DOI] [PubMed] [Google Scholar]
- 4. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J 2014; 35: 455–469. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol 2007; 99: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen D, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 7. McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 2004; 109: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 8. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53: 582–588. [DOI] [PubMed] [Google Scholar]
- 9. Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis 2011; 54: 144–153. [DOI] [PubMed] [Google Scholar]
- 10. Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J 2012; 33: 2135–2142. [DOI] [PubMed] [Google Scholar]
- 11. de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, Bhandari S, Clark AL, Cleland JG. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J 2006; 27: 569–581. [DOI] [PubMed] [Google Scholar]
- 12. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, Van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail 2016; 18: 588–598. [DOI] [PubMed] [Google Scholar]
- 13. Unger ED, Dubin RF, Deo R, Daruwalla V, Friedman JL, Medina C, Beussink L, Freed BH, Shah SJ. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2016; 18: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray JJV. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail 2018; 6: 489–498. [DOI] [PubMed] [Google Scholar]
- 15. Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, Solomon SD. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015; 17: 510–517. [DOI] [PubMed] [Google Scholar]
- 16. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, Van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000; 102: 203–210. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38: 955–962. [DOI] [PubMed] [Google Scholar]
- 18. Mahon NG, Blackstone EH, Francis GS, Starling RC 3rd, Young JB, Lauer MS. The prognostic value of estimated creatinine clearance alongside functional capacity in ambulatory patients with chronic congestive heart failure. J Am Coll Cardiol 2002; 40: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 19. Gori M, Senni M, Gupta DK, Charytan DM, Kraigher‐Krainer E, Pieske B, Claggett B, Shah AM, Santos ABS, Zile MR, Voors AA, McMurray JJV, Packer M, Bransford T, Lefkowitz M, Solomon SD, for the PARAMOUNT Investigators . Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3442–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ott C, Schneider MP, Raff U, Ritt M, Striepe K, Alberici M, Schmieder RE. Effects of manidipine vs. amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol 2013; 75: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 22. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group , de Backer G, Heagerty AM, Agewall S, Bochud M, Borghi C, Boutouyrie P, Brguljan J, Bueno H, Caiani EG, Carlberg B, Chapman N, Cífková R, Cleland JGF, Collet JP, Coman IM, de Leeuw PW, Delgado V, Dendale P, Diener HC, Dorobantu M, Fagard R, Farsang C, Ferrini M, Graham IM, Grassi G, Haller H, Hobbs FDR, Jelakovic B, Jennings C, Katus HA, Kroon AA, Leclercq C, Lovic D, Lurbe E, Manolis AJ, McDonagh TA, Messerli F, Muiesan ML, Nixdorff U, Olsen MH, Parati G, Perk J, Piepoli MF, Polonia J, Ponikowski P, Richter DJ, Rimoldi SF, Roffi M, Sattar N, Seferovic PM, Simpson IA, Sousa‐Uva M, Stanton AV, van de Borne P, Vardas P, Volpe M, Wassmann S, Windecker S, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet JP, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa‐Uva M, Zamorano JL, Tsioufis C, Lurbe E, Kreutz R, Bochud M, Rosei EA, Jelakovic B, Azizi M, Januszewics A, Kahan T, Polonia J, van de Borne P, Williams B, Borghi C, Mancia G, Parati G, Clement DL, Coca A, Manolis A, Lovic D, Benkhedda S, Zelveian P, Siostrzonek P, Najafov R, Pavlova O, de Pauw M, Dizdarevic‐Hudic L, Raev D, Karpettas N, Linhart A, Olsen MH, Shaker AF, Viigimaa M, Metsärinne K, Vavlukis M, Halimi JM, Pagava Z, Schunkert H, Thomopoulos C, Páll D, Andersen K, Shechter M, Mercuro G, Bajraktari G, Romanova T, Trušinskis K, Saade GA, Sakalyte G, Noppe S, DeMarco DC, Caraus A, Wittekoek J, Aksnes TA, Jankowski P, Polonia J, Vinereanu D, Baranova EI, Foscoli M, Dikic AD, Filipova S, Fras Z, Bertomeu‐Martínez V, Carlberg B, Burkard T, Sdiri W, Aydogdu S, Sirenko Y, Brady A, Weber T, Lazareva I, Backer TD, Sokolovic S, Jelakovic B, Widimsky J, Viigimaa M, Pörsti I, Denolle T, Krämer BK, Stergiou GS, Parati G, Trušinskis K, Miglinas M, Gerdts E, Tykarski A, de Carvalho Rodrigues M, Dorobantu M, Chazova I, Lovic D, Filipova S, Brguljan J, Segura J, Gottsäter A, Pechère‐Bertschi A, Erdine S, Sirenko Y, Brady A. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 23. Ott C, Schlaich MP, Schmidt BM, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin improves basal nitric oxide activity of the renal vasculature in patients with hypercholesterolemia. Atherosclerosis 2008; 196: 704–711. [DOI] [PubMed] [Google Scholar]
- 24. Friedrich S, Schmieder RE. Review of direct renin inhibition by aliskiren. J Renin‐Angiotensin‐Aldosterone Syst: JRAAS 2013; 14: 193–196. [DOI] [PubMed] [Google Scholar]
- 25. Schmieder RE, Veelken R, Schobel H, Dominiak P, Mann JF, Luft FC. Glomerular hyperfiltration during sympathetic nervous system activation in early essential hypertension. J Am Soc Nephrol: JASN 1997; 8: 893–900. [DOI] [PubMed] [Google Scholar]
- 26. Schneider MP, Schneider A, Jumar A, Kistner I, Ott C, Schmieder RE. Effects of folic acid on renal endothelial function in patients with diabetic nephropathy: results from a randomized trial. Clin Sci 2014; 127: 499–505. [DOI] [PubMed] [Google Scholar]
- 27. Delanaye P, Melsom T, Ebert N, Back SE, Mariat C, Cavalier E, Björk J, Christensson A, Nyman U, Porrini E, Remuzzi G. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Delanaye P, Ebert N, Melsom T, Gaspari F, Mariat C, Cavalier E, Björk J, Christensson A, Nyman U, Porrini E, Remuzzi G, Ruggenenti P, Schaeffner E, Soveri I, Sterner G, Eriksen BO, Bäck SE. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin Kidney J 2016; 9: 682–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dixon JJ, Lane K, Dalton RN, Turner C, Grounds RM, MacPhee IA, Philips BJ. Validation of a continuous infusion of low dose iohexol to measure glomerular filtration rate: randomised clinical trial. J Transl Med 2015; 13: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dixon JJ, Lane K, Dalton RN, Turner C, MacPhee IAM, Chis Ster I, Philips BJ. Continuous infusion of low‐dose iohexol measures changing glomerular filtration rate in critically ill patients. Crit Care Med 2018; 46: e190–e197. [DOI] [PubMed] [Google Scholar]
- 31. Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest 1951; 30: 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delles C, Klingbeil AU, Schneider MP, Handrock R, Weidinger G, Schmieder RE. Direct comparison of the effects of valsartan and amlodipine on renal hemodynamics in human essential hypertension. Am J Hypertens 2003; 16: 1030–1035. [DOI] [PubMed] [Google Scholar]
- 33. Group KDIGOKCW . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012; 3: 1–150. [DOI] [PubMed] [Google Scholar]
- 34. Forfar JC. Neuroendocrine activation in congestive heart failure. Am J Cardiol 1991; 67: 3C–5C. [DOI] [PubMed] [Google Scholar]
- 35. Swedberg K. Importance of neuroendocrine activation in chronic heart failure. Impact on treatment strategies. Eur J Heart Fail 2000; 2: 229–233. [DOI] [PubMed] [Google Scholar]
- 36. van Kimmenade RR, Januzzi JL Jr, Baggish AL, Lainchbury JG, Bayes‐Genis A, Richards AM, Pinto YM. Amino‐terminal pro‐brain natriuretic peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol 2006; 48: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 37. Viswanathan G, Gilbert S. The cardiorenal syndrome: making the connection. Int J Nephrol 2010; 2011: 283137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waldum B, Os I. The cardiorenal syndrome: what the cardiologist needs to know. Cardiology 2013; 126: 175–186. [DOI] [PubMed] [Google Scholar]
- 39. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 40. Anderson S, Rennke HG, Zatz R. Glomerular adaptations with normal aging and with long‐term converting enzyme inhibition in rats. Am J Physiol 1994; 267: F35–F43. [DOI] [PubMed] [Google Scholar]
- 41. Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP. Senescence and the renal vasculature in normal man. Circ Res 1974; 34: 309–316. [DOI] [PubMed] [Google Scholar]
- 43. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis 2010; 17: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]


