Abstract
Aims
Sacubitril/valsartan combines renin–angiotensin–aldosterone system inhibition with amplification of natriuretic peptides. In addition to well‐described effects, natriuretic peptides exert direct effects on pulmonary vasculature. The effect of sacubitril/valsartan on pulmonary artery pressure (PAP) has not been fully defined.
Methods and results
This was a retrospective case‐series of PAP changes following transition from angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) to sacubitril/valsartan in patients with heart failure reduced ejection fraction and a previously implanted CardioMEMS™ sensor. Pre‐sacubitril/valsartan and post‐sacubitril/valsartan PAPs were compared for each patient by examining averaged consecutive daily pressure readings from 1 to 5 days before and after sacubitril/valsartan exposure. PAP changes were also compared between patients based on elevated trans‐pulmonary gradients (trans‐pulmonary gradient ≥ 12 mmHg) at time of CardioMEMS™ sensor implantation. The cohort included 18 patients, 72% male, mean age 60.1 ± 13.6 years. There was a significant decrease in PAPs associated with transition from ACEI/ARB to sacubitril/valsartan. The median (interquartile range) pre‐treatment and post‐treatment change in mean, systolic and diastolic PAPs were −3.6 (−9.8, −0.7) mmHg (P < 0.001), −6.5 (−15.0, −2.0) mmHg (P = 0.001), and −2.5 (−5.7, −0.7) (P = 0.001), respectively. The decrease in PAPs was independent of trans‐pulmonary gradient (F(1,16) = 0.49, P = 0.49).
Conclusions
In this retrospective case series, transition from ACEI/ARB to sacubitril/valsartan was associated with an early and significant decrease in PAPs.
Keywords: Heart failure, Pulmonary hypertension, Remote monitoring, Heart failure reduced ejection fraction, Implantable monitors, Neprilysin
Background
Sacubitril/valsartan embodies a novel neurohormono‐modulatory approach to the treatment of heart failure with reduced ejection fraction (HFrEF). It combines renin–angiotensin–aldosterone system antagonism with amplification of the natriuretic peptide system, conferring many beneficial compensatory effects in HFrEF. 1 The superior clinical response to sacubitril/valsartan is generally attributed to peripheral vasodilation, reverse remodelling, and sustained natriuresis. 2 , 3
Natriuretic peptides are also expressed in the pulmonary vascular bed and have been shown to mediate multiple pulmonary vascular effects including vasorelaxation, antiproliferation, and reverse remodelling. 4 , 5 Active natriuretic peptide levels are increased by the neprilysin inhibitor sacubitril. Although pulmonary hypertension (PH) is associated with worse clinical outcomes in HFrEF, the clinical actions of sacubitril/valsartan on pulmonary vascular pressure have not yet been described.
Aims
In this study, we used the CardioMEMS™ system (Abbott Vascular, Inc.), a wireless haemodynamic monitoring device enabling ambulatory assessment of pulmonary artery pressure (PAP), to measure the effect of sacubitril/valsartan initiation on PAPs in patients with HFrEF previously treated with ACEI or ARB.
Methods
This was a retrospective case‐series of ambulatory HFrEF patients who had previously received a CardioMEMS device, in whom sacubitril/valsartan was initiated at the discretion of the treating physician. Haemodynamic data at device implantation and the timing of conversion from ACEI or ARB to sacubitril/valsartan at the 24 mg/26 mg dosage were abstracted from the medical record. Up to three consecutive daily PAP readings from 1 to 5 days before and after sacubitril/valsartan initiation were collected from the Merlin website and averaged to yield pre‐values and post‐values for each subject. Patient records were audited to verify that other interventions that might have influenced PAPs measurement (e.g. addition of new diuretic drugs and change in diuretic dose) had not occurred during the observation period.
Haemodynamic data pre‐sacubitril/valsartan and post‐sacubitril/valsartan exposure were compared via Wilcoxon signed‐rank testing. Patients were also separated into two groups based on trans‐pulmonary gradient (TPG) at CardioMEMS implantation, with TPG ≥ 12 suggestive of mixed pre‐capillary and post‐capillary PH and TPG < 12 consistent with post‐capillary PH. Between‐group comparisons of implant haemodynamics were performed with Wilcoxon rank‐sum testing. Within‐group comparisons of pre‐exposure and post‐exposure were made via Wilcoxon signed‐rank testing. A two‐way repeated‐measures ANOVA was performed to assess the effect of TPG on the magnitude of changes in pulmonary artery mean pressures (PAMP). Data management and statistical analyses were conducted using STATA 14.2. The study was approved by the Institutional Review Board.
Results
We identified 18 subjects who had previously received a CardioMEMS device and were subsequently transitioned from ACEI or ARB to sacubitril/valsartan. All had a left ventricular ejection fraction <40% (mean 23.4 ± 6.9), 28% were women, and the mean age was 60.1 ± 13.6 years (Table 1 ). Relevant comorbidities included atrial fibrillation/flutter (44%) and chronic obstructive pulmonary disease (75%). Medication regimens, apart from transition to sacubitril/valsartan, remained unchanged during the observation period.
Table 1.
Baseline characteristics
| All subject N = 18 | TPG < 12 n = 12 | TPG ≥ 12 n = 6 | |
|---|---|---|---|
| Age | 60.1 ± 13.6 | 59.1 ± 13.4 | 32.0 ± 15.0 |
| BMI | 32.1 ± 11.6 | 31.5 ± 11.6 | 33.4 ± 12.7 |
| EF | 23.4 ± 6.9 | 22.2 ± 6.9 | 26.0 ± 6.8 |
| PCWP | 21 [17,29] | 22 [18,32] | 21 [12, 23] |
| eGFR | 48 [37, 59] | 50 [40,60] | 35 [30, 40] |
| Atrial fibrillation | 7 (44) | 5 (42) | 2 (33) |
| Beta‐blocker | 14 (89) | 10 (83) | 4 (67) |
| Hydralazine | 2 (13) | 1 (8.3) | 1 (17) |
| Nitrate | 2 (13) | 1 (8.3) | 1 (17) |
| MCA | 10 (63) | 6 (50) | 4 (67) |
| Loop diuretic | 15 (94) | 11 (92) | 4 (67) |
| Thiazide diuretic | 5 (31) | 3 (25) | 2 (33) |
BMI, body mass index; EF, ejection fraction; eGFR, estimated glomerular filtration rate; MCA, mineralocorticoid antagonist; PCWP, pulmonary capillary wedge pressure; TPG, trans‐pulmonary gradient.
Descriptive statistics summarizing baseline demographic, haemodynamic, and medication data in all patients and in groups defined by TPG ≥ 12. Data are presented as mean ± standard deviation when normally distributed and as median [interquartile range] when not normally distributed. Medication frequency is presented as number (percentage). Haemodynamic data were obtained at time of CardioMEMS implantation.
At device implantation, median [interquartile range (IQR)] PAMP and pulmonary capillary wedge pressure were 33 (28, 37) mmHg and 21 (17, 29) mmHg, respectively (Table 1 ). There were 47 independent measurements prior and 51 measurements following the first sacubitril/valsartan dose. Two patients on ACEI were started on sacubitril/valsartan after a 24 h washout period. The remainder were on an ARB and transitioned directly to sacubitril/valsartan.
There was a significant decrease in PAMP, pulmonary artery systolic pressure, and pulmonary artery diastolic pressure associated with sacubitril/valsartan initiation (Table 2 , Figure 1 ). The median (IQR) changes in PAMP, pulmonary artery systolic pressure, and pulmonary artery diastolic pressure after sacubitril/valsartan initiation were −3.6 (−9.8, −0.7) mmHg (P < 0.001), −6.5 (−15.0, −2.0) mmHg (P = 0.001), and −2.5 (−5.7, −0.7) (P = 0.001), respectively.
Table 2.
Pulmonary pressure changes
| All patients N = 18 | TPG ≤ 12 (n = 12) | TPG > 12 (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre‐initiation | Median change | P value | Pre‐initiation | Median change | P value | Pre‐initiation | Median change | P value | |
| PAMP | 43 (34, 50) | −3.6 (−9.8, −0.7) | <0.001 | 40 (33, 46) | −2.5 (−10.4, −0.7) | 0.013 | 48 (34, 55) | −4.5 (−9.8, −3.3) | 0.028 |
| PASP | 61 (50, 67) | −6.5 (−15.0, −2.0) | 0.001 | 57 (46, 65) | −5.0 (−14.3, −1.7) | 0.015 | 66 (50, 76) | −7.8 (−17.0, −4.3) | 0.035 |
| PADP | 28 (22, 37) | −2.5 (−5.7, −0.7) | 0.001 | 26 (23, 32) | −2.5 (−6.3, −0.5) | 0.013 | 35 (21, 38) | −2.8 (−4.5, −1.0) | 0.028 |
PADP, pulmonary artery diastolic pressure; PAMP, pulmonary artery mean pressure; PASP, pulmonary artery systolic pressure; TPG, trans‐pulmonary gradient.
Pulmonary artery pressure data pre‐sacubitril/valsartan initiation, in all patients and in groups defined by trans‐pulmonary gradient (TPG) ≥12. Data are presented as median (interquartile range). P values were obtained from Wilcoxon signed‐rank testing.
Figure 1.
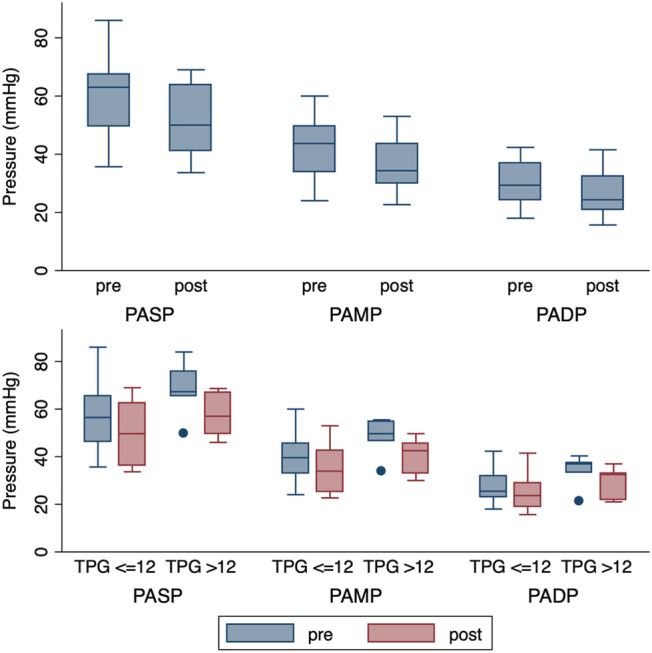
Pulmonary artery pressure changes after sacubitril/valsartan exposure. A box plot illustration of pulmonary artery pressures pre‐sacubitril/valsartan and post‐sacubitril/valsartan initiation. The box ranges indicate 25th/50th/75th quartiles. Dots indicate outside values. The plot above indicates pulmonary artery pressures pre and post initiation while the plot below indicates pulmonary artery pressures pre‐initiation and post‐initiation, divided into groups based on TPG ≥ 12. PADP, pulmonary artery diastolic pressure; PAMP, pulmonary artery mean pressure; PASP, pulmonary artery systolic pressure; TPG, trans‐pulmonary gradient.
At CardioMEMS implantation, six patients had a TPG ≥ 12. The median (IQR) pulmonary vascular resistance (PVR) at baseline was significantly higher in this group compared with the rest of the cohort: 357 (320, 548) vs. 137 (73, 172) dynes respectively, P = 0.001. Pulmonary capillary wedge pressure did not differ significantly between the high and low TPG groups (21 (12, 23) vs. 22 (18, 32) mmHg, respectively, P = 0.32).
Patients demonstrated declines in PAPs after sacubitril/valsartan initiation regardless of their baseline TPG, with median (IQR) PAMP change of −4.5 (−9.8, −3.3) mmHg in the high TPG group and a median (IQR) change of −2.5 (−10.4, −0.7) mmHg in the low TPG group (Table 2 , Figure 1 ). Based on repeated‐measures ANOVA, there was no significant association between TPG group and magnitude of PAMP changes (F(1,16) = 0.49, P = 0.49).
Eleven (64.7%) patients, including the majority (5/6) of the high TPG patients, demonstrated a ‘hyperacute response’, which we defined as a >1 standard deviation reduction in PAMP within 48 h of sacubitril/valsartan initiation. Baseline characteristics of this sub‐group did not differ significantly from the other patients, and there was no significant difference in PAPs or PVR as measured at right heart catheterization between the groups.
Discussion
In this observational study, the combination drug sacubitril/valsartan significantly and acutely reduced PAPs in ambulatory patients with HFrEF and elevated PAPs. This effect was seen in patients with low TPG and relatively normal PVR and in those with elevated TPG and PVR.
The prevalence of PH in HF is reported to be 40–75% as diagnosed with right heart catheterization. 6 , 7 PH in patients with HFrEF is associated with considerably increased morbidity and mortality. 8 This association has been specifically confirmed in CardioMEMS‐implanted HF patients, where those with concomitant PH were found to have higher rates of HF‐related hospitalizations and mortality compared with those patients without. 6 While the presence of PH with HFrEF has prognostic importance, no PH directed therapies have been demonstrated to clearly improve clinical outcomes in these patient. 7
Preclinical HF models have shown reverse remodelling, improved myocardial structure and function, and reduced left ventricular end‐diastolic pressure in animals exposed to sacubitril/valsartan compared with either valsartan or placebo. 9 , 10 These effects have also been demonstrated among HFrEF patients treated with sacubitril/valsartan. 11 Other unique effects of this drug combination are its early and sustained effect on increased natriuresis and diuresis, through increased levels of natriuretic peptides. 1 These mechanisms may explain the reduction in PAPs observed in our cohort through an amelioration of left heart‐related, post‐capillary PH. However, there is also considerable evidence supporting a direct role of natriuretic peptides and neprilysin inhibition on pulmonary vascular tone.
Natriuretic peptides are secreted and degraded in the lungs, and a number of studies have shown that their concentration is increased in the setting of PH. 12 In a study using a rodent model of hypoxemia‐induced PH, sacubitril/valsartan was shown to significantly reduce right ventricular pressure and right ventricular dilatation. 13 Zern et al. described five inotrope‐dependent HFrEF patients listed for heart transplant in whom sacubitril/valsartan initiation resulted in reduced PVR within 24 h. 14 A proof of concept trial of acute neprilysin inhibition for PAH demonstrated a swift 14% fall in PVR. 15 An analogous response may underlie the acute declines seen in our study. The strong correlation between PH and the risk of morbidity and mortality in HF may broaden the implication of these findings. 6 , 7
This study is limited chiefly by its small size and retrospective nature. The observation window was short in duration, and due to adjustments in other therapies over the long term, we could not determine if these changes were sustained. Additionally, the pre‐capillary and post‐capillary PH vs. post‐capillary PH distinction was made at the time of CardioMEMS implantation, and some patients designated as post‐capillary PH may have progressed to combined pre‐capillary and post‐capillary PH prior to sacubitril/valsartan initiation. Despite such limitations, our findings suggest an attribute of sacubitril/valsartan deserving of more investigation.
In summary, an acute fall in PAPs was demonstrated with initial transition to sacubitril/valsartan from ACEI or ARB. Additional studies are needed to define the clinical relevance of this observation and to elucidate its therapeutic implications.
Conflict of interest
Dr David M Shavelle is a consultant for Abbott Vascular, Inc. All other authors report no conflict of interest and have no financial disclosures. This research received no external funding support.
Acknowledgements
The authors wish to thank Claire Boylan RN for her help with this project.
Tran, J. S. , Havakuk, O. , McLeod, J. M. , Hwang, J. , Kwong, H. Y. , Shavelle, D. , Zile, M. R. , Elkayam, U. , Fong, M. W. , and Grazette, L. P. (2021) Acute pulmonary pressure change after transition to sacubitril/valsartan in patients with heart failure reduced ejection fraction. ESC Heart Failure, 8: 1706–1710. 10.1002/ehf2.13225.
Jeffrey S. Tran and Ofer Havakuk contributed equally to this study.
References
- 1. Menendez JT. The mechanism of action of LCZ696. Card Fail Rev 2016; 2: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Committees P‐HIa . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 3. Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, Ogawa H, Kim‐Mitsuyama S. LCZ696, angiotensin II receptor–neprilysin inhibitor, ameliorates high‐salt‐induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens 2015; 28: 1409–1417. [DOI] [PubMed] [Google Scholar]
- 4. Arjona AA, Hsu CA, Wrenn DS, Hill NS. Effects of natriuretic peptides on vascular smooth‐muscle cells derived from different vascular beds. Gen Pharmacol 1997; 28: 387–392. [DOI] [PubMed] [Google Scholar]
- 5. Zhao L, Long L, Morrell NW, Wilkins MR. NPR‐A–deficient mice show increased susceptibility to hypoxia‐induced pulmonary hypertension. Circulation 1999; 99: 605–607. [DOI] [PubMed] [Google Scholar]
- 6. Benza RL, Raina A, Abraham WT, Adamson PB, Lindenfeld J, Miller AB, Bourge RC, Bauman J, Yadav J. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 2015; 34: 329–337. [DOI] [PubMed] [Google Scholar]
- 7. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk‐Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rickenbacher PR, Trindade PT, Haywood GA, Vagelos RH, Schroeder JS, Willson K, Prikazsky L, Fowler MB. Transplant candidates with severe left ventricular dysfunction managed with medical treatment: characteristics and survival. J Am Coll Cardiol 1996; 27: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 9. Trivedi RK, Polhemus DJ, Li Z, Yoo D, Koiwaya H, Scarborough A, Goodchild TT, Lefer DJ. Combined angiotensin receptor–neprilysin inhibitors improve cardiac and vascular function via increased NO bioavailability in heart failure. J Am Heart Assoc 2018; 7: e008268. 10.1161/JAHA.117.008268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor–neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin‐induced diabetic mice. Eur J Heart Fail 2016; 18: 386–393. [DOI] [PubMed] [Google Scholar]
- 11. Almufleh A, Marbach J, Chih S, Stadnick E, Davies R, Liu P, Mielniczuk L. Ejection fraction improvement and reverse remodeling achieved with sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am J Cardiovasc Dis 2017; 7: 108–113. [PMC free article] [PubMed] [Google Scholar]
- 12. Liczek M, Panek I, Damianski P, Jeczen O, Jazwiec J, Kuna P, Panek M. Neprilysin inhibitors as a new approach in the treatment of right heart failure in the course of chronic obstructive pulmonary disease. Adv Respir Med 2018; 86: 257–259. [DOI] [PubMed] [Google Scholar]
- 13. Andersen S, Axelsen JB, Ringgaard S, Nyengaard JR, Hyldebrandt JA, Bogaard HJ, de Man FS, Nielsen‐Kudsk JE, Andersen A. Effects of combined angiotensin II receptor antagonism and neprilysin inhibition in experimental pulmonary hypertension and right ventricular failure. Int J Cardiol 2019; 293: 203–210. [DOI] [PubMed] [Google Scholar]
- 14. Zern EK, Cheng S, Wolfson AM, Hamilton MA, Zile MR, Solomon SD, Kittleson MM. Angiotensin receptor–neprilysin inhibitor therapy reverses pulmonary hypertension in end‐stage heart failure patients awaiting transplantation. Circ Heart Fail 2020; 13: e006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hobbs AJ, Moyes AJ, Baliga RS, Ghedia D, Ochiel R, Sylvestre Y, Dore CJ, Chowdhury K, Maclagan K, Quartly HL, Sofat R, Smit A, Schreiber BE, Coghlan GJ, MacAllister RJ. Neprilysin inhibition for pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial. Br J Pharmacol 2019; 176: 1251–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]


