Abstract
Aims
The mortality in cardiogenic shock (CS) is high. The role of specific mechanical circulatory support (MCS) systems is unclear. We aimed to compare patients receiving Impella versus ECLS (extracorporal life support) with regard to baseline characteristics, feasibility, and outcomes in CS.
Methods and results
This is a retrospective cohort study including CS patients over 18 years with a complete follow‐up of the primary endpoint and available baseline lactate level, receiving haemodynamic support either by Impella 2.5 or ECLS from two European registries. The decision for device implementation was made at the discretion of the treating physician. The primary endpoint of this study was all‐cause mortality at 30 days. A propensity score for the use of Impella was calculated, and multivariable logistic regression was used to obtain adjusted odds ratios (aOR).
In total, 149 patients were included, receiving either Impella (n = 73) or ECLS (n = 76) for CS. The feasibility of device implantation was high (87%) and similar (aOR: 3.14; 95% CI: 0.18–56.50; P = 0.41) with both systems. The rates of vascular injuries (aOR: 0.95; 95% CI: 0.10–3.50; P = 0.56) and bleedings requiring transfusions (aOR: 0.44; 95% CI: 0.09–2.10; P = 0.29) were similar in ECLS patients and Impella patients. The use of Impella or ECLS was not associated with increased odds of mortality (aOR: 4.19; 95% CI: 0.53–33.25; P = 0.17), after correction for propensity score and baseline lactate level. Baseline lactate level was independently associated with increased odds of 30 day mortality (per mmol/L increase; OR: 1.29; 95% CI: 1.14–1.45; P < 0.001).
Conclusions
In CS patients, the adjusted mortality rates of both ECLS and Impella were high and similar. The baseline lactate level was a potent predictor of mortality and could play a role in patient selection for therapy in future studies. In patients with profound CS, the type of device is likely to be less important compared with other parameters including non‐cardiac and neurological factors.
Keywords: Cardiogenic shock, Mechanical circulatory support, Extracorporeal life support, ECMO, Impella
Introduction
Cardiogenic shock (CS) is still associated with a high mortality rate, especially after cardiac arrest. 1 , 2 CS due to acute myocardial infarction (AMI‐CS) and cardiac arrest CS (CA‐CS) constitute the two major causes of the disease. 3 Despite advances in medical management including the administration of inotropes, vasopressors, and the introduction of dedicated shock teams, mortality remains high. Expectations are therefore currently put in mechanical circulatory support (MCS) systems, which may help to improve haemodynamic condition without vasoconstrictive side effects that frequently occur after the administration of catecholamines. 1 , 4 , 5 , 6 , 7 , 8
In the seventies and eighties, the intra‐aortic balloon pump (IABP) was used in nearly all AMI‐CS patients, but recently, the IABP‐SHOCK II trial showed no benefit of IABP as compared with medical treatment. 9 The European Society of Cardiology (ESC) guidelines have downgraded its use to a class III recommendation for routine use. 10 However, new other promising MCS were developed and successfully tested in smaller non‐randomized registries reporting outcomes of CS patients. 8 , 11
Extracorporal life support, ECLS, also known as veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO), has become a treatment option in CS patients, especially after percutaneous insertion has become feasible. 12 However, it is still unclear whether ECLS has any beneficial effect on mortality rate. 7
The micro‐axial Impella pump provides blood flow from the left ventricle into the ascending aorta and, thereby, augments mean arterial pressure and cardiac index. Using the Impella, flow rates of up to 5.5 L/min depending on the specific device diameter are feasible. Although the Impella lacks the oxygenation support in respiratory failure compared with ECLS, experimental studies reported left ventricular unloading and improved blood flow to the coronary arteries in Impella patients, which could be especially beneficial in CS patients. 13 Again, small randomized trials, large observational studies, and meta‐analyses have thus far failed to show a benefit of Impella versus control. 14 , 15 , 16 , 17 , 18 , 19 It is, however, very likely that patient selection for MCS is very crucial in order to show any benefit of MCS.
As demonstrated by recent studies, besides clinical judgements and established scores, lactate level is a useful parameter for stratifying shock patients. It is directly correlated with mortality rate and functional outcome in critically ill patients both with and without MCS. 20 , 21 , 22 , 23
There are only a few small‐sized studies comparing Impella versus ECLS in CS patients. 24 , 25 , 26 , 27 We, therefore, aimed to compare Impella versus ECLS patients in a pooled data analysis from two registries with regard to baseline characteristics, feasibility, outcomes, and predictors of mortality with particular focus on baseline lactate concentration.
Methods
Study population and data collection
Patients with CS over 18 years receiving haemodynamic support either by Impella or ECLS from two European registries, the Impella‐EUROSHOCK registry and the German Lifebridge registry, were included in this analysis. 8 , 12 Patients were included from 2005 to 2014. With regard to the Impella‐EUROSHOCK registry, shock criteria were defined at the discretion of the treating physician based on the SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) criteria. 28 In order to maximize statistic validity, only patients with a complete follow‐up of the primary endpoint and available baseline lactate level were included in the study. Importantly, the decision for MCS application was made at the discretion of the treating physician. Both CS patients with AMI‐CS and CA‐CS were included. AMI‐CS and CA‐CS were defined by the treating physician using a case report form (CRF).
The primary endpoint of the study was all‐cause mortality at 30 days. Left ventricular ejection fraction was obtained using bedside echo on the day of admission. Ethical approval was obtained from the ethical committees of the participating centers. 8 , 12
Device and procedure
Extracorporal life support implantation was performed via percutaneous femoral access using a 15 to 17 French arterial cannula and 17 to 21 French venous cannulae according to the instructions of the manufacturer using retrograde perfusion cannula. The Impella 2.5 was inserted through a 13 French femoral arterial sheath and retrogradely positioned in the left ventricle. A pigtail‐tip aims to avoid myocardial injury and ensures a stable position in the LV. The maximum flow provided by the Impella 2.5 device is 2.5 L/min.
Secondary endpoints
The technical feasibility of device implantation was graded by the implanting physician as ‘easy’, ‘suitable’, or ‘difficult’ using a CRF. In this study, ‘easy’ and ‘suitable’ were considered ‘feasible’. Renal failure, multi‐organ failure, vascular injury during implantation, and bleeding requiring transfusion were clinically judged and graded using a CRF, and no formal prespecified protocol was applied for these endpoints which were clinically judged by the treating physicians.
Statistical analysis
Statistical analyses were performed using SPSS statistical software, version 26.0 (IBM Corp. Released 2019, Armonk, NY, USA). Baseline patient characteristics, procedural data, complications, and outcomes were analysed for the overall cohort. In this analysis, all patients with data on baseline lactate concentration and 30 day mortality were included. Missing values were imputed by multiple imputation. In total, five imputations were calculated. Then, a propensity score on the use of Impella was calculated using logistic regression from the covariates sex, age, concomitant diseases (diabetes, arterial hypertension, hyperlipidaemia, chronic renal insufficiency, atrial fibrillation, PAD, previous AMI, and previous CABG), smoking status, mean arterial pressure (MAP), ejection fraction (EF), BMI, presentation after CPR, shock after myocardial infarction, use of inotropes and vasopressors, heart rate, and use of mechanical ventilation for each imputed dataset. Results from analyses were pooled for inference using Rubin's rules. 29 The propensity scores were applied using the Across approach. 30 Categorical variables are expressed by numbers and percentages. For continuous variables, data are expressed as median ± interquartile range (IQR). Differences between independent groups have been calculated using one‐way ANOVA. Logistic regression was used to evaluate associations with the primary and secondary endpoints. Odds ratios (OR) and adjusted odds ratios (aOR) with 95% confidence intervals (CI) were obtained. For a multivariable logistic regression model, confounders with a P‐value <0.10 in the univariate analysis were included, and then a backward variable elimination was performed. If not stated explicitly, aORs refer to propensity score adjustment. Additionally, multivariable models using propensity score plus lactate and propensity score plus lactate plus complications (vascular injury and bleeding needing transfusion) were built. The elimination criterion was a P‐value of more than 0.10. P‐values <0.05 were considered statistically significant.
Results
In total, 149 patients were included in this retrospective analysis, receiving either Impella (n = 73) or ECLS (n = 76) for CS. Patients receiving Impella were at a similar age as patients receiving ECLS (63 ± 19 vs. 63 ± 22; P = 0.18; Table 1 ). There were no relevant differences in preexisting diseases between patients receiving Impella versus ECLS, except for less diabetes in Impella patients (27% vs. 59%; P < 0.001; Table 1 ).
Table 1.
Baseline characteristics of Impella versus ECLS (extracorporal life support) patients
| Impella (n = 73) | ECLS (n = 76) | P‐value | |
|---|---|---|---|
| Age (years) | 63 (±19) | 63 (±22) | 0.18 |
| Female | 10 (14) | 11 (15) | 1.00 |
| BMI | 27 (±5) | 27 (±4) | 0.52 |
| Concomitant diseases | |||
| Arterial hypertension | 45 (62) | 50 (66) | 0.63 |
| Diabetes mellitus | 21 (29) | 46 (61) | <0.001 |
| Current smoker | 22 (30) | 24 (32) | 0.86 |
| Hyperlipoproteinaemia | 25 (34) | 23 (30) | 0.63 |
| COPD | 11 (15) | 21 (28) | 0.11 |
| Renal insufficiency | 18 (25) | 11 (14) | 0.18 |
| Atrial fibrillation | 15 (21) | 18 (24) | 0.69 |
| Peripheral artery disease | 10 (14) | 14 (18) | 0.41 |
| Previous CABG | 5 (7) | 11 (14) | 0.20 |
| Previous AMI | 9 (12) | 15 (20) | 0.22 |
| Within 90 day | 11 (15) | 18 (24) | 0.20 |
Continuous variables (age and BMI) are given as median ± IQR. Categorial variables are given as N (%).
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease.
Patients in the Impella group had in trend higher rates of low left ventricular ejection fraction (EF < 35%) before device implantation than patients in the ECLS group (78% vs. 58%), but haemodynamic parameters such as heart rate and blood pressure did not differ between the two groups (Table 2 ). Baseline lactate levels were higher in ECLS patients (12.0 ± 12 vs. 4.10 ± 6.30; P < 0.001). ECLS patients were also more often in the highest lactate level quartile (>14.7 mmol/L; 39% vs. 10%; P < 0.001).
Table 2.
Clinical characteristics, haemodynamic parameters, and lactate concentrations prior to MCS (mechanical circulatory support) implantation
| Impella (n = 73) | ECLS (n = 76) | P‐value | |
|---|---|---|---|
| Previous CPR | 27 (37) | 55 (72) | <0.001 |
| Acute myocardial infarction | 65 (89) | 11 (14) | <0.001 |
| Medical haemodynamic support | |||
| Vasopressor use | 62 (85) | 64 (84) | 0.001 |
| Inotropes use | 56 (77) | 58 (76) | 0.04 |
| Haemodynamic parameters | |||
| Mean arterial pressure <65 mmHg | 34 (47) | 33 (43) | 0.82 |
| Heart rate (b.p.m.) | 102 (±26) | 92 (±48) | 0.11 |
| Left ventricular ejection fraction <35% | 57 (78) | 44 (58) | 0.06 |
| Mechanical ventilation | 57 (78) | 56 (74) | 0.61 |
| Lactate (mmol/L) | |||
| Baseline concentration | 4.10 (±6.30) | 12.0 (±12) | <0.001 |
| Baseline lactate (quartiles) | <0.001 | ||
| 0–3.5 mmol/L | 31 (42%) | 6 (8%) | |
| >3.5–8.3 mmol/L | 24 (33%) | 15 (20%) | |
| >8.3–14.7 mmol/L | 11 (15%) | 25 (33%) | |
| >14.7 mmol/L | 7 (10%) | 30 (39%) | |
Continuous variables (heart rate and lactate baseline concentrations) are given as median ± IQR. Categorial variables are given as N (%).
CPR, cardiopulmonary reanimation; ECLS, extracorporal life support.
The rates of previous CPR were higher in ECLS patients (72% vs. 39%; P < 0.001), whereas the rates of CS due to acute myocardial infarction were higher in the Impella group (89% vs. 14%; P < 0.001).
Feasibility, safety, and complications
Feasibility of device implantation was high (‘easy’ or ‘suitable’ in 88%) with both systems and similar between Impella and ECLS (93% vs. 80%; aOR: 3.14; 95% CI: 56.50; P = 0.41). After propensity score adjustment, rates of vascular injuries (25% vs. 12%; aOR: 0.59; 95% CI: 0.10–3.50; P = 0.56) and rates of bleedings requiring transfusions (64% vs. 27%; aOR: 0.44; 95% CI: 0.09–2.10; P = 0.29) were similar in ECLS and Impella patients (Table 3 ).
Table 3.
Comparison of the primary and secondary endpoints between Impella versus ECLS (extracorporal life support) patients
| Event rate | Unadjusted analysis | Propensity‐adjusted analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Impella, n (%) | ECLS, n (%) | OR | 95%CI | P‐value | aOR | 95%CI | P‐value | |
| 30 day mortality | 51 (70) | 63 (83) | 0.48 | 0.22–1.04 | 0.06 | 1.06 | 0.17–6.75 | 0.95 |
| Procedural endpoints | ||||||||
| Vascular injury | 9 (12) | 19 (25) | 0.42 | 0.17–1.01 | 0.054 | 0.59 | 0.10–3.50 | 0.56 |
| Procedural feasibility | 68 (93) | 61 (80) | 3.06 | 1.02–9.16 | 0.046 | 3.14 | 0.18–56.50 | 0.41 |
| Clinical endpoints (judged by CRF) | ||||||||
| Haemolysis | 15 (21) | 17 (22) | 0.91 | 0.31–2.72 | 0.86 | 0.63 | 0.08–5.23 | 0.65 |
| Renal failure | 25 (34) | 29 (38) | 0.85 | 0.42–1.72 | 0.66 | 0.52 | 0.07–3.72 | 0.49 |
| Bleeding requiring transfusion | 20 (27) | 49 (64) | 0.20 | 0.10–0.41 | <0.001 | 0.44 | 0.09–2.10 | 0.29 |
| Multi‐organ failure | 28 (38) | 25 (33) | 1.26 | 0.60–2.62 | 0.54 | 1.62 | 0.18–14.62 | 0.64 |
Reported are unadjusted ORs and propensity‐score adjusted aORs with respective 95% CI and P‐values.
CRF, case report‐form; ECLS, extracorporal life support.
Outcomes
There was a trend towards higher 30 day mortality in ECLS patients (83% vs. 70%; OR: 2.09; 95% CI: 0.22–1.04; P = 0.06; Table 3 ) in univariable analysis. After correction for (i) propensity score (aOR: 1.06; 95% CI: 0.17–6.75; P = 0.95), (ii) propensity score plus lactate concentration at baseline (aOR: 4.19; 95% CI: 0.53–33.25; P = 0.17), and (iii) propensity score plus lactate plus procedural feasibility and vascular injury (aOR: 4.37; 95% CI: 0.51–37.27; P = 0.17), the use of Impella versus ECLS was not associated with significantly different odds for 30 day mortality.
In univariable analysis, higher lactate level at baseline was associated with increased odds of 30 day mortality (per mmol/L increase; OR 1.24; 95% CI: 1.12–1.37; P < 0.001) at high predictiveness (AUC: 0.79; 95% CI: 0.71–0.87). Further, baseline lactate level was associated with 30 day mortality in a sensitivity analysis assessing only ECLS (OR: 1.34; 95% CI: 1.12–1.60; P = 0.001) and Impella (OR: 1.21; 95% CI: 1.03–1.42; P = 0.02), as well as patients with previous CPR (OR: 1.20; 95% CI: 1.05–1.36; P = 0.006) and patients with acute myocardial infarction (OR: 1.16; 95% CI: 1.02–1.32; P = 0.03). In a multivariable logistic regression model, baseline lactate level was independently associated with increased odds of 30 day mortality after correction for Impella use and propensity score (per mmol/L increase; OR: 1.29; 95% CI: 1.14–1.45; P < 0.001).
In sensitivity analysis (n = 112) excluding patients in the highest lactate quartile (n = 37), there were no differences between ECLS and Impella patients (68% vs. 72%; OR: 0.84; 95% CI: 0.37–1.93; P = 0.69) in 30 day mortality. In further sensitivity analysis evaluating only patients with lactate concentration below the median lactate concentration (≤8.3 mmol/L, i.e. patients in the two lowest baseline lactate concentrations; n = 76), 30 day mortality was similar (62% vs. 57%; OR: 1.21; 95% CI: 0.44–3.72; P = 0.71) between Impella and ECLS patients. This finding was consistent in all lactate quartiles (Q1: 55% vs. 33%; P = 0.34; Q2: 71% vs. 67%; P = 0.78; Q3: 100% vs. 86%; P = 0.99; Q4: 86% vs. 100%; P = 0.99; Figure 1 ).
Figure 1.
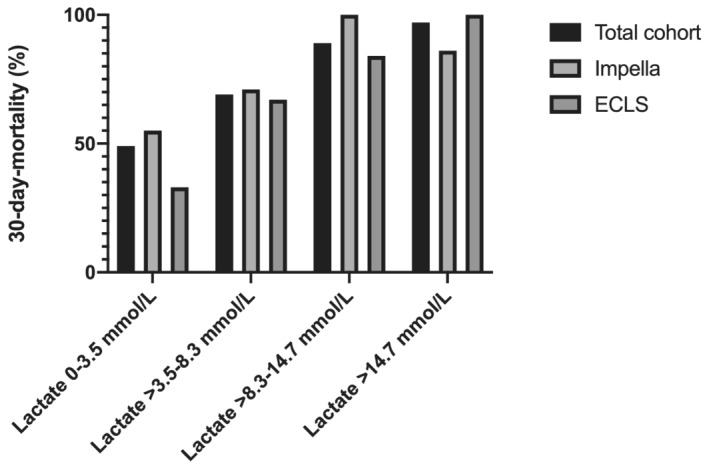
The patients were split in four groups based on quartiles of initial lactate concentration (Q1: 0–3.5 mmol/L; Q2: >3.5–8.3 mmol/L; Q3: >8.3–14.7 mmol/L; Q4: >14.7 mmol/L). Mortality in Q4 and Q3 was 97% and 89%, respectively, and in Q1 49% and in Q2 69% (P < 0.05). Mortality was similar between Impella and ECLS in all sub‐groups (Q1: 55% vs. 33%; P = 0.34; Q2: 71% vs. 67%; P = 0.78; Q3: 100% vs. 86%; P = 0.99; Q4: 86% vs. 100%; P = 0.99).
Discussion
In this study, mortality in CS patients was high, regardless of the type of MCS applied. Still, the technical feasibility of both the Impella and ECLS device was reported to be high. The baseline lactate level was predictive for mortality in all sub‐groups that were assessed. In this retrospective analysis of two European registries in patients with CS, treatment with Impella versus ECLS did not result in a difference in 30 day mortality after correction for lactate values. However, given the high mortality in this real‐world population, the main finding of this study is that in unselected patients with profound CS, the type of device is likely to be less important compared with other parameters including non‐cardiac and neurological factors.
Cardiogenic shock is associated with high mortality. However, patients included in this analysis had a particularly high mortality rate. Even after excluding patients with lactate concentration in the highest quartile (above 14.7 mmol/L; n = 112) at baseline, the mortality was 70%. In this regard, this study cohort differs from previous studies comparing ECLS versus Impella in CS. 24 , 25 , 26 , 27
In the recent study of Karami et al. comparing Impella CP/5.0 (n = 90) versus ECLS in CS patients (n = 38), the mortality was 53% and 49% in the two groups, respectively. 24 Similarly, Schiller et al. reported intensive care unit mortality rates of 35% and 37% for ECLS and Impella patients. 26 Also, in earlier studies, mortality rates around 50% were reported. 25 , 27 We can only speculate that patients included in our analysis were sicker and in more profound shock with subsequent more severe organ hypoperfusion. This notion is supported by the high baseline lactate concentrations of patients included in this analysis, with a median lactate of 8.3 mmol/L, possibly due to the high number of patients needing CPR.
Still, considering the high mortality, lactate was a useful predictor of mortality in this study cohort. In patients with baseline lactate levels in the highest quartile (>14.7 mmol/L; n = 37), mortality was 97% (36/37 patients). Together with clinical judgement, lactate levels could help physicians to assess CS patients before any MCS implantation. Very high lactate levels (e.g. >15 mmol/L) are likely a surrogate parameter for already established advanced end‐organ hypoperfusion, hypoxaemia, and threatening end organ failure. In these situations, MCS application might be considered futile and palliative.
In this regard, the timing of initiation of MCS could be critical. In a meta‐analysis based on observational data, Impella application before reperfusion therapy decreased mortality. 31 In other observational studies, early MCS implantation was associated with increased survival and reduced infarct size and was cardioprotective. 32 , 33 Recently, in AMI patients, Impella application even before reperfusion was reported to be feasible, but no data on efficacy are available. 34 For this study cohort, no data on timing was available.
Adjusted mortality rates did not differ between ECLS and Impella patients in this study, which is in accordance with previous retrospective studies comparing these two MCS devices. 24 , 25 , 26 , 27 Further, although this study cohort differs from other studies with regard to devices (Impella 2.5 versus Impella CP/5.0) and severity of sickness of the included patients, the mortality rate remained similar after matching patients based on lactate and main aetiology of CS. Therefore, although this study cohort differs significantly from previous studies with regard to baseline characteristics, it supports the notion that individual patient selection may be crucial and the choice of MCS device should be considered only secondary. 23 , 35 Anyway, case reports indicate the potential strengths of MCS in overcoming CS, for instance due to fulminant myocarditis. 36
Theoretically, higher rates of vascular injury in ECLS could be explained due to the larger cannulae used for ECLS implantation. However, in the propensity score adjusted analysis, there were no differences in the secondary endpoints between Impella and ECLS. Importantly, feasibility was high for both MCS devices. Of note, this study included only patients receiving the Impella 2.5 device, and results with regard to outcomes, but also feasibility and rates of vascular injuries, could differ in newer devices such as the Impella CP or Impella 5.0.
Extracorporal life support and Impella, although both are potentially promising concepts for CS patients, lack definitive proof for reducing mortality. Meta‐analyses of randomized trials and large observational studies failed to show a survival benefit for these MCS which are frequently used. 7 , 18 Therefore, adequately powered trials for both ECLS (EUROSHOCK (NCT03813134) and ECLS‐SHOCK (NCT02544594) and (NCT03637205)]; and ANCHOR (NCT04184635) and Impella (DanGer shock trial) are ongoing and eagerly awaited. The DanGer shock trial will combine selective patient inclusion criteria excluding comatose patients, with a newer and potent MCS device, the Impella CP. 37
Ultimately, however, the data situation regarding the use of MCS per se remains inclusive. There is no randomized evidence of superiority over conservative treatment for either ECLS or Impella. So, while our study tries to evaluate the advantages and disadvantages of ECLS versus Impella, randomized evidence of MCS versus conservative treatment is certainly the most pressing scientific challenge. It may be possible that precise patient selection increases the effectiveness of MCS, but this is by no means a trivial task but a huge scientific and especially clinical challenge. The structured collection and interpretation of lactate in a clinical context could help in patient selection, especially if this biomarker is integrated with other information.
Another therapy option for patients in CS due to severe heart failure is the implantation of a permanent ventricular assist device (VAD). Due to the invasiveness of the operation, VADs are more frequently applied for patients in more stable clinical conditions, for example, as bridge‐to‐transplantation. 38 There is also a lack of randomized evidence for the use of VAD in CS.
Both Impella and ECLS are theoretically favourable in distinct situations. Impella implantation necessitates smaller access site cannulae/sheaths, which could help to avoid vascular injuries. Further, Impella reduces left ventricular pressure and could be more cardioprotective, especially if applied early. 31 , 32 , 34 Theoretically, Impella might, therefore, be considered advantageous over ECLS in patients with AMI and CS.
On the other hand, ECLS could improve outcomes in patients with respiratory or biventricular failure. 23 Furthermore, combining ECLS with venting strategies may also reduce the drawbacks of the afterload increase with ECLS. 39 However, given the scarce evidence, these considerations remain speculative. The new CS classification of the Society for Cardiovascular Angiography and Intervention (SCAI) and clinical judgement, including early neurological evaluation and lactate levels, could help to select patients who would potentially benefit from MCS. 40
Of note, recently, Impella application was even associated with adverse outcome in a large recent retrospective analysis. 41 Also, even the Impella 5.0, which is theoretically more potent in increasing cardiac output compared with Impella 2.5 and Impella CP, failed to improve haemodynamics. 42 Therefore, the whole concept of MCS is challenged, and randomized studies comparing both Impella and ECLS versus medical treatment are warranted.
Limitations
This study has several limitations, which need to be acknowledged. First, this is a retrospective study comparing patients receiving distinct devices at the treating physicians' discretion. Given local policies, individual preferences, and experience, one of the devices might be practically unavailable for specific patients, which could reduce the comparability of the patients investigated here. Second, detailed data on post‐cardiac arrest care were lacking due to the retrospective nature of the study. Third, only patients on Impella 2.5 were included in this analysis, and using more potent Impella devices could lead to distinct outcomes. Fourth, no data on the functional and neurological outcomes of the survivors is available. Fifth, the timing of MCS implantation was left at the respective treating physicians' decision, and no further information about timing is available for this study cohort. Sixth, the patients in this retrospective analysis had high mortality, even in the setting of CS, which exposes this analysis theoretically to selection bias. However, this analysis provides real‐world data on outcomes of CS patients with MCS. Further, the high mortality could dilute any beneficial effect on the outcome of any of the two MCS. Seventh, although all patients were treated according to guidelines, and all patients with AMI‐CS underwent revascularization, data on revascularization success are lacking. Eighth, we imputed missing data and, therefore, refrained from propensity score matching as the best way to match data from multiple imputation; this is a subject to discussion. 30 Also, propensity scores were calculated for each imputed dataset on relevant baseline variables. However, relevant confounders could have been missed, and propensity adjustment can never replace randomization. Still, our data could help thesis generation. Ninth, the specific characteristics of CA in CA‐CS patients are unknown—although centres were asked to include CS patients, ‘borderline’ patients with refractory CS after CA could influence the outcomes of ECLS patients. Tenth, the reason for CS in the Impella group was AMI in 89%, whereas that occurred in only 14% in the ECLS group. The cause of CA as well as the exact criteria, defining CS in the German Lifebridge registry, remain unknown, thus limiting the validity of the comparison. Eleventh, some of the obtained CIs were wide, and the study could be underpowered to detect some subtle differences between ECLS and Impella. Twelfth, due to the retrospective character of the study, missing baseline lactate levels led to the exclusion of patients and, therefore, an incomplete follow‐up, what could cause a bias and limit the validity of the analyses.
However, we think that the study adds insight to available knowledge, supporting the notion that the choice of a particular MCS is less important than patients selection.
Still, we think that this analysis of severely sick CS patients adds a new perspective to the available literature comparing Impella versus ECLS in CS. But most importantly, it confirms the similar outcomes of Impella versus ECLS seen in previous studies in an unselected patient cohort and, therefore, supports the call for randomized data in highly selected patients to evaluate the effects of MCS in CS.
Conclusion
In CS patients, the adjusted mortality rates of both ECLS and Impella were high and similar. The baseline lactate level was a potent predictor of mortality and could play a role in patient selection for future studies. In patients with profound CS, the type of device is likely to be less important compared with other parameters including non‐cardiac and neurological factors.
Conflict of interest
The authors have nothing to declare.
Funding
No specific funding was received for this study.
Wernly, B. , Karami, M. , Engström, A. E. , Windecker, S. , Hunziker, L. , Lüscher, T. F. , Henriques, J. P. , Ferrari, M. W. , Binnebößel, S. , Masyuk, M. , Niederseer, D. , Abel, P. , Fuernau, G. , Franz, M. , Kelm, M. , Busch, M. C. , Felix, S. B. , Thiele, H. , Lauten, A. , and Jung, C. (2021) Impella versus extracorporal life support in cardiogenic shock: a propensity score adjusted analysis. ESC Heart Failure, 8: 953–961. 10.1002/ehf2.13200.
References
- 1. Mebazaa A, Combes A, van Diepen S, Hollinger A, Katz JN, Landoni G, Hajjar LA, Lassus J, Lebreton G, Montalescot G, Park JJ. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 2. Feistritzer HJ, Desch S, de Waha S, Jobs A, Zeymer U, Thiele H. German contribution to development and innovations in the management of acute myocardial infarction and cardiogenic shock. Clin Res Cardiol 2018; 107: 74–80. [DOI] [PubMed] [Google Scholar]
- 3. Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, Cecconi M, Choi DJ, Cohen Solal A, Christ M, Masip J, Arrigo M, Nouira S, Ojji D, Peacock F, Richards M, Sato N, Sliwa K, Spinar J, Thiele H, Yilmaz MB, Januzzi J. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med 2016; 42: 147–163. [DOI] [PubMed] [Google Scholar]
- 4. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 5. Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014; 35: 156–167. [DOI] [PubMed] [Google Scholar]
- 6. Sayer GT, Baker JN, Parks KA. Heart rescue: the role of mechanical circulatory support in the management of severe refractory cardiogenic shock. Curr Opin Crit Care 2012; 18: 409–416. [DOI] [PubMed] [Google Scholar]
- 7. Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engström AE, Lagrand WK, Cherpanath TGV, Driessen AHG, de Mol BAJM, Henriques JPS. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta‐analysis. Intensive Care Med 2016; 42: 1922–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauten A, Engström AE, Jung C, Empen K, Erne P, Cook S, Windecker S, Bergmann MW, Klingenberg R, Lüscher TF, Haude M. Percutaneous left‐ventricular support with the Impella‐2.5‐assist device in acute cardiogenic shock: results of the Impella‐EUROSHOCK‐registry. Circ Heart Fail 2013; 6: 23–30. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Böhm M, Ebelt H, Schneider S, Werdan K, Schuler G. Intra‐aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP‐SHOCK II): final 12 month results of a randomised, open‐label trial. Lancet 2013; 382: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 10. Backhaus T, Fach A, Schmucker J, Fiehn E, Garstka D, Stehmeier J, Hambrecht R, Wienbergen H. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST‐segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol 2018; 107: 371–379. [DOI] [PubMed] [Google Scholar]
- 11. Schiller P, Hellgren L, Vikholm P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno‐arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur Heart J Acute Cardiovasc Care 2018. 2048872618799745. [DOI] [PubMed] [Google Scholar]
- 12. Masyuk M, Abel P, Hug M, Wernly B, Haneya A, Sack S, Sideris K, Langwieser N, Graf T, Fuernau G, Franz M, Westenfeld R, Kelm M, Felix SB, Jung C. Real‐world clinical experience with the percutaneous extracorporeal life support system: results from the German Lifebridge((R)) Registry. Clin Res Cardiol 2020; 109: 46–53. [DOI] [PubMed] [Google Scholar]
- 13. Remmelink M, Sjauw KD, Henriques JP, de Winter RJ, Koch KT, van der Schaaf RJ, Vis MM, Tijssen JG, Piek JJ, Baan J Jr. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv 2007; 70: 532–537. [DOI] [PubMed] [Google Scholar]
- 14. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott‐Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra‐aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008; 52: 1584–1588. [DOI] [PubMed] [Google Scholar]
- 15. Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BAJM, Tijssen JGP, Henriques JPS. Percutaneous mechanical circulatory support versus intra‐aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287. [DOI] [PubMed] [Google Scholar]
- 16. Karatolios K, Chatzis G, Markus B, Luesebrink U, Ahrens H, Dersch W, Betz S, Ploeger B, Boesl E, O'Neill W, Kill C, Schieffer B. Impella support compared to medical treatment for post‐cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 2018; 126: 104–110. [DOI] [PubMed] [Google Scholar]
- 17. Alushi B, Douedari A, Froehlig G, Knie W, Wurster TH, Leistner DM, Staehli BE, Mochmann HC, Pieske B, Landmesser U, Krackhardt F, Skurk C. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart 2019; 6: e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning JM, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke JT, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius‐Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schäfer A, Westermann D. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019; 139: 1249–1258. [DOI] [PubMed] [Google Scholar]
- 19. Wernly B, Seelmaier C, Leistner D, Stähli BE, Pretsch I, Lichtenauer M, Jung C, Hoppe UC, Landmesser U, Thiele H, Lauten A. Mechanical circulatory support with Impella versus intra‐aortic balloon pump or medical treatment in cardiogenic shock—a critical appraisal of current data. Clin Res Cardiol 2019; 108: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 20. Masyuk M, Wernly B, Lichtenauer M, Franz M, Kabisch B, Muessig JM, Zimmermann G, Lauten A, Schulze PC, Hoppe UC, Kelm M, Bakker J, Jung C. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med 2019; 45: 55–61. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno‐arterial‐ECMO (SAVE)‐score. Eur Heart J 2015; 36: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 22. Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Bréchot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, Anselmi A, Combes A. The ENCOURAGE mortality risk score and analysis of long‐term outcomes after VA‐ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016; 42: 370–378. [DOI] [PubMed] [Google Scholar]
- 23.Health care systems in the European Union countries.
- 24. Karami M, den Uil CA, Ouweneel DM, Scholte NT, Engström AE, Akin S, Lagrand WK, Vlaar AP, Jewbali LS, Henriques JP. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur Heart J Acute Cardiovasc. Care 2019. 2048872619865891. [DOI] [PubMed] [Google Scholar]
- 25. Chamogeorgakis T, Rafael A, Shafii AE, Nagpal D, Pokersnik JA, Gonzalez‐Stawinski GV. Which is better: a miniaturized percutaneous ventricular assist device or extracorporeal membrane oxygenation for patients with cardiogenic shock? ASAIO J 2013; 59: 607–611. [DOI] [PubMed] [Google Scholar]
- 26. Schiller P, Hellgren L, Vikholm P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno‐arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur Heart J Acute Cardiovasc Care 2019; 8: 329–337. [DOI] [PubMed] [Google Scholar]
- 27. Lamarche Y, Cheung A, Ignaszewski A, Higgins J, Kaan A, Griesdale DEG, Moss R. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg 2011; 142: 60–65. [DOI] [PubMed] [Google Scholar]
- 28. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, Picard MH, Menegus MA, Boland J, Dzavik V, Thompson CR, Wong SC, Steingart R, Forman R, Aylward PE, Godfrey E, Desvigne‐Nickens P, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
- 29. Leyrat C, Seaman SR, White IR, Douglas I, Smeeth L, Kim J, Resche‐Rigon M, Carpenter JR, Williamson EJ. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res 2019; 28: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Granger E, Sergeant JC, Lunt M. Avoiding pitfalls when combining multiple imputation and propensity scores. Stat Med 2019; 38: 5120–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flaherty MP, Khan AR, O'Neill WW. Early initiation of impella in acute myocardial infarction complicated by cardiogenic shock improves survival: a meta‐analysis. JACC Cardiovasc Interv 2017; 10: 1805–1806. [DOI] [PubMed] [Google Scholar]
- 32. Loehn T, O'Neill WW, Lange B, Pfluecke C, Schweigler T, Mierke J, Waessnig N, Mahlmann A, Youssef A, Speiser U, Strasser RH. Long term survival after early unloading with Impella CP((R)) in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018. 2048872618815063. [DOI] [PubMed] [Google Scholar]
- 33. Esposito ML, Zhang Y, Qiao X, Reyelt L, Paruchuri V, Schnitzler GR, Morine KJ, Annamalai SK, Bogins C, Natov PS, Pedicini R, Breton C, Mullin A, Mackey EE, Patel A, Rowin E, Jaffe IZ, Karas RH, Kapur NK. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J Am Coll Cardiol 2018; 72: 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kapur NK, Alkhouli MA, DeMartini TJ, Faraz H, George ZH, Goodwin MJ, Hernandez‐Montfort JA, Iyer VS, Josephy N, Kalra S, Kaki A. Unloading the left ventricle before reperfusion in patients with anterior ST‐segment‐elevation myocardial infarction. Circulation 2019; 139: 337–346. [DOI] [PubMed] [Google Scholar]
- 35. Thiele H, Ohman EM, de Waha‐Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019; 40: 2671–2683. [DOI] [PubMed] [Google Scholar]
- 36. Fox H, Farr M, Horstkotte D, Flottmann C. Fulminant myocarditis managed by extracorporeal life support (Impella(R) CP): a rare case. Case Rep Cardiol 2017; 2017: 9231959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Udesen NJ, Møller JE, Lindholm MG, Eiskjær H, Schäfer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, Schmidt H. Rationale and design of DanGer shock: Danish‐German cardiogenic shock trial. Am Heart J 2019; 214: 60–68. [DOI] [PubMed] [Google Scholar]
- 38. Gummert JF, Haverich A, Schmitto JD, Potapov E, Schramm R, Falk V. Permanent implantable cardiac support systems. Dtsch Arztebl Int 2019; 116: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, di Santo P, Mathew R, So DY, Takeda K, Garan AR, Karmpaliotis D, Takayama H, Kirtane AJ, Hibbert B. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 2019; 73: 654–662. [DOI] [PubMed] [Google Scholar]
- 40. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S, Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019; 94: 29–37. [DOI] [PubMed] [Google Scholar]
- 41. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, Berkowitz A, Masoudi FA, Messenger JC, Parzynski CS, Ngufor C, Girotra S, Amin AP, Shah ND, Desai NR. Association of use of an intravascular microaxial left ventricular assist device vs intra‐aortic balloon pump with in‐hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2020; 323: 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bochaton T, Huot L, Elbaz M, Delmas C, Aissaoui N, Farhat F, Mewton N, Bonnefoy E, IMPELLA‐STIC investigators . Mechanical circulatory support with the Impella(R) LP5.0 pump and an intra‐aortic balloon pump for cardiogenic shock in acute myocardial infarction: the IMPELLA‐STIC randomized study. Arch Cardiovasc Dis 2020; 113: 237–243. [DOI] [PubMed] [Google Scholar]


