Abstract
Aims
This study aimed to assess the utility of contemporary clinical risk scores and explore the ability of two biomarkers [growth differentiation factor‐15 (GDF‐15) and soluble ST2 (sST2)] to improve risk prediction in elderly patients with cardiogenic shock.
Methods and results
Patients (n = 219) from the multicentre CardShock study were grouped according to age (elderly ≥75 years and younger). Characteristics, management, and outcome between the groups were compared. The ability of the CardShock risk score and the IABP‐SHOCK II score to predict in‐hospital mortality and the additional value of GDF‐15 and sST2 to improve risk prediction in the elderly was evaluated. The elderly constituted 26% of the patients (n = 56), with a higher proportion of women (41% vs. 21%, P < 0.05) and more co‐morbidities compared with the younger. The primary aetiology of shock in the elderly was acute coronary syndrome (84%), with high rates of percutaneous coronary intervention (87%). Compared with the younger, the elderly had higher in‐hospital mortality (46% vs. 33%; P = 0.08), but 1 year post‐discharge survival was excellent in both age groups (90% in the elderly vs. 88% in the younger). In the elderly, the risk prediction models demonstrated an area under the curve of 0.75 for the CardShock risk score and 0.71 for the IABP‐SHOCK II score. Incorporating GDF‐15 and sST2 improved discrimination for both risk scores with areas under the curve ranging from 0.78 to 0.84.
Conclusions
Elderly patients with cardiogenic shock have higher in‐hospital mortality compared with the younger, but post‐discharge outcomes are similar. Contemporary risk scores proved useful for early mortality risk prediction also in the elderly, and risk stratification could be further improved with biomarkers such as GDF‐15 or sST2.
Keywords: Cardiogenic shock, Elderly, Risk prediction, Biomarker, GDF‐15, sST2
Introduction
The management of cardiogenic shock (CS) in the elderly poses a clinical challenge. On one hand, the elderly are at the highest risk for adverse outcomes and therefore have the greatest potential to benefit from treatment, but, on the other hand, they are vulnerable to treatment‐related complications. Furthermore, there is remarkable individual variation in functional and cognitive reserves among this age group, presenting a hurdle to the objective evaluation of the prognosis in acute settings.
Early assessment of shock severity is crucial in order to single out patients at high risk of death. Accurate risk stratification could guide the treatment and help in the allocation of clinical resources, through identification of patients most likely to benefit from the highly intense and costly treatment options. Age itself elevates the risk of myocardial infarction‐related CS, and advanced age is an additional known risk factor for CS mortality. 1 , 2 Risk prediction models have been introduced to facilitate risk assessment and the prediction of mortality in the acute phase of CS. Two risk prediction scores, the CardShock risk score and the IABP‐SHOCK II score, have been developed specifically for CS and have shown good performance in both early risk stratification and prediction of short‐term mortality. 1 , 3 , 4 However, the utility of these risk score models in the elderly remains unclear. Biomarkers have become significant prognostic tools in many cardiovascular diseases. 5 , 6 Most recently, growth differentiation factor‐15 (GDF‐15) and soluble ST2 (sST2) have been found to be valuable in risk stratification in heart failure and CS, 7 , 8 , 9 , 10 but data on elderly patients with CS in the contemporary era remain scarce.
We examined the clinical picture, management, and outcomes of patients aged ≥75 years in a prospective, multicentre study on CS. Our aim was to compare the key features between survivors and non‐survivors and to assess the performance of the contemporary risk prediction scores in the elderly. Finally, we investigated the ability of GDF‐15 and sST2 to improve early risk stratification in this age group.
Methods
The CardShock study (NCT01374867 at https://www.ClinicalTrials.gov is a prospective, observational, multicentre study on CS, including both acute coronary syndrome (ACS)‐related and non‐ACS‐related aetiologies. Nine tertiary hospitals in eight European countries participated between October 2010 and December 2012, enrolling 219 patients. The detailed design and the primary results of the study have been published elsewhere. 1
Inclusion criteria and data collection
Besides an acute cardiac cause, the inclusion criteria consisted of systolic blood pressure <90 mmHg (after adequate fluid challenge) for 30 min, or need for vasopressor therapy to maintain systolic blood pressure >90 mmHg, and signs of hypoperfusion (altered mental status/confusion, cold periphery, oliguria <0.5 mL/kg/h for the previous 6 h, or blood lactate >2 mmol/L). The exclusion criterion was shock caused either by ongoing hemodynamically significant arrhythmias or by cardiac or non‐cardiac surgery. Patients had to be over 18 years old, and they had to be included within 6 h of the identification of the shock.
Baseline characteristics and previous medical history were recorded. Biochemical and clinical findings, as well as haemodynamic parameters, were documented at detection of shock and at pre‐specified time points until 96 h after inclusion. Patients were treated according to local practice in each hospital, and treatment procedures were registered. The primary outcome was all‐cause in‐hospital mortality. In addition, 1 year mortality was assessed. After hospital discharge, three patients were lost to follow‐up. In the mortality analyses, their cases were censored at the time of hospital discharge. Written informed consent was obtained from the patient or, according to local regulations, from a close person or a relative. Vital status during follow‐up was determined through direct contact with the patient or next of kin or through population and hospital registers. The study was approved by the following local ethics committees: Athens: Ethics Committee of Attikon University Hospital; Barcelona: Health Research Ethics Committee of the Hospital de Sant Pau; Brescia: Ethics Committee of the Province of Brescia; Brno: Ethics Committee of University Hospital Brno; Helsinki: The Ethics Committee, Department of Medicine, The Hospital District of Helsinki and Uusimaa; Porto: Ethics Committee of São João Hospital Center/Porto Medical School; Rome: Ethical Committee Sant'Andrea Hospital; Warsaw: Local Bioethics Committee of the Institute of Cardiology; and Copenhagen: the study was approved by the Danish Protection Agency with reference number GEH‐2014‐013 and I‐Suite number 02731. The study was conducted in accordance with the Declaration of Helsinki.
Serial blood sampling was performed at baseline and thereafter at 12 h intervals up to 48 h, and plasma samples were stored in aliquots frozen at −80 (−70)°C until assayed. Creatinine, C‐reactive protein, high‐sensitivity troponin T (hsTnT), N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and GDF‐15 (Roche Diagnostics, Basel, Switzerland) were analysed centrally at ISLAB (Kuopio, Finland). sST2 was measured at INSERM UMR‐S 942 (Paris, France) using a quantitative sandwich monoclonal enzyme‐linked immunosorbent assay (Presage sST2 Assay; Critical Diagnostics, San Diego, CA, USA). Arterial blood lactate and pH were analysed locally. Estimated glomerular filtration rate (eGFR) was calculated from creatinine values using the Chronic Kidney Disease Epidemiology Collaboration equation. 11 Acute kidney injury was defined and staged according to the Kidney Disease: Improving Global Outcomes criteria based on creatinine value. 12
Risk prediction models
We assessed the ability of two published risk prediction models, the CardShock risk score and the IABP‐SHOCK II score, to predict in‐hospital mortality in the elderly.
The CardShock risk score consists of seven variables measured at admission in patients with CS of various aetiologies (age >75 years; eGFR; blood lactate; confusion on admission; left ventricular ejection fraction <40%; previous myocardial infarction or coronary artery bypass grafting; and ACS aetiology) with a maximum of 9 points. The score categorizes the patients into low‐risk (0–3 points), intermediate‐risk (4–5 points), and high‐risk (6–9 points) groups. 1
The IABP‐SHOCK II score is derived from the Intra‐aortic Balloon Pump in Cardiogenic Shock II (IABP‐SHOCK II) study in patients with CS due to ACS who are undergoing percutaneous coronary intervention (PCI). It consists of six variables (age >73 years; prior stroke; glucose at admission >10.6 mmol/L; creatinine at admission >132.6 mmol/L; thrombolysis in myocardial infarction flow grade <3 after PCI; and arterial blood lactate at admission >5 mmol/L) giving a maximum of 9 points. The patients can be classified according to the points into low‐risk (0–2 points), intermediate‐risk (3–4 points), and high‐risk (5–9 points) categories. 3
Statistical analysis
We categorized the patients by age into (i) ≥75 years old (elderly group) and (ii) <75 years old (younger group). Elderly patients were further compared with respect to their in‐hospital survival status (survivors vs. non‐survivors). Results are presented here as number (n) and percentage (%); mean with standard deviation (SD); or median with inter‐quartile range, as appropriate. Group comparisons were performed using the χ 2 test and Fisher's exact test for categorical variables and Student's t‐test and Mann–Whitney U test for continuous variables. The Kaplan–Meier method was used to elucidate the timing of events during 30 day and 1 year follow‐up in relation to age group, and statistical assessment was performed using the log‐rank test.
To assess the ability of the CardShock risk score and IABP‐SHOCK II score to predict in‐hospital mortality in the elderly, and to evaluate the additional value of GDF‐15 and sST2 on the risk prediction models, receiver operating characteristic curve analysis was performed. We used previously defined cut‐off values of the biomarkers (GDF‐15 > 7000 ng/L and sST2 > 500 ng/mL) for this analysis. 7 , 8 The distribution of the elderly patients and observed mortality within risk categories of both risk prediction models were calculated. The additional value of the biomarkers in mortality prediction was assessed via the likelihood ratio test for nested models. For comparison, the CardShock risk score was further validated in an external cohort of patients with CS with unselected aetiology from a single‐centre prospective study. The validation was performed in all patients as well as separately in the elderly (≥75 years) and the younger (<75 years) patients. Logistic regression was used to investigate the interaction between age and risk prediction models. A two‐sided P‐value <0.05 was regarded as statistically significant. All statistical analyses were performed with SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics, presentation, and treatment of cardiogenic shock in the elderly
Table 1 outlines the baseline characteristics of the elderly and the younger patients. The elderly constituted 26% (n = 56) of the patients, with a mean age of 81 ± 4 years. They were more frequently female (41% vs. 21%, P = 0.003) and had more co‐morbidities overall, with generalized arteriosclerosis being particularly high compared with younger patients. Overall, ACS (81%) was the principal aetiology of shock, and its frequency did not differ between the age groups.
TABLE 1.
Clinical characteristics, medical history, and shock aetiology
| ≥75 years | <75 years | Elderly survivors | Elderly non‐survivors | |||
|---|---|---|---|---|---|---|
| n = 56 (26%) | n = 163 (74%) | P‐value | n = 30 (54%) | n = 26 (46%) | P‐value | |
| Age (years) |
81 (4) 80 (78–83) |
62 (9) 63 (57–69) |
<0.001 0.003 |
81 (5) | 81 (4) | 0.7 |
| Female, n (%) | 23 (41) | 34 (21) | 0.003 | 13 (43) | 10 (39) | 0.6 |
| BMI (kg/m2) | 26 (5) | 27 (4) | 0.2 | 26 (5) | 26 (4) | 0.9 |
| Resuscitated, n (%) | 16 (29) | 46 (28) | 1.0 | 7 (23) | 9 (35) | 0.4 |
| Medical history, n (%) | ||||||
| Hypertension | 39 (70) | 93 (57) | 0.10 | 21 (70) | 18 (69) | 1.0 |
| Diabetes | 17 (30) | 44 (27) | 0.6 | 8 (27) | 9 (35) | 0.5 |
| History of MI/CABG | 19 (34) | 38 (23) | 0.12 | 7 (23) | 12 (46) | 0.07 |
| Prior stroke/TIA | 8 (14) | 12 (7) | 0.12 | 4 (13) | 4 (15) | 1.0 |
| PAD | 11 (20) | 10 (6) | 0.003 | 3 (10) | 8 (31) | 0.05 |
| Chronic heart failure | 7 (13) | 29 (18) | 0.4 | 2 (7) | 5 (19) | 0.2 |
| History of AF | 11 (20) | 21 (13) | 0.2 | 4 (13) | 7 (27) | 0.2 |
| Renal insufficiency | 11 (20) | 14 (9) | 0.03 | 4 (13) | 7 (27) | 0.2 |
| Smoking history | 24 (43) | 111 (68) | <0.001 | 14 (47) | 10 (39) | 0.6 |
| Shock aetiology, n (%) | ||||||
| ACS | 47 (84) | 130 (80) | 0.5 | 25 (83) | 22 (85) | 1.0 |
| STEMI | 37 (66) | 112 (69) | 0.7 | 21 (70) | 16 (62) | 0.5 |
ACS, acute coronary syndrome; AF, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass grafting; MI, myocardial infarction; PAD, peripheral artery disease; STEMI, ST‐elevation myocardial infarction; TIA, transient ischaemic attack.
Data are presented as numbers and percentages (%), means (SD), and median (inter‐quartile range).
The two age groups were largely similar with respect to clinical presentation and biochemical findings, as summarized in Table 2 , except for a few differences. Compared with the younger group, the elderly were less likely to have sinus rhythm at baseline, and a higher proportion of them had left ventricular ejection fraction >40%. The elderly group had worse renal function (creatinine and eGFR) at admission but with a similar incidence of acute kidney injury as the younger patients. Biomarker levels of congestion/cardiac stress (NT‐proBNP) were higher in the elderly, whereas the extent of myocardial injury as measured by hsTnT did not differ between the groups [peak hsTnT 3680 (1270–14 281) ng/L in the elderly vs. 3849 (927–12 585) ng/L in the younger; P = 0.8]. The extent of coronary artery disease assessed by coronary angiogram in the elderly (one‐vessel disease 20%; multivessel disease 59%; and left main disease 11%) was comparable with that of the younger group.
TABLE 2.
Clinical presentation and biochemical findings at baseline
| ≥75 years | <75 years | Elderly survivors | Elderly non‐survivors | |||
|---|---|---|---|---|---|---|
| n = 56 (26%) | n = 163 (74%) | P‐value | n = 30 (54%) | n = 26 (46%) | P‐value | |
| Systolic blood pressure (mmHg) | 78 (11) | 77 (15) | 0.7 | 81 (10) | 75 (11) | 0.08 |
| Heart rate (b.p.m.) | 86 (31) | 92 (27) | 0.2 | 78 (29) | 93 (33) | 0.10 |
| Sinus rhythm, n (%) | 30 (54) | 128 (79) | 0.001 | 20 (67) | 11 (42) | 0.09 |
| Left ventricular ejection fraction (LVEF) (%) | 36 (15) | 32 (14) | 0.12 | 41 (15) | 31 (13) | 0.03 |
| LVEF < 40%, n (%) | 28 (50) | 107 (66) | 0.03 | 13 (43) | 15 (58) | 0.3 |
| Confusion, n (%) | 44 (79) | 104 (64) | 0.06 | 21 (70) | 23 (89) | 0.09 |
| Oliguria, n (%) | 35 (63) | 86 (53) | 0.19 | 11 (37) | 24 (92) | <0.001 |
| Biochemical findings | ||||||
| Haemoglobin (g/L) | 124 (21) | 130 (23) | 0.12 | 124 (19) | 123 (21) | 0.6 |
| CRP (mg/L) | 19 (3–65) | 14 (5–49) | 0.9 | 19 (5–74) | 8 (2–65) | 0.3 |
| Creatinine (μmol/L) | 121 (96–159) | 96 (73–138) | 0.004 | 116 (92–137) | 127 (106–173) | 0.3 |
| eGFR (mL/min/1.73 m2) | 44 (32–62) | 70 (45–95) | <0.001 | 44 (32–66) | 35 (30–56) | 0.3 |
| ALT (U/L) | 37 (20–86) | 46 (20–101) | 0.4 | 29 (19–71) | 50 (22–90) | 0.4 |
| Glucose (mmol/L) | 12.7 (9.0–15.6) | 10.3 (7.6–16.1) | 0.3 | 11.2 (7.9–14.4) | 12.7 (9.4–19.0) | 0.9 |
| hsTnT (ng/L) | 1568 (358–4174) | 2307 (403–5418) | 0.7 | 1479 (212–6249) | 1568 (441–4140) | 0.9 |
| Peak hsTnT (ng/L) | 3680 (1270–14 281) | 3849 (927–12 585) | 0.8 | 3521 (1194–8036) | 4291 (1345–16 665) | 0.4 |
| NT‐proBNP (ng/L) | 4322 (1599–16 786) | 2367 (400–8082) | 0.02 | 4965 (838–18 786) | 3706 (1609–16 547) | 0.9 |
| Arterial pH | 7.29 (7.18–7.37) | 7.32 (7.21–7.39) | 0.2 | 7.31 (7.25–7.41) | 7.23 (7.10–7.31) | 0.02 |
| Lactate (mmol/L) | 2.8 (2.0–7.9) | 2.9 (1.6–5.3) | 0.16 | 2.3 (1.6–3.3) | 6.4 (2.8–8.6) | <0.001 |
| Lactate >5 mmol/L, n (%) | 20 (36) | 44 (27) | 0.2 | 5 (17) | 15 (58) | 0.001 |
| GDF‐15 (ng/L at 12 h) (n = 154) | 10 871 (6562–30 074) | 8440 (3846–16 033) | 0.05 | 6912 (3901–13 309) | 29 647 (9775–42 406) | <0.001 |
| sST2 (ng/mL at 12 h) (n = 154) | 602 (372–1244) | 633 (343–1028) | NS | 482 (311–923) | 907 (543–1574) | 0.2 |
ALT, alanine aminotransferase; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor‐15; hsTnT, high‐sensitivity troponin T; NS, not significant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; sST2, soluble ST2.
Data are presented as numbers and percentages (%), means (SD), and median (inter‐quartile range).
The management of CS, including invasive assessment by coronary angiography and coronary interventions, mechanical ventilation, and use of vasoactive pharmacotherapy, was similar between the age groups, as shown in Table 3 . The use of intra‐aortic balloon pump was more common in the younger group (61% vs. 39%, P = 0.004). No differences in the prescription of antithrombotic medication were observed between the age groups (data not shown).
TABLE 3.
Treatment of the shock, length of hospital stay, and outcomes
| ≥75 years | <75 years | Elderly survivors | Elderly non‐survivors | |||
|---|---|---|---|---|---|---|
| n = 56 (26%) | n = 163 (74%) | P‐value | n = 30 (54%) | n = 26 (46%) | P‐value | |
| Treatment, n (%) | ||||||
| Angiogram | 45 (80) | 137 (84) | 0.2 | 28 (93) | 17 (65) | 0.009 |
| PCI a | 39 (87) | 110 (80) | 0.3 | 23 (82) | 16 (94) | 0.2 |
| TIMI flow <3 prior PCI (n = 167) | 38 (95) | 114 (90) | 0.5 | 22 (92) | 15 (100) | 0.5 |
| CABG a | 1 (2) | 8 (6) | 0.5 | 1 (4) | 0 | — |
| IABP | 22 (39) | 100 (61) | 0.004 | 10 (33) | 12 (46) | 0.3 |
| Invasive mechanical ventilation | 31 (55) | 106 (65) | 0.2 | 11 (37) | 20 (77) | 0.003 |
| Use of vasoactive medication, n (%) | ||||||
| Noradrenaline | 40 (71) | 124 (76) | 0.5 | 19 (63) | 21 (81) | 0.15 |
| Dobutamine | 25 (45) | 84 (52) | 0.4 | 10 (33) | 15 (58) | 0.07 |
| Adrenaline | 13 (23) | 33 (20) | 0.6 | 3 (10) | 10 (39) | 0.01 |
| Levosimendan | 12 (21) | 41 (25) | 0.6 | 7 (23) | 5 (19) | 0.7 |
| Outcomes | ||||||
| Incidence of AKI, n (%) (n = 154) | 15 (36) | 32 (29) | 0.4 | 1 (4) | 14 (74) | <0.001 |
| Length of hospital stay (days) b | 10 (5–18) | 14 (8–27) | 0.07 | |||
| In‐hospital mortality, n (%) | 26 (46) | 54 (33) | 0.08 | |||
| One year mortality (n = 216), n (%) | 29 (52) | 67 (41) | 0.17 | |||
| One year mortality (among hospital survivors, n = 139), n (%) | 3 (10) | 13 (12) | 1.0 | |||
AKI, acute kidney injury; CABG, coronary artery bypass grafting; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary angiogram; TIMI, thrombolysis in myocardial infarction flow.
Data are presented as numbers and percentages (%), means (SD), and median (inter‐quartile range).
Proportion of those who underwent angiogram.
Reported only for those who survived to hospital discharge.
Outcomes and comparing elderly survivors and non‐survivors
The elderly had numerically higher in‐hospital (46% vs. 33%, P = 0.08) and 1 year (52% vs. 41%; P = 0.17) mortality. Kaplan–Meier survival curves for in‐hospital mortality for all patients and 1 year mortality for those surviving hospitalization stratified by the age group are shown in Figure 1 . Of note, among patients discharged alive, 1 year survival was comparable between the age groups (Figure 1 and Table 3 ). Causes of death did not differ between the groups (Supporting Information, Table S1 ). In addition, an exploratory analysis of the patients with ACS aetiology undergoing PCI (n = 142) showed no difference in in‐hospital (41% vs. 38%, P = 0.7) and 1 year (44% vs. 46%, P = 0.8) mortality rates between the elderly and the younger groups.
Figure 1.
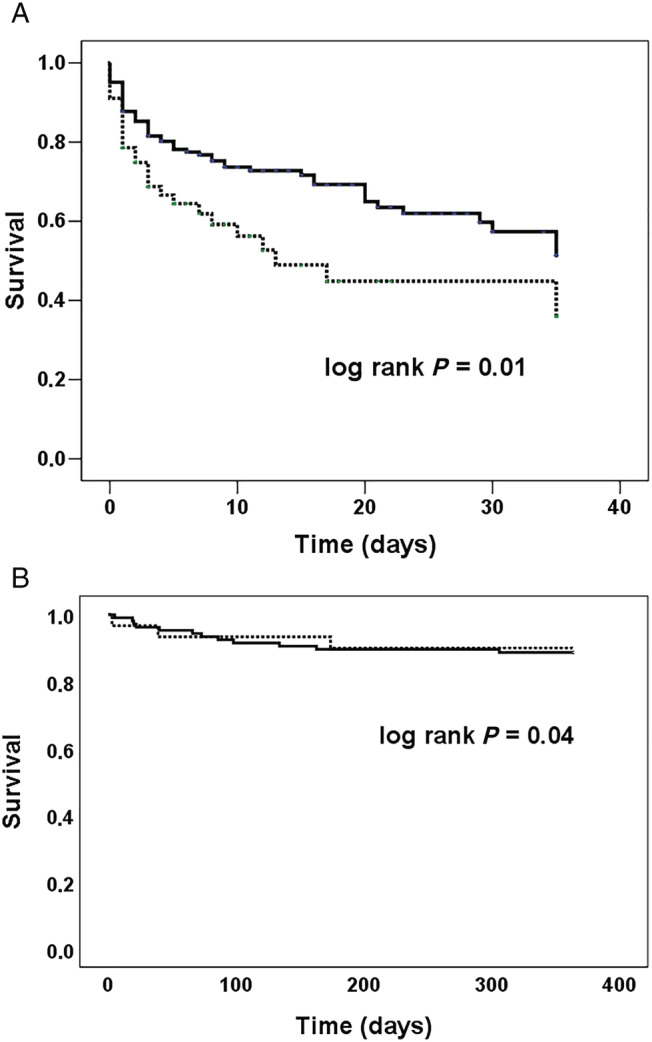
Survival in patients with cardiogenic shock by the age group. Kaplan–Meier survival curves for all‐cause mortality in the elderly (≥75 years old) (dashed line) and the younger (<75 years old) (solid line) patients with cardiogenic shock. (A) In‐hospital mortality (46% in the elderly and 33% in the younger) for all patients. (B) One year mortality (10% in the elderly and 12% in the younger) for those surviving hospitalization.
Elderly survivors and non‐survivors were largely similar with respect to age, sex, medical history, and shock aetiology (Table 1 ). In contrast, the non‐survivors suffered from more severe shock already at baseline, requiring more intense respiratory and haemodynamic support, as depicted in Tables 2 and 3 . Interestingly, non‐survivors had significantly higher GDF‐15 levels compared with survivors (Table 2 ). Survivors were more likely to undergo coronary angiogram (93% vs. 65%, P = 0.009). The revascularization rate was, however, still very high (94% had PCI) among non‐survivors undergoing angiogram (Table 3 ).
Performance of risk scores for prediction of in‐hospital mortality in the elderly
Compared with the younger patients, the CardShock and IABP‐SHOCK II risk scores more frequently categorized the elderly patients into the intermediate‐risk and high‐risk groups and less frequently into the low‐risk group (Figure 2 ). Both scores were useful for mortality risk prediction in the elderly; in the low‐risk category, the outcome was favourable, and the mortality increased with higher risk category (Figure 2 ). The elderly demonstrated a higher CardShock risk score [5.6 (SD 1.5) vs. 4.0 (1.8); P < 0.001] and IABP‐SHOCK II score [4.8 (SD 1.4) vs. 3.2 (1.4); P < 0.001] compared with the younger. The difference remained significant even after excluding the age variable from the model [CardShock risk score 4.6 (1.4) vs. 4.0 (1.8); P = 0.01 and IABP‐SHOCK II score 3.8 (1.4) vs. 3.2 (1.4); P = 0.02].
Figure 2.
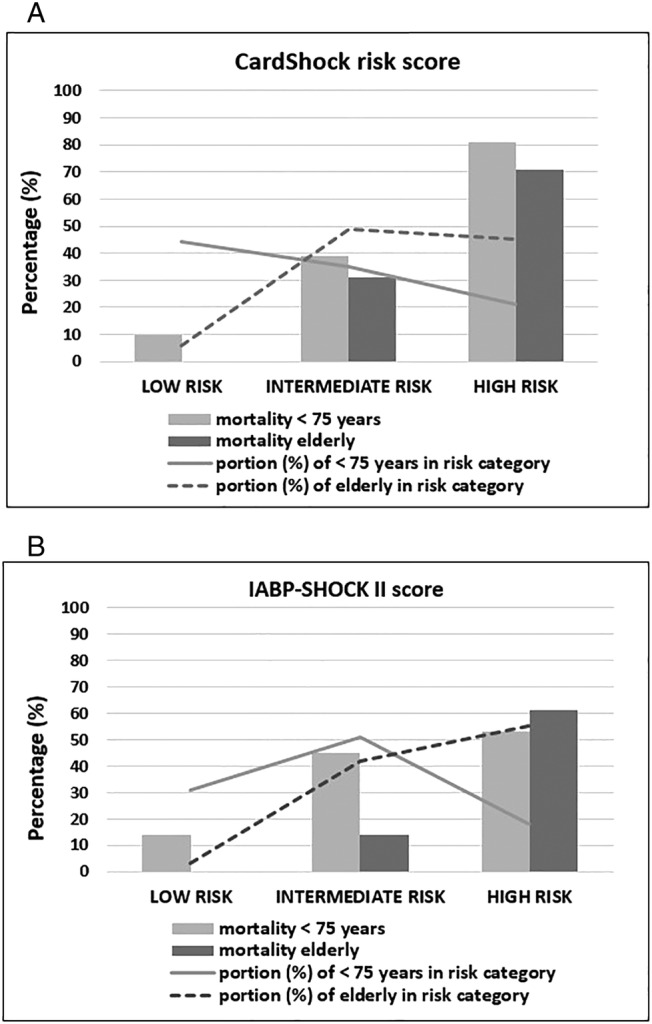
In‐hospital mortality by the risk categories in the elderly and the younger with cardiogenic shock. Distribution of the patients (%; bars) and in‐hospital mortality (%, dashed lines) according to the risk category (low, intermediate, and high) in the elderly (≥75 years) and in the younger (<75 years) patients with cardiogenic shock in (A) CardShock risk score and (B) IABP‐SHOCK II score.
The elderly survivors had a lower risk profile than non‐survivors according to both risk models (CardShock risk score 4.9 vs. 6.4; P < 0.001 and IABP‐SHOCK II 4.4 vs. 5.6; P = 0.009). While both risk models categorized the majority of the non‐survivors (65% in CardShock risk score and 85% in IABP‐SHOCK II) into the high‐risk group, a significant proportion of the elderly survivors were classified as intermediate‐risk patients (Figure 3 ). Age did not have an effect on the ability of the CardShock risk score or the IABP‐SHOCK II score to predict outcome (P interaction 0.82 and 0.47, respectively).
Figure 3.
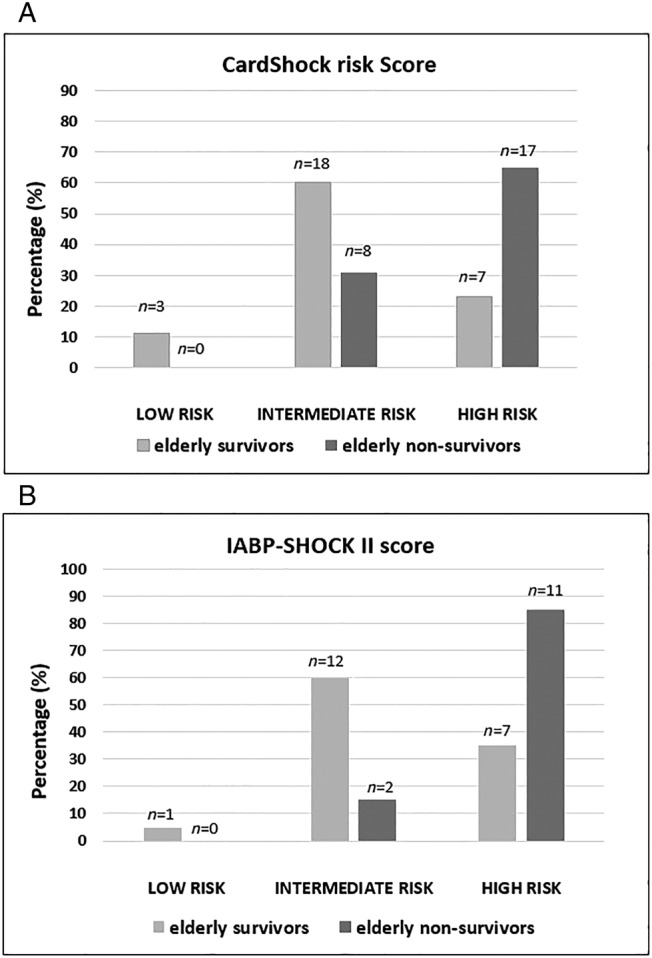
Elderly survivors and non‐survivors and the risk model categories. Distribution (%) of the elderly (≥75 years) in‐hospital survivors and non‐survivors in different risk categories in (A) CardShock risk score (n = 53) and (B) IABP‐SHOCK II score (n = 33). Numbers above the bars indicate the number of the patients in each category.
Among the elderly, the CardShock risk score had an area under the curve (AUC) of 0.75 (vs. 0.82 in the younger group) and the IABP‐SHOCK II score an AUC of 0.71 (vs. 0.73 in the younger group) for prediction of in‐hospital mortality. Each of the biomarkers increased the discrimination of both the CardShock and IABP‐SHOCK II risk scores (Table 4 ). Adding the combination of GDF‐15 and sST2 to the risk prediction models did not improve discrimination compared with adding only one biomarker at a time (Table 4 ).
TABLE 4.
AUC for the CardShock risk score and for the IABP‐SHOCK II score in combination with GDF‐15 or sST2 or both biomarkers to discriminate between in‐hospital survivors and non‐survivors in the elderly (≥75 years old) and in the younger (<75 years old)
| ≥75 years | <75 years | |||||
|---|---|---|---|---|---|---|
| Model | AUC (95% CI) | χ 2 a | P a | AUC (95% CI) | χ 2 a | P a |
| CardShock risk score | 0.75 (0.60–0.91) (n = 40) | 0.82 (0.74–0.90) (n = 106) | ||||
| +GDF‐15 | 0.82 (0.69–0.95) | 4.92 | 0.03 | 0.85 (0.78–0.92) | 4.67 | 0.03 |
| +sST2 | 0.80 (0.66–0.93) | 2.56 | 0.1 | 0.83 (0.76–0.91) | 1.08 | 0.3 |
| +GDF‐15 + sST2 | 0.81 (0.68–0.94) | 5.76 | 0.06 | 0.85 (0.78–0.93) | 4.76 | 0.09 |
| IABP‐SHOCK II score | 0.71 (0.47–0.94) (n = 28) | 0.73 (0.61–0.84) (n = 81) | ||||
| +GDF‐15 | 0.84 (0.69–0.99) | 8.78 | 0.003 | 0.81 (0.71–0.90) | 8.64 | 0.003 |
| +sST2 | 0.78 (0.59–0.96) | 5.14 | 0.02 | 0.79 (0.69–0.89) | 4.75 | 0.03 |
| +GDF‐15 + sST2 | 0.83 (0.68–0.98) | 9.56 | 0.008 | 0.81 (0.72–0.90) | 9.42 | 0.009 |
AUC, area under the curve; CI, confidence interval; GDF‐15, growth differentiation factor‐15; sST2, soluble ST2.
χ 2 and P‐values are shown for comparison of nested models.
For comparison, the CardShock risk score had an AUC of 0.75 [95% confidence interval (CI) 0.69–0.80] for all patients (n = 262), 0.77 (95% CI 0.67–0.87) for patients ≥75 years old (n = 83), and 0.75 (95% CI 0.68–0.82) for patients <75 years old (n = 179) for predicting in‐hospital mortality in the validation cohort.
Discussion
Within this prospective, multicentre study on CS with unselected aetiology, the elderly constituted one‐fourth of the population. They had a higher in‐hospital mortality rate compared with the younger, despite active revascularization. However, those surviving to hospital discharge had a favourable long‐term prognosis. Contemporary CS risk prediction scores showed good ability for mortality risk stratification also in the elderly. The discriminative performance of the scores could be further improved by biomarkers such as GDF‐15 and sST2.
The elderly constituted 26% of the patients with CS in this study. In prior studies, the proportion of the elderly has varied between 29% and 37%. 2 , 13 , 14 In our study, both the clinical presentation and management strategies were similar in the elderly and the younger patients with CS. Considering the predominance of ACS aetiology also in the elderly, the PCI rate was very reasonable; indeed, it was higher than in previous studies. The PCI rate among the elderly patients with CS in previous studies varied between 26% and 51% depending on the study period and the study design including hospital facilities. 2 , 14 The elderly had 40% higher in‐hospital mortality compared with the younger. However, the outcome at 1 year in the elderly hospital survivors was surprisingly good and comparable with the younger. Other studies have reported similar rates of short‐term and 1 year mortality in the elderly. 2 , 13 , 15
All the centres in our study were tertiary hospitals with an on‐site catheterization laboratory, which might have had an influence on patient profiles. The most frail elderly patients judged to have poor prognosis may not have been transferred to tertiary centres. Consequently, mortality in all elderly patients may be higher than we found in our study. The decision whether to transfer the patient or not is made by the physician in charge, often based on the patient's clinical condition.
Accurate risk prediction is essential in the critically ill and in the elderly in particular. Considering the complex pathophysiology and clinical picture of CS especially in the elderly, tools for objective risk stratification are needed to help treatment decisions at all levels of care. The favourable prognosis in discharged elderly patients with CS highlights the importance of well‐balanced treatment decisions early during the in‐hospital phase. We evaluated two contemporary risk scores developed specifically in CS and found satisfactory performance in the elderly. Both scores have recently been externally validated in patients with CS. 4 , 16 , 17 Furthermore, according to our additional validation, CardShock risk score performed well in the elderly in the study population cohort as well. The risk scores are easy to use, and they can be applied early in the management of patients with CS. These risk scores could serve as useful tools for clinicians taking care of elderly patients with CS in their daily practice. In addition, they provide objective risk stratification and may therefore be an aid in allocating resources adequately.
In the current study, the discriminative ability of the risk scores was, however, somewhat lower in the elderly compared with the younger. Age is included as a variable in both scores, and certain other variables, such as renal function and prior manifestation of atherosclerosis, reflect the greater underlying burden of diseases in the elderly. Consequently, most elderly patients are classified into intermediate‐risk or high‐risk groups. Although the majority of the non‐survivors were appropriately categorized as high risk, many elderly survivors were in the intermediate‐risk group, leaving room for improved risk classification.
We found that incorporating biomarkers into the risk prediction improved mortality risk discrimination in the elderly. Considering the complex pathophysiology of CS biomarkers such as GDF‐15, a marker of oxidative stress and associating with biomarkers of hypoperfusion in CS, 8 and sST2, a marker of cardiac stress and inflammation, 18 likely provide additional prognostic information related to CS that is not captured by traditional risk score variables, which could be particularly useful in the elderly. In view of the ageing population, more studies on accurate risk stratification and the optimal management of elderly patients with CS seem warranted.
Limitations
There are some limitations to be acknowledged. First, although the number of the patients in the prospective CardShock study was reasonable, the proportion of elderly was limited, creating some statistical uncertainty in between‐group comparisons. This is a common problem for most studies in CS. 19 Nevertheless, only a small number of patients were included in the analyses of the risk scores' performance and biomarkers. The number of the patients available differed between the CardShock and the IABP‐SHOCK II risk scores. This is mostly due to the variable thrombolysis in myocardial infarction flow in the IABP‐SHOCK II score, which was missing in patients who did not undergo coronary angiogram (e.g. non‐ACS aetiology). Only a few patients were excluded for other missing variables. Furthermore, GDF‐15 and sST2 concentrations were not available from all patients limiting the number of the patients included in the analyses assessing the additional prognostic value of the biomarkers. Secondly, age is one of the variables in both scores giving one point to the elderly automatically. This may contribute to higher score levels and lesser dispersion of the scores among the elderly potentially diminishing the predictive capability of the risk models. Thirdly, having been developed in this study population (patients with CS with different aetiologies), CardShock risk score will perform better in this patient population compared with IABP‐SHOCK II score, which was developed in a different patient population (patients with CS due to ACS). Nevertheless, IABP‐SHOCK II score performed well in this study population as well. Finally, all treatment decisions were at the discretion of the physician in charge. Nevertheless, this study reflects real‐life practice in European tertiary care hospitals, and the choice of treatment strategy was made after careful evaluation based on each individual patient's global health and clinical presentation.
Conclusions
A quarter of patients with CS are elderly. Despite being similar to younger patients in terms of clinical presentation and active revascularization, elderly patients have a higher in‐hospital mortality rate. Those surviving to hospital discharge, however, were comparable with younger discharges in having a good long‐term prognosis. Contemporary mortality risk prediction scores are useful for risk stratification also in the elderly. The added value of biomarker‐based risk stratification in elderly patients with CS needs to be confirmed in larger, prospective cohorts.
Conflict of interest
J.L. has received fees for lectures and advisory board meetings from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Roche Diagnostics, and Vifor Pharma. J.P. received honoraria for advisory meetings and lectures from Orion Pharma and Roche Diagnostics. V.C. received consulting honoraria from CVie Therapeutics Limited, Servier, and Windtree Therapeutics. All other authors have no conflicts to declare.
Funding
This work was supported by Aarne Koskelo Foundation (Aarne Koskelon Säätiö), the Finnish Cardiac Foundation (Suomen Kardiologinen Seura), and the state funding for university‐level health research. M.H. received a personal scholarship from the Department of Emergency Medicine and Services of Helsinki University Hospital (HUS Akuutti) and a personal grant from Aarne Koskelo Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Table S1. Causes of death as reported by local investigators. Note: more than 1 cause of death per patient was accepted.
Figure S1. Distribution of the age in the elderly. Histogram of the distribution of the age in the elderly patients.
Acknowledgements
The authors thankfully acknowledge the contribution of the CardShock Study investigators in all participating hospitals and the GREAT network. Laboratory kits for analysis of NT‐proBNP, hsTnT, and GDF‐15 were kindly provided by Roche Diagnostics, Basel, Switzerland.
Hongisto, M. , Lassus, J. , Tarvasmäki, T. , Sionis, A. , Sans‐Rosello, J. , Tolppanen, H. , Kataja, A. , Jäntti, T. , Sabell, T. , Lindholm, M. G. , Banaszewski, M. , Silva Cardoso, J. , Parissis, J. , Di Somma, S. , Carubelli, V. , Jurkko, R. , Masip, J. , Harjola, V.‐P. , and for the CardShock Study Investigators and the GREAT Network (2021) Mortality risk prediction in elderly patients with cardiogenic shock: results from the CardShock study. ESC Heart Failure, 8: 1398–1407. 10.1002/ehf2.13224.
[Correction added on 11 February 2021, after first online publication: The name of the author Jordi Sans Roselló has been corrected in this version.]
References
- 1. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, Di Somma S, Tolppanen H, Zeymer U, Thiele H, Nieminen MS, Mebazaa A. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17: 501–509. [DOI] [PubMed] [Google Scholar]
- 2. Aissaoui N, Puymirat E, Juilliere Y, Jourdain P, Blanchard D, Schiele F, Guéret P, Popovic B, Ferrieres J, Simon T, Danchin N. Fifteen‐year trends in the management of cardiogenic shock and associated 1‐year mortality in elderly patients with acute myocardial infarction: the FAST‐MI programme. Eur J Heart Fail 2016; 18: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 3. Poss J, Koster J, Fuernau G, Eitel I, de Waha S, Ouarrak T, Lassus J, Harjola VP, Zeymer U, Thiele H, Desch S. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 4. Miller RJH, Southern D, Wilton SB, James MT, Har B, Schnell G, van Diepen S, Grant ADM. Comparative prognostic accuracy of risk prediction models for cardiogenic shock. J Intensive Care Med 2019; 14: 885066619878125. [DOI] [PubMed] [Google Scholar]
- 5. Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L. A biomarker‐based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J 2018; 39: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demissei BG, Cotter G, Prescott MF, Felker MG, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi‐time point‐based risk stratification strategy in acute heart failure: results from the RELAX‐AHF trial. Eur J Heart Fail 2017; 19: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 7. Tolppanen H, Rivas‐Lasarte M, Lassus J, Sadoune M, Gayat E, Pulkki K, Arrigo M, Krastinova E, Sionis A, Parissis J, Spinar J, Januzzi J, Harjola VP, Mebazaa A. Combined measurement of soluble ST2 and amino‐terminal pro‐B‐type natriuretic peptide provides early assessment of severity in cardiogenic shock complicating acute coronary syndrome. Crit Care Med 2017; 45: e666–e673. [DOI] [PubMed] [Google Scholar]
- 8. Hongisto M, Kataja A, Tarvasmaki T, Holopainen A, Javanainen T, Jurkko R, Jäntti T, Kimmoun A, Levy B, Mebazaa A, Pulkki K, Sionis A, Tolppanen H, Wollert K, Harjola VP, Lassus J. Levels of growth differentiation factor 15 and early mortality risk stratification in cardiogenic shock. J Card Fail 2019; 25: 894–901. [DOI] [PubMed] [Google Scholar]
- 9. Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am J Cardiol 2015; 115: 26B–31B. [DOI] [PubMed] [Google Scholar]
- 10. Wang J, Tan GJ, Han LN, Bai YY, He M, Liu HB. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol 2017; 14: 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ad‐hoc working group of ERBP , Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast‐induced nephropathy. Nephrol Dial Transplant 2012; 27: 4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim HS, Farouque O, Andrianopoulos N, Yan BP, Lim CCS, Brennan AL, Reid CM, Freeman M, Charter K, Black A, New G, Ajani AE, Duffy SJ, Clark DJ. Survival of elderly patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock. JACC Cardiovasc Interv 2009; 2: 146–152. [DOI] [PubMed] [Google Scholar]
- 14. Gasior M, Slonka G, Wilczek K, Gierlotka M, Ruzyllo W, Zembala M, Osadnik T, Dubiel J, Zdrojewski T, Kalarus Z, Polonski L. Comparison of invasive and non‐invasive treatment strategies in older patients with acute myocardial infarction complicated by cardiogenic shock (from the Polish Registry of Acute Coronary Syndromes—PL‐ACS). Am J Cardiol 2011; 107: 30–36. [DOI] [PubMed] [Google Scholar]
- 15. Aissaoui N, Puymirat E, Simon T, Bonnefoy‐Cudraz E, Angoulvant D, Schiele F, Benamer H, Quandalle P, Prunier F, Durand E, Berard L, Blanchard D, Danchin N. Long‐term outcome in early survivors of cardiogenic shock at the acute stage of myocardial infarction: a landmark analysis from the French registry of acute ST‐elevation and non‐ST‐elevation myocardial infarction (FAST‐MI) Registry. Crit Care 2014; 18: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rueda F, Borràs E, García‐García C, Iborra‐Egea O, Revuelta‐López E, Harjola VP, Cediel G, Lassus J, Tarvasmäki T, Mebazaa A, Sabidó E, Bayés‐Genís A. Protein‐based cardiogenic shock patient classifier. Eur Heart J 2019; 40: 2684–2694. [DOI] [PubMed] [Google Scholar]
- 17. Rivas‐Lasarte M, Sans‐Roselló J, Collado‐Lledó E, González‐Fernández V, Noriega FJ, Hernández‐Pérez FJ, Fernández‐Martínez J, Ariza A, Lidón RM, Viana‐Tejedor A, Segovia‐Cubero J, Harjola VP, Lassus J, Thiele H, Sionis A. External validation and comparison of the CardShock and IABP‐SHOCK II risk scores in real‐world cardiogenic shock patients. Eur Heart J Acute Cardiovasc Care 2020; 31 Epub ahead of print. PMID: 32004078. [DOI] [PubMed] [Google Scholar]
- 18. Weinberg E, Shimpo M, Keulenaer G, MacGillivray C, Tominaga SI, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin‐1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002; 106: 2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy B, Clere‐Jehl R, Legras A, Morichau‐Beauchant T, Leone M, Frederique G, Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon P. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2018; 72: 173–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Causes of death as reported by local investigators. Note: more than 1 cause of death per patient was accepted.
Figure S1. Distribution of the age in the elderly. Histogram of the distribution of the age in the elderly patients.


