Abstract
Increased risk of cardiovascular complications during and post‐COVID‐19 infection is more and more recognized—including myocarditis, arrhythmias, and myocardial infarctions (MIs). The mechanisms leading to these complications are direct virus‐induced injuries, as well as potential thrombotic and inflammatory‐induced mechanisms. To the latter, inflammatory plaque instability and plaque rupture are discussed entities contributing to MI‐induced post‐COVID‐19 complications. Our case report describes the first time, when a temporary impairment of LVEF in the COVID‐19‐convalescence phase unmasks a silent MI due to coronary plaque rupture by using invasive (OCT) and non‐invasive (CMR) modalities. Myocardial infarction might be an important differential diagnosis to consider in deteriorating patients with COVID‐19, especially if dyspnoea persists after acute infection.
Keywords: CMR, convalescence, COVID‐19, heart, magnetic resonance, myocarditis, myocardial infarction, OCT, recovery, SARS‐CoV‐2, thrombosis
Introduction
Increased risk of cardiovascular complications during and post‐COVID‐19 infection is more and more recognized—including myocarditis, arrhythmias, and myocardial infarctions (MIs). 1 , 2 , 3 , 4 The mechanisms leading to these complications are direct virus‐induced injuries, as well as potential thrombotic and inflammatory‐induced mechanisms. To the latter, inflammatory plaque instability and plaque rupture are discussed entities contributing to MI‐induced post‐COVID‐19 complications. 2 , 3
We are reporting the case of a 74‐year‐old man, who presented with slight cough after a diagnosed SARS‐CoV‐2 infection 6 weeks ago. Besides known and sufficient treated arterial hypertension (ramipril 5 mg twice a day) and hyperlipoproteinaemia (simvastatin 40 mg once a day) as the only pre‐existing diseases, the patient was completely asymptomatic before COVID‐19. A positive PCR for the COVID‐19 virus and confirmation of a diagnosis of COVID‐19‐induced pneumonia at chest‐CT and MRI (bilateral pleural effusion, distinct peripheral consolidation, and reticulations), the patient was hospitalized without intubation for 11 days. During rehabilitation, there was an unexplained drop of left ventricular ejection fraction (LVEF) to 41%. The patient was transferred to our hospital with suspected post‐COVID‐19 myocarditis and presented without severe clinical symptoms (laboratory results: hs‐troponin slightly elevated with 21 ng/L; CRP normal with 3.5 mg/L and NT‐pro‐BNP normal with 69 ng/L; normal ECG without signs of ischaemia). Moreover, the LVEF (measured using Simpson's biplane method) returned to normal (72%) at presentation at our institution. For further evaluation, coronary angiography and cardiac magnetic resonance (CMR) 5 was recommended for the first time.
The coronary angiogram (Figure 1 ) revealed a two‐vessel coronary artery disease with severe (80% diameter) reduction (A demonstrates normal left main stem; B indicates high‐grade stenosis) of the mid‐left anterior descending (LAD) artery and intermediate circumflex artery stenosis (TIMI III). It also presented a ruptured plaque with lipid core and thrombus (C) at optical coherence tomography at LAD, which was not seen at distal LAD (D). The patient was treated by placement of a stent into the LAD. Four weeks later, hs‐troponin found to be in the normal range.
Figure 1.
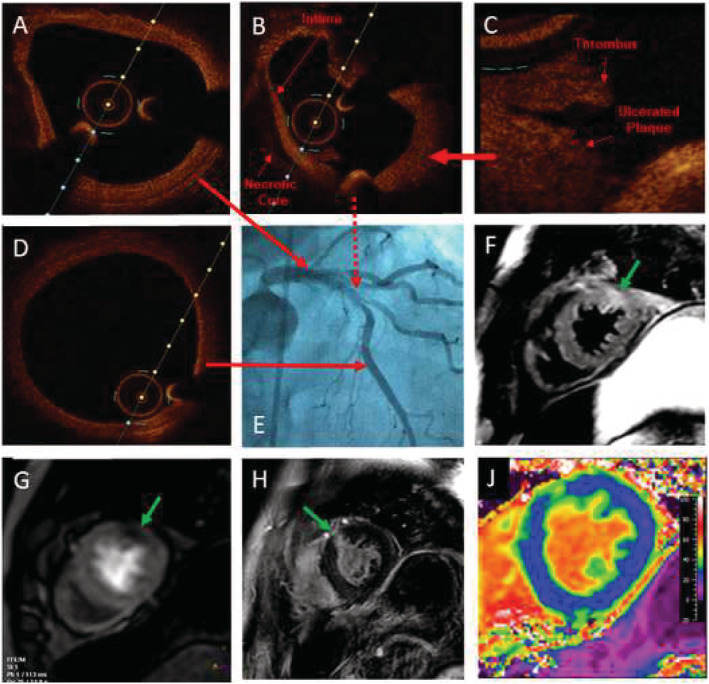
Coronary angiogram including OCT and CMR at COVID‐19‐convalescence phase.
CMR demonstrated normal LVEF and slightly hypertrophied myocardium with myocardial oedema at anterior/lateral wall, seen on T2‐weighted sequences at mid and apical short‐axis‐orientation (SAX) (indicated by green arrow in F). After contrast administration, perfusion abnormalities (green arrow at G) and late gadolinium enhancement (LGE) of 75% transmurality were detected in the same region (green arrow in H). In T2‐mapping sequences, T2 was increased up to 59 ms (normal 35–51 ms at 3.0T); native T1‐mapping was elevated up to 1398 ms (normal: 1173–1334 ms at 3.0T); extracellular volume (ECV) map (J), based on a T1‐mapping sequence before and after contrast, revealed significantly increased ECV anterior (indicated by green colour) vs. remote myocardium (blue colour in J).
This case report describes the first time when a temporary impairment of LVEF in the COVID‐19‐convalescence phase unmasks a silent MI due to coronary plaque rupture. Myocardial infarction might be an important differential diagnosis to consider in deteriorating patients with COVID‐19, especially if dyspnoea persists after acute infection.
Conflict of interest
C.T., M.S.A., and S.K. have received research support by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research) and personal fees from Servier, outside the current work. All other authors have nothing to disclose.
Funding
S.K. received research support from Philips Healthcare. C.T. and S.K. received research support by the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research).
Acknowledgements
None.
Open Access funding enabled and organized by Projekt DEAL.
Tschöpe, C. , Sherif, M. , Anker, M. S. , Geisel, D. , Kuehne, T. , and Kelle, S. (2021) COVID‐19‐convalescence phase unmasks a silent myocardial infarction due to coronary plaque rupture. ESC Heart Failure, 8: 971–973. 10.1002/ehf2.13186.
Carsten Tschöpe and Mohammad Sherif contributed equally to this study.
References
- 1. van Linthout S, Klingel K, Tschöpe C. SARS‐CoV‐2‐related myocarditis‐like syndromes Shakespeare's question: what's in a name? Eur J Heart Fail 2020; 22: 922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, Cacucci M, Assanelli E, Bellini B, Russo F, Ielasi A, Tespili M, Danzi GB, Vandoni P, Bollati M, Barbieri L, Oreglia J, Lettieri C, Cremonesi A, Carugo S, Reimers B, Condorelli G, Chieffo A. ST‐elevation myocardial infarction in patients with COVID‐19: clinical and angiographic outcomes. Circulation 2020; 141: 2113–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J 2020; 41: 1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of Covid‐19 in New York city. N Engl J Med 2020; 382: 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelle S, Bucciarelli‐Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S, Raimondi F, Weinsaft JW, Wong TC, Carr J. Society for cardiovascular magnetic resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID‐19 infection. J Cardiovasc Magn Reson 2020; 22: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]


