Abstract
Aims
Determine the feasibility of implementing a heart failure (HF) management strategy that (i) uses a device‐based, remote, dynamic, multimetric risk stratification model to predict the risk of HF events and (ii) uses a standardized, centrally administered, ambulatory medication intervention protocol to reproducibly and safely decrease elevated risk scores.
Methods and results
Prospective, non‐randomized, single‐arm, multicenter feasibility study (Intervene‐HF) was conducted in HF patients implanted with a cardiac resynchronization therapy with implantable cardio defibrillator (CRT‐D) with TriageHF risk score feature. Certified HF nurses (CHFN) in the Medtronic Care Management Services Program implemented an ambulatory medication intervention strategy by following a standardized guided action pathway triggered by risk‐based alert. When CHFN received notification of increased risk score (HF care alert), they implemented a 3 day course of diuretic up‐titration (PRN) previously prescribed by a physician. Safety was monitored daily. Recovery after PRN was defined as ≥70% recovery of impedance toward baseline levels. Sixty‐six patients followed for 8.2 ± 3.9 months had 49 HF care alerts. Twenty‐three of 49 alerts did not receive PRN due to protocol‐mandated criteria. Twenty‐six of 49 alerts received PRN, 22 were completed, and 19 led to impedance recovery. Four interventions were stopped for safety without leading to an adverse event (AE). One of 26 PRNs was followed by a HF event. Eighty‐five per cent (22/26) of PRNs were completed without an AE; 69% (18/26) met the recovery criteria.
Conclusions
The Intervene‐HF study supports the feasibility of testing, in a large randomized clinical trial, an ambulatory medication intervention strategy that is physician‐directed, CHFN‐implemented, and based on individualized device risk stratification.
Keywords: Heart failure, Congestive, Remote metric
Introduction
Ambulatory risk stratification models based on multiple parameters combined into an integrated device diagnostic risk score have been developed to predict heart failure (HF) events. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 However, standardized companion management strategies guided by these integrated diagnostic scores have not been developed. Ideally, the management strategies would follow a remote, ambulatory care pathway that would recognize increased risk before the appearance of or worsening of symptoms, allow active intervention that would prevent the progression to acute decompensated HF, increase efficiency and productivity of healthcare provider teams, and avoid management‐induced adverse events (AEs).
One possible care pathway would follow a physician‐directed, nurse‐implemented, ambulatory, medication intervention strategy. In this conceptual strategy, several sequential steps would be taken. A risk score would be calculated based on data from an implanted diagnostic device. For each patient, an HF physician would a priori prescribe a PRN medication response to an increased risk score. A commercial, centralized, certified HF nurse (CHFN) service would monitor for ambulatory, remotely assessed, device‐triggered, risk score alerts, determine an actionable increased risk, determine whether the increased risk can/should be intervened upon, implement the medication response plan, and examine the effect of treatment by examining the recovery effect on remotely measured risk parameters. The CHFN would not contact the HF provider team unless the risk score elevation could not be treated with PRN treatment plan or if the PRN treatment did not result in an appropriate reduction in the risk score. The risk status alerts would be silent to the patient.
The purpose of the INTERVENE‐HF study (Integrated Diagnostics Driven Diuretic and Chronic Medication Management for Heart Failure) was to determine whether it is feasible to apply one example of a physician‐directed, nurse‐implemented, ambulatory, medication intervention strategy (schematically shown in Figure 1 ) in HF patients with an increased HF risk score. The novel aspects of this feasibility study include (i) use of a risk metric not based on directly measured pressures; (ii) use of a centralized CHFN service to receive and evaluate HF risk alerts, apply and monitor a standardized guided PRN action pathway; and (iii) use a recovery criteria that is responsive over the 3 day PRN to determine success of one PRN, the need for a second PRN, or need for referral to HF care team. Ultimately, this manuscript will help provide the rationale, design, and feasibility data necessary to develop a pivotal randomized clinical study of the proposed novel patient management strategy.
Figure 1.
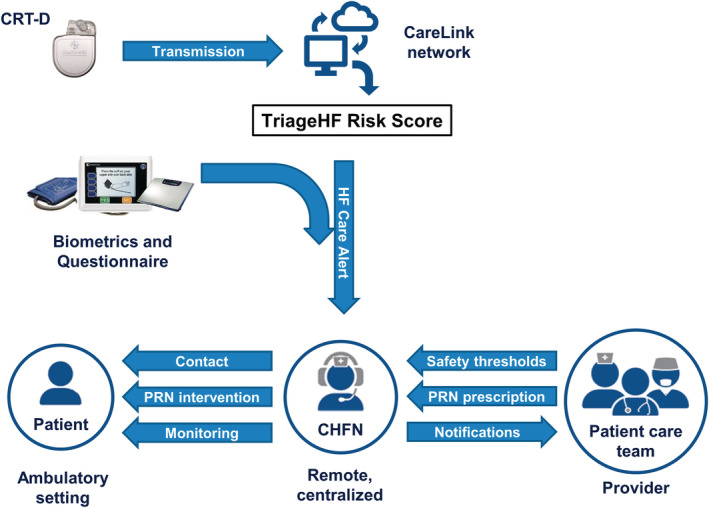
Intervene‐HF study information flow chart. This strategy entails a physician‐directed, nurse‐implemented, ambulatory, medication intervention described in detail in the manuscript text.
Methods
Patients
Seventy‐nine participants were enrolled in the INTERVENE‐HF study between May 2016 and September 2018 from 10 US sites (Supporting Information, Table S1 ).
Study design
This study was a prospective, non‐randomized, single‐arm, multicenter feasibility study conducted in HF patients. Inclusion and exclusion criteria are listed in Table S2 . Investigators prescribed an individualized medication intervention plan consisting of up to two rounds of transient up titration of volume management medication referred to as PRN and established subject‐specific weight loss and blood pressure safety thresholds that triggered the cessation of a PRN medication intervention. The PRN prescription for each patient was based on the investigator's specific and in depth knowledge of the patient and previous experience altering medication prescription during prior episode of volume overload or acute decompensation. Patients were provided with a CommanderFLEX™ monitoring device to collect weight, blood pressure, HF symptoms. CHFNs used these data at the time they received a HF care alert as part of the guided action pathway. CHFNs monitored these data daily during the PRN intervention as part of the safety assessment.
Patients were monitored and managed remotely by CHFNs at Medtronic Care Management Services using the patient assessment PRN medication intervention algorithm (Figure 2 ). These CHFNs were employed by Medtronic Inc. The CHFNs did not participate in data analysis of the results reported in this manuscript. The CHFNs monitored for HF care alerts, categorized overall subject risk status, augmented risk status determination based on biometric data when necessary, determined if a PRN medication intervention was warranted, executed the PRN medication interventions, monitored the patients' weight and blood pressure during an intervention, and performed dietary counselling and medication adherence reinforcement. The CHFNs also notified sites of PRN medication intervention and device‐diagnostic‐based information ( Table S3 ).
Figure 2.
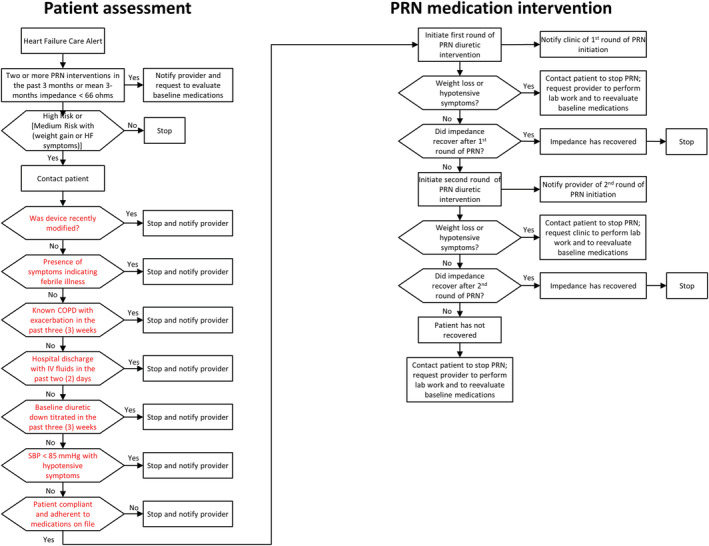
Guided PRN medication intervention pathway. Patients were assessed by certified heart failure nurses to determine whether the increased risk could/should be intervened upon using the ‘patient assessment’ algorithm shown. If a PRN medication intervention was appropriate to be instituted, the ‘PRN medication intervention’ algorithm was followed by certified heart failure nurses.
Heart failure care alert
When the OptiVol fluid index crossed a predefined threshold (60 ohm‐days), the CRT‐D device transmitted a remote wireless alert to the Medtronic CareLink Network (Figure 1 ). The dynamic TriageHF risk score was then calculated on the CareLink network as previously described. 4 , 12 , 13 Briefly, the TriageHF risk score was generated by combining the following parameters into a single risk score: intrathoracic impedance, heart rate variability, atrial fibrillation burden, ventricular rate during atrial fibrillation, percent CRT pacing, ventricular tachycardia episodes/shocks, night‐time heart rate, and activity. All of these individual parameters were categorized into individual risk levels, and then, these risk levels were combined in a Bayesian Belief Network framework to generate the TriageHF risk score. 4 , 12 The TriageHF risk score was expressed on a scale of 0% to 100% probability of having a HF event within 6 months. Low risk was defined as any probability score <5.4%, medium risk was defined as a probability range from ≥5.4% to <20%, and a high risk was defined as a probability ≥20%. In the current study, once the numerical TriageHF risk score was categorized as ‘medium’ or ‘high’ risk status corresponding to the likelihood of having a HF event in the next 30 days, an HF care alert notification was then transmitted to the CHFNs. The CHFNs aggregated both the information from the care link communication and the biometric data transmitted by the patient.
The HF care alert lead to an evaluation of eligibility for PRN intervention by CHFNs under the following circumstances:
-
1‐
If TriageHF risk score was high, PRN intervention eligibility was evaluated, regardless of the biometric data examining patient weight and HF symptom status.
-
2‐
If TriageHF risk score was medium, the biometric data were then used to determine which patients should undergo PRN intervention evaluation. If biometric data were available and there were no weight changes or symptom changes, PRN intervention evaluation was not performed based on the relatively lower patient risk for acute decompensated heart failure (ADHF). If biometric data were available and there were significant changes in weight or symptoms, PRN intervention evaluation was performed based on the relatively higher patient risk for ADHF. If biometric data were not available, PRN intervention evaluation was performed assuming the presence of a relatively higher patient risk for ADHF. Weight gain of less than two pounds in 1 day or five pounds in 7 days was considered significant. Monitored HF symptoms were dyspnoea with exertion, nocturnal dyspnoea, and lower extremity oedema.
Guided PRN medication intervention pathway
Patient assessment
The implementation of the PRN medication intervention followed a guided intervention pathway (Figure 2 ). CHFNs contacted the patient to determine whether there were alternative, non‐HF‐related reasons, that could explain the presence of the ‘high risk’ status and to determine whether it was safe to implement the PRN intervention (specific issues in red highlighted boxes in the left column of Figure 2 ). If there were no contraindications, CHFNs followed the ‘PRN medication intervention’ pathway outlined in the right column in Figure 2 .
PRN medication intervention
After establishing that a patient was eligible for PRN intervention, CHFNs provided directions for the self‐administration of the physician‐prescribed PRN medications. The PRN intervention (Figure 3 ) consisted of up to two rounds of a 3 day course of medication up titration for volume management (usually diuretic) with daily safety monitoring. After completion of the first round of PRN medications (Day 4 post‐initiation), CHFNs evaluated the efficacy of the PRN intervention by examining an intrathoracic impedance‐based recovery criterion. ‘Recovery’ was defined as occurring when the impedance recovered to ≥70% of the baseline value (Figure 4 ). If the intrathoracic impedance had recovered by Day 4, the PRN intervention was judged to be successful and the CHFNs contacted the patient to direct them to stop taking their PRN medications. If the recovery was not successful by Day 4 and there were no contraindications (such as excessive weight loss or symptomatic hypotension), a second round of PRN medications was initiated, and the recovery criteria was re‐evaluated on Day 8.
Figure 3.
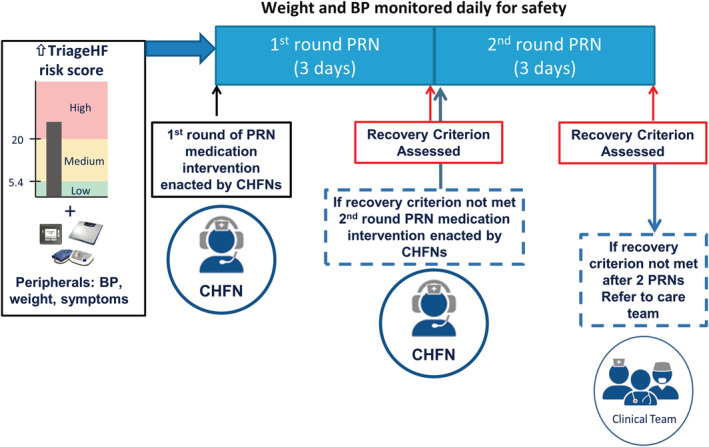
Schematic representation of guided action pathway. A heart failure care alert based on integrated TriageHF risk score and peripheral biometric data were used by CHFNs to implement PRNs and monitor safety and attainment of recovery criterion (recovery criterion presented in Figure 4). CHFNs interaction with and notification of clinical provider team is also denoted. BP, blood pressure; CHFNs, certified heart failure nurses.
Figure 4.
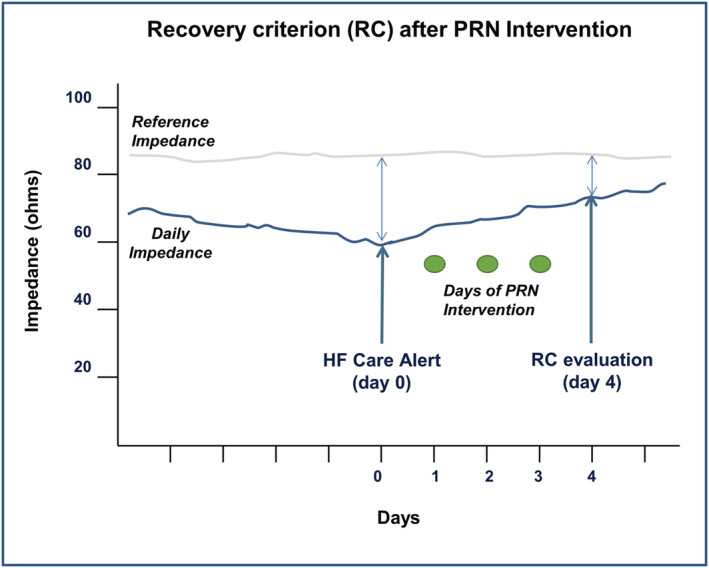
Recovery criterion resulting from PRNs. Recovery criterion after PRN medication intervention was based on the presence of a ≥70% recovery of impedance (daily impedance) toward baseline values (reference impedance). HF, heart failure.
In two previous publications, Zile, Sharma, Johnson et al. 11 and Zile, Sharma, Baicu et al. 14 , the predictive value of direct measurements of intrathoracic impedance, measured at baseline and measured as change from baseline, for predicting HFH events and mortality, was demonstrated. In addition, its advantages compared with Optivol fluid index were also highlighted. In particular, the very short time frame (hours) in which direct measurements of intrathoracic impedance responds to changes in intravascular volume vs. the longer time frame (days) for Optivol fluid index was emphasized. It was based on these data that the methods used in INTERVENE‐HF to judge the effectiveness of the PRN intervention were chosen.
Safety evaluation during PRN intervention
To ensure patient safety, CHFNs monitored biometric data and symptom status. Patient‐specific safety thresholds were chosen by the physician prior to patient initiation into the study. If a patient‐specific safety threshold was crossed, the CHFN contacted the patient to evaluate clinical status and, where indicated, stopped the PRN intervention, notified the provider, requested that appropriate laboratory assessment be performed, and that baseline medications be re‐evaluated.
Outcome evaluations
Feasibility of approach was defined by examining the following criteria: (i) attaining the impedance recovery criterion after completion of the PRN medication intervention, (ii) the subject had no HF event in the next 14 days after completion of the PRN medication intervention, and (iii) the subject had not experienced any AE that was related to the PRN medication intervention. An intervention was considered effective if all three criteria were met. An HF event was defined as an intervention that required IV diuretics, ultrafiltration, or their equivalent or an inpatient hospitalization. Given the small sample size used in this study, a limited effectiveness assessment of the PRN was calculated as the number of recovered PRN medication interventions without a HF event within 14 days and without a PRN‐related AE divided by the total number of PRN medication interventions.
Avoidance of harm was defined by examining the following criteria: a PRN medication intervention was (i) completed without safety issues, (ii) did not result in any PRN treatment‐related AE. An intervention was considered safe if both the criteria were met. Given the small sample size used in this study, a limited safety assessment of the PRN intervention was calculated as the number of PRN medication interventions without PRN‐related AE divided by the total number of PRN medication interventions.
Statistical analysis
For each PRN intervention, the effectiveness and safety were evaluated as a binary outcome variable, where 1 = effective/safe and 0 = not effective/not safe. During the course of the study, a subject could experience more than one intervention. Therefore, a generalized linear model with the generalized estimating equation (GEE) method was used to analyse the correlated binary data. The effectiveness and safety rates and their 95% confidence interval (CI) were calculated based on the log of odds estimated using the GEE. Note that the 95% CI from the GEE may not be a good estimate due to the small sample size of the study. Change in the 3 day average impedance before and after each PRN intervention was evaluated using the Wilcoxon signed‐rank test. Mean 30 day TriageHF risk score and impedance before intervention were compared between cases recovered after one PRN, two PRNs vs. not recovered cases using the Wilcoxon rank sum test.
Results
Patient demographics
Seventy‐nine patients were enrolled in the INTERVENE‐HF study (Figure 5 ). Eleven patients did not meet eligibility or screening criteria, and two patients withdrew. Sixty‐six patients fulfilled the eligibility and screening criteria, had the fluid alert feature activated, and were followed by CHFNs. These 66 patients had demographic features typical of patients with heart failure with reduced ejection fraction (Table 1 ). Total follow‐up time was 8.2 ± 3.9 months. Forty‐five patients completed the study, 21 patients did not complete the study because 14 withdrew, 3 were lost to follow‐up, 2 died, and 2 had their CRT device explanted. Reasons for withdrawal before study completion: eight subjects were withdrawn due to being started on therapy not conducive to PRN diuretic therapy (e.g. IV inotropes, haemodialysis, weekly medication changes in clinic, and diuretics therapy managed by a physician not part of study team); six subjects were withdrawn by the investigators due to patient request (three) or geographic relocation (three).
Figure 5.
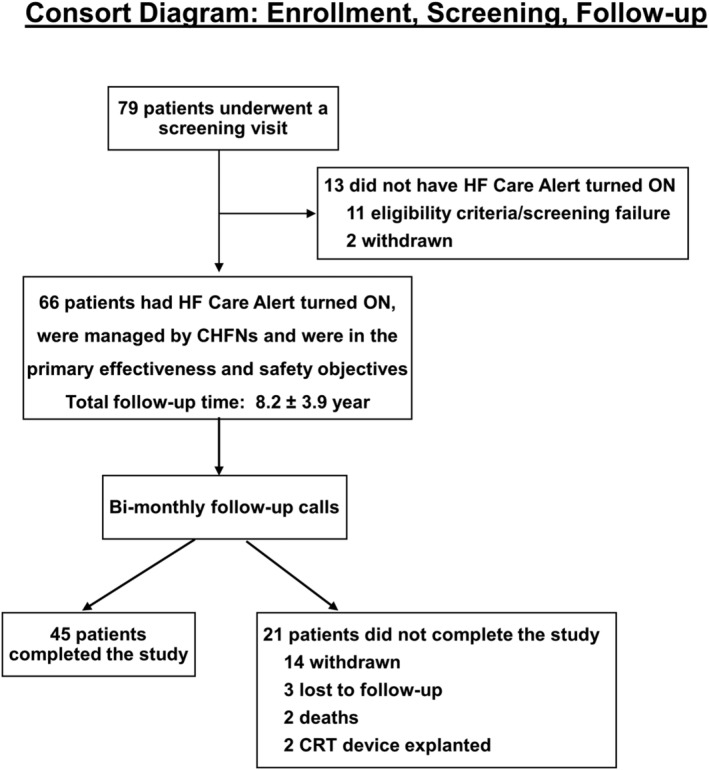
Consort diagram: enrolment, screening, and follow‐up. The number of patients enrolled, screen/eligibility failed, length of follow‐up, completed follow‐up, and exited patients are listed. HF, heart failure.
Table 1.
Baseline patient characteristics
| Demographics | |
| Number of subjects | 66 |
| Age (years) | 73 ± 9, range 46–92 |
| Male | 46 (70%) |
| Caucasian | 57 (86%) |
| Laboratory findings | |
| BMI (kg/m2) | 28.1 ± 6.4 |
| Systolic BP (mmHg) | 119.6 ±17.7 |
| Diastolic BP (mmHg) | 70.6 ± 9.4 |
| GFR (mL/min per 1.73 m2) | 57.2 ± 17.6 |
| Haemoglobin (g/dL) | 12.8 ± 1.6 |
| Potassium (mmol/L) | 4.4 ± 0.4 |
| Sodium (mmol/L) | 139.9 ± 2.8 |
| NYHA class | |
| Class II | 27 (41%) |
| Class III | 39 (59%) |
| Medical history | |
| Ischemic cardiomyopathy | 38 (58%) |
| Non‐ischemic cardiomyopathy | 21 (32%) |
| Hypertrophic cardiomyopathy | 1 (1%) |
| Hypertension | 42 (64%) |
| Myocardial infarction | 27 (41%) |
| Peripheral vascular disease | 18 (27%) |
| Atrial fibrillation | 40 (61%) |
| Atrial flutter | 7 (11%) |
| Chronic obstructive pulmonary disease | 13 (20%) |
| Diabetes mellitus | 28 (42%) |
| Chronic renal dysfunction | 16 (24%) |
| Stroke | 8 (12%) |
| Cardiovascular medications at baseline | |
| ACE‐I/ARB/ARNI | 48 (73%) |
| Beta blockers | 57 (86%) |
| Diuretics | 55 (83%) |
| MRAs | 19 (29%) |
| Vasodilators | 17 (26%) |
| Digitalis compounds | 16 (24%) |
| Anti‐arrhythmic drugs | 15 (23%) |
| Calcium channel blockers | 3 (4%) |
| HCN channel blockers | 2 (3%) |
| HF events in previous 6 months | |
| Yes | 13 (20%) |
| No | 53 (80%) |
ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor‐neprilysin inhibitors; BMI, body mass index; BP, blood pressure; GFR, glomerular filtration rate; HCN, hyperpolarization‐activated cyclic nucleotide‐gated; MRA, mineralocorticoid receptor antagonist.
Data are mean ± standard deviation or number (%).
Heart failure care alerts and PRN interventions
Of 66 eligible patients, 37 did not have any HF care alerts (Figure 6 ). CHFNs received 49 HF care alerts from 29 patients. Twenty‐three HF care alerts in 19 patients did not result in a PRN intervention for the following reasons: five were ruled out by protocol mandated criteria (such as patient presenting symptoms of febrile illness, pneumonia, or chronic obstructive pulmonary disease exacerbation), six were ruled out due to safety concerns (such as patient having received IV fluids in the past 2 days or baseline diuretic medication change in the past 3 weeks), five were ruled out due to medication related issues (such as patient non‐adherence with baseline diuretic medication or inaccurate medication information on file), two patients were unable to reach, three had a medium TriageHF risk and were known to be without symptoms, and two were ruled out for other reasons (refer to Table 2 for details).
Figure 6.
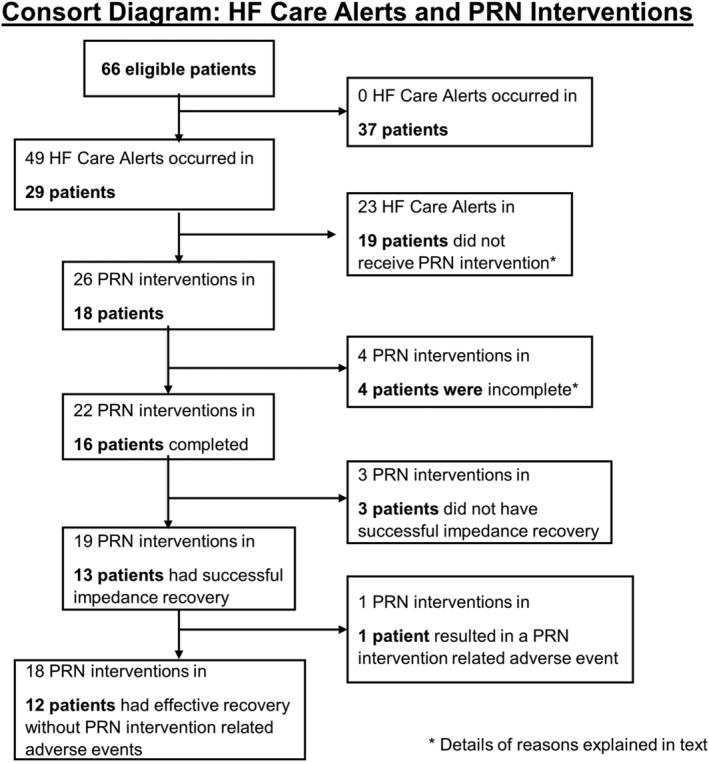
Consort diagram: HF care alerts and PRN interventions. The number of HF care alerts occurring in the number of patients, their PRN medication interventions, impedance recoveries, and outcomes are listed. HF, heart failure.
Table 2.
Twenty‐three risk alerts that were not followed by a PRN medication intervention
| Protocol‐related rule‐outs (n = 5) |
2 Subject presenting symptoms of febrile illness 2 Subject presenting symptoms of pneumonia 1 Subject presenting symptoms of COPD exacerbation |
| Safety‐related rule‐outs (n = 6) |
3 Baseline diuretics were changed in the past 3 weeks 1 Subject received IV fluids for greater than or equal to 1 day in past 2 days 1 Subject presenting third degree burns from radiation treatment 1 Subject on continuous inotropic infusion (dobutamine drip) |
| Medication‐related rule‐outs (n = 5) |
2 Subjects self‐administered PRN diuretic medication 2 MCMS had inaccurate medication information on file 1 Subject non‐compliant with baseline medications |
| Medium triage risk score with no symptoms (n = 3) | |
| Unable to reach (n = 2) | |
| Other (n = 2) |
1 Upcoming exit visit 1 Clinical site instructed MCMS not to intervene |
The PRN medications used in INTERVENE‐HF consisted of 55% of the prescribed PRN round 1 medications and 42% of PRN round 2 medications were double the daily baseline diuretics dose. The distribution of the medications used during the PRN interventions were 56% furosemide, 13% bumetanide, 10% metolazone, 6% torsemide, 6% spironolactone, and 10% other.
Twenty‐six PRN interventions were performed in 18 patients; four interventions in four patients were not completed because they met one of the safety thresholds (n = 3) or due to the appearance of new symptoms of febrile illness before the second round of PRN medication was initiated (n = 1); 22 PRN interventions in 16 patients were completed. Nineteen PRN interventions in 13 patients had successful impedance recovery; 15 after one PRN intervention, and 4 after 2 PRN interventions. Eight successful PRN interventions occurred after high risk alert, 11 successful PRN interventions occurred after medium risk alert, 75% of successfully treated high risk alerts were asymptomatic and 45% of successfully treated medium risk alerts were asymptomatic. Three PRN interventions in three patients were stopped early because of excessive weight loss accompanied by muscle cramps or dizziness. Three PRN interventions in three patients did not achieve the pre‐defined impedance recovery. One completed PRN intervention with impedance recovery was associated with a non‐serious PRN treatment‐related AE. Therefore, 69% (18/26) of the PRN interventions were effective. The GEE‐estimated effectiveness rate was 70.8% (95% CI: 52.4–84.2%).
Of the 26 PRN interventions, 3 were stopped because of excessive weight loss accompanied with muscle cramps or dizziness, 19 PRN interventions in 13 patients had successful impedance recovery, and 1 PRN intervention in 1 patient resulted in a non‐serious PRN related AE. The patient self‐reported two falls that did not result in any injury that occurred during a PRN intervention; neither fall required a hospitalization or clinic visit. Eighteen PRN interventions in 12 patients had an effective recovery without PRN related AE. No completed PRN intervention with impedance recovery was followed by a HF event in the next 30 days or decreased renal function (Table 3 ). Eight of the 37 patients who did not have any HF care alert developed a HF event. Therefore, 85% (22/26) of the PRN interventions were safe. The GEE‐estimated safety rate was 80.3% (95% CI: 76.5–83.6%).
Table 3.
Safety profile of subjects by intervention groups
| Subjects (n = 66) | HF care alert received & PRN intervention performed (n = 18) | HF care alert received but PRN intervention not performed (n = 9) | No HF care alert received (n = 39) |
|---|---|---|---|
| Bloodwork at baseline | |||
| GFR (mL/min/1.73 m2) | 51.9 ± 17.9 | 46.1 ± 12.6 | 62 ± 16.5 (n = 38) |
| Haemoglobin (gL/dL) | 13.4 ± 1.6 | 11.7 ± 1.7 | 13 ± 1.5 |
| Potassium (mmol/L) | 4.2 ± 0.4 | 4.2 ± 0.3 | 4 ± 0.5 |
| Sodium (mmol/L) | 139.7 ± 2.5 | 139.7 ± 4.4 | 140 ± 2.4 |
| Bloodwork at exit | |||
| GFR (mL/min per 1.73 m2) | 50.7 ± 19.8 (n = 14) | 44.3 ± 18.3 (n = 7) | 60 ± 19.6 (n = 29) |
| Creatinine (mg/dL) | 1.4 ± 0.6 (n = 14) | 1.9 ± 0.5 (n = 4) | 1 ± 0.4 (n = 27) |
| Haemoglobin (g/dL) | 12.3 ± 2.4 (n = 14) | 10.5 ± 2.1 (n = 7) | 13 ± 2.2 (n = 30) |
| BUN (mg/dL) | 30.3 ± 16.9 (n = 12) | 41.0 ± 10.6 (n = 3) | 22 ± 6.1 (n = 26) |
| Potassium (mmol/L) | 4.2 ± 0.6 (n = 17) | 4.1 ± 0.4 (n = 7) | 4 ± 0.5 (n = 32) |
| Sodium (mmol/L) | 139.3 ± 2.9 (n = 17) | 136.9 ± 2.9 (n = 7) | 140 ± 3.0 (n = 32) |
BUN, blood urea nitrogen; GFR, glomerular filtration rate; HF, heart failure.
Potential determinants to successful impedance recovery after PRN intervention
Overall, the PRN medication interventions had a significant effect on increasing the intrathoracic impedance. There was a 4.73 Ω increase in the impedance averaged over the 3 days that followed the PRN intervention compared with impedance averaged over the 3 days that preceded the PRN intervention (P < 0.0001).
Three possible determinants that may have affected the successful impedance recovery were examined: mean 30 day TriageHF risk score before intervention, mean 30 day impedance before intervention, and proportion of subjects with a history of atrial fibrillation. No statistical differences between these three factors were detected in these measurements (Table S4). However, there may be a trend suggesting that the lower intrathoracic impedance may be associated with higher therapy resistance. There were no trends between baseline TriageHF risk score and atrial fibrillation frequency and response to PRN treatment.
Impediments to implementation of management protocol
Factors that presented challenges to the implementation of the PRN medication intervention guided action pathway included: (i) clarity of and adherence to the baseline medical regimen, (ii) inability to reach patient by phone, (iii) patient not having the prescribed PRN medication available, and (iv) clarity of and compliance with protocol. HF care alerts occurred in 4 patients that were noncompliant with medications, 3 patients that had discordant medication records, 2 patients were not reachable by phone, 1 patient had delayed contact, 1 patient did not have PRN medications available to take, and 13 patients had missing or incomplete biometric or symptoms data. CHFNs took actions to resolve the compliance or discrepancy issues. The average time between a CHFNs receipt of a HF care alert and the initiation of a PRN medication intervention was 1.23 days, with 24/26 (92%) of PRN medication interventions (in 17 patients) initiated in less than 2 days after the HF care alert was received by CHFNs.
Discussion
Patients with heart failure with reduced ejection fraction, despite use of guideline‐directed medical treatment, have frequent recurrences of ADHF. Two of the factors that make it difficult to anticipate and prevent ADHF recurrences include (i) the limited/infrequent outpatient evaluations performed in routine practice and (ii) the fact that changes in symptoms and signs of HF occur late in the course of decompensation well after diastolic filling pressures and intravascular volume have increased. To address these issues, multisensory, multimetric‐integrated dynamic risk scores using implantable devices (that are a critical part of guideline‐directed medical treatment such as CRT, International Classification of Diseases, and pacer) have been designed to supply continuous (or at least daily), ambulatory, remote data that can be uploaded to a secure website and viewed by health care providers. 9 , 11 , 14 , 15
However, whether and how these risk scores are actionable in a safe, reliable, and effective manner that does not ‘overburden’ providers remain open questions. Such a risk‐based management must overcome several challenges including (i) communication, (ii) intervention selection, (iii) avoidance of under/over treatment. For example, once there has been a change in the risk metric, it must be determined that the cause of this change is an actionable HF‐related issue. To do this, communication between the provider and patient must be accomplished. The provider must then prescribe an action and finally determine whether and when this action should be discontinued because it successfully prevented ADHF. Each of these steps requires communication with the patient. These communication steps are difficult to accomplish, time‐consuming, and subject to failure.
The INTERVENE‐HF study represents the initial step in the development of one approach to the challenges presented above. This study was designed to provide the rationale, design, and feasibility data necessary to develop a pivotal randomized clinical study of the proposed novel patient management strategy. INTERVENE‐HF explored an approach that was based on a physician‐directed, ambulatory medication intervention strategy, remotely implemented by CHFNs following a guided action pathway triggered by a risk based alert. The initial steps accomplished during the INTERVENE‐HF study included addressing the following questions: (i) could the system transmit assessment of risk, (ii) could appropriate patients be properly selected, (iii) could PRN management be reliably implemented, (iv) could success/failure of PRN be adequately monitored, and (v) could PRN induced AEs be avoided? It was never the intention of INTERVENE‐HF nor was the sample size sufficient to determine whether this management strategy would reduce the frequency of ADHF events or meet rigorous statistical endpoints of safety or effectiveness. Determination of an ADHF outcome requires a large, randomized, control study; however, before such a trial could be designed, the questions stated above had to be addressed. However, data from the INTERVENE‐HF trial do support the conclusion that each of the five challenges listed above could be accomplished by the physician‐directed, ambulatory medication intervention strategy implemented by CHFNs following a guided action pathway triggered by risk based alert. In addition, INTERVENE‐HF did meet its objectives of providing the rationale, design, and feasibility data necessary to develop a pivotal randomized clinical study. Two such randomized clinical trials are ongoing: the ‘Multiple Cardiac Sensors for the Management of Heart Failure (Manage‐HF, NCT03237858)’ study and ‘Algorithm Using LINQ Sensors for Evaluation and Treatment of Heart Failure’ (Alleviate‐HF, NCT04452149). Data from INTERVENE‐HF were pivotal to the Alleviate‐HF study design.
Not only have the successes of the INTERVENE‐HF study demonstrated how to overcome the challenges listed above, they may also highlight some of the reasons previous studies have resulted in neutral or negative outcomes and why very few other studies have successfully reduced HF outcomes. 14 , 15 , 16 , 17 , 18 The reasons included lack of evidence that remote data were acted upon as judged by the number of medication changes made during the course of a trial, need for a defined intervention algorithm, unwillingness to make management decisions dependent on changes in device supplied data and independent of the need for the presence of patient symptoms, and the lack of an ability to remotely determine the success (or failure) of a PRN medication intervention in a timely fashion.
For example, in the Champion trial, in patients who were managed based on the results of haemodynamic monitoring with a Cardiomems device, there were 9.1 medication changes/patient/6 months compared with the control group managed with usual care who had 3.8 medication changes/patient/6 months. 18 This addition of 5.3 changes/patient/6 months in the treatment group was associated with a significant reduction in morbidity and mortality in these HF patients. By contrast, in the OptiLink HF study, both the treatment group (management informed by the using the OptiVol fluid index) and the usual care group had a limited number of medication changes, with only 0.37 additional medication changes/patient/6 months follow‐up in the treatment group. 14 OptiLink HF did not meet its primary endpoint. In the Intervene‐HF study, there were 1.1 medication changes/patient/6 months follow‐up; these results demonstrated that remote monitoring of risk metrics that reflect haemodynamic changes (but do not directly measure haemodynamic pressures) can be used to determine the safe and effective application of PRN interventions.
In addition, in the INTERVENE‐HF study, determination of the success of the first PRN, the need for a second PRN, and the success of the second PRN medication intervention were based on changes in one selected metric within the integrated diagnostic set of metrics measured, specifically intrathoracic impedance. This choice was based on the rate at which this individual measurement responded to the PRN intervention. Data from the current and previous studies demonstrated this advantage, including rapid recovery time in days, facilitating safety assessment, and limiting the number of PRN interventions needed. 14 By definition and intrinsic to Bayesian methodology, integrated aggregated metrics such as TriageHF, Heartlogic, and OptiVol fluid index have a longer ‘hysteresis’ recovery taking 7–14 days to recover, thus making them less ideal for this purpose. Other single metrics may also be suited for a recovery criterion such as heart rate, respiratory rate, activity, position, and S3 or S4 intensity. However, none of these individual metrics have yet been examined during recovery after PRN medication intervention and therefore need further studies.
While INTERVENE‐HF utilized centralized CHFNs, the workflow and algorithms utilized in this study may be implemented at any HF centre with trained HF nursing staff.
Limitations
The most important limitations of this study include the use of a limited samples size, the lack of a randomized, blinded design, and that the study was not designed to examine mortality and morbidity outcomes. However, this was a feasibility, proof of concept, pilot study that successfully addressed a number of crucial issues that can now be incorporated in future studies that will address each of these limitations.
Conclusion
The INTERVENE‐HF study demonstrated that it is feasible to apply an individualized risk stratification based ambulatory medication intervention strategy that is physician‐directed and CHFN‐implemented to HF patients. Several critical developmental steps were successfully accomplished during the INTERVENE‐HF study: we demonstrated that (i) the system could transmit assessment of risk, (ii) appropriate patients could be properly selected, (iii) PRN management could be reliably implemented, (iv) success/failure of PRN could be adequately monitored, (v) PRN‐induced AEs be could avoided. In this study, PRN medication interventions based on TriageHF risk scores were used in absence of a need for the presence of HF symptoms and without the development of AEs. Measurements of changes in intrathoracic impedance were developed to provide recovery criterion that indicated success of the PRN medication intervention. The INTERVENE‐HF study provides the rationale, design and feasibility data necessary to develop a pivotal randomized clinical study of the proposed novel patient management strategy.
Conflict of interest
Drs Zile, Costanzo, Butler, Stapleton, Sadhu, Jimenez served as consultants to Medtronic. Zhang, Ippolito, Sharma, Warman, Hobbs, and Streeter are Medtronic Inc. employees.
Funding
This study was funded by Medtronic, Inc., Minneapolis, Minnesota, USA.
Clinical Trials.gov
Integrated Diagnostics Driven Diuretic and Chronic Medication Management for Heart Failure. NCT02698241.
Supporting information
Data S1. Supporting information
Acknowledgements
The authors thank Nancy McLellan, Jennifer Wehking, and Shauna Johnson (Medtronic Care Management Services) for their management of patients through the study intervention protocol.
Zile, M. R. , Costanzo, M. R. R. , Ippolito, E. M. , Zhang, Y. , Stapleton, R. , Sadhu, A. , Jimenez, J. , Hobbs, J. , Sharma, V. , Warman, E. N. , Streeter, L. , and Butler, J. (2021) INTERVENE‐HF: feasibility study of individualized, risk stratification‐based, medication intervention in patients with heart failure with reduced ejection fraction. ESC Heart Failure, 8: 849–860. 10.1002/ehf2.13231.
References
- 1. Whellan DJ, Ousdigian KT, Al‐Khatib SM, Pu W, Sarkar S, Porter CB, Pavri BB, O'Connor CM, PARTNERS Study Investigators . Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) study. J Am Coll Cardiol 2010; 55: 1803–1810. [DOI] [PubMed] [Google Scholar]
- 2. Sarkar S, Koehler J. A dynamic risk score to identify increased risk for heart failure decompensation. IEEE Trans Biomed Eng 2012; 60: 147–150. [DOI] [PubMed] [Google Scholar]
- 3. Whellan DJ, Sarkar S, Koehler J, Small RS, Boyle A, Warman EN, Abraham WT. Development of a method to risk stratify patients with heart failure for 30‐day readmission using implantable device diagnostics. Am J Cardiol 2013; 111: 79–84. [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WHW, Abraham WT, Sharma V, Santini M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J 2013; 34: 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gula LJ, Wells GA, Yee R, Koehler J, Sarkar S, Sharma V, Skanes AC, Sapp JL, Redfearn DP, Manlucu J, Tang ASL. A novel algorithm to assess risk of heart failure exacerbation using ICD diagnostics: validation from RAFT. Heart Rhythm 2014; 11: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 6. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, An Q, Averina V, Stolen CM, Thakur PH, Thompson JA. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. J Am Coll Cardiol 2017; 27: 216–225. [DOI] [PubMed] [Google Scholar]
- 7. Burri H, da Costa A, Quesada A, Ricci RP, Favale S, Clementy N, Boscolo G, Villalobos FS, di Mangoni S, Stefano L, Sharma V, Boriani G. Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. EP Europace 2018; 20: e69–e77. [DOI] [PubMed] [Google Scholar]
- 8. Virani SA, Sharma V, McCann M, Koehler J, Tsang B, Zieroth S. Prospective evaluation of integrated device diagnostics for heart failure management: results of the TRIAGE‐HF study. ESC Heart Fail 2018; 5: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C, Thakur PH, An Q, Wehrenberg S, Hammill EF, Zhang Y. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circ Heart Fail 2018; 11: e004669. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed FZ, Taylor JK, Green C, Moore L, Goode A, Black P, Howard L, Fullwood C, Zaidi A, Seed A, Cunnington C, Motwani M. Triage‐HF Plus: a novel device‐based remote monitoring pathway to identify worsening heart failure. ESC Heart Fail 2020; 7: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zile MR, Sharma V, Johnson JW, Warman EN, Baicu CF, Bennett TD. Prediction of all‐cause mortality based on the direct measurement of intrathoracic impedance. Circ Heart Fail 2016; 9: e002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zile MR, Koehle J, Sarkar S, Butler J. Prediction of worsening heart failure events and all‐cause mortality using an individualized risk stratification strategy. ESC Heart Failure in press; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Käab S, Huegl B, Brachmann J, OptiLink HF Study Investigators . Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016; 37: 3154–3163. [DOI] [PubMed] [Google Scholar]
- 14. Zile MR, Sharma V, Baicu CF, Koehler J, Tang AS. Prediction of heart failure hospitalizations based on the direct measurement of intrathoracic impedance. ESC Heart Fail 2020; 7: 3040–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM Jr, Magalski A, Zile MR, Smith AL, Smart FW, O'Shaughnessy MA, Jessup ML, Sparks B, Naftel DL, Stevenson LW, COMPASS‐HF Study Group . Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS‐HF study. J Am Coll Cardiol 2008; 51: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 16. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Muñoz Aguilera R, Lunati M, Yu CM, Gerritse B, Borggrefe M, for the DOT‐HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011; 124: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 17. Adamson PB, Gold MR, Bennett T, Bourge RC, Stevenson LW, Trupp R, Stromberg K, Wilkoff BL, Costanzo MR, Luby A, Aranda JM, Heywood JT, Baldwin HA, Aaron M, Smith A, Zile M. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients with Chronic Heart Failure (REDUCEhf) trial. Congest Heart 2011; 17: 248–254. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomized controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


