Abstract
Aims
Readmission after hospitalization for acute decompensated heart failure (HF) remains a major public health problem. Use of remote dielectric sensing (ReDS) to measure lung water volume allows for an objective assessment of volume status and may guide medical optimization for HF. We hypothesized that the use of ReDS would lower 30 day readmission in patients referred to rapid follow‐up (RFU) clinic after HF discharge.
Methods and results
We conducted a retrospective analysis of the use of ReDS for patients scheduled for RFU within 10 days post‐discharge for HF at Mount Sinai Hospital between 1 July 2017 and 31 July 2018. Diuretics were adjusted using a pre‐specified algorithm. The association between use of ReDS and 30 day readmission was evaluated. A total of 220 patients were included. Mean age was 62.9 ± 14.7 years, and 36.4% were female. ReDS was performed in 80 (36.4%) and led to medication adjustment in 52 (65%). Use of ReDS was associated with a lower rate of 30 day cardiovascular readmission [2.6% vs. 11.8%, hazard ratio (HR): 0.21; 95% confidence interval (CI): 0.05–0.89; P = 0.04] and a trend towards lower all‐cause readmission (6.5% vs. 14.1%, HR: 0.43; 95% CI: 0.16–1.15; P = 0.09) as compared with patients without a ReDS assessment.
Conclusions
ReDS‐guided HF therapy during RFU after HF hospitalization may be associated with lower risk of 30 day readmission.
Keywords: Heart failure, Readmissions, Remote dielectric sensing, Congestion
Introduction
Hospitalization for decompensated heart failure (HF) marks a turning point in patients' lives. More than half of hospitalized HF patients will be re‐hospitalized or die within 6 months of discharge. With an increasing prevalence of HF [regardless of ejection fraction (EF)], direct costs associated with care of hospitalized HF patients are projected to cross $70 billion by 2030. 1 , 2 , 3 , 4 Accordingly, the Center for Medicare & Medicaid Services have targeted hospitals with financial penalties for failing to achieve a 30 day readmission rate in line with national averages. 5
Despite marked advancements in the treatment of HF, reducing HF readmissions remains a challenge. Studies of telemedicine, utilizing remote monitoring of daily weights, blood pressure, heart rate, and symptoms, combined with nurse health coaching interventions, have yielded inconsistent results with respect to demonstrating improvements in HF hospitalization and mortality. 6 , 7 , 8 Early follow‐up appointments after hospital discharge, however, have been associated with lower rates of readmission for HF. 9 More recently, remote monitoring of pulmonary arterial pressures using a fully implantable pressure sensor has been shown to reduce hospitalizations for patients with HF with preserved (HFpEF) or reduced EF (HFrEF) 10 , 11 ; but limited access, reimbursement challenges, and the need for additional staffing have slowed the widespread adoption of this technology.
Use of a novel, non‐invasive electromagnetic energy‐based technology to directly measure lung water may be an effective strategy to lower HF hospitalizations. Remote dielectric sensing (ReDS; Sensible Medical Innovations, Ltd., Netanya, Israel) measures the dielectric properties of tissues, which are mainly determined by the pulmonary fluid content. Use of this system in a point‐of‐care (POC) environment can aid providers in the assessment of a patient's volume status after hospital discharge to guide further adjustment of diuretic therapy. As such, we hypothesized that among patients referred to a HF nurse practitioner‐led clinic within 10 days of hospital discharge for HF, use of ReDS POC testing would be associated with a lower rate of 30 day cardiovascular (CV) and all‐cause hospital readmissions as compared with no ReDS use.
Methods
We conducted a retrospective observational cohort study including adult patients (age ≥ 18 years) who presented to a HF rapid follow‐up (RFU) clinic within 10 days of discharge after a hospitalization for HF between 1 July 2017 and 31 July 2018 at the Mount Sinai Hospital in New York, New York. Patients with HFpEF and HFrEF were included (Figure 1 ). Baseline characteristics, co‐morbid conditions, ReDS application, medication changes, 30 day readmission, and cause of readmission were collected for all patients. The study was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai Hospital.
Figure 1.
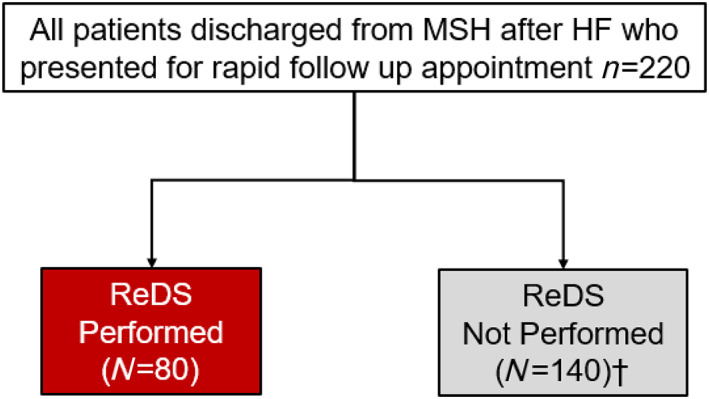
CONSORT diagram. HF, heart failure; ReDS, remote dielectric sensing.
The RFU clinic is staffed by a specialized HF nurse practitioner with physician oversight. During this visit, HF signs and symptoms were assessed; guideline‐directed medical therapy (GDMT) was reviewed and optimized; adherence, lifestyle changes, and education were reinforced. These changes and interventions were communicated to the primary care physician or cardiologist.
ReDS testing was considered for all patients as part of routine care in RFU clinic. ReDS technology has been described previously. 11 , 12 The system employs low‐power electromagnetic signals emitted into the body to measure the dielectric properties of tissues. The ReDS™ Wearable System consists of two sensors in a wearable vest positioned on the front and back of the patient's thorax without requiring direct skin contact, allowing measurements to be performed through light clothing. These signals are emitted through the right mid‐thorax and received posteriorly after passing through tissue. The characteristics of the received signals are affected by the fluid content of tissue in the path of the signal. The wearable vest is connected by a cable to a bedside monitor console. Measurements are provided after 90 s and recorded in the patient's chart. Normal intrathoracic fluid content (between 20% and 35%) has been validated using different quantitative imaging modalities including computed tomography, nuclear magnetic resonance imaging, and positron emission tomography. 11 , 12 Readings over 35% represent increased intrathoracic fluid content.
All changes to GDMT and diuretic therapy during the RFU visit were made in accordance with the American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) HF guideline, 13 tempered by vital signs, history, and signs and symptoms of HF. For those with ReDS readings obtained during clinic visits, GDMT and diuretic therapy were adjusted based upon a pre‐specified algorithm (Table 1 ).
Table 1.
Pre‐specified algorithm to adjust guideline‐directed medical therapy on the basis of the obtained ReDS values during a follow‐up visit
| ReDS Reading (value, %) | Action |
|---|---|
| ≤20% | Hold diuretics |
| 21–35% | Maintain current diuretic dosing and optimize guideline‐directed medical therapy |
| 36–45% | Increase diuretics and return to rapid follow‐up in 1 week |
| ≥46% | Consider outpatient intravenous loop diuretic or hospitalization |
The two primary endpoints were 30 day CV and all‐cause hospital readmission after index HF hospitalization. Secondary endpoints were all‐cause death and the combined outcome of all‐cause death or re‐hospitalization within 30 days. Follow‐up time was based on the last point of contact either via telephone or office appointment.
Baseline characteristics in patients with ReDS vs. without ReDS assessment were compared using χ 2 and Fisher's exact tests for categorical variables and Student's t‐tests for continuous measures as appropriate. Event rates were estimated with the Kaplan–Meier method, and univariate Cox regression models were used to evaluate the unadjusted association between ReDS and 30 day adverse events after the index hospital discharge. The instantaneous risk of re‐hospitalization within 30 days was computed using Epanechnikov kernel smoothed hazard function. The independent association between use of ReDS and 30 day CV and all‐cause readmission was evaluated with multivariable Cox regression modelling and reported with adjusted hazard ratios (HRs) and 95% confidence interval (CIs). All covariates with a level of significance < 0.20 on univariate analysis were sequentially included in the multivariable model, and only those with a level of significance of <0.10 were included in the final model. A complete list of covariates included in each multivariable logistic regression model is reported in the tables. Co‐linearity was assessed by the variance inflation factor, which is an index that measures how much the variance of an estimated regression coefficient is increased because of collinearity. The proportionality assumption for the Cox models was verified using the Schoenfeld residuals method. Discrimination of the model was quantified with C‐statistic. Two‐sided P‐values < 0.05 were considered to indicate statistical significance. All statistical analyses were performed with the use of STATA software, version 14.0 (Stata Corp., College Station, Texas).
Results
Baseline characteristics and remote dielectric sensing assessments
A total of 220 patients presented to RFU clinics within 10 days of HF hospitalization. The median time to RFU appointment after discharge was 6 days [inter‐quartile range (IQR), 5 to 8 days]. The mean age was 62.9 ± 14.6 years, and 36.4% were women. Most patients were hospitalized at least two times in the preceding year, 65.5% had HFrEF, and 68% were New York Heart Association (NYHA) Class III and IV (Table 2 ). A total of 80 patients (36.4%) had ReDS readings during their visit, and the median ReDS value was 33 (IQR, 30 to 38). Reasons ReDS was not performed included body habitus (n = 28; 20%), wearable defibrillator vest (n = 6; 4.3%), presence of port for infusion of inotropes or other medication (n = 4; 2.9%), and unknown in the remaining 102. Aside from a higher body mass index in those who did not have a ReDS reading obtained, there were no significant baseline differences between patients who had a ReDS reading and those who did not (Table 2 ). The rate of HF medication adjustment was higher in patients who had a ReDS assessment than in those who did not have a ReDS reading (69.1% vs. 55.7%; univariate odds ratio: 1.77; 95% CI: 1.01–3.12; P = 0.047).
Table 2.
Baseline clinical characteristics and medication use on hospital discharge among patients who presented for RFU visit, stratified by ReDS use vs. no ReDS use
| Overall (n = 220) | ReDS (n = 80) | No ReDS (n = 140) | P‐value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 62.9 ± 14.7 | 65.2 ± 13.2 | 61.5 ± 15.4 | 0.08 |
| Male sex | 139 (63.2%) | 53 (66.3%) | 86 (61.4%) | 0.48 |
| Body mass index, kg/m2 | 28.8 ± 7.0 | 27.3 ± 5.1 | 29.7 ± 7.7 | 0.02 |
| White race | 46 (20.9%) | 17 (21.3%) | 29 (20.7%) | 0.93 |
| Arterial hypertension | 156 (72.2%) | 60 (75.0%) | 96 (70.6%) | 0.49 |
| Diabetes mellitus | 90 (41.7%) | 33 (41.3%) | 57 (41.9%) | 0.92 |
| Renal insufficiency | 49 (61.3%) | 49 (61.3%) | 77 (55.0%) | 0.37 |
| Chronic obstructive pulmonary disease | 77 (35.0%) | 28 (35.0%) | 49 (35.0%) | 1.00 |
| Atrial fibrillation | 90 (40.9%) | 28 (35.0%) | 62 (44.3%) | 0.18 |
| Coronary artery disease | 112 (52.1%) | 44 (55.0%) | 68 (50.4%) | 0.51 |
| Smoking status | 0.38 | |||
| Never smoker | 86 (43.2%) | 32 (43.2%) | 54 (43.2%) | |
| Current smoker | 22 (11.1%) | 11 (14.9%) | 11 (8.8%) | |
| Former smoker | 91 (45.7%) | 31 (41.9%) | 60 (48.0%) | |
| New York Heart Association class | 0.35 | |||
| I | 1 (0.5%) | 0 (0.0%) | 1 (0.8%) | |
| II | 53 (26.1%) | 19 (25.7%) | 34 (26.4%) | |
| III | 126 (62.1%) | 50 (67.6%) | 76 (58.9%) | |
| IV | 23 (11.3%) | 5 (6.8%) | 18 (14.0%) | |
| Left ventricular ejection fraction < 40% | 143 (66.2%) | 56 (70.9%) | 87 (63.5%) | 0.27 |
| Number of prior hospitalizations | 2.1 ± 1.9 | 2.0 ± 1.7 | 2.2 ± 2.1 | 0.41 |
| Brain natriuretic peptide, pg/mL | 917.2 ± 1083.3 | 979.4 ± 1300 | 881.0 ± 937.9 | 0.52 |
| Medications at hospital discharge | ||||
| ARNIs | 35 (15.9%) | 12 (15.0%) | 23 (16.4%) | 0.78 |
| ACE inhibitors or ARBs | 109 (49.6%) | 44 (55.0%) | 65 (46.4%) | 0.22 |
| Mineralocorticoid receptor antagonists | 92 (41.8%) | 37 (46.3%) | 55 (39.3%) | 0.31 |
| Beta‐blockers | 185 (84.1%) | 67 (83.8%) | 118 (84.3%) | 0.92 |
| Calcium channel blockers | 17 (7.7%) | 9 (11.3%) | 8 (5.7%) | 0.14 |
| Loop diuretics | 190 (86.4%) | 69 (86.3%) | 121 (86.4%) | 0.97 |
| Thiazide diuretics | 12 (5.5%) | 3 (3.8%) | 9 (6.4%) | 0.40 |
| Inotropes | 14 (6.4%) | 2 (2.5%) | 12 (8.6%) | 0.08 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ARNIs, angiotensin receptor‐neprilysin inhibitors; ReDS, remote dielectric sensing.
Results are reported as n (%) or mean ± standard deviations.
Outcomes
Over a median follow‐up time of 211 days (IQR, 123 to 354 days), there were 12 deaths and 44 all‐cause hospitalizations. When restricted to a 30 day post‐discharge period, there were 24 hospitalizations yielding a 30 day all‐cause re‐hospitalization rate of 11% (Table 3 ), the majority of which were due to CV causes (8.2% for the whole cohort and 18/24 hospitalizations). The majority of CV hospitalizations were due to HF (n = 12/18, 66.7%). The median time to first re‐hospitalization was 27 days (IQR, 19 to 49 days); the highest risk for re‐hospitalization was observed between 20 and 25 days post‐discharge (Figure 2 ). Among patients who had a ReDS assessment, median time to re‐hospitalization was 40 compared with 22 days among patients without a ReDS assessment.
Table 3.
Causes of 30 day hospital readmission (n = 24)
| Re‐hospitalization for cardiovascular causes | 18 (75.0%) |
| Heart failure‐related hospitalization | 12/18 (66.7%) |
| Non‐heart failure‐related hospitalization | 6/18 (33.3%) |
| Re‐hospitalization for non‐cardiovascular causes | 6 (25.0%) |
Figure 2.
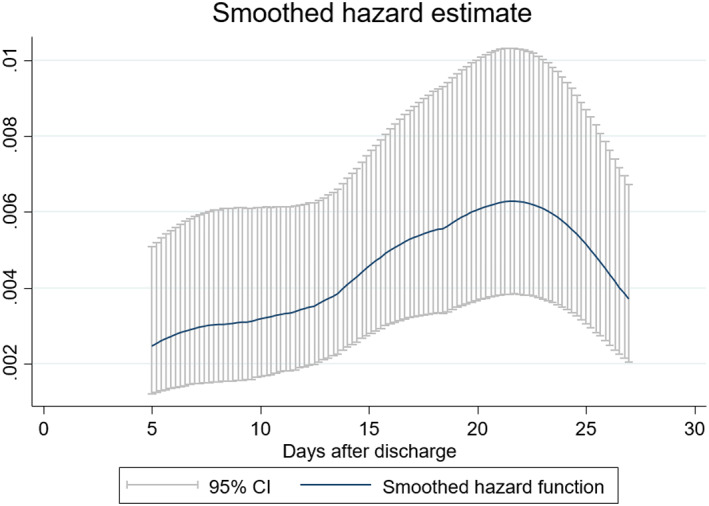
Daily hazard of any re‐hospitalization within 30 days post‐hospital discharge.
Rates of 30 day adverse events in patients with a ReDS vs. without ReDS assessment are reported in Table 4 and Figure 3 . A ReDS assessment was associated with significantly lower 30 day CV readmissions (2.6% vs. 11.8%; HR: 0.21; 95% CI: 0.05–0.89, P = 0.04) (Figure 4A ) with a trend towards lower rates of 30 day all‐cause readmissions (6.5% vs. 14.1%; HR: 0.43; 95% CI: 0.16–1.15, P = 0.09) (Figure 4B ). A ReDS assessment was also associated with a significantly lower combined endpoint of 30 day death or CV readmissions (HR 0.29 95: CI 0.08–0.99, P = 0.047) (Figure 4C ), whereas there was a trend towards an association with lower combined endpoint of 30 day death or all‐cause readmissions (HR 0.41; 95% CI 0.15–1.09, P = 0.07) (Figure 4D ).
Table 4.
Rates of death and re‐hospitalization at 30 days after hospital discharge
| ReDS (n = 80) | No ReDS (n = 139) | ARD (%) | Hazard ratio (95% CI) | P‐value | |
|---|---|---|---|---|---|
| All‐cause death | 1.3% (1) | 1.5% (2) | −0.2 | 0.86 (0.08–9.50) | 0.90 |
| All‐cause re‐hospitalization | 6.5% (5) | 14.0% (19) | −7.5 | 0.43 (0.16–1.16) | 0.09 |
| Cardiovascular re‐hospitalization | 2.6% (2) | 11.8% (16) | −9.2 | 0.21 (0.05–0.90) | 0.04 |
| All‐cause death or re‐hospitalization | 6.5% (5) | 14.7% (20) | −8.2 | 0.41 (0.15–1.09) | 0.08 |
| All‐cause death or cardiovascular re‐hospitalization | 3.9% (3) | 12.5% (17) | −8.6 | 0.29 (0.09–0.99) | 0.049 |
ARD, absolute risk difference; CI, confidence interval; RFU, rapid follow‐up; ReDS, remote dielectric sensing.
Results are reported as Kaplan–Meier estimates (number of events).
Figure 3.
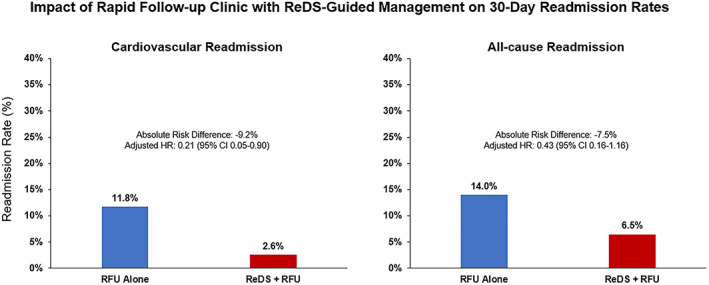
Impact of rapid follow‐up clinic with ReDS‐guided management on 30 day readmission rates. ReDS, remote dielectric sensing; RFU, rapid follow‐up.
Figure 4.
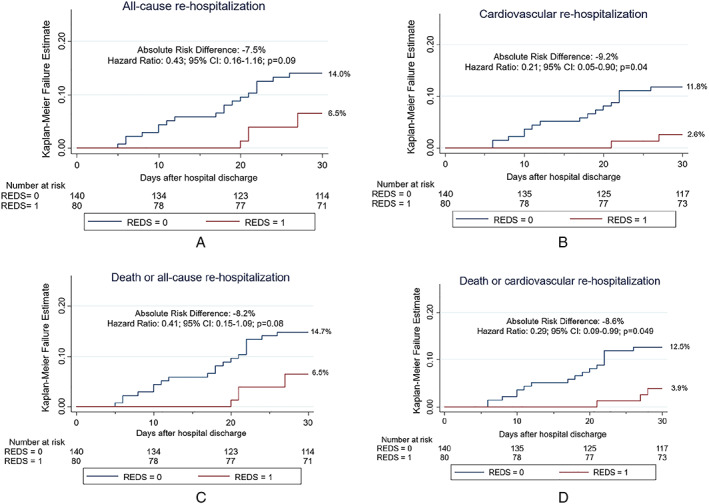
Rates of death and re‐hospitalization within 30 days in patients with vs. without ReDS during the rapid follow‐up visit. (A) Any re‐hospitalization. (B) Cardiovascular re‐hospitalization. (C) Death or any re‐hospitalization. (D) Death or cardiovascular re‐hospitalization. ReDS, remote dielectric sensing.
Multivariable logistic regression models for all‐cause and CV readmissions at 30 days are reported in Table 5 . The covariates associated with 30 day CV readmissions were number of prior hospitalizations, male sex, atrial fibrillation, and use of ReDS. The only factor associated with of 30 day all‐cause readmissions after adjustment was the number of prior hospitalizations.
Table 5.
Predictors of 30 day cardiovascular and all‐cause re‐hospitalizations by multivariable Cox regression modelling
| Adjusted HR (95% CI) | P‐value | |
|---|---|---|
| Cardiovascular re‐hospitalization | ||
| ReDS | 0.17 (0.03–0.87) | 0.03 |
| Male sex | 6.87 (1.53–30.91) | 0.01 |
| Atrial fibrillation | 2.76 (1.02–7.47) | 0.05 |
| Number of prior hospitalizations a | 1.31 (1.12–1.53) | 0.001 |
| Brain natriuretic peptide, per 100 ng/mL | 1.03 (1.00–1.06) | 0.09 |
| All‐cause re‐hospitalization | ||
| ReDS | 0.47 (0.17–1.27) | 0.14 |
| Male sex | 2.30 (0.90–5.89) | 0.08 |
| Atrial fibrillation | 2.17 (0.96–4.91) | 0.06 |
| Number of prior hospitalizations a | 1.24 (1.08–1.41) | 0.002 |
HR, hazard ratio; ReDS, remote dielectric sensing.
C‐statistics for the cardiovascular re‐hospitalization model: 0.82.
Per number of prior hospitalization increase; C‐statistics for the all‐cause re‐hospitalization model: 0.69.
Discussion
In this retrospective single‐centre study, we explored the impact of performing ReDS assessments after HF hospitalization on 30 day outcomes. The findings of our analysis are as follows: (i) among patients who presented to an RFU clinic after admission for HF, the rate of all‐cause 30 day re‐hospitalization was approximately 11%, and (ii) the use of ReDS technology during a RFU clinic visit was associated with lower risk of 30 day CV re‐hospitalization and a trend towards lower risk of 30 day all‐cause re‐hospitalization as compared with those of patients in whom ReDS was not utilized.
Reducing readmissions for HF is a major public health focus. Early follow‐up post‐discharge for HF has been shown to improve outcomes for patients admitted with HF 9 and is recommended by the ACC/AHA/HFSA guidelines for the management of HF. 13 Accordingly, in our cohort of patients, the RFU visit within 10 days of discharge alone was associated with lower rates of 30 day readmission compared with the national average of approximately 19% noted in a previous Get With the Guidelines Registry analysis. Our cohort included a high‐risk HF population with over two‐thirds describing NYHA Class III to IV symptoms.
Congestion is the main cause for HF decompensation and hospitalization, manifested as dyspnoea, orthodema, and/or bendopnea. 6 , 14 , 15 Inadequate decongestion during hospitalization for HF and recurrent congestion post‐discharge are postulated to partially explain the high rates of hospital readmission. 10 , 14 , 16 Yet detecting congestion by physical exam and laboratory markers in the absence of symptoms can be challenging and is subject to limitations. 17 Specifically, signs, symptoms, and weight changes can be poor surrogates for filling pressures and therefore unreliable predictors of HF readmission. 18 As such, the use of ReDS wearable vest as a simple non‐invasive tool to guide optimization of volume status presents considerable appeal.
This retrospective analysis of over 200 racially diverse patients in a vulnerable post HF hospitalization discharge period reveals that the use of ReDS technology is safe and may be associated with lower risk of 30 day readmissions. A thoracic fluid content reading was provided to the nurse practitioner, allowing for optimization of volume status using a pre‐specified algorithm to adjust diuretics. The use of this non‐invasive POC approach in an outpatient setting was associated with lower 30 day readmissions compared with patients without ReDS assessments. We hypothesize that this benefit may be mediated by the increased number of medication changes made in the patients for whom ReDS assessments were performed. The use of a standardized algorithm to optimize volume status and inhibitors of the renin–angiotensin–aldosterone system may have contributed to the observed statistically significant reduction in CV readmissions. Although not formally captured, our anecdotal experience suggested that the ReDS measurement seemed to create a ‘teachable moment’ to review medications, compliance, and dietary restrictions. Further study is needed to reveal whether use of ReDS in this setting improves adherence and subsequent follow‐up.
There are several limitations that warrant discussion. First, owing to the retrospective, single‐centre design of this study, our findings have to be considered hypothesis generating. Second, the small sample size precluded a non‐parsimonious multivariable adjustment of our predictive models; therefore, our effect estimates are potentially subject to significant confounding bias, and, importantly, we could not perform an analysis in sub‐groups of clinical interest such as HFrEF vs. HFpEF. Third, not all patients hospitalized for HF at Mount Sinai Hospital may have been identified for RFU clinic, raising the possibility of selection bias. Further, among those presenting for RFU clinic, ReDS readings were performed in less than 40% of patients, and there was no pre‐defined protocol as to which patients underwent ReDS readings. Reasons as to why ReDS readings were not performed were often not listed in the chart, which may have also introduced bias. Despite these limitations, the fact that patients who had ReDS assessments performed were similar to those who did not have ReDS assessments performed is reassuring. We did not capture readmissions to other hospitals; however, the Mount Sinai Health system is large and far reaching in this geographic location with multiple hospitals.
Conclusions
Early readmission after hospitalization for HF remains a major clinical challenge. Use of POC testing with ReDS technology after hospital discharge for HF allowed optimization of medications and appeared to be associated with lower risk of 30 day CV readmissions in a high‐risk cohort. Further experience with POC ReDS testing may provide insights into the frequency of congestion early after HF discharge, reduction in hospital readmission, and optimization of diuretic therapy and GDMT. Our preliminary findings warrant evaluation in a randomized controlled trial to evaluate the clinical impact of using ReDS technology after HF hospitalizations to reduce early readmissions.
Funding Information
Fundació Privada Daniel Bravo Andreu (ES) Icahn School of Medicine at Mount Sinai
Conflict of interest
There are no financial or industry relationships that pose a conflict of interest with the submitted article. Dr. Sean Pinney has received consulting fees from Abbott, CareDx, Medtronic, and Procyrion. Dr. Jesus Alvarez‐Garcia has received a research grant from Private Foundation Daniel Bravo Andreu.
Lala, A. , Barghash, M. H. , Giustino, G. , Alvarez‐Garcia, J. , Konje, S. , Parikh, A. , Ullman, J. , Keith, B. , Donehey, J. , Mitter, S. S. , Trivieri, M. G. , Contreras, J. P. , Burkhoff, D. , Moss, N. , Mancini, D. M. , and Pinney, S. P. (2021) Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Failure, 8: 1047–1054. 10.1002/ehf2.13026.
Anuradha Lala and Maya H. Barghash equally contributed to this work.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Green SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Kostam MA, Tang WH, Felker GM, Shah MR, Braunwald E. Rationale and design of the ATHENA‐HF Trial: aldosterone targeted neurohormonal combined with natriuresis therapy in heart failure. JACC Heart Fail 2016; 4: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desai AS, Stevenson LW. There must be a better way: piloting alternate routes around heart failure hospitalizations. J Am Coll Cardiol 2013; 61: 127–130. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013; 61: 391–403. [DOI] [PubMed] [Google Scholar]
- 5. Vader JM, LaRue SJ, Stevens SR, Mentz RJ, DaVore AD, Lala A, Groarke JD, Abou Ezzeddine OF, Dunlay SM, Grodin JL, Davila‐Roman VG, de Las Fuentes L. Timing and causes of readmission after acute heart failure hospitalization—insights from the Heart Failure Network Trials. J Card Fail 2016; 22: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abraham WT, Perl L. Implantable hemodynamic monitoring for heart failure patients. J Am Coll Cardiol 2017; 70: 389–398. [DOI] [PubMed] [Google Scholar]
- 7. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel group, un‐masked trial. Lancet 2018; 391: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 8. Galinier M, Roubille F, Berdague P, Brierre G, Cantie P, Dary P, Ferradou JM, Fondard O, Labarre JP, Mansourati J, Picard F, Ricci JE, Salvat M, Tartière L, Ruidavets JB, Bongard V, Delval C, Lancman G, Pasche H, Ramirez‐Gil JF, Pathak A, OSICAT Investigators . Telemonitoring versus standard of care in heart failure: a randomised multicentre trial. Eur J Heart Fail 2020; 22: 985–994 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. McAlister FA, Youngson E, Kaul P, Ezekowitz JA. Early follow‐up after a heart failure exacerbation: the importance of continuity. Circ Heart Fail 2016; 9: e003194. [DOI] [PubMed] [Google Scholar]
- 10. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 11. Amir O, Azzam ZS, Gaspar T, Faranesh‐Abboud S, Andria N, Burkhoff D, Abbo A, Abraham WT. Validation of remote dielectric sensing (ReDS) technology for quantification of lung fluid status: comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int J Cardiol 2016; 221: 841–846. [DOI] [PubMed] [Google Scholar]
- 12. Amir O, Ben‐Gal T, Weinstein JM, Schliamser J, Burkhoff D, Abbo A, Abraham WT. Evaluation of remote dielectric sensing (ReDS) technology‐guided therapy for decreasing heart failure re‐hospitalizations. Int J Cardiol 2017; 240: 279–284. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBridge PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 14. Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, Abou Ezzeddine OF, DeVore AD, Khazanie P, Redfield MM, Goldsmith SR, Bart BA, Anstrom KJ, Felker GM, Hernandez AF, Stevenson LW. Relief and recurrence of congestion during and after hospitalization for acute heart failure: Insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE‐AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS‐HF) . Circ Heart Fail 2015; 8: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thibodeau JT, Turer AT, Gualano SK, Ayers CR, Velez‐Martinez M, Mishkin JD, Patel PC, Mammen PP, Markham DW, Levine BD, Drazner MH. Characterization of a novel symptom of advanced heart failure: bendopnea. JACC Heart Fail 2014; 2: 24–31. [DOI] [PubMed] [Google Scholar]
- 16. Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, Atar D, Teerlink JR, Califf RM, Massie BM, Hasselblad V, Starling RC, O'Connor CM, Ponikowski P. Predictors of early dyspnoea relief in acute heart failure and the association with 30‐day outcomes: findings from ASCEND‐HF. Eur J Heart Fail 2013; 15: 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groarke JD, Stevens SR, Mentz RJ, Cooper LB, Vader JM, Abou Ezzeddine OF, Grodine JL, Joyce E, Anstrom KJ, Felker GM, Redfield MM, Stevenson LW, Lala A. Clinical significance of early fluid and weight change during acute heart failure hospitalization. J Card Fail 2018; 24: 542–549. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]


