Abstract
The prevalence of non‐alcoholic fatty liver disease (NAFLD) in heart failure (HF) preserved left ventricular ejection fraction (HFpEF) patients could reach 50%. Therefore, NAFLD is considered an emerging risk factor. In 20% of NAFLD patients, the condition progresses to non‐alcoholic steatohepatitis (NASH), the aggressive form of NAFLD characterized by the development of fibrosis in the liver, leading to cirrhosis. The purpose of this review is to provide an overview of the relationships between NAFLD and HFpEF and to discuss its impact in clinical setting. Based on international reports published during the past decade, there is growing evidence that NAFLD is associated with an increased incidence of cardiovascular diseases, including impaired cardiac structure and function, arterial hypertension, endothelial dysfunction, and early carotid atherosclerosis. NAFLD and HFpEF share common risk factors, co‐morbidities, and cardiac outcomes, in favour of a pathophysiological continuum. Currently, NAFLD and NASH are principally managed with non‐specific therapies targeting insulin resistance like sodium‐glucose co‐transporter‐2 inhibitors and liraglutide, which can effectively treat hepatic and cardiac issues. Studies including HFpEF patients are ongoing. Several specific NAFLD‐oriented therapies are currently being developed either alone or as combinations. NAFLD diagnosis is based on a chronic elevation of liver enzymes in a context of metabolic syndrome and insulin resistance, with fibrosis scores being available for clinical practice. In conclusion, identifying HF patients at risk of NAFLD is a critically important issue. As soon as NAFLD is confirmed and its severity determined, patients should be proposed a management focused on symptoms and co‐morbidities.
Keywords: Non‐alcoholic steatohepatitis, Non‐alcoholic fatty liver disease, Heart failure with preserved ejection fraction, Fibrosis, Insulin resistance, Atherosclerosis
Introduction
Nearly half of patients with heart failure (HF) have a preserved left ventricular ejection fraction (HFpEF), 1 historically referred to as ‘diastolic heart failure’. Over the past decade, several studies have shown that HFpEF most likely results from the complex interplay of multiple impairments in ventricular diastolic and systolic reserve function, heart rate reserve and rhythm, atrial dysfunction, stiffening of the ventricles and vasculature, impaired vasodilatation, pulmonary hypertension, endothelial dysfunction, and abnormalities in the periphery, including skeletal muscle. 1 Many of these abnormalities are not apparent at rest but are noted when the cardiovascular (CV) system is stressed. The limitations in CV reserve then interact with systemic processes to cause symptoms of dyspnoea and fatigue, which ultimately culminate in systemic and pulmonary venous congestion, muscle wasting, and loss of functionality and independence, escalating the need for HF care. In many respects, this situation represents an exaggerated version of phenomena associated with normal CV aging. 2 , 3 Obesity and type 2 diabetes mellitus (T2DM) are more prevalent in HFpEF than in HF with reduced ejection fraction (HFrEF). 4 Besides, no effective treatment for HFpEF is currently available.
Non‐alcoholic fatty liver disease (NAFLD), the hepatic outcome of metabolic abnormalities such as obesity, insulin resistance, or T2DM and dyslipidaemia, affects about 25% of the general population worldwide and can be found in both men and women. NAFLD, which can progress to a more aggressive form known as non‐alcoholic steatohepatitis (NASH), is the most prominent cause of chronic liver disease 5 and is on the point of becoming the first indication for liver transplantation in the USA. 6 NAFLD has been shown to increase the risk of atherosclerosis, cardiomyopathy, and arrhythmia, as well as CV morbidity and mortality, supporting a need for primary and secondary prevention of CV disease (CVD) in patients with NAFLD. 7 A large proportion of patients with NAFLD and NASH have an accompanying atrial and ventricular myopathy, which often manifests clinically as atrial fibrillation and HFpEF. 8 Conversely, NAFLD is frequently found in patients with HFpEF. 9 A recent prospective study in outpatients with HFpEF undergoing abdominal imaging indicates that the prevalence of NAFLD could reach 50% and that half of the NAFLD patients could have a NAFLD fibrosis score (NFS) indicative of advanced fibrosis. 10 A high NFS has been found to be associated with a higher risk of all‐cause mortality for symptomatic patients hospitalized for decompensated HFpEF. 11 Because NAFLD is characterized by many co‐morbidities common in HFpEF and also by proinflammatory adipocytokines causing derangements of the adjoining myocardium and resulting in atrial fibrillation, 8 one should consider NAFLD as an emerging risk factor for HFpEF complications. Yet little has been documented about the routine management of patients with HFpEF at risk of NAFLD.
This review aims to draw an overview of the relationships between NAFLD and HFpEF based on the current understanding of their pathophysiological interactions and to discuss its impact on cardiology clinical practice.
Pathogenesis, natural history, and diagnosis of non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis
Liver steatosis is the hallmark feature of NAFLD. Under conditions of a sedentary lifestyle, high fat diet, obesity, and/or insulin resistance, the accumulation of triglyceride (TG) within hepatocytes (steatosis) leads to an increased influx of lipids into the liver and decreased lipid disposal. Plasma non‐esterified fatty acids (FA), the main sources of FA (60%), come from adipose tissue (where inhibition of lipolysis is impaired), de novo lipogenesis‐derived metabolites (25%), and dietary FA through the uptake of intestinally derived chylomicron (15%). In addition, FA β‐oxidation and export of TG by very‐low‐density lipoprotein are impaired, leading to the synthesis and storage of TG within hepatocytes as lipid droplets. 12
A subset of patients (20%) are expected to develop NASH, defined by the additional presence of hepatocyte ballooning degeneration and lobular inflammation. NASH represents the aggressive form of the disease with progressive development of fibrosis in the liver, leading to cirrhosis (5%) and liver‐related complications (hepatic decompensation and hepatocellular carcinoma). 13 Insulin resistance, accumulation of lipotoxic metabolites and bile acids within hepatocytes, oxidative stress, release of cytokines, and other inflammatory mediators, as well as gut microbiota, are believed to promote the transition from steatosis to liver inflammation (NASH) and fibrosis through mitochondrial dysfunction, endoplasmic reticulum stress, cell death, and immune cell infiltration. 14 , 15 , 16 While several preclinical models based on varying genetic or dietary manipulations have been developed to better characterize the disease, none of them entirely mimics the pathogenesis of human NAFLD. 17
Among all the liver lesions described in NAFLD, liver fibrosis is the only one shown to be independently associated with patient outcomes. 18 A recent meta‐analysis has demonstrated that prognosis of NAFLD is impaired in F2 fibrosis stage with exponential increase when transitioning to stage F3 (bridging fibrosis), then F4 (cirrhosis). 19 Patients with cirrhosis have higher rates of mortality and liver‐related complications than those with bridging fibrosis, whereas vascular events and non‐hepatic cancers are the commonest complications in those with bridging fibrosis. 20
The diagnosis of NAFLD is firstly based on the presence of fatty liver on imaging (ultrasound and MRI) and the chronic elevation of transaminases and/or gamma‐glutamyl transpeptidase (GGT) in a context of metabolic syndrome and insulin resistance, and after excluding other causes of chronic liver disease including excessive alcoholic consumption. 21 , 22 The gold standard for the evaluation of NAFLD severity (NASH, fibrosis, and cirrhosis) remains liver biopsy using the classification of NASH‐CRN. 21 , 22 However, this procedure is invasive and cannot be used as a first‐line screening or diagnosis because of the high prevalence of the disease. Blood tests combine clinical parameters and blood markers of fibrosis in more or less complex algorithms and are often used as a first‐line strategy. The two most validated simple blood tests are the NAFLD fibrosis score and the FIB4 index. 23 , 24 Elastometry devices (FibroScan, ARFI, Supersonic, ElastoMRI, etc.) measure liver stiffness using elastic waves, perform better than blood fibrosis tests, and may be used as a second‐line strategy in patients in whom the blood test cannot exclude advanced fibrosis. Liver biopsy may be considered only if the result is likely to impact the management, for instance in case of doubt about another cause of chronic liver disease or about cirrhosis.
Association of non‐alcoholic fatty liver disease and cardiovascular disease
There is growing evidence that NAFLD is a multisystem disease affecting extra‐hepatic organs. 25 , 26 Above all, NAFLD is associated with an increased incidence of CVD, independently of established CV risk factors. 7 , 27 , 28 A post hoc analysis of a single‐centre prospective register based on 1965 patients undergoing routine screening colonoscopy recently showed that CV risk, as assessed by the Framingham risk score, was higher in patients with NAFLD (8.7 ± 6.4 vs. 5.4 ± 5.2%; P < 0.001), supporting that NAFLD might independently improve prediction of long‐term risk for CVD. 29
Compared with subjects without liver disease, patients with NAFLD have impaired cardiac structure and function 30 and develop arterial hypertension, 31 endothelial dysfunction, 32 and early carotid atherosclerosis 33 shown to appear 5 to 10 years earlier than in subjects without NAFLD. These features are commonly associated with HF and lead to increased CV risk. 27 Besides, in subjects with NASH, the presence of severe fibrosis/cirrhosis (F3–F4) is also associated with the most severe CVD compared with those having no or low fibrosis (F0–F2). 34
Relationship between heart failure preserved left ventricular ejection fraction and non‐alcoholic steatohepatitis
Heart failure preserved left ventricular ejection fraction is described as a multisystem disease with diastolic and systolic reserve anomalies, endothelial dysfunction, atrial dysfunction, inadequate chronotropic reserve, ventricular and vessel rigidity, and pulmonary hypertension. 1 , 35 In parallel with investigations aimed to describe the complex structural damage of the heart, the concept of phenomapping has emerged, which considers several clinical entities combining risk factors and co‐morbidities such as high blood pressure, diabetes, and obesity. 3 A patient phenotype characterized by a high prevalence of obesity supports the concept of a pathophysiological continuum between HFpEF and NAFLD (Figure 1 ).
Figure 1.
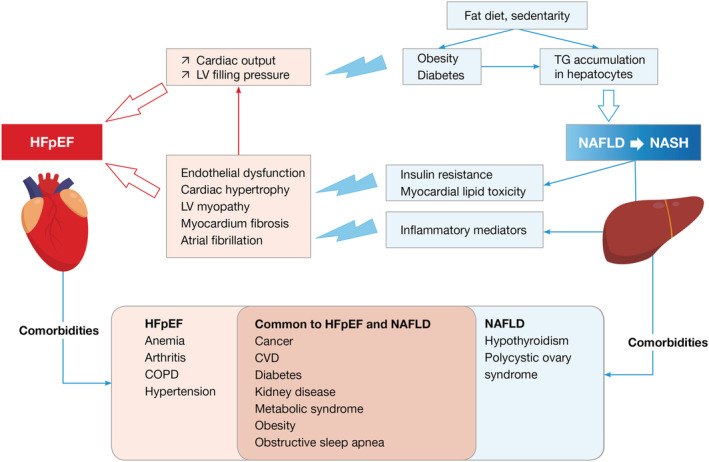
Pathophysiological relationship and common co‐morbidities in NAFLD and HFpEF. The scheme summarizes the main pathophysiological pathways resulting from NAFLD or NASH reported to enhance (thunderlights) heart dysfunctions associated with HFpEF. The part below indicates common co‐morbidities shared by both conditions.
The pathophysiological continuum between NAFLD and HFpEF is attributable, at least in part, to the secretion of adipokines and pro‐inflammatory cytokines. 7 First, central perivisceral adipose tissue has intense endocrine activity with a strong autocrine and paracrine effect. Adipokines contribute to insulin resistance and the formation of steatosis and consequently NASH. Leptin is produced by fatty tissue, heart tissue, and the digestive system. At the level of the hepatic tissue, it has a profibrotic activity via the activation of PI3K promoting the synthesis of osteopontin. 36 On the other hand, it leads to cardiac hypertrophy and endothelial dysfunction. Secondly, there is a similar pro‐inflammatory cytokine spectrum between NAFLD and HFpEF. For instance, pro‐inflammatory macrophages (M1) release cytokines such as TNF‐A and IL‐6 that contribute to hepatocyte injuries and NAFLD, while damaged hepatocytes release IL‐33 that promotes profibrogenic effect via the IL‐33 receptor (ST2) and galectin 3 , 37 In the heart, IL‐33 is released in response to the stretching of myocardial fibres. Binding to its up‐regulated soluble form ST2 receptor, in response to stretch myocyte, inflammation, and myocardial fibrosis, leads to hypertrophy of cardiomyocytes and heart fibrosis similarly to the action of galectin 3 , 38 A prospective real‐life study of HFpEF patients with NAFDL found an important association between more advanced HF and hepatic fibrosis stage, and that patients with advanced fibrosis had an increase in left atrial diameter and ≥grade 2 diastolic dysfunction. 10 A similar relationship was observed in HF patients hospitalized for the treatment of decompensated HFpEF. 11
Impact of co‐existing non‐alcoholic steatohepatitis on heart diseases
Myocardial abnormalities
Strong evidence links NAFLD with functional and structural myocardial abnormalities in individuals with or without coexisting features of metabolic syndrome. 39 Echocardiographic assessments in normotensive, non‐diabetic patients with NAFLD showed a markedly impaired diastolic function, a mild alteration of LV structure, and the associated impairment of LV function and myocardial relaxation. 39 , 40 The assessment of mitral inflow velocity showed a lower E‐wave and e′ velocity on tissue Doppler imaging (TDI), a lower E/A ratio, and a higher E/e′ ratio, suggesting higher LV filling pressure in NAFLD patients compared with controls, in the absence of morbid obesity, hypertension, or diabetes. There was no significant difference in ejection fraction (EF) between groups. However, several studies using speckle tracking echocardiography (STE) have shown an impairment of LV global strain, confirming the subclinical and early alteration of systolic function in that population. 41 These adverse structural alterations and cardiac dysfunction were confirmed in a recent meta‐analysis based on 16 studies. 42 Compared with non‐NAFLD subjects, NAFLD patients had concentric cardiac remodelling, including increased LA size, higher LV volume, wall thickness, and LV mass indices.
An interesting MRI‐based study assessed the effect of different ectopic fat depots on LV diastolic function in nondiabetic men with NAFLD and free of CVD. 43 Hepatic steatosis and visceral adipose tissue (VAT) were associated with significant changes in LV structure and function. Hepatic TGs and VAT correlated with the degree of LV diastolic function, and surprisingly, no significant correlations were found between indices of diastolic function and myocardial TG. Another study examined the impact of both hepatic steatosis and fibrosis on diastolic heart dysfunction in relation to myocardial glucose uptake with 18FDG‐PET in the general population. 44 Hepatic steatosis and fibrosis were associated with diastolic heart dysfunction and were correlated with decreased myocardial glucose uptake. On the other hand, patients without NAFLD were more likely to have higher myocardial glucose uptake. Finally, diastolic dysfunction in NAFLD was found to be linked to impaired exercise capacity with a significant decreased peak VO2, and the severity of impairment in exercise capacity and diastolic function was directly related to the stage of liver disease. 45 Moreover, NAFLD has been shown to be strongly and independently associated with aortic valve stenosis and mitral annulus calcification (singly or in combination) in patients with T2DM, suggesting that NAFLD could have an impact on valve calcification. 46
Cardiac arrhythmias
Atrial fibrillation (AF) is the most common arrhythmia, and its prevalence and impact on NASH remain debatable. 47 While some studies did not find an over‐risk (based on Framingham or Pomerania cohorts), most of them suggest a risk of increased morbidity and mortality. 48 , 49 , 50 Overall, patients with NASH, with or without cirrhosis, develop twice as many arrhythmias from AF as patients without NASH. 47 , 51 , 52
This non‐fortuitous association may involve a large number of factors, including, in the first place, cardiac anomalies like subclinical myocardial remodelling independent of the usual risk factors and structural changes such as size and left atrium (LA) volume, impaired intra‐atrial conduction, LV mass and thickness, or impaired diastolic function. 53 , 54 , 55 Inherent liver abnormalities, such as fibrosis evaluated by subclinical hepatic thickness on MRI, are also expected to directly correlate with structural and functional cardiac anomalies such as LV hypertrophy, LA dysfunction, or myocardial fibrosis. The role of systemic abnormalities, such as inflammation, known to be responsible for changes in metabolism and heart function has also been proposed. In this respect, studies measuring GGT, a marker of liver damage and oxidative stress independently of atrial arrhythmia, have shown that systemic factors are preponderant when compared with hepatic factors and specific aminotransferase‐associated liver damage alone. 56 The more common factors like obesity, insulin resistance, and diabetes also interfere, so it is not easy to differentiate the proper role of NASH from the frequently associated CV risk factors regarding the impact on morbidity and mortality.
Regarding clinical outcomes, patients with NASH have more hospitalizations with longer lengths of stay, but only 62.5% are effectively treated in compliance with the current recommendations concerning anticoagulation. 47
Coronary artery disease
Coronary artery disease has been reported as highly prevalent in patients with NAFLD benefiting from coronary angiogram 57 and in patients undergoing liver transplantation. 58 Based on systematic coronary angiograms performed in patients with CAD risk factors and/or aged more than 50 years, CAD was detected in 36.8% of patients with the highest prevalence (52.8%) in those with NASH‐associated cirrhosis. 57 In the multivariate analysis, NASH remained the only one aetiology significantly associated with CAD.
The link between NASH and cardiac dysfunction involves several pathophysiologic pathways. The common pathway of the two conditions could be mediated by macrophage, as macrophage infiltration is known to play a central role in the initiation of atherosclerosis and then could also be involved in the initiation of NASH. Inflammatory processes could also explain the relationship between NAFLD and coronary disease. Coronary calcic score and various inflammatory biomarkers have been explored by imaging in 3876 volunteers with neither known chronic liver disease nor CVD. 59 In this unselected population, the prevalence of unknown NAFLD was 17%. While no inflammatory biomarker was correlated with a coronary calcic score in patients with CAD, IL‐6 was the only biomarker independently associated with elevated CAC. This study illustrates the need for a better characterization of the pathophysiological links between NASH and CAD.
Two recent meta‐analyses including patients with NAFLD have confirmed an increased prevalence of subclinical atherosclerosis in those patients 60 and that NAFLD was associated with a higher risk of increased carotid artery intima‐media thickness/plaques, arterial stiffness, CAC, and endothelial dysfunction. 61 Interestingly, the results of a study including 1473 individuals within the Cardiometabolic risk, Epicardial fat, and Subclinical Atherosclerosis Registry (CAESAR) suggest that epicardial fat could be more specifically involved. 62
The role of NAFLD as a major risk factor in clinical settings 63 has been retrospectively shown through a post hoc analysis of the large IMPROVE‐IT trial including patients with stabilized acute coronary syndrome (ACS). 64 In this study, a dual lipid‐lowering therapy (ezetimibe/simvastatin) was found to be efficient only in patients with higher risks of recurrent major CV events, which underlines that NAFLD could help better select patients who could benefit from the dual therapy.
Perspectives in cardiovascular disease‐associated non‐alcoholic steatohepatitis management
Results with therapies targeting insulin resistance
No specific pharmacological treatment is approved for NAFLD/NASH to date, which may be due in part to the complex and multimodal pathophysiology of NAFLD/NASH and also the complexities in clinical trial design and patient enrollment. 9 In this context, treatments of obesity (including adjuvant vitamin E) have been suggested to both improve NASH and reduce CV dysfunction. 65 , 66 , 67 A systematic review based on 29 randomized clinical trials showed that pioglitazone and liraglutide were associated with improvement of histologic features of NAFLD, with a mild effect on liver fibrosis with pioglitazone. 67 However, this analysis points out the impact of pioglitazone on weight gain and the contra‐indication in patients with (or at risk of) HF. As for liraglutide, studies in patients with HFrEF showed an association with more serious adverse cardiac events, and there is no available data or ongoing studies in patients with HFpEF. 68 , 69 Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors reduce HF hospitalization of T2DM patients with established CVD or who are at risk of CVD. 68 Furthermore, the SGLT2 inhibitor licogliflozin has been shown to improve NASH patients' liver biochemistries, with a good safety profile. 70 Two ongoing placebo‐controlled randomized trials are currently assessing the effects of the SGLT2 inhibitors empagliflozin 71 and dapagliflozin (NCT03619213) on CV outcomes in HFpEF patients.
Overall, available data on the effects of drugs acting on insulin resistance are from studies assessing CV endpoints in patients with NASH. Furthermore, the increased prevalence of obesity in HFpEF patients when compared with those with HFrEF may override the improved glycaemic control. 69 Data results that include HFpEF patients are eagerly awaited.
Advances in the development of non‐alcoholic steatohepatitis specific therapy
Many drugs targeting different pathways are under investigation as monotherapies or combinations (Table 1 ). The most advanced options in terms of development target the farnesoid X nuclear receptor (FXR), apoptosis signal‐regulating kinase 1 (ASK‐1), chemokine receptors CCR2/CCR5, and dual peroxisome proliferator‐activated receptor (PPAR)‐α/δ.
Table 1.
Main clinical trials of NASH‐oriented drugs in development
| Drugs | Mechanisms of action | Stage of development and main outcomes | |
|---|---|---|---|
| Inflammatory/fibrosis modulators | |||
| Cenicriviroc | CCR2/CCR5 antagonist | Phase III (CENTAUR) | Significant antifibrotic effect |
| Selonsertib | ASK‐1 inhibitor | Phase III (STELLAR) | Primary endpoint (fibrosis improvement) not met |
| Belapectin | Galectin‐3 inhibitor | Phase IIb | Primary endpoint (no reduction in HVPG) not met |
| Metabolic modulators | |||
| Elafibranor | Dual PPAR‐α/δ agonist | Phase III (RESOLVE‐IT) | Primary endpoint (NASH resolution without fibrosis worsening) not met |
| Multimodal drugs | |||
| Obeticholic acid | FXR agonist | Phase III (REGENERATE) | Histological improvement—clinical assessment ongoing |
| Cilofexor | FXR agonist | Phase II | Reductions in hepatic steatosis and liver biochemistry |
| Combination‐based therapies | |||
| Cilofexor + selonsertib | Phase II (ATLAS) | Primary endpoint (fibrosis improvement) not met | |
| Cilofexor + firsocostat a | Phase II (ATLAS) | Primary endpoint (fibrosis improvement) not met | |
| Selonsertib + firsocostat a | Phase II (ATLAS) | Primary endpoint (fibrosis improvement) not met | |
| Cenicriviroc + tropifexor | Phase IIb (TANDEM) | Ongoing—primary endpoint: safety and tolerability | |
| Tropifexor + licogliflozin | Phase II (ELIVATE) | Ongoing—NASH resolution + no fibrosis worsening, or fibrosis improvement | |
| Semaglutide b + cilofexor | Phase II | Ongoing—primary endpoint: safety and tolerability | |
| Semaglutide b + firsocostat a | Phase II | Ongoing—primary endpoint: safety and tolerability | |
ASK‐1, apoptosis signal‐regulating kinase 1; FXR, farnesoid X nuclear receptor; PPAR, peroxisome proliferator activated receptor.
Inhibitor of the acetyl‐CoA carboxylase.
Glucagon‐like peptide‐1 (GLP‐1) receptor agonist.
FXR agonists have a multimodal effect on NASH with anti‐steatotic, anti‐inflammatory, and antifibrotic actions. Obeticholic acid, an FXR agonist already approved in the treatment of primary biliary cholangitis, has been studied in adults with NASH and fibrosis stages F2–F3. 72 In a placebo‐controlled phase III trial, the endpoint of fibrosis improvement was achieved by a significantly higher proportion of patients in obeticholic acid groups, with adverse effects (mainly pruritus) generally mild or moderate. The assessment of clinical outcomes is ongoing. In a phase II randomized trial performed in non‐cirrhotic patients with NASH, cilofexor, a nonsteroidal FXR agonist, was found to provide significant reductions in hepatic steatosis and liver biochemistry. 73
Regarding fibrosis modulators of interest, cenicriviroc inhibits CeC chemokine receptor types 2 and 5 (CCR2 and CCR5), which are implicated in the progression of NASH and liver fibrosis. In the CENTAUR randomized placebo‐controlled study performed in adults with NASH, cenicriviroc was associated with a significant antifibrotic effect (reduction in PRO‐C3 levels and ELF scores) coupled with a safety profile comparable with that of placebo. 74
While being considered as a promising anti‐NASH drug, the dual (PPAR)‐α/δ agonist elafibranor 75 failed to show efficiency on NASH resolution (NCT02704403). Other pharmacologic drugs targeting pathways of interest for the treatment of NAFLD and NASH, like stearoyl‐CoA desaturase 1 or human fibroblast growth factor, are being evaluated.
Finally, one can expect higher benefits when combining drugs targeting different pathways. However, in a phase II study, none of the three dual therapies with cilofexor, selonsertib, and firsocostat (an inhibitor of the acetyl‐CoA carboxylase) was found to improve fibrosis (NCT03449446). Ongoing phase II trials are evaluating the safety and efficacy of other combinations including the FXR agonist tropifexor with cenicriviroc 76 or licogliflozin (NCT04065841), and the combinations of cilofexor and semaglutide with (triple therapy) or without (dual therapy) firsocostat (NCT03987074).
Current challenges in non‐alcoholic fatty liver disease detection in clinical practice
So far, there is no specific treatment for HFpEF nor any approved NAFLD‐oriented specific therapies. The high degree of uncertainty about the involvement of some of the incriminated mechanisms makes evaluating and developing specific therapies difficult. However, given the high proportion of advanced cirrhosis in HFpEF patients with NAFLD, 10 the current challenge for cardiologists is to detect NAFLD early so it can be managed with approved non‐specific pharmacologic or non‐pharmacologic strategies associated with heart disease treatment. In the absence of validated biomarkers, cardiologists must be aware of risk factors like T2DM or metabolic syndrome, two conditions that warrant a detection primarily based on liver enzymes measurements (Figure 2 ). In patients with abnormal tests, and after another possible cause such as alcohol or medication (e.g. corticoids and methotrexate) consumption has been eliminated, patients should undergo larger blood tests consistent with a possible liver pathology, which should include coagulation parameters and markers of viral infections (HBV/HCV). In the case of increased liver enzymes, a liver echography will be considered.
Figure 2.
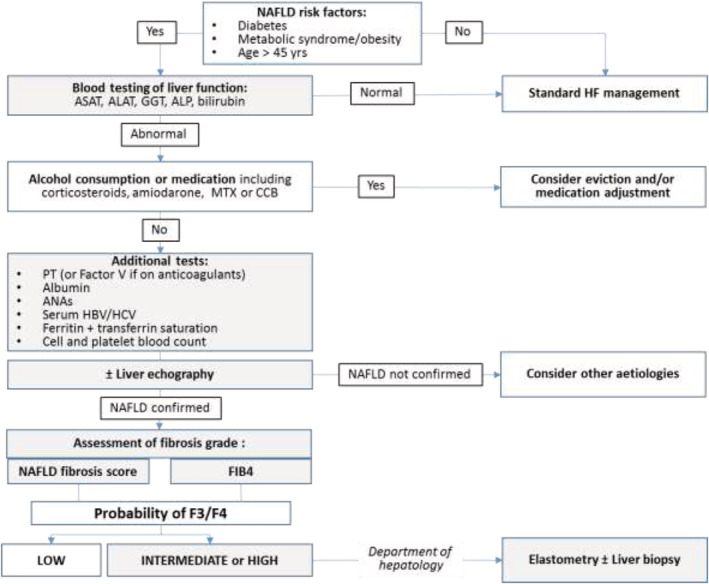
Proposed algorithm for the screening of NAFLD in patients with HFpEF.
Once the condition of a NAFLD is confirmed, fibrosis severity must be assessed by using available simple free blood tests such as the NAFLD fibrosis score or FIB4. Both scores allow for determining the low, intermediate, or high probability of F3/F4 (Figure 3 ). In the case of intermediate or high risks, patients should be followed by a specialized hepatology department to perform liver elastometry and appropriate therapy. The use of NFS as proposed to assess liver stiffness and make prognoses for HFpEF patients warrants further studies on large populations. 11
Figure 3.
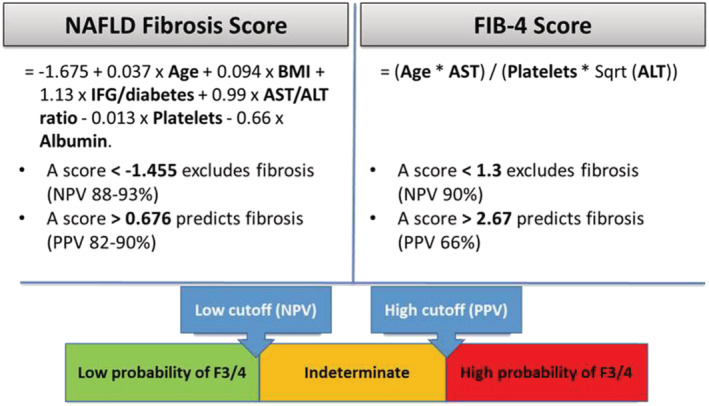
Fibrosis scores available for clinical practice. Values are from the EASL‐ALEH Clinical Practice Guidelines 20 and Boursier et al. (2016) 21 .
In conclusion, even though the successful trials required to consider a cost‐effectiveness screening for NAFLD in HFpEF are not currently available, cardiologists must be aware of the importance of identifying HFpEF patients at risk of fibrosis. In this respect, a close collaboration between cardiologists and hepatologists must be encouraged. Finally, one can advocate for clinical research addressing cardiological endpoints in patients recruited in clinical trials targeting NAFLD and on the other side metabolic and hepatology endpoints in patients included in cardiological research.
Conflict of interest
J.E.R. declares personal fees from AstraZeneca, Novartis, and Vifor. M.Gu. declares personal fees from Intercept. Other authors have nothing to declare.
Acknowledgements
We thank Hervé Bismut (Geminicis, Montrouge, France) for helping to prepare the manuscript. The authors also wish to thank Diana Dahan and Aimeric Moncelly for their assistance in logistic management. Novartis provided financial support for logistic management but was not involved in the writing of the manuscript.
Itier, R. , Guillaume, M. , Ricci, J.‐E. , Roubille, F. , Delarche, N. , Picard, F. , Galinier, M. , and Roncalli, J. (2021) Non‐alcoholic fatty liver disease and heart failure with preserved ejection fraction: from pathophysiology to practical issues. ESC Heart Failure, 8: 789–798. 10.1002/ehf2.13222.
References
- 1. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011; 32: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014; 11: 507–515. [DOI] [PubMed] [Google Scholar]
- 3. Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin 2014; 10: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O'Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014; 64: 2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golabi P, Paik J, Hwang JP, Wang S, Lee HM, Younossi ZM. Prevalence and outcomes of non‐alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int 2019; 39: 748–757. [DOI] [PubMed] [Google Scholar]
- 6. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. gastroenterology 2020. pii: S0016–5085(20)30223–7; 158: 1851–1864. [DOI] [PubMed] [Google Scholar]
- 7. Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 948–963. [DOI] [PubMed] [Google Scholar]
- 8. Packer M. Atrial fibrillation and heart failure with preserved ejection fraction in patients with nonalcoholic fatty liver disease. Am J Med 2020; 133: 170–177. [DOI] [PubMed] [Google Scholar]
- 9. Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: Current and emerging. Prevalence and staging of non‐alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. J Hepatol 2018; 68: 362–375.29122694 [Google Scholar]
- 10. Miller A, McNamara J, Hummel SL, Konerman MC, Tincopa MA. Prevalence and staging of non‐alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. Sci Rep 2020; 10: 12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology 2016; 150: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 14. Marra F, Svegliati‐Baroni G. Lipotoxicity and the gut‐liver axis in NASH pathogenesis. J Hepatol 2018; 68: 280–295. [DOI] [PubMed] [Google Scholar]
- 15. Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017; 65: 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple‐hit pathogenesis of non‐alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 17. Santhekadur PK, Kumar DP, Sanyal AJ. Preclinical models of non‐alcoholic fatty liver disease. J Hepatol 2018; 68: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hagström H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol 2017; 67: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 19. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017; 65: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, Eslam M, Gonzalez‐Fabian L, Alvarez‐Quiñones Sanz M, Conde‐Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero‐Gomez M. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology 2018; 155: 443–457 e17. [DOI] [PubMed] [Google Scholar]
- 21. EASL . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016; 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 22. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 23. European association for study of the liver, asociacion latinoamericana para el estudio del higado . EASL‐ALEH Clinical Practice Guidelines: Non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015; 63: 237–264. [DOI] [PubMed] [Google Scholar]
- 24. Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J, Oberti F, Charbonnier M, Fouchard‐Hubert I, Rousselet MC, Calès P, de Lédinghen V. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non‐alcoholic fatty liver disease. J Hepatol 2016; 65: 570–578. [DOI] [PubMed] [Google Scholar]
- 25. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62: S47–S64. [DOI] [PubMed] [Google Scholar]
- 26. Adams LA, Anstee QM, Tilg H, Targher G. Non‐alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017; 66: 1138–1153. [DOI] [PubMed] [Google Scholar]
- 27. Gastaldelli A, Kozakova M, Hojlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, Balkau B, RISC Investigators . Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009; 49: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 28. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363: 1341–1350. [DOI] [PubMed] [Google Scholar]
- 29. Niederseer D, Wernly S, Bachmayer S, Wernly B, Bakula A, Huber‐Schönauer U, Semmler G, Schmied C, Aigner E, Datz C. Diagnosis of non‐alcoholic fatty liver disease (NAFLD) is independently associated with cardiovascular risk in a large austrian screening cohort. J Clin Med 2020; 9 pii: E1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, MacGowan GA, Anstee QM, Taylor R, Day CP, Trenell MI. Cardiac structure and function are altered in adults with non‐alcoholic fatty liver disease. J Hepatol 2013; 58: 757–762. [DOI] [PubMed] [Google Scholar]
- 31. Sung KC, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow‐up, and risk of incident hypertension. J Hepatol 2014; 60: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 32. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005; 42: 473–480. [DOI] [PubMed] [Google Scholar]
- 33. Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima‐media thickness in nonalcoholic fatty liver disease. Am J Med 2008; 121: 72–78. [DOI] [PubMed] [Google Scholar]
- 34. Petta S, Argano C, Colomba D, Cammà C, Di Marco V, Cabibi D, Tuttolomondo A, Marchesini G, Pinto A, Licata G, Craxì A. Epicardial fat, cardiac geometry and cardiac function in patients with nonalcoholic fatty liver disease: association with the severity of liver disease. J Hepatol 2015; 62: 928–933. [DOI] [PubMed] [Google Scholar]
- 35. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 36. Coombes JD, Choi SS, Swiderska‐Syn M, Manka P, Reid DT, Palma E, Briones‐Orta MA, Xie G, Younis R, Kitamura N, Della Peruta M, Bitencourt S, Dollé L, Oo YH, Mi Z, Kuo PC, Williams R, Chokshi S, Canbay A, Claridge LC, Eksteen B, Diehl AM, Syn WK. Osteopontin is a proximal effector of leptin‐mediated non‐alcoholic steatohepatitis (NASH) fibrosis. Biochim Biophys Acta 1862; 2016: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pejnovic N, Jeftic I, Jovicic N, Arsenijevic N, Lukic ML. Galectin‐3 and IL‐33/ST2 axis roles and interplay in diet‐induced steatohepatitis. World J Gastroenterol 2016; 22: 9706–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wettersten N, Maisel AS. Biomarkers for heart failure: an update for practitioners of internal medicine. Am J Med 2016; 129: 560–567. [DOI] [PubMed] [Google Scholar]
- 39. Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol 2006; 40: 949–955. [DOI] [PubMed] [Google Scholar]
- 40. Fotbolcu H, Yakar T, Duman D, Karaahmet T, Tigen K, Cevik C, Kurtoglu U, Dindar I. Impairment of the left ventricular systolic and diastolic function in patients with non‐alcoholic fatty liver disease. Cardiol J 2010; 17: 457–463. [PubMed] [Google Scholar]
- 41. VanWagner LB, Wilcox JE, Colangelo LA, Lloyd‐Jones DM, Carr JJ, Lima JA, Lewis CE, Rinella ME, Shah SJ. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population‐based study. Hepatology 2015; 62: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borges‐Canha M, Neves JS, Libânio D, Von‐Hafe M, Vale C, Araújo‐Martins M, Leite AR, Pimentel‐Nunes P, Carvalho D, Leite‐Moreira A. Association between nonalcoholic fatty liver disease and cardiac function and structure‐a meta‐analysis. Endocrine 2019; 66: 467–476. [DOI] [PubMed] [Google Scholar]
- 43. Granér M, Nyman K, Siren R, Pentikäinen MO, Lundbom J, Hakkarainen A, Lauerma K, Lundbom N, Nieminen MS, Taskinen MR. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ Cardiovasc Imaging 2015; 8: e001979. [DOI] [PubMed] [Google Scholar]
- 44. Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K, Youn JC, Yun M, Park JY, Shim CY, Lee BW, Kang SM, Ha JW, Cha BS, Kang ES. Association of non‐alcoholic steatohepatitis with subclinical myocardial dysfunction in non‐cirrhotic patients. J Hepatol 2018; 68: 764–772. [DOI] [PubMed] [Google Scholar]
- 45. Canada JM, Abbate A, Collen R, Billingsley H, Buckley LF, Carbone S, Trankle CR, Idowu MO, Kadariya D, Van Tassell B, Sanyal AJ, Siddiqui MS. Relation of hepatic fibrosis in nonalcoholic fatty liver disease to left ventricular diastolic function and exercise tolerance. Am J Cardiol 2019; 123: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mantovani A, Ballestri S, Lonardo A, Targher G. Cardiovascular disease and myocardial abnormalities in nonalcoholic fatty liver disease. Dig Dis Sci 2016; 61: 1246–1267. [DOI] [PubMed] [Google Scholar]
- 47. Whitsett M, Wilcox J, Yang A, Zhao L, Rinella M, VanWagner LB. Atrial fibrillation is highly prevalent yet undertreated in patients with biopsy‐proven nonalcoholic steatohepatitis. Liver Int 2019; 39: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Long MT, Yin X, Larson MG, Ellinor PT, Lubitz SA, McManus DD, Magnani JW, Staerk L, Ko D, Helm RH, Hoffmann U, Chung RT, Benjamin EJ. Relations of liver fat with prevalent and incident atrial fibrillation in the framingham heart study. J Am Heart Assoc 2017; 6: e005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2018; 15: 425–439. [DOI] [PubMed] [Google Scholar]
- 50. Ostovaneh MR, Ambale‐Venkatesh B, Fuji T, Bakhshi H, Shah R, Murthy VL, Tracy RP, Guallar E, Wu CO, Bluemke DA, Lima JAC. Association of liver fibrosis with cardiovascular diseases in the general population: the multi‐ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging 2018; 11: e007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mantovani A, Nascimbeni F. Is it time to include non‐alcoholic fatty liver disease in the current risk scores for atrial fibrillation? Dig Liver Dis 2018; 50: 626–628. [DOI] [PubMed] [Google Scholar]
- 52. Wijarnpreecha K, Boonpheng B, Thongprayoon C, Jaruvongvanich V, Ungprasert P. The association between non‐alcoholic fatty liver disease and atrial fibrillation: a meta‐analysis. Clin Res Hepatol Gastroenterol 2017; 41: 525–532. [DOI] [PubMed] [Google Scholar]
- 53. Sirbu O, Floria M, Dascalita P, Sorodoc V, Sorodoc L. Non‐alcoholic fatty liver disease—from the cardiologist perspective. Anatol J Cardiol 2016; 16: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non‐alcoholic fatty liver disease. World J Gastroenterol 2014; 20: 1724–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ozveren O, Izgi C, Eroglu E, Simsek MA, Turer A, Kucukdurmaz Z, Cinar V, Degertekin M. Doppler tissue evaluation of atrial conduction properties in patients with non‐alcoholic fatty‐liver disease. Ultrason Imaging 2016; 38: 225–235. [DOI] [PubMed] [Google Scholar]
- 56. Alonso A, Misialek JR, Amiin MA, Hoogeveen RC, Chen LY, Agarwal SK, Loehr LR, Soliman EZ, Selvin E. Circulating levels of liver enzymes and incidence of atrial fibrillation: the atherosclerosis risk in communities cohort. Heart 2014; 100: 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong VW, Wong GL, Yeung JC, Fung CY, Chan JK, Chang ZH, Kwan CT, Lam HW, Limquiaco J, Chim AM, Yu CM, Chan HL. Long‐term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology 2016; 63: 754–763. [DOI] [PubMed] [Google Scholar]
- 58. Patel SS, Nabi E, Guzman L, Abbate A, Bhati C, Stravitz RT, Reichman T, Matherly SC, Driscoll C, Lee H, Luketic VA, Sterling RK, Sanyal AJ, Patel V, Levy M, Siddiqui MS. Coronary artery disease in decompensated patients undergoing liver transplantation evaluation. Liver Transpl 2018; 24: 333–342. [DOI] [PubMed] [Google Scholar]
- 59. Simon TG, Trejo MEP, McClelland R, Bradley R, Blaha MJ, Zeb I, Corey KE, Budoff MJ, Chung RT. Circulating interleukin‐6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the multi‐ethnic study of atherosclerosis. Int J Cardiol 2018; 259: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kapuria D, Takyar VK, Etzion O, Surana P, O'Keefe JH, Koh C. Association of hepatic steatosis with subclinical atherosclerosis: systematic review and meta‐analysis. Hepatol Commun 2018; 2: 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou YY, Zhou XD, Wu SJ, Fan DH, Van Poucke S, Chen YP, Fu SW, Zheng MH. Nonalcoholic fatty liver disease contributes to subclinical atherosclerosis: a systematic review and meta‐analysis. Hepatol Commun 2018; 2: 376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim BJ, Cheong ES, Kang JG, Kim BS, Kang JH. Relationship of epicardial fat thickness and nonalcoholic fatty liver disease to coronary artery calcification: from the CAESAR study. J Clin Lipidol 2016; 10: 619–626. [DOI] [PubMed] [Google Scholar]
- 63. Mantovani A, Mingolla L, Rigolon R, Pichiri I, Cavalieri V, Zoppini G, Lippi G, Bonora E, Targher G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol 2016; 225: 387–391. [DOI] [PubMed] [Google Scholar]
- 64. Simon TG, Corey KE, Cannon CP, Blazing M, Park JG, O'Donoghue ML, Chung RT, Giugliano RP. The nonalcoholic fatty liver disease (NAFLD) fibrosis score, cardiovascular risk stratification and a strategy for secondary prevention with ezetimibe. Int J Cardiol 2018; 270: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blazina I, Selph S. Diabetes drugs for nonalcoholic fatty liver disease: a systematic review. Syst Rev 2019; 8: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ganguli S, DeLeeuw P, Satapathy SK. A review of current and upcoming treatment modalities in non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Hepat Med 2019; 11: 159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti‐hyperglycaemic drugs in patients with non‐alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab 2020. pii: S1262–3636(20)30002–1; 46: 427–441. [DOI] [PubMed] [Google Scholar]
- 68. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 69. Paulus WJ, Dal Canto E. Distinct myocardial targets for diabetes therapy in heart failure with preserved or reduced ejection fraction. JACC Heart Fail 2018; 6: 1–7. [DOI] [PubMed] [Google Scholar]
- 70. Harrison SA, Manghi FP, Smith WB, Alpenidze D, Aizenberg D, Burggraaf K, Chen CY, Zuckerman E, Ravussin E, Charatcharoenwitthaya P, Cheng PN. LIK066 (licogliflozin), an SGLT1/2 inhibitor, robustly decreases alt and improves markers of hepatic and metabolic health in patients with non‐alcoholic fatty liver disease: interim analysis of a 12‐week, randomized, placebo‐controlled, phase 2a study. 2019. AASLD 2019. Abst LO7.
- 71. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, EMPEROR‐Preserved Trial Committees and Investigators . Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 72. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano‐Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez‐Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ, REGENERATE Study Investigators . Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase III trial. Lancet 2019; 394: 2184–2196. [DOI] [PubMed] [Google Scholar]
- 73. Patel K, Harrison SA, Elkashab M, Trotter JF, Herring R, Rojter S, Kayali Z, Wong VW, Greenbloom S, Jayakumar S, Shiffman ML, Freilich B, Lawitz EJ, Gane E, Harting E, Xu J, Billin AN, Chung C, Djedjos CS, Subramanian GM, Myers RP, Middleton MS, Rinella M, Noureddin M. Cilofexor, a nonsteroidal FXR agonist, in non‐cirrhotic patients with nonalcoholic steatohepatitis: a phase 2 randomized controlled trial. Hepatology 2020; 72: 58–71. [DOI] [PubMed] [Google Scholar]
- 74. Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S, Fischer L, Loomba R, Abdelmalek MF, Tacke F. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase IIb CENTAUR study. Hepatology 2020; Jan 13; 72: 892–905. [DOI] [PubMed] [Google Scholar]
- 75. Boeckmans J, Natale A, Rombaut M, Buyl K, Rogiers V, De Kock J, Vanhaecke T, Rodrigues RM. Anti‐NASH drug development hitches a lift on PPAR agonism. Cells 2019; 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, Loomba R, Ratziu V, Kochuparampil J, Fischer L, Vaidyanathan S, Anstee QM. A randomized, double‐blind, multicenter, phase IIb study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp Clin Trials 2020; 88: 105889. [DOI] [PubMed] [Google Scholar]


