Abstract
Aims
Concern has been raised that treatment with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the expression of angiotensin‐converting enzyme 2 (ACE2), which acts as the entry receptor for SARS‐CoV‐2, and lead to an increased risk of death from SARS‐CoV‐2. We aimed to address this concern by evaluating the in vivo relationship of treatment with ACE inhibitors and angiotensin receptor blockers (ARB) with circulating plasma concentrations of ACE2 in a large cohort of patients with established cardiovascular disease (n = 1864) or cardiovascular risk factors (n = 2144) but without a history of heart failure.
Methods and results
Angiotensin‐converting enzyme 2 was measured in 4008 patients (median age 68, 33% women, 31% on ACE‐inhibitors, 31% on ARB) using the SOMAscan proteomic platform (SomaLogic Inc, Colorado, USA). Plasma concentration of ACE2 was comparable in 1250 patients on ACE inhibitors (mean 5.99) versus patients without ACE inhibitors (mean 5.98, P = 0.54). Similarly, plasma concentration of ACE2 was comparable in 1260 patients on ARB (mean 5.99) versus patients without ARB (mean 5.98, P = 0.50). Plasma concentration of ACE2 was comparable in 2474 patients on either ACE inhibitors or ARB (mean 5.99) versus patients without ACE inhibitors or ARB (mean 5.98, P = 0.31). Multivariable quantile regression model analysis confirmed the lack of association between treatment with ACE inhibitors or ARB and ACE2 concentrations. Body mass index showed the only positive association with ACE2 plasma concentration (effect 0.015, 95% confidence interval 0.002 to 0.028, P = 0.024).
Conclusions
In a large cohort of patients with established cardiovascular disease or cardiovascular risk factors but without heart failure, ACE inhibitors and ARB were not associated with higher plasma concentrations of ACE2.
Keywords: SARS‐CoV‐2, Covid‐19, ACE, ARB, RAAS, ACE2, Plasma levels
Background
The majority of deaths from the new severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) occurred in patients with cardiovascular disease, who often were on chronic treatment with angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB). 1 This epidemiological association together with experimental evidence that treatment with ACE inhibitors and ARB may increase the expression of angiotensin‐converting enzyme 2 (ACE2), which acts as the entry receptor for SARS‐CoV‐2, has led to concern that ACE inhibitors and ARB may causally contribute to an increased risk of death from SARS‐CoV‐2 via increasing ACE2. 2
Aims
We aimed to address this concern by evaluating the in vivo effect of treatment with ACE inhibitors and ARB on circulating plasma concentrations of ACE2 in a large cohort of patients with established cardiovascular disease (n = 1864) or cardiovascular risk factors (n = 2144), but without a history of heart failure (NCT01838148, ClinicalTrials.gov). 3
Methods
This analysis was performed in a large prospective diagnostic study (ClinicalTrials.gov, NCT01838148) designed to advance the early detection of inducible myocardial ischaemia. Consecutive adult patients with symptoms possibly related to inducible myocardial ischaemia, who were referred for stress and rest myocardial perfusion imaging with single‐photon emission computed tomography combined with computed tomography (MPI‐SPECT/CT) to the University Hospital Basel (Basel, Switzerland), were recruited. All patients provided written informed consent. Clinical information, including patient characteristics, medication, symptoms, and cardiovascular history, was documented by physicians using standardized questionnaires and all medical files available. The study was approved by the local ethics committee and carried out according to the principles of the Declaration of Helsinki.
Of the 4219 patients enrolled in the study, ACE2 was measured in 4008 patients (median age 68 [interquartile range 60 to 76], 33% women, 31% on ACE inhibitors, 31% on ARB, baseline characteristics; see Table 1 ) using the SOMAscan proteomic platform version 4 (SomaLogic Inc, Colorado, USA). 4 In brief, SOMAscan uses a proprietary DNA‐based aptamer technology to bind to the target protein with high specificity and transforms individual protein concentrations into a corresponding modified aptamer concentration. Therefore, resulting concentrations [relative fluorescent units (RFU)] are relative and directly proportional to the amount of target protein in the initial sample. Additional analytical validation was performed in this study by utilizing routine cardiovascular biomarker measurements [n‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), Spearman ρ = 0.63 and growth differentiation factor 15 (GDF‐15), Spearman ρ = 0.93, both P < 0.001, both Roche Diagnostics] for correlation analysis with SOMAscan results. The natural logarithm of ACE2 measurements was used for all analyses.
Table 1.
Baseline characteristics of the overall study cohort and stratified by the use of ACEi or ARB
| Variable | Overall | No ACEi or ARB | ACEi or ARB | P |
|---|---|---|---|---|
| n | 4008 | 1534 | 2474 | |
| Age (years) | 68.0 [60.0, 76.0] | 66.0 [57.0, 74.0] | 69.0 [61.0, 76.0] | <0.001 |
| Female | 1316 (33) | 584 (38) | 732 (30) | <0.001 |
| eGFR (mL/min/1.73 m2) | 81.0 [61.4, 93.0] | 85.6 [69.7, 96.5] | 77.6 [57.1, 91.0] | <0.001 |
| BMI (kg/m2) | 27.1 [24.4, 30.5] | 26.1 [23.7, 29.4] | 27.7 [24.9, 31.2] | <0.001 |
| Medical history | ||||
| Atrial fibrillation | 561 (14) | 172 (11) | 389 (16) | <0.001 |
| Diabetes | 995 (25) | 240 (16) | 755 (31) | <0.001 |
| Arterial hypertension | 3227 (81) | 762 (50) | 2465 (100) | <0.001 |
| Chronic obstructive pulmonary disease | 324 (8) | 108 (7) | 216 (9) | 0.057 |
| Myocardial infarction | 1156 (29) | 237 (15) | 919 (37) | <0.001 |
| Coronary artery disease | 1864 (47) | 480 (31) | 1384 (56) | <0.001 |
| Percutaneous coronary intervention | 1420 (35) | 358 (23) | 1062 (43) | <0.001 |
| Coronary artery bypass graft | 532 (13) | 140 (9) | 392 (16) | <0.001 |
| Medication | ||||
| ACE inhibitors | 1250 (31) | ‐ | 1250 (51) | <0.001 |
| Angiotensin receptor blockers | 1260 (31) | ‐ | 1260 (51) | <0.001 |
| Beta‐blockers | 2226 (56) | 659 (43) | 1567 (63) | <0.001 |
| Diuretics | 1546 (39) | 230 (15) | 1316 (53) | <0.001 |
| Calcium channel blockers | 926 (23) | 215 (14) | 711 (29) | <0.001 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Results
Plasma concentration of ACE2 was comparable in 1250 patients on ACE inhibitors (mean 5.99) versus patients without ACE inhibitors (mean 5.98, P = 0.54; Figure 1 ). Similarly, plasma concentration of ACE2 was comparable in 1260 patients on ARB (mean 5.99) versus patients without ARB (mean 5.98, P = 0.50). Also, plasma concentration of ACE2 was comparable in 2474 patients on either ACE inhibitors or ARB (mean 5.99) versus patients without ACE inhibitors or ARB (mean 5.98, P = 0.31).
Figure 1.
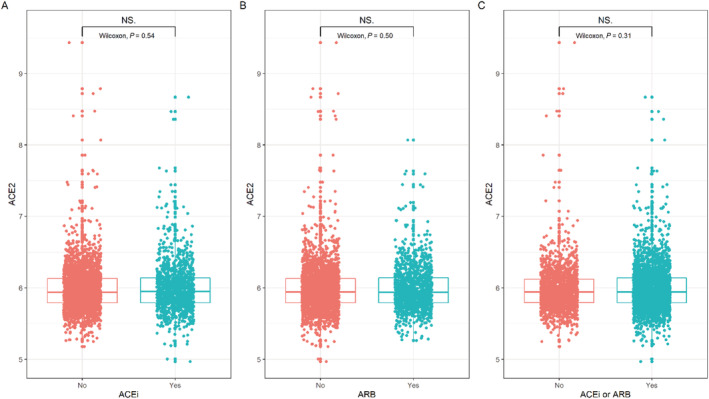
ACE2 concentrations in patients stratified by use of ACEi and/or ARB. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ACE2, angiotensin‐converting enzyme 2; NS, non‐significant.
Multivariable quantile regression model analysis adjusted for age, sex, body mass index, history of atrial fibrillation, diabetes, arterial hypertension, chronic obstructive pulmonary disease, myocardial infarction, coronary artery disease, percutaneous coronary intervention, and coronary artery bypass graft confirmed the lack of association between treatment with ACE inhibitors or ARB and ACE2 concentrations.
Body mass index was positively associated with ACE2 plasma concentration (effect 0.015, 95% CI: 0.002 to 0.028, P = 0.024), while age (−0.049, 95% CI: −0.065 to −0.034, P < 0.001), history of atrial fibrillation (−0.034, 95% CI: −0.062 to −0.006, P = 0.024), history of PCI (−0.041, 95% CI: −0.080 to −0.001, P = 0.042), and history of CABG (−0.052, 95% CI: −0.081 to −0.022, P < 0.001) were all negatively associated with ACE2 concentrations in the model.
Discussion/conclusion
This large observational study found no association between treatment with ACE inhibitors and/or ARB and circulating plasma concentrations of ACE2 in patients with established cardiovascular disease or cardiovascular risk factors, but without overt heart failure, representing the population most severely affected by SARS‐CoV‐2. This finding extends and corroborates similar observations regarding the lack of associations between treatment with ACE inhibitors and/or ARB on ACE2 plasma concentrations in two independent cohorts of patients with overt heart failure. 5 , 6 The first study included 1485 men and 537 women with heart failure in the derivation cohort and 1123 men and 575 women in the validation cohort, all enrolled in Europe. Median age was 69 years for men and 75 years for women. The second study included 2248 patients with heart failure enrolled in the USA. 5 , 6 In contrast to the European heart failure study, 5 this study did not find male sex to be a significant predictor for ACE2 plasma levels. This might in part be explained by an upregulation of ACE2 expression in heart‐failure patients, 7 potentially more so in men than in women. 8 Overall, these biomarker studies are in agreement with observational clinical studies documenting a lack of increased risk of severe illness in patients with COVID‐19 treated with an ACE inhibitor or ARB. 9 However, ACE2 regulation by RAAS blockade might be different in patients with active COVID‐19 compared with patients without, and further research is needed on this topic.
Taken together with emerging epidemiological data, 1 the three in vivo studies on ACE2 plasma concentrations support the concept that treatment with ACE inhibitors or ARB should not be withheld from patients at risk for infection with SARS‐CoV‐2, although this will need to be ultimately clarified by clinical trials. 10 , 11
Some limitations should be considered. First, liquid chromatography coupled to mass spectrometry is the gold standard for plasma ACE2 analysis. Therefore, it is reassuring that SOMAscan measurements were shown to be largely in accordance with proteome and transcriptome measurements and the ACE2 aptamer specifically has been validated. 6 , 12 Second, like previous studies, we measured circulating ACE2 plasma concentrations. The equilibrium between circulating and membrane‐bound ACE2 remains, to our knowledge, incompletely understood.
In conclusion, in a large cohort of patients with established cardiovascular disease or cardiovascular risk factors but without heart failure, ACE inhibitors and ARB were not associated with higher plasma concentrations of ACE2.
Conflict of interest
Dr. Zimmermann reports research grants from the Freiwillige Akademische Gesellschaft Basel. Dr. Walter reports research grants from the Swiss Heart Foundation (FF19097 and F18111) and the Swiss Academy of Medical Sciences and Bangerter Foundation. Dr. Mueller has received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Astra Zeneca, Beckman Coulter, Biomerieux, BRAHMS, Critical Diagnostics, Roche, Siemens, Singulex, and Sphingotec, as well as speaker/consulting honoraria from Abbott, Alere, Astra Zeneca, Bayer, Biomerieux, Boehringer Ingelheim, BMS, BRAHMS, Cardiorentis, Novartis, Roche, Sanofi, Siemens, and Singulex. All other authors declare that they have no conflict of interest with this study. All authors critically reviewed the manuscript and approved the final version for submission. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript.
Funding
This study was supported by the KTI, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University Basel, the University Hospital Basel, Abbott, Roche, Schiller, Somalogic, and Singulex.
Zimmermann, T. , Walter, J. E. , Lopez‐Ayala, P. , Strebel, I. , Amrein, M. , Koechlin, M. , Honegger, U. , Mueller, C. , and BASEL VIII Investigators (2021) Influence of renin‐angiotensin‐aldosterone system inhibitors on plasma levels of angiotensin‐converting enzyme 2. ESC Heart Failure, 8: 1717–1721. 10.1002/ehf2.13249.
Clinical Trial Registration: NCT01838148.
References
- 1. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid‐19. N Engl J Med 2020; 382: 2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N‐H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walter J, du Fay de Lavallaz J, Koechlin L, Zimmermann T, Boeddinghaus J, Honegger U, Strebel I, Twerenbold R, Amrein M, Nestelberger T, Wussler D, Puelacher C, Badertscher P, Zellweger M, Fahrni G, Jeger R, Kaiser C, Reichlin T, Mueller C. Using high‐sensitivity cardiac troponin for the exclusion of inducible myocardial ischemia in symptomatic patients. Ann Intern Med 2020; 172: 175–185. [DOI] [PubMed] [Google Scholar]
- 4. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA. Development and validation of a protein‐based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA ‐ J Am Med Assoc 2016; 315: 2532–2541. [DOI] [PubMed] [Google Scholar]
- 5. Sama IE, Ravera A, Santema BT, van Goor H, ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J 2020; 41: 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chirinos JA, Cohen JB, Zhao L, Hanff T, Sweitzer N, Fang J, Corrales‐Medina V, Ammar R, Morley M, Zamani P, Bhattacharya P, Brandimarto J, Jia Y, Basso MD, Wang Z, Ebert C, Ramirez‐Valle F, Schafer PH, Seiffert D, Gordon DA, Cappola T. Clinical and proteomic correlates of plasma ACE2 (angiotensin‐converting enzyme 2) in human heart failure. Hypertension 2020; 76: 1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up‐regulated in the human failing heart. BMC Med 2004; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, GTEx Consortium , Laboratory, Data Analysis &Coordinating Center (LDACC)—Analysis Working Group , Statistical Methods groups—Analysis Working Group , Enhancing GTEx (eGTEx) groups , NIH Common Fund , NIH/NCI , NIH/NHGRI , NIH/NIMH , NIH/NIDA , Biospecimen Collection Source Site—NDRI , Biospecimen Collection Source Site—RPCI , Biospecimen Core Resource—VARI , Brain Bank Repository—University of Miami Brain Endowment Bank , Leidos Biomedical—Project Management , ELSI Study , Genome Browser Data Integration &Visualization—EBI , Genome Browser Data Integration &Visualization—UCSC Genomics Institute, University of California Santa Cruz , Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur D. Landscape of X chromosome inactivation across human tissues. Nature 2017; 550: 244–248.29022598 [Google Scholar]
- 9. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin–angiotensin–aldosterone system inhibitors and risk of Covid‐19. N Engl J Med 2020; 382: 2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen JB, Hanff TC, Corrales‐Medina V, William P, Renna N, Rosado‐Santander NR, Rodriguez‐Mori JE, Spaak J, Andrade‐Villanueva J, Chang TI, Barbagelata A, Alfonso CE, Bernales‐Salas E, Coacalla J, Castro‐Callirgos CA, Tupayachi‐Venero KE, Medina C, Valdivia R, Villavicencio M, Vasquez CR, Harhay MO, Chittams J, Sharkoski T, Byrd JB, Edmonston DL, Sweitzer N, Chirinos JA. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) trial protocol. J Clin Hypertens 2020; 22: 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopes RD, Macedo AVS, Moll‐Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, de Souza AS, de Albuquerque DC, Mazza L, Santos MF, Salvador NZ, Gibson CM, Granger CB, Alexander JH, de Souza OF. Continuing versus suspending angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)—the BRACE CORONA trial. Am Heart J 2020; 226: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Billing AM, Ben Hamidane H, Bhagwat AM, Cotton RJ, Dib SS, Kumar P, Hayat S, Goswami N, Suhre K, Rafii A, Graumann J. Complementarity of SOMAscan to LC‐MS/MS and RNA‐seq for quantitative profiling of human embryonic and mesenchymal stem cells. J Proteomics 2017; 150: 86–97. [DOI] [PubMed] [Google Scholar]


