Abstract
Aims
This study sought to compare healthcare quality and 30 day, 90 day, and 1 year mortality rates among patients admitted to secondary and tertiary hospitals for heart failure (HF) in Beijing.
Methods and results
This study retrospectively enrolled patients hospitalized with a primary discharge diagnosis of HF during January 2014 to December 2015, from five tertiary and four secondary hospitals, in Beijing, China. Mortality data were extracted from Beijing Death Surveillance Database. HF healthcare quality indices were used to evaluate in‐hospital care. Associations between hospital level and mortality rates were assessed using generalized linear mixed models, adjusting for patients' baseline characteristics and intra‐hospital correlation. Data from 1413 patients (median [interquartile range] age = 74 [65–80] years, 52.7% female) from secondary hospitals and 1250 patients (median [interquartile range] age = 72 [61–79] years, 43.3% female) from tertiary hospitals were collected. Rates of left ventricular ejection fraction assessment (73.2% vs. 90.1%) and combined use of β‐blockers and angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (30.1% vs. 49.3%) were lower in secondary hospitals than those in tertiary hospitals, respectively. Patients admitted to secondary hospitals had a higher 90 day mortality [10.8% vs. 5.0%; adjusted odds ratio (OR): 2.06; 95% confidence interval (CI): 1.10–3.84, P = 0.024 and a higher 1 year mortality rate [21.0% vs. 12.1%; adjusted OR: 1.64; 95% CI: 1.02–2.62, P = 0.039], but 30 day mortality rates were not significantly different (5.5% vs. 3.0%; adjusted OR: 1.49; 95% CI: 0.63–3.52, P = 0.368).
Conclusions
Worse quality of care for patients with HF in secondary hospitals was associated with higher 90 day and 1 year mortality rates. Improving care quality in secondary hospitals is crucial to improve prognosis of patients they served.
Keywords: Heart failure, Healthcare quality, Mortality, China
Introduction
Heart failure (HF) is becoming an overwhelming social burden alongside the rapid population aging and changing profile of cardiovascular risk in China. 1 , 2 , 3 China ranks the highest among low‐income and middle‐income countries for its burden of HF. 4 , 5 Despite the availability of evidence‐based therapies to prevent death and re‐hospitalization in HF patients, 6 gaps between practice and guidelines remain large, especially in middle‐income countries like China. 7 , 8 , 9 , 10 , 11 Left ventricular ejection fraction (LVEF) assessment, use of β‐blocker, use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) for patients with left ventricular systolic dysfunction (LVSD), and provision of post‐discharge appointments have been recommended as metrics to measure healthcare quality of care for patients admitted with HF. 12 , 13 Data from the United States show variation in caring of patients with HF decreased from 25% in 2006 to 8% in 2011 after quality improvement activities. 14 Although wide heterogeneity in caring of patient with HF among different hospitals has been reported in China, 11 differences in the quality of care across different hospital levels have rarely been compared. Moreover, despite suboptimal in‐hospital care for patients with HF in China, inpatient mortality rates have been lower in China compared with data reported from other countries. 10 Considering the social convention of not dying in hospital, out‐of‐hospital death information has become essential to evaluate quality of in‐hospital care and short‐term outcomes of HF patients. Our study aims to compare quality of care in secondary hospitals and that in tertiary hospitals in Beijing, China, and the association with 30 day, 90 day, and 1 year mortality rates among patients hospitalized with HF.
Method
Study design and data collection
We invited all the affiliated hospitals and teaching hospitals of the Capital Medical University, including 10 tertiary hospitals and 13 secondary hospitals, of which five typical tertiary hospitals and four typical secondary hospitals agreed to participate in this study. All these participating hospitals provided inpatient care to HF patients within existing cardiovascular departments. In China, hospitals are graded into community health centres, secondary hospitals and tertiary hospitals, according to their functionality, size and specialization. Tertiary hospitals generally have a large number of beds and provide comprehensive medical services. Secondary hospitals usually provide general medical service to catchment areas of 300 000–500 000 local inhabitants. Characteristics of participating tertiary hospitals and secondary hospitals are shown in Table A1. All patients aged 18 years or older and hospitalized with a primary discharge diagnosis of HF during 1 January 2014 to 31 December 2015 were included in this study. For patients hospitalized twice or more in the participating hospitals, only the most recent inpatient hospitalization was included in the analysis.
Data were collected through chart review by trained staff. Information on patients' demographic characteristics, medical history, clinical presentation, laboratory examination, echocardiography, in‐hospital treatments, discharge diagnoses, and medications at discharge were collected. We used insurance types as a proxy for patients' socio‐economic status. The database was linked to the Death Surveillance System in Beijing to obtain all patient's survival information after admission. Ethical approvals were acquired from each participating hospital. Informed consent was waived in this study.
Metrics used in the measurement of healthcare quality in patients with heart failure
We reported three inpatient performance measures: (i) proportion of patients who had an LVEF assessment, (ii) proportion of β‐blocker prescription at discharge in patients with LVSD, defined as LVEF < 40%, and (iii) proportion of ACEI or ARB prescription at discharge in patients with LVSD. All these indices were recommended by the American Heart Association/American College of Cardiology/American Medical Association HF performance measurement guideline. 13 We also evaluated two additional quality measures: (i) proportion of patients with B‐type natriuretic peptide (BNP) /N‐terminal pro BNP tested and (ii) proportion of mineralocorticoid receptor antagonists (MRAs) prescription at discharge in patients with LVSD 12 , 13 , 15 (Table 1 ). For measures of drug prescriptions at discharge, patients with contraindication or patients who died during their hospitalization were excluded.
Table 1.
Comparison of impatient performance measures between the ACCF/AHA/AMA‐PCPI 2011 Guideline and the current study
| What ACCF/AHA/AMA‐PCPI 2011 Guideline advised | What was measured in the current study |
|---|---|
| LVEF assessment | LVEF assessment |
| β‐blocker therapy a for LVSD | β‐blocker therapy a for LVSD |
| ACEI/ARB therapy a for LVSD | ACEI/ARB therapy a for LVSD |
| Post‐discharge appointment for HF patients | — |
| BNP/NT‐proBNP test | |
| MRAs therapy a for LVSD |
Abbreviations: ACCF, American College of Cardiology Foundation; AHA, American Heart Association; AMA‐PCPI, American Medical Association–Physician Consortium for Performance Improvement; ACEI, angiotensin‐converting enzyme inhibitors; AMA, American Medical Association; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; HF, heart failure; LVEF, left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; MRA, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Drugs were prescribed at hospital discharge. Patients with contraindication or patients who died during their hospitalization were excluded.
Clinical outcomes
Patients' mortality data were acquired from the Beijing Death Surveillance System through data linkage, which records survival status of all Beijing citizens. Patients with the same name initials, sex, and birth dates in the health record and in the surveillance system were considered as the same person. Patients not recorded in the death surveillance system were considered as alive. A 30 day mortality rate was defined as the proportion of patients who died from any reason within 30 days after being admitted to a hospital with HF as the primary discharge diagnosis. The 90 day and 1 year mortality rates were defined as death from any cause within 90 days or within 1 year after hospital admission.
Statistical analysis
Patients' baseline characteristics, clinical outcomes and hospital performance measures were analysed to assess differences between secondary and tertiary hospitals. We summarized skewed continuous variables as median with interquartile ranges, normally distributed data as mean with standard deviations and categorical variables as frequencies and percentages. Student's t test/Wilcoxon rank‐sum test, and χ 2 test were used to compare continuous or categorical data between two hospital groups.
Propensity score‐weighted hierarchical generalized linear mixed models with logit link were performed to compare 30 day, 90 day, and 1 year mortality rates between secondary and tertiary hospitals. Propensity scores, which represent the probability of being admitted to tertiary hospital compared with a secondary hospital, were acquired using a logistic model that adjusted for 11 patient‐level risk factors that associated with patients' outcomes, 16 including age, sex, LVEF, New York Heart Association class, serum creatinine, diabetes, systolic blood pressure, body mass index, HF duration, current smoking, and chronic obstructive pulmonary disease. Weights of models were derived from the inverse of propensity score or the inverse of 1 minus propensity score. 17 Additional adjustment of patient‐level risk factors that used in the propensity score calculation was also performed in modelling process. Although these covariates were already in the propensity score model, this double‐adjustment approach reduced selection bias as thoroughly as possible following the principle of ‘double robustness’. 18 Four models were set up sequentially. The 11 patient‐level risk factors mentioned earlier were adjusted in Model 1. The variables including heart rate >100 beats per minute, anaemia (haemoglobin <130 g/L in male patients, haemoglobin <120 g/L in female patients), medical history of coronary artery disease (defined as a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft surgery), cardiomyopathy, valvular heart disease, and stroke were further adjusted in Model 2. Health insurance and marriage were added to previously adjusted risk factors for Model 3. Discharge prescription of β‐blocker, ACEI/ARB, and MRAs were added to previous risk factors for Model 4. Clustering of patients and their care within hospitals was treated as random effects in all hierarchical generalized linear mixed models. Odds ratios (ORs) and their 95% confidence intervals (CIs) were presented to show the associations of predictors and clinical outcomes.
Modes were used to impute missing values of categorical variables, and medians were imputed for missing values of continuous variables. Haemoglobin level had the highest proportion of missing values at 5%, which were replaced with the median value of 132 g/L. A two‐sided P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SAS software version 9.4 (SAS Institution Inc., Cary, NC, USA).
Results
Baseline characteristics
We enrolled 2663 patients hospitalized due to HF (1413 from four secondary hospitals and 1250 from five tertiary hospitals). Table 2 outlines the baseline characteristics of the patients. Patients hospitalized in secondary hospitals were older (median age: 74 years vs. 72 years, P < 0.001) and with larger proportion of female patients (52.7% vs. 43.3%, P < 0.001) than those hospitalized in tertiary hospitals. A significantly higher proportion of patients in secondary hospitals (55.5% vs. 4.4%, P < 0.001) was covered by the New Rural Cooperative Medical Scheme (NRCMS, which covered rural residents in China). Prevalence of coronary artery disease, cardiomyopathy, atrial fibrillation, hypertension, diabetes, anaemia, and kidney dysfunction (estimated glomerular filtration rate <60 mL/min/1.73m2) were higher in patients hospitalized in tertiary hospitals.
Table 2.
Patients' baseline characteristics by hospital levels
| Patient characteristics | Total (N = 2663) | Patients admitted to secondary hospitals (N = 1413) | Patients admitted to tertiary hospitals (N = 1250) | P value |
|---|---|---|---|---|
| Age, median (IQR), years | 73 (63, 80) | 74 (65, 80) | 72 (61, 79) | <0.001 |
| Female, N (%) | 1286/2663 (48.3%) | 745/1413 (52.7%) | 541/1250 (43.3%) | <0.001 |
| Married, N (%) | 2516/2663 (94.5%) | 1334/1413 (94.4%) | 1182/1250 (94.6%) | 0.865 |
| Insurance, N (%) | ||||
| Free medical care (urban: 100% covered) | 163/2663 (6.1%) | 5/1413 (0.4%) | 158/1250 (12.6%) | <0.001 |
| URBMI/UEBMI (urban: partially covered) | 1389/2663 (52.2%) | 569/1413 (40.3%) | 820/1250 (65.6%) | |
| NRCMS (rural: partially covered) | 839/2663 (31.5%) | 784/1413 (55.5%) | 55/1250 (4.4%) | |
| 100% out of pocket | 77/2663 (2.9%) | 35/1413 (2.5%) | 42/1250 (3.4%) | |
| Other (i.e. commercial insurance) | 195/2663 (7.3%) | 20/1413 (1.4%) | 175/1250 (14.0%) | |
| NYHA classification, N (%) | ||||
| I | 8/2663 (0.3%) | 3/1413 (0.2%) | 5/1250 (0.4%) | <0.001 |
| II | 29/2663 (1.1%) | 9/1413 (0.6%) | 20/1250 (1.6%) | |
| III | 1323/2663 (49.7%) | 484/1413 (34.2%) | 839/1250 (67.1%) | |
| IV | 1303/2663 (48.9%) | 917/1413 (64.9%) | 386/1250 (30.9%) | |
| Left bundle branch block, N (%) | 183/2663 (6.9%) | 125/1413 (8.8%) | 58/1250 (4.6%) | <0.001 |
| Disease history, N (%) | ||||
| CAD a | 787/2663 (29.6%) | 339/1413 (24%) | 448/1250 (35.8%) | <0.001 |
| Cardiomyopathy | 313/2663 (11.8%) | 111/1413 (7.9%) | 202/1250 (16.2%) | <0.001 |
| Valvular heart disease | 359/2663 (13.5%) | 192/1413 (13.6%) | 167/1250 (13.4%) | 0.863 |
| AF | 591/2663 (22.2%) | 274/1413 (19.4%) | 317/1250 (25.4%) | <0.001 |
| Stroke | 559/2663 (21%) | 316/1413 (22.4%) | 243/1250 (19.4%) | 0.064 |
| Hypertension | 1852/2663 (69.5%) | 959/1413 (67.9%) | 893/1250 (71.4%) | 0.046 |
| Diabetes mellitus | 886/2663 (33.3%) | 397/1413 (28.1%) | 489/1250 (39.1%) | <0.001 |
| Anaemia | 508/2663 (19.1%) | 235/1413 (16.6%) | 273/1250 (21.8%) | <0.001 |
| COPD | 251/2663 (9.4%) | 161/1413 (11.4%) | 90/1250 (7.2%) | <0.001 |
| Ever smoking tobacco, N (%) | 1222/2663 (45.9%) | 626/1413 (44.3%) | 596/1250 (47.7%) | 0.081 |
| Ever drinking alcohol, N (%) | 658/2663 (24.7%) | 351/1413 (24.8%) | 307/1250 (24.6%) | 0.867 |
| eGFR, N (%) | ||||
| <30 mL/min/1.73 m2 | 125/2663 (4.7%) | 62/1413 (4.4%) | 63/1250 (5%) | <0.001 |
| 30–59 mL/min/1.73 m2 | 464/2663 (17.4%) | 230/1413 (16.3%) | 234/1250 (18.7%) | |
| 60–89 mL/min/1.73 m2 | 856/2663 (32.1%) | 415/1413 (29.4%) | 441/1250 (35.3%) | |
| ≥90 mL/min/1.73 m2 | 1218/2663 (45.7%) | 706/1413 (50%) | 512/1250 (41%) | |
| LVEF, N (%) | ||||
| <40% | 600/2160 (27.8%) | 287/1034 (27.8%) | 313/1126 (27.8%) | 0.994 |
| 40–49% | 399/2160 (18.5%) | 192/1034 (18.6%) | 207/1126 (18.4%) | |
| ≥50% | 1161/2160 (53.8%) | 555/1034 (53.7%) | 606/1126 (53.8%) | |
| SBP, median (IQR) (mmHg) | 130 (120, 145) | 130 (120, 150) | 130 (115, 140) | <0.001 |
| Heart rate, median (IQR) (beats per min) | 80 (70, 92) | 82 (70, 96) | 76 (68, 88) | <0.001 |
| BMI, median (IQR) (kg/m2) | 23.7 (22.1, 26.7) | 23.4 (22.0, 26.1) | 24.6 (22.5, 27.3) | <0.001 |
| TG, median (IQR) (mmol/L) | 1.0 (0.8, 1.4) | 1.0 (0.8, 1.4) | 1.1 (0.8, 1.5) | 0.010 |
| TC, median (IQR) (mmol/L) | 3.8 (3.3, 4.5) | 3.8 (3.3, 4.5) | 3.8 (3.2, 4.5) | 0.171 |
| LDL‐C, median (IQR) (mmol/L) | 2.2 (1.8, 2.8) | 2.3 (1.9, 2.9) | 2.2 (1.7, 2.8) | <0.001 |
| HDL‐C, median (IQR), (mmol/L) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | 0.052 |
| ICD implantation after admission, N (%) | 5/2663 (0.2%) | 0/1413 (0%) | 5/1250 (0.4%) | 0.017 |
| CRT implantation after admission, N (%) | 1/2663 (0.03%) | 0/1413 (0%) | 1/1250 (0.1%) | 0.288 |
| Pacemaker implantation after admission, N (%) | 47/2663 (1.8%) | 2/1413 (0.1%) | 45/1250 (3.6%) | <0.001 |
| Hospital stays (days) | 8 (6, 12) | 8 (6, 11) | 9 (7, 13) | <0.001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; CRT, cardiac resynchronization therapy; HDL‐c, high‐density lipoprotein cholesterol; ICD, implantable cardioversion defibrillation; IQR, interquartile range; LBBB, left bundle branch block; LDL‐c, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NRCMS, New Rural Cooperative Medical system; NYHF classification, New York Heart Association classification; SBP, systolic blood pressure; TC, total cholesterol; TG, total triglycerides; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Residents' Basic Medical Insurance.
Defined as a history of diagnosed old myocardial infarction or percutaneous coronary intervention or coronary‐artery bypass graft.
Performance measures
Table 3 demonstrates rates of individual performance measures by hospital level. Although proportions of patients having BNP/N‐terminal pro BNP tested were similar in secondary and tertiary hospitals (85.9% vs. 87.7%, P = 0.180), a lower proportion of patients in secondary hospitals had LVEF evaluated (73.2% vs. 90.1%, P < 0.001). A lower proportion of patients with LVSD wad prescribed β‐blockers or combined β‐blockers and ACEI/ARB at discharge in secondary hospitals (44.3% vs. 72.4%, and 30.1% vs. 49.3%, respectively, both P < 0.001). Although not statistically significant, proportion of patients receiving ACEI/ARB was also lower in secondary hospitals (57.1% vs. 64.4%, P = 0.070). In contrast, a slightly higher proportion of eligible patients in secondary hospitals were prescribed MRAs at discharge (85.9% vs. 80.3%, P = 0.072).
Table 3.
Comparison of performance measures in eligible patients by hospital levels, n/N (%)
| Performance measures | Secondary hospital | Tertiary hospital | P value |
|---|---|---|---|
| N = 1413 | N = 1250 | ||
| BNP/NT‐proBNP test | 1214/1413 (85.9%) | 1096/1250 (87.7%) | 0.180 |
| LVEF assessment | 1034/1413 (73.2%) | 1126/1250 (90.1%) | <0.001 |
| β‐blocker therapya for LVSD | 127/285 (44.6%) | 218/301 (72.4%) | <0.001 |
| ACEI/ARB therapya for LVSD | 161/282 (57.1%) | 192/298 (64.4%) | 0.070 |
| Combined β‐blocker + ACEI/ARB therapya for LVSD | 85/282 (30.1%) | 147/298 (49.3%) | <0.001 |
| MRAs therapya for LVSD | 243/283 (85.9%) | 240/299 (80.3%) | 0.072 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BNP, B‐type natriuretic peptide; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MRAs, mineralocorticoid receptor antagonists; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Drugs were prescribed at hospital discharge. Patients with contraindication or patients died in hospital were excluded.
Clinical outcomes
Before adjustment, mortality rates at 30 days, 90 days, and 1 year were significantly higher in secondary hospitals than their counterparts in tertiary hospitals (5.5% vs. 3.0%, P = 0.001; 10.8% vs. 5.0%, P < 0.001; and 21.0% vs. 12.1%, P < 0.001). In Model 1, patients in secondary hospitals had a higher risk of 90 day mortality (OR: 2.06, 95% CI: 1.10–3.84, P = 0.024) and 1 year mortality (OR: 1.64, 95% CI: 1.02–2.62, P = 0.039), but not statistically significant lower risk of 30 day mortality (OR: 1.49, 95% CI: 0.63–3.52, P = 0.368). The associations remained consistent in Models 2, 3, and 4 (Figure 1 ).
Figure 1.
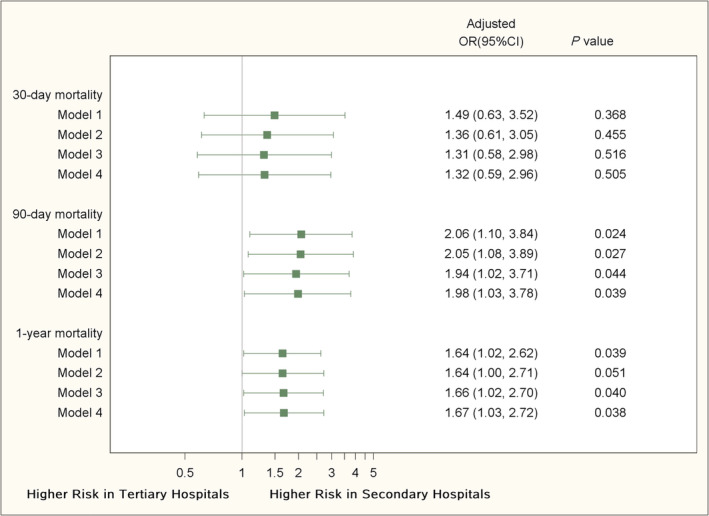
Patients' clinical outcomes by hospital levels. Model 1: adjusting for intra‐hospital correlation and 11 patient‐level risk factors (age, left ventricular ejection fraction, New York Heart Association classification, serum creatinine, diabetes, systolic blood pressure, body mass index, heart failure duration, current smoker, chronic obstructive pulmonary disease, and male gender); Model 2: adjusting for intra‐hospital correlation, previous 11 patient‐level risk factors in model 1, and heart rate >100 beats per minute, anaemia, medical history of coronary artery disease, cardiomyopathy, valvular heart disease, stroke; Model 3: adjusting for intra‐hospital correlation, previous 11 patient‐level risk factors in Model 1, and heart rate >100 beats per minute, anaemia, medical history of coronary artery disease, cardiomyopathy, valvular heart disease, stroke, health insurance and marriage; Model 4: adjusting for intra‐hospital correlation, previous 11 patient‐level risk factors in Model 1, and heart rate >100 beats per minute, anaemia, medical history of coronary artery disease, cardiomyopathy, valvular heart disease, stroke, health insurance, marriage, and prescription of β‐blocker, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker and mineralocorticoid receptor antagonists.
Discussion
In this retrospective study in four secondary and five tertiary hospitals in Beijing, China, we found that compared with those admitted to tertiary hospitals, HF patients admitted to secondary hospitals received worse in‐hospital care, and experienced higher 90 day and 1 year mortality rates. This associations remained significant after adjusting intra‐hospital correlation and patients' baseline characteristics.
A recent nationwide study has shown wide heterogeneity in the quality of care for HF among hospitals in China. 11 Our data show that in‐hospital care in Beijing were much better than the median level of the whole country on LVEF assessment during hospitalization (secondary hospitals: 73.2%/tertiary hospitals: 90.1% vs. nationwide median rate: 66.7%), and β‐blocker therapy for LVSD patients at discharge (secondary hospitals: 44.6%/tertiary hospitals: 72.4% vs. nationwide median rate: 14.8%), despite the measure of ACEI/ARB therapy for LVSD patients were similar to or slightly higher than the nationwide median level (secondary hospitals: 57.1%/tertiary hospitals: 64.4% vs. nationwide median rate: 57.1%). 11 However, the room for improvement in our participating hospitals was large when compared with data reported from Europe centres, where rates of β‐blocker prescription and ACEI/ARB prescription were over 80%. 19 Moreover, a specific attention should be focused on the quality of HF care in secondary hospitals.
The association between hospital‐level quality of care and mortality has been reported in previous studies outside of China. 14 , 20 For example, in a Danish nationwide study, researchers used echocardiography, New York Heart Association classification, treatment with ACEI/ARB, β‐blockers, physical training and patient education to construct a composite process performance measure to represent overall quality of HF care, and found the association between performance measures and 1‐year mortality that followed a dose–response pattern. 20 In our study, 90 day and 1 year mortality rates of 10.8% and 21.0%, respectively, were observed in patients hospitalized in secondary hospitals, almost twice the corresponding rates of people hospitalized in tertiary institutions (90 day mortality: 5.0%; 1 year mortality: 12.1%). After controlling individual‐level confounders, patients admitted to tertiary hospitals had a 51% lower risk of 90 day death and a 39% lower risk of 1 year mortality death.
In China, many severe HF patients prefer not to die in hospital and are discharged home under the care of their families for palliation. Therefore, it possible that 30 day mortality rate might be a better short‐term metric for hospital performance evaluation 21 , 22 , 23 , 24 instead of in‐hospital mortality. However, in the current study, although risk of adjusted 30‐day mortality difference was not significantly different between secondary hospitals and tertiary hospitals, the direction and magnitude of association was similar with longer time periods. These results showed a worse short‐term outcome among secondary hospital inpatients, although these results were imprecise.
Considering the complexity of care, it is difficult to explain the reasons for the quality gap between different levels of hospitals. Performance measures in this study which were recommended by the guideline and other literature, 12 , 13 , 15 were chosen in terms of usefulness, accuracy, feasibility, and measurability. However, there were other factors affecting healthcare quality and patients' clinical outcome as well, but were rarely documented and hard to measure, such as quality of discharge instructions provided, bedside manner of physician and nurse, patients' confidence of overcoming disease, compliance of post‐hospital treatment, patients' socio‐economic position, and local insurance policies.
Implications for health disparities
Differences in health insurance and extent of coverage may partially account for the heterogeneity of hospital‐level healthcare quality. 11 China's government instituted universal health insurance coverage to improve access to and affordability of public healthcare, and social health insurance programmes have successfully covered more than 97% Chinese residents. 25 However, different social health insurance programmes have different reimbursement policies, which influences patients' care‐seeking behaviour from different levels of hospitals. 26 For example, in Beijing, urban employers are covered by Urban Employ Based Medical Insurance, under which reimbursement for inpatient care in secondary hospitals and tertiary hospitals are similar [92% (in secondary hospital) vs. 90% (in tertiary hospital) between RMB 30 000 and 40 000 (US $4471–$5961); 97% vs. 95% above RMB 40 000 (US $5961) 27 ]. On the other hand, rural residents are covered by the NRCMS, under which the reimbursement rates for inpatient care in secondary hospitals and tertiary hospitals are quite different [70% (in secondary hospital) vs. 60% (in tertiary hospital) between RMB 20 000 and 50 000 (US $2980–$7452); 80% vs. 67% above RMB 50 000 (US $7452) 27 ]. The current study shows that 55.5% secondary hospital inpatients were covered by NRCMS, while corresponding number was 4.4% in tertiary hospitals. Rural HF residents, especially female patients with low socio‐economic status, had limited accessibility to high‐quality medical treatment, which may exacerbate health inequities. Future research should be conducted to reduce variation of health service in different hospitals.
Limitations
Several limitations of this study should be addressed. First, only patients with severe HF symptoms are given in‐hospital treatment, and those patients with mild symptoms are usually treated on an outpatient basis instead; thus, the study population does not represent patients with HF in all stages. Second, despite patient education about lifestyle, diet and medication was considered as an important component of providing qualified care for patients, 28 we did not use this process measure to assess hospitals' healthcare quality, mainly because it was difficult to evaluate by chart review. Third, the medical history of disease, especially non‐circulatory diseases, might be incompletely reported and documented. Fourth, only mortality data can be obtained from Beijing's Death Surveillance System through data linkage, and information of readmission and cardiac function were not available. In addition, there are limitations to causal inference about the relationship between quality and outcomes because the observational nature of our data, but the results are congruent with other published studies. 29 Although we adjusted for insurance and other patient‐level features by propensity score weighting and multivariate models, there may still be measured and unmeasured confounding that were not included in our multivariable models.
Conclusions
In conclusion, compared with tertiary hospitals, secondary hospitals in China had worse adherence to HF clinical guidelines, and patients discharged from secondary hospitals had worse mid‐term survival. Differences of healthcare quality and patients' outcomes by hospital level may exacerbate health inequities. Efforts should be made to improve quality of care across different hospital levels in China, and especially within secondary hospitals, to ensure that all patients receive high‐quality healthcare.
Conflict of interest
Chang‐Sheng Ma has received honoraria from Bristol‐Myers Squibb (BMS), Pfizer, Johnson & Johnson, Boehringer‐Ingelheim (BI), and Bayer for giving lectures. Jian‐Zeng Dong has received honoraria from Johnson & Johnson for giving lectures. Mark D. Huffman has received support from the American Heart Association, Verily, and AstraZeneca for work unrelated to this research and salary support from the American Medical Association for his role as an associate editor for JAMA Cardiology, and he also plans to submit patents for heart failure polypills. The George Institute for Global Health has a patent and licence and has received investment funding with intent to commercialize fixed‐dose combination therapy through its social enterprise business, George Medicines. The remaining authors have no disclosures to report.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFC1301002 and 2020YFC2004803), and grant from the Beijing Municipal Commission of Science and Technology (D171100006817001).
Acknowledgements
We thank the participants, the project staff, and the Beijing Centre for Disease Prevention and Control for access to Death Surveillance System.
Appendix A.
Please insert Table A1 here
Table A1.
Hospital characteristicsa
| Beds | Number of health workers | Number of outpatient visits (thousands) | Number of visits to emergency room (thousands) | Number of hospital discharges (thousands) | Number of inpatient surgical procedures (thousands) | Average number of clinical visits by each doctor | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number | Turnover rate (%) | Total | Qualified doctors | Qualified nurses | Else | ||||||
| Tertiary hospitals | |||||||||||
| A | 1287 | 110.86 | 3538 | 1236 | 1848 | 454 | 2577.4 | 142.0 | 66.1 | 39.2 | 2259.6 |
| B | 1265 | 95.04 | 2722 | 860 | 1406 | 456 | 2341.0 | 180.5 | 57.5 | 20.2 | 2994.6 |
| C | 801 | 85.13 | 1706 | 599 | 879 | 228 | 1291.3 | 65.9 | 23.1 | 11.1 | 2486.4 |
| D | 1162 | 93.83 | 1660 | 786 | 671 | 203 | 1305.3 | 101.2 | 40.0 | 22.0 | 1905.5 |
| E | 1598 | 94.78 | 2887 | 966 | 1295 | 626 | 2324.2 | 303.2 | 80.1 | 57.9 | 2700.4 |
| Secondary hospitals | |||||||||||
| A | 971 | 79.01 | 1318 | 393 | 652 | 273 | 1135.8 | 68.8 | 27.4 | 5.1 | 3116.8 |
| B | 780 | 82.61 | 988 | 376 | 442 | 170 | 1054.6 | 144.4 | 21.2 | 7.8 | 3058.7 |
| C | 943 | 91.87 | 1283 | 448 | 687 | 148 | 1155.8 | 155.2 | 36.8 | 9.8 | 3010.4 |
| D | 1009 | 89.83 | 2027 | 767 | 935 | 325 | 1826.5 | 218.7 | 35.8 | 12.6 | 2737.4 |
aData collected from health statistics of Beijing, in 2015.
He, L. , Dong, Z.‐J. , Du, X. , Jiang, C. , Chen, N. , Xia, S.‐J. , Hou, X.‐X. , Yu, H.‐R. , Lv, Q. , Yu, R.‐H. , Long, D.‐Y. , Bai, R. , Liu, N. , Sang, C.‐H. , Jiang, C.‐X. , Li, S.‐N. , Huffman, M. D. , Dong, J.‐Z. , and Ma, C.‐S. (2021) Healthcare quality and mortality among patients hospitalized for heart failure by hospital level in Beijing, China. ESC Heart Failure, 8: 1186–1194. 10.1002/ehf2.13178.
Dr Liu He and Dr Zhao‐Jie Dong contributed equally to this work.
Contributor Information
Xin Du, Email: duxinheart@sina.com.
Chang‐Sheng Ma, Email: chshma@vip.sina.com.
References
- 1. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, Wei T, Yu J, Wu Z, Gao Y, Han X, Zhang X, Wen S, Anker SD, Filippatos G, Fonarow GC, Gan T, Zhang R, China HFI. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in china: results from the China Heart Failure (China‐HF) Registry. J Card Fail 2017; 23: 868–875. [DOI] [PubMed] [Google Scholar]
- 3. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014; 171: 368–376. [DOI] [PubMed] [Google Scholar]
- 5. Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ 2017; 20: 549–553. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016; 68: 1476–1488. [DOI] [PubMed] [Google Scholar]
- 7. Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K. Heart failure care in low‐ and middle‐income countries: a systematic review and meta‐analysis. PLoS Med 2014; 11: e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lund LH, Carrero JJ, Farahmand B, Henriksson KM, Jonsson A, Jernberg T, Dahlstrom U. Association between enrolment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 9. Canepa M, Franssen FME, Olschewski H, Lainscak M, Bohm M, Tavazzi L, Rosenkranz S. Diagnostic and therapeutic gaps in patients with heart failure and chronic obstructive pulmonary disease. JACC Heart Fail 2019; 7: 823–833. [DOI] [PubMed] [Google Scholar]
- 10. Yu Y, Gupta A, Wu C, Masoudi FA, Du X, Zhang J, Krumholz HM, Li J, China PCG. Characteristics, management, and outcomes of patients hospitalized for heart failure in China: the China PEACE retrospective heart failure study. J Am Heart Assoc 2019; 8: e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta A, Yu Y, Tan Q, Liu S, Masoudi FA, Du X, Zhang J, Krumholz HM, Li J. Quality of care for patients hospitalized for heart failure in China. JAMA Netw Open 2020; 3: e1918619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonow RO, Bennett S, Casey DE Jr, Ganiats TG, Hlatky MA, Konstam MA, Lambrew CT, Normand SL, Pina IL, Radford MJ, Smith AL, Stevenson LW, Bonow RO, Bennett SJ, Burke G, Eagle KA, Krumholz HM, Lambrew CT, Linderbaum J, Masoudi FA, Normand SL, Ritchie JL, Rumsfeld JS, Spertus JA. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol 2005; 46: 1144–1178. [DOI] [PubMed] [Google Scholar]
- 13. Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE Jr, Goodlin SJ, Grady KL, Hundley RF, Jessup M, Lynn TE, Masoudi FA, Nilasena D, Pina IL, Rockswold PD, Sadwin LB, Sikkema JD, Sincak CA, Spertus J, Torcson PJ, Torres E, Williams MV, Wong JB. ACCF/AHA/AMA‐PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association‐Physician Consortium for Performance Improvement. Circulation 2012; 125: 2382–2401. [DOI] [PubMed] [Google Scholar]
- 14. Nuti SV, Wang Y, Masoudi FA, Bratzler DW, Bernheim SM, Murugiah K, Krumholz HM. Improvements in the distribution of hospital performance for the care of patients with acute myocardial infarction, heart failure, and pneumonia, 2006–2011. Med Care 2015; 53: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 16. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart F . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 17. Agoritsas T, Merglen A, Shah ND, O'Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: users' guides to the medical literature. JAMA 2017; 317: 748–759. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emdin CA, Conrad N, Kiran A, Salimi‐Khorshidi G, Woodward M, Anderson SG, Mohseni H, Dargie HJ, Hardman SM, McDonagh T, McMurray JJ, Cleland JG, Rahimi K. Variation in hospital performance for heart failure management in the National Heart Failure Audit for England and Wales. Heart 2017; 103: 55–62. [DOI] [PubMed] [Google Scholar]
- 20. Nakano A, Vinter N, Egstrup K, Svendsen ML, Schjodt I, Johnsen SP. Association between process performance measures and 1‐year mortality among patients with incident heart failure: a Danish nationwide study. Eur Heart J Qual Care Clin Outcomes 2019; 5: 28–34. [DOI] [PubMed] [Google Scholar]
- 21. Fonarow GC, Peterson ED. Heart failure performance measures and outcomes: real or illusory gains. JAMA 2009; 302: 792–794. [DOI] [PubMed] [Google Scholar]
- 22. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB, OPTIMIZE‐HF Investigators and Hospitals . Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA 2007; 297: 61–70. [DOI] [PubMed] [Google Scholar]
- 23. Pandey A, Patel KV, Liang L, DeVore AD, Matsouaka R, Bhatt DL, Yancy CW, Hernandez AF, Heidenreich PA, de Lemos JA, Fonarow GC. Association of hospital performance based on 30‐day risk‐standardized mortality rate with long‐term survival after heart failure hospitalization: an analysis of the get with the Guidelines‐Heart Failure Registry. JAMA Cardiol 2018; 3: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pandey A, Golwala H, Xu H, DeVore AD, Matsouaka R, Pencina M, Kumbhani DJ, Hernandez AF, Bhatt DL, Heidenreich PA, Yancy CW, de Lemos JA, Fonarow GC. Association of 30‐day readmission metric for heart failure under the hospital readmissions reduction program with quality of care and outcomes. JACC Heart Fail 2016; 4: 935–946. [DOI] [PubMed] [Google Scholar]
- 25. Meng Q, Fang H, Liu X, Yuan B, Xu J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet 2015; 386: 1484–1492. [DOI] [PubMed] [Google Scholar]
- 26. Li X, Lu J, Hu S, Cheng KK, De Maeseneer J, Meng Q, Mossialos E, Xu DR, Yip W, Zhang H, Krumholz HM, Jiang L, Hu S. The primary health‐care system in China. Lancet 2017; 390: 2584–2594. [DOI] [PubMed] [Google Scholar]
- 27.Available at: http://ybj.beijing.gov.cn/. Accessed at September 28, 2019.
- 28. Magnani JW, Mujahid MS, Aronow HD, Cene CW, Dickson VV, Havranek E, Morgenstern LB, Paasche‐Orlow MK, Pollak A, Willey JZ. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation 2018; 138: e48–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rush CJ, Campbell RT, Jhund PS, Petrie MC, McMurray JJV. Association is not causation: treatment effects cannot be estimated from observational data in heart failure. Eur Heart J 2018; 39: 3417–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]


