Abstract
Aims
Most devices for treating ambulatory Class II and III heart failure are linked to electrical pulses. However, a steady electric potential gradient is also necessary for appropriate myocardial performance and may be disturbed by structural heart diseases. We investigated whether chronic application of electrical microcurrent to the heart is feasible and safe and improves cardiac performance. The results of this study should provide guidance for the design of a two‐arm, randomized, controlled Phase II trial.
Methods and results
This single‐arm, non‐randomized pilot study involved 10 patients (9 men; mean age, 62 ± 12 years) at two sites with 6 month follow‐up. All patients had New York Heart Association (NYHA) Class III heart failure and non‐ischaemic dilated cardiomyopathy, with left ventricular ejection fraction (LVEF) <35%. A device was surgically placed to deliver a constant microcurrent to the heart. The following tests were performed at baseline, at hospital discharge, and at six time points during follow‐up: determination of LVEF and left ventricular end‐diastolic/end‐systolic diameter by echocardiography; the 6 min walk test; and assessment of NYHA classification and quality of life (36‐Item Short‐Form Health Survey questionnaire). Microcurrent application was feasible and safe; no device‐related or treatment‐related adverse events occurred. During follow‐up, rapid and significant signal of efficacy (P < 0.005) was present with improvements in LVEF, left ventricular end‐diastolic diameter, left ventricular end‐systolic diameter, and distance walked. For eight patients, NYHA classification improved from Class III to Class I (for seven, as early as 14 days post‐operatively); for one, to Class II; and for one, to Class II/III. 36‐Item Short‐Form Health Survey questionnaire scores also improved highly significantly.
Conclusions
Chronic application of microcurrent to the heart is feasible and safe and leads to a rapid and lasting improvement in heart function and a near normalization of heart size within days. The NYHA classification and quality of life improve just as rapidly.
Keywords: Heart failure, Electrical microcurrent, Electro‐osmosis, Electric potential gradient
Introduction
Therapy for heart failure is applied in a stepwise graduated manner. 1 , 2 If, despite optimal pharmacological therapy, cardiac function continues to decline, treatment with devices must be considered. 3 , 4 However, devices for cardiac resynchronization therapy (CRT) or cardiac contractility modulation can be considered for only a minority of patients due to restrictions that have emerged in clinical studies (QRS complex duration) or the application's inherent principle (triggered pulse delivery and therefore regular rhythm dependent). 1 , 2 Mechanical cardiac support systems are considered in the event of disease progression towards the end stage of the cardiomyopathic disease. 5 , 6 , 7 , 8
Patients who require CRT are moderately symptomatic with reduced left ventricular (LV) ejection fraction and New York Heart Association (NYHA) Class II or III heart failure. Mortality rates among NYHA Class III patients are as high as 26% during the 20 months after diagnosis or as high as 76% within 8 years. 9 , 10 Indeed, an enormous therapeutic gap exists for these patients presenting a target population with an unmet medical need. On the one hand, they do not qualify for the implantation of these electrical devices with a variable treatment effect. On the other hand, due to the risks of adverse events (AEs) with ventricular assist devices, these patients' heart failure symptoms are often not severe enough to justify the surgical implantation of such a system. 6 , 11 , 12 , 13 , 14 , 15 , 16
Here, we report the results of a Phase I dual‐site first‐in‐human study implanting a device that delivers a permanent non‐excitatory subthreshold microcurrent directly to the heart, completely independent of electrocardiogram status. The application of this intervention is based on the premise that an electrical potential gradient plays a central role in maintaining sufficient myocardial function. The study enrolled ambulatory NYHA Class III non‐ischaemic patients with a significantly reduced LVEF and 6 min walk under 250 m and was designed to provide information for a later randomized controlled Phase II trial.
Methods
Trial design and oversight
The study (Clinical Trials Register DRKS00015708) was performed between May 2019 and April 2020. A total of 10 patients were enrolled in a single‐arm, open‐label study at two sites in Europe. The study protocol was approved by the institutional ethics boards (Approval No. 1168/2018; 31/4) and by the competent national authorities (Approval No. 515‐05‐00067‐18‐1; 84/04). All patients provided written informed consent.
Primary endpoints were feasibility and safety in terms of incidence of AEs; secondary endpoints were all‐cause mortality, signs of efficacy as improvement of cardiac performance demonstrated by LVEF, LV end‐diastolic diameter (LVEDd), and LV end‐systolic diameter (LVEDs) as recorded by quantitative echocardiography, the 6 min walk test, NYHA classification, and health‐related quality of life as determined by the 36‐Item Short‐Form Health Survey (SF‐36) questionnaire.
Main inclusion criteria were heart failure with reduced ejection fraction caused by non‐ischaemic dilated cardiomyopathy (NYHA Class III), LVEF of 35% or less despite optimized medical management with maximum tolerated dose not modified for longer than 30 days, and heart failure of less than 5 years in duration.
Main exclusion criteria were previous cardiac surgical procedures and the presence of any other implantable electronic device.
Each patient was surgically implanted with a cardiac microcurrent device (C‐MIC; Berlin Heals, Berlin, Germany) capable of applying a permanent non‐excitatory subthreshold microcurrent directly to the heart via a coil electrode placed in the right ventricular (RV) cavity and a patch electrode placed intrapericardially on the epicardium of the left ventricle. The therapy current was set to the same fixed value in all patients. At the time of implantation, none of the patients enrolled in the study had already been implanted with another device such as CRT or implantable cardioverter defibrillator. The design of the device allows for simultaneous use along with implantable cardioverter defibrillators, pacemakers, or other devices when there is a reasonable medical indication.
The 6 month follow‐up period required examinations before discharge from the hospital, on Day 10 after implantation, and at 2 and 4 weeks and 2, 4, and 6 months after implantation. At each visit, an interval medical history, including NYHA classification and medications taken, was obtained. A health‐related quality‐of‐life questionnaire (SF‐36), transthoracic echocardiography, and a 6 min hall walk distance were administered at each visit, as was an interrogation of the implant via its wireless connection (Medical Implant Communication Service Adapter) to check for correct therapy current, battery lifetime, and further technical parameters to ensure proper device function. The device was deactivated at the end of the study.
Device description
The C‐MIC device that supplies the microcurrent is comparable with a regular pacemaker. The RV lead does not differ from leads of internal defibrillators. The patch for the LV epicardium is a thin, highly flexible flat electrode with a conductive side (Figure 1 ). The implantation procedure of the patch leads is like that used in placing epicardial leads for CRT device when access via the coronary sinus fails. The device can be programmed with an external radio module (Figure 2 ).
Figure 1.
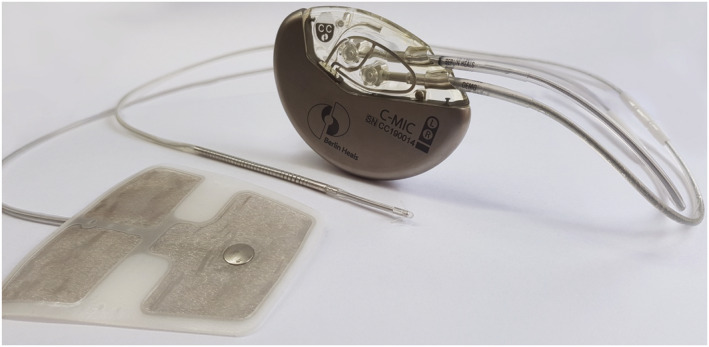
Implantable device with its left ventricular patch and right ventricular coil leads.
Figure 2.
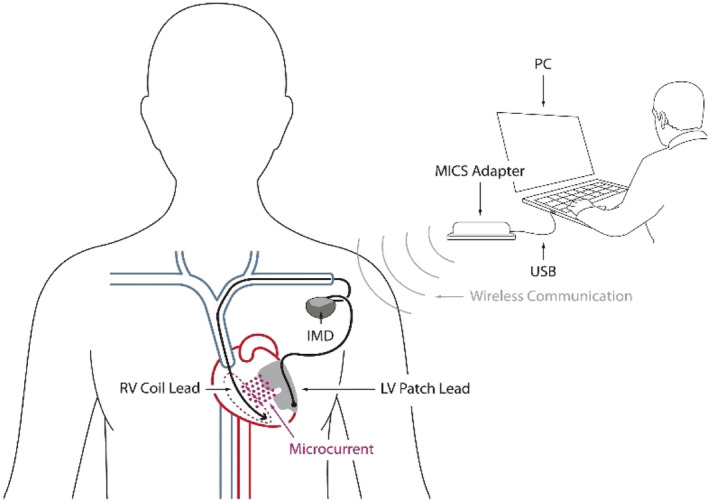
Schematic of system configuration. IMD, implantable microcurrent device; LV, left ventricular; MICS, Medical Implant Communication Service; PC, personal computer; RV, right ventricular; USB, universal serial bus.
Implant procedure
With the patient in the supine position and under general anaesthesia, a subcutaneous pocket for the implantable device is created at a left subclavian position. After access to the subclavian vein has been obtained, the RV lead is placed transvenously into the RV under fluoroscopic guidance. The soft anchored tip of the lead is fixed in the trabecula of the RV apex. A left‐sided incision 5 to 6 cm long is created in the fourth intercostal space. A longitudinal incision is made into the pericardium, and an LV patch lead is inserted into the pericardial space, with its conductive surface facing the epicardium of the LV free wall. The patch is then attached to the pericardium and immobilized with four sutures, one at each corner. The pericardial incision is closed with single sutures. After the lead is tunnelled to the subclavian pocket, both the LV patch lead and the RV lead are connected to the implantable device, which is then inserted into the pocket and fixed into position. The wounds of both the pocket and the chest incision are closed. The device is activated 24 h after the implantation procedure.
Follow‐up
The investigators recorded all AEs and serious AEs. Adjudication of the AEs was carried out by an external data monitoring committee.
Two‐dimensional echocardiography was performed at baseline and at each follow‐up visit to assess changes in LVEF, LVEDd, and LVEDs. LVEF was calculated according to Simpson's rule.
Patients completed the 6 min walk test at each follow‐up visit according to a standardized procedure. 17 Quality of life was assessed with the SF‐36 questionnaire.
Statistics
Data analysis was performed according to the intention‐to‐treat principle. Primary statistical analyses were performed with descriptive statistics. Statistical analyses of longitudinal changes used paired‐samples t‐tests to compare baseline values and follow‐up values at 14 days and at 6 months after device implantation when the Shapiro–Wilk test confirmed normal distribution of the samples. When samples were not normally distributed or data are available in an ordinal scale, the Wilcoxon signed‐rank test was applied. Statistical significance was set at the level of P < 0.05. The mixed‐model repeated measurement analysis tested the measuring points to baseline by multiple comparisons. All statistical analyses were performed with the SAS statistical software package (SAS, Cary, USA, NC).
Results
Study population
The baseline demographic characteristics and optimized heart failure medications of the 10 patients who underwent microcurrent device implantation are presented in Table 1 . Follow‐up of patients in the trial averaged 6 months (185 ± 7 days; range, 170–196). Table 2 presents baseline and follow‐up clinical characteristics at 14 days and at 6 months after implantation. There was zero mortality in the study population throughout the follow‐up period. For one patient, a misplaced patch lead (placement anterolaterally, predominantly over the right ventricle) was determined by X‐ray after surgery, which causes according to Ohm's law a low or at least an insufficient current flow through the LV myocardium, because patch and RV leads over the RV wall are in too close proximity.
Table 1.
Baseline demographic characteristics and anti‐heart failure medication
| Variable | Patients data (mean ± SD) (N = 10) |
|---|---|
| Age (years) | 53.3 ± 11.4; range, 29–67 |
| Women (%), men (%) | 1 (10), 9 (90) |
| White race (%) | 10 (100) |
| Body weight (kg) | 95.3 ± 15.3; range, 62–128 |
| Body height (cm) | 175.4 ± 7.9; range, 166–190 |
| Body mass index (kg/m2) | 30.9 ± 4.1; range, 22.5–35.9 |
| History of heart failure (years) | 2.1 ± 1.0; range, 1–4 |
| Atrial fibrillation—no. patients (%) | 1 (10)—paroxysmal |
| Medication—no. of patients (%) | |
| Aldosterone antagonist | 10 (100) |
| ACE inhibitor | 7 (70) |
| ARNI | 1 (10) |
| Beta‐blocker | 10 (100) |
| Diuretic | 8 (80) |
| Calcium channel blocker | 1 (10) |
ACE, angiotensin‐converting enzyme; ARNI, angiotensin receptor–neprilysin inhibitor; SD, standard deviation.
Table 2.
Baseline and follow‐up clinical characteristics
| Variable | Baseline | 14 days | P value | 6 months | P value |
|---|---|---|---|---|---|
| N = 10 | N = 10 | N = 10 | |||
| Systolic blood pressure (mmHg) | 117.4 ± 15.2 | 109.3 ± 8.6 | 0.128 | 124.1 ± 15.2 | 0.114 |
| Diastolic blood pressure (mmHg) | 73.3 ± 6.8 | 67.9 ± 7.4 | 0.201 | 73.5 ± 9.7 | 0.956 |
| QRS complex duration (ms) | 98.9 ± 14; r, 86–134 | 96.2 ± 9.9 | 0.902 | 104.1 ± 11.9; r, 96–134 | 0.078 |
| LVEF (%) | 31.8 ± 3.9; r, 26–35 | 39.8 ± 6.9 | 0.001 | 41.9 ± 9.0; r, 29–54 | 0.005 |
| LVEDd (mm) | 63.9 ± 3.2; r, 60–68 | 56.4 ± 3.3 | <0.001 | 57.6 ± 4.9; r, 53–68 | 0.005 |
| LVEDs (mm) | 50.9 ± 7.0; r, 40–59 | 43.3 ± 5.1 | 0.007 | 44.4 ± 7.0; r, 37–62 | 0.002 |
| 6 min walk distance (m) | 210.3 ± 38.5; r, 149–270 | 404.3 ± 49.0 | <0.001 | 418.5 ± 47.4; 300–493 | <0.001 |
| NYHA class, no. of patients (%) | III, 10 (100) | I, 7 (70) | 0.004 | I, 8 (80) | 0.002 |
| I/II, 1 (10) | II, 1 (10) | ||||
| II, 1 (10) | II/III, 1 (10) | ||||
| III, 1 (10) | |||||
| SF‐36 total score | |||||
| PCS | 41.0 ± 4.1 | 53.1 ± 3.4 | <0.001 | 57.2 ± 2.9 | <0.001 |
| MCS | 31.6 ± 8.6 | 57.7 ± 4.8 | <0.001 | 59.3 ± 4.8 | <0.001 |
LVEDd, left ventricular end‐diastolic diameter; LVEDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; MCS, Mental Component Summary; NYHA, New York Heart Association; PCS, Physical Component Summary; r, range; SF‐36, 36‐Item Short‐Form Health Survey questionnaire.
Values are presented as mean ± SD.
Primary endpoint
The implantation procedure was uneventful in all patients. Weaning from ventilator was possible for all patients immediately after the surgical procedure ended. There was no relevant pericardial effusion in any of the patients. No device or microcurrent treatment‐related AEs occurred.
Because the applied current is extremely weak—similar in strength to that of physiologically occurring currents in living biological systems—AEs caused by the current were not expected or observed. The patients did not feel the current nor could they distinguish whether the current was off or on. No arrhythmias or nerve stimulation events were detected. On the contrary, in a patient with paroxysmal atrial fibrillation at baseline, the atrial fibrillation disappeared in a few weeks after microcurrent therapy.
Adverse events
The primary endpoint was met, with documented feasibility and safety in all patients. In total, 17 AEs in nine patients were recorded. Fifteen AEs were classified as mild: 13 of these were only transient and disappeared until the second post‐operative day, and the remaining two AEs (left diaphragmatic elevation and gastro‐oesophageal reflux) in the same patient were not detectable at the 4 month visit. The patient with a misplaced LV lead experienced two serious AEs: worsening of heart failure and need for in‐hospital drug (levosimendan) support 50 and 97 days after device placement. The transient mild AEs were atrial fibrillation (in one patient, post‐operatively only), sinus bradycardia, pain, cough, skin bleeding at the wound site, mild atelectasis of the lung, small intrapleural effusion, gout, subcutaneous emphysema, and tingling in the fingers.
Secondary endpoint
The secondary endpoint showed significant signs of efficacy across the cohort in all determined parameters such as LVEF, LV end‐diastolic/end‐systolic diameter, 6 min walk test, NYHA classification, and quality of life total scores.
Echocardiographic parameter
Left ventricular ejection fraction, LVEDd, and LVEDs were measured by two‐dimensional echocardiogram at baseline and at all follow‐up visits (Figure 3 A and 3 B ). Compared with baseline values, all three variables showed a statistically significantly improvement at all follow‐up visits. Table 2 shows the numerical values (with statistical significance) for all three variables at baseline and at the 2 week and 6 month follow‐up visits.
Figure 3.
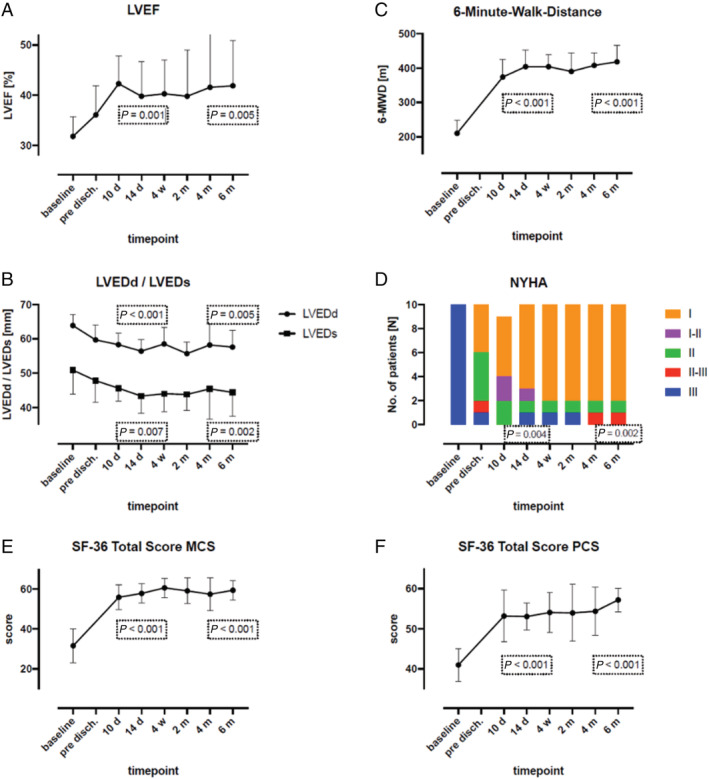
(A–F) Changes in the mean values of all examined variables between baseline and 6 month follow‐up (end of study). Compared with baseline values, all measured variables exhibited highly significant differences, except for values determined immediately before patient discharge. 6‐MWD, 6 min walk distance; d, days; LVEDd, left ventricular end‐diastolic diameter; LVEDs, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; m, months; MCS, Mental Component Summary; NYHA, New York Heart Association; PCS, Physical Component Summary; pre disch., pre‐discharge; SF‐36, 36‐Item Short‐Form Health Survey questionnaire; w, weeks.
Exercise capacity—6 min walk test
Apart from the follow‐up visit on Day 3, the 6 min walk test was performed at all regular visits. Table 2 shows the average baseline values and the average distances at 14 days and 6 months after implantation, with the statistical significance of the differences. Compared with the measured baseline distance, all differences in distances recorded during the follow‐up period are highly statistically significant (Figure 3 C ).
New York Heart Association classification
All patients were in NYHA Class III heart failure at baseline. At Month 6, eight patients were in NYHA Class I, one patient was in Class II, and one patient was in Class II/III heart failure (Figure 3 D ). As compared with the baseline classification, the improvements in NYHA classification at all visits were highly significant. Table 2 shows the NYHA classification of the patients' heart failure (with the calculated P value) at 14 days and at 6 months after implantation.
Health‐related quality‐of‐life questionnaire, 36‐Item Short‐Form Health Survey
The results of the health‐related quality‐of‐life questionnaire SF‐36 showed a highly significant improvement in the two total scores—Physical and Mental Component Summary—at all visits from baseline throughout the follow‐up period. Figure 3 E and 3 F depicts the course of the scores over the study period. Table 2 presents the numeric values at baseline and at 2 weeks and 6 months after device implantation.
Discussion
In this first‐in‐human pilot study, the application of a permanent non‐excitatory subthreshold electrical microcurrent directly to the heart in moderately symptomatic patients (NYHA III) with reduced LVEF was feasible and safe and was associated with clinically important signals of efficacy—such as improvements in cardiac performance, NYHA classification, and quality of life.
This application of microcurrent is based on the consideration that an unaltered physiological endogenous steady potential gradient (electrical field) within cells or organs is a precondition for regeneration of a disturbed function, a consideration that applies especially to electrically active organs, such as the heart, when their function is compromised. 18 , 19 , 20 , 21 , 22 The intensity of the microcurrent was specifically chosen to induce an electric field at physiologically occurring strength that was believed to compensate for the disturbed or missing potential gradient. External microcurrent is being used more and more frequently at various strengths and in various forms for physical and regenerative therapy. 23 However, chronic long‐term application of a non‐pulsating direct current for treating a significantly diseased internal organ has not been previously reported.
After 14 days of microcurrent treatment, the average LVEF of patients with NYHA Class III heart failure improved by 8 percentage points, and the mean 6 min walking distance increased by nearly 100%. In addition, within this short treatment period, mean LVEDd decreased significantly (by more than 7 mm). Highly significant improvement in NYHA class and quality of life, as determined by the SF‐36 questionnaire, also occurred rapidly after the initiation of therapy. The improvements in cardiac function over baseline were sustained at 6 months after implantation. Furthermore, an improvement in mitral valve regurgitation in nine out of nine examined patients could be determined (P = 0.016). In one patient, mitral valve regurgitation was not examined. No signs of efficacy were observed for the single patient with a misplaced patch lead. We can hypothesize that this lack of improvement was caused by insufficient flow of current through the LV myocardium rather than by the microcurrent and the observed deterioration corresponded to the natural course of the heart failure.
Given the limitations of this current study (open‐label, single‐arm, 10‐patient, non‐randomized), it cannot be excluded the possibility of a bias, in particular as in this pilot study objective parameters for an improvement of cardiac performance are missing. However, it is not impossible that microcurrent may have had a beneficial effect on cardiac function and in turn on exercise capacity, NYHA classification, and quality of life of patients. 11 , 13 , 14 , 24 , 25 , 26 , 27 , 28 In particular, the rapid improvement is an observation that requires further reflections.
Myocardial oedema
Supported by experimental evidence, biophysicists have long proposed that developmental, regenerative, maintenance, and signalling processes are controlled by intracellular and extracellular endogenous electrical fields. 19 , 20 , 29 Endogenous electrical microcurrent is responsible for a large number of effects. It can modulate inflammation by down‐regulating pro‐inflammatory cytokines and regulating macrophage function and T‐cell activation. 30 , 31 , 32 , 33
The myocardial application of microcurrent as a compensatory measure for a distorted endogenous potential gradient cannot not be expected to result in reverse remodelling as a primary mechanism of improved cardiac function within 2 week period demonstrated in this study. 34 , 35 , 36 The development of myocardial oedema significantly compromises heart function even with only a minimal increase (of only a few percentage points) in interstitial fluid volume. 37 , 38 , 39 The filtration rate for fluid moving out of the coronary microvasculature exchange vessels into the interstitium is described by the Starling–Landis equation. Besides depending on hydrostatic and plasma colloid osmotic pressure differences, this fluid filtration rate also depends on the myocardial microvascular permeability. The intraluminal glycocalyx layer of the fluid exchange vessels is highly negatively charged. Maintaining this negative charge is crucial for preventing the formation of oedema. Changing the negativity or neutralizing the negative charge of the glycocalyx will modify the microvascular permeability and, in turn, induce the formation of myocardial oedema. 38 , 40 , 41
In physics, the term electro‐osmosis is used to describe the phenomenon of the movement of a liquid caused by an electric field parallel to surfaces, for example, capillaries. 42 In biology, electroosmotic flow is a known transport mechanism that may involve small channel structures of heterogeneous tissue, such as gap junctions or capillaries, and can be found in particular in areas of high electrical activity. 43 , 44 One precondition for electroosmotic flow is an electric field parallel to the surface in which the flow should be induced. 42 , 45 , 46 Therefore, the microvascular fluid balance depends on an intact endogenous electric potential gradient. A disturbed endogenous field leads, via a disturbance of the glycocalyx and electro‐osmosis, to a disturbance of the fluid balance and to the formation of myocardial oedema. 38 , 40 , 41
The rapid effect of microcurrent on cardiac performance may be derived from the effects of electro‐osmosis. In this study, disturbed or absent endogenous electrical fields are being replaced by an external microcurrent at physiological strength. One of the characteristics of electro‐osmosis is that it generates measurable effects at the moment the microcurrent is turned on. This characteristic could explain why such a rapid effect occurs after the activation of the microcurrent device.
Of course, as long as there are no conclusive animal experimental data on this issue and with the current knowledge about the effect of microcurrent, we cannot exclude other mechanisms responsible for the observed findings than the mentioned electro‐osmosis.
Effects of chronic myocardial oedema have not been studied in depth nor is it exactly known to what extent myocardial oedema contributes to the pathology of heart failure. Some evidence suggests that excitation–contraction uncoupling induced by myocardial oedema causes contractile dysfunction and that myocardial oedema stimulates an exuberant collagen synthesis in the interstitium and induces fibrosis. 47 , 48
Limitations
The lack of randomization and the small number of patients with its possibility of a bias are clear limitations to draw final conclusions from this pilot study data and must be addressed in further studies. Future randomized studies will determine if the observed beneficial effect is significant and sustained in the patients who are maintained on evidence‐based heart failure therapies over the long term. In addition, it has to be determined if this early response with improved indexes of LV size and function at 2 weeks that is sustained at 6 months can be durable and robust in the patients who are maintained on evidence‐based heart failure therapies over the long term. 49 Finally, although the primary endpoints of the study were feasibility and safety, the lack of an objective parameter such as N‐terminal pro‐brain natriuretic peptide to confirm the improvement in cardiac performance is a shortcoming. N‐terminal pro‐brain natriuretic peptide should be included in the study design of follow‐up studies.
Conclusions
This study provides first signs of evidence that applying microcurrent directly to the heart is feasible and safe and can induce clinically significant improvements in moderately symptomatic patients as a target population with unmet medical needs. We found a signal of an early significant improvement in cardiac performance. The underlying mechanism may be restoration of endogenous electric potential gradients improving myocardial function. The rapidity with which these improvements occur suggests that microcurrent, mediated by electro‐osmosis, could have a direct effect the myocardial oedema that often accompanies heart failure. The restoration of myocardial function within this short period of time could have major implications for patients with progressive heart failure who experience a vicious cycle of congestive decompensation, neurohormonal activation, and myocardial dysfunction. As far as we know today, the therapeutic intervention of applying microcurrent can be administered to all patients regardless of electrocardiogram findings. Thus, the microcurrent device‐based approach may prove to be a disruptive therapeutic intervention beyond the current standard of care and deserves further clinical investigation.
Conflict of Interest
The authors P. Goettel, K. Brandes and J. Mueller are employees of Berlin Heals, the company that developed the device.
Funding
The study was funded by Berlin Heals.
Kosevic, D. , Wiedemann, D. , Vukovic, P. , Ristic, V. , Riebandt, J. , Radak, U. , Brandes, K. , Goettel, P. , Duengen, H.‐D. , Tahirovic, E. , Kottmann, T. , Voss, H. W. , Zdravkovic, M. , Putnik, S. , Schmitto, J. D. , Mueller, J. , Rame, J. E. , and Peric, M. (2021) Cardio‐microcurrent device for chronic heart failure: first‐in‐human clinical study. ESC Heart Failure, 8: 962–970. 10.1002/ehf2.13242.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2013; 128: e240–327. [DOI] [PubMed] [Google Scholar]
- 3. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, Misier AR, Curnis A, Bocker D, Remppis A, Kautzner J, Stuhlinger M, Leclerq C, Taborsky M, Frigerio M, Parides M, Burkhoff D, Hindricks G. Randomized, double blind study of non‐excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008; 29: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009; 361: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 5. Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, Loebe M, Moazami N, Long JW, Stehlik J, Kasirajan V, Haas DC, O'Connell JB, Boyle AJ, Farrar DJ, Rogers JG, ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients. J Am Coll Cardiol 2015; 66: 1747–1761. [DOI] [PubMed] [Google Scholar]
- 6. Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde‐McLean RC, DeVore AD, Pamboukian SV, Thibodeau JT, Teuteberg JJ, Cadaret L, Xie R, Taddei‐Peters W, Naftel DC, Kirklin JK, Stevenson LW, Stewart GC. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant 2019; 38: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stehlik J, Mountis M, Haas D, Palardy M, Ambardekar AV, Estep JD, Ewald G, Russell SD, Robinson S, Jorde U, Taddei‐Peters WC, Jeffries N, Richards B, Khalatbari S, Spino C, Baldwin JT, Mann D, Stewart GC, Aaronson KD, REVIVAL Investigators . Quality of life and treatment preference for ventricular assist device therapy in ambulatory advanced heart failure: a report from the REVIVAL study. J Heart Lung Transplant 2020; 39: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Potapov EV, Antonides C, Crespo‐Leiro MG, Combes A, Färber G, Hannan MM, Kukucka M, de Jonge N, Loforte A, Lund LH, Mohacsi P, Morshuis M, Netuka I, Özbaran M, Pappalardo F, Scandroglio AM, Schweiger M, Tsui S, Zimpfer D, Gustafsson F. 2019 EACTS Expert Consensus on long‐term mechanical circulatory support. Eur J Cardiothorac Surg 2019; 56: 230–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld J, Januzzi JL. Clinical implications of the New York Heart Association classification. J Am Heart Assoc. 2019; 8: e014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biton Y, Rosero S, Moss A, Zareba W, Kutyifa V, Baman J, Barsheshet A, McNitt S, Polonsky B, Goldenberg I. Long‐term survival with implantable cardioverter‐defibrillator in different symptomatic functional classes of heart failure. Am J Cardiol 2018; 121: 615–620. [DOI] [PubMed] [Google Scholar]
- 11. Noor MR, Lane RE, Dar O. Cardiac Resynchronization Therapy for Heart Failure. In Raja S., (ed). Cardiac Surgery. Cham: Springer International Publishing; 2020. 10.1007/978-3-030-24174-2_66 [DOI] [Google Scholar]
- 12. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, Sherfesee L, Wells GA, Tang ASL. An individual patient meta‐analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013; 34: 3547–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balla C, Cappato R. When to choose cardiac resynchronization therapy in chronic heart failure: type and duration of the conduction delay. Eur Heart J Suppl 2019; 21: B31–B35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hernandez N, Huang DT. Updated clinical evidence for effective cardiac resynchronization therapy in congestive heart failure and timing of implant. Card Electrophysiol Clin 2019; 11: 55–65. [DOI] [PubMed] [Google Scholar]
- 15. Starling RC, Estep JD, Horstmanshof DA, Milano CA, Stehlik J, Shah KB, Bruckner BA, Lee S, Long JW, Selzman CH, Kasirajan V, Haas DC, Boyle AJ, Chuang J, Farrar DJ, Rogers JG, ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients. JACC Heart Fail 2017; 5: 518–527. [DOI] [PubMed] [Google Scholar]
- 16. Samman‐Tahhan A, Hedley JS, McCue AA, Bjork JB, Georgiopoulou VV, Morris AA, Butler J, Kalogeropoulos AP. INTERMACS profiles and outcomes among non‐inotrope‐dependent outpatients with heart failure and reduced ejection fraction. JACC Heart Fail 2018; 6: 743–753. [DOI] [PubMed] [Google Scholar]
- 17. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 18. McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005; 85: 943–978. [DOI] [PubMed] [Google Scholar]
- 19. Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol 1985; 101: 2023–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci 2009; 122: 4267–4276. [DOI] [PubMed] [Google Scholar]
- 21. McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays 1997; 19: 819–826. [DOI] [PubMed] [Google Scholar]
- 22. Liu Q, Song B. Electric field regulated signaling pathways. Int J Biochem Cell Biol 2014; 55: 264–268. [DOI] [PubMed] [Google Scholar]
- 23. Naclerio F, Seijo M, Karsten B, Brooker G, Carbone L, Thirkell J, Larumbe‐Zabala E. Effectiveness of combining microcurrent with resistance training in trained males. Eur J Appl Physiol 2019; 119: 2641–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abraham WT, Kuck K‐H, Goldsmith RL, Lindenfeld JA, Reddy VY, Carson PE, Mann DL, Saville B, Parise H, Chan R, Wiegn P, Hastings JL, Kaplan AJ, Edelmann F, Luthje L, Kahwash R, Tomassoni GF, Gutterman DD, Stagg A, Burkhoff D, Hasenfuß G. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail 2018; 6: 874–883. [DOI] [PubMed] [Google Scholar]
- 25. Mentz RJ, Butler J. Cardiac contractility modulation: the next cardiac resynchronization therapy or another renal sympathetic denervation? J Card Fail 2015; 21: 24–26. [DOI] [PubMed] [Google Scholar]
- 26. Müller D, Remppis A, Schauerte P, Schmidt‐Schweda S, Burkhoff D, Rousso B, Gutterman D, Senges J, Hindricks G, Kuck KH. Clinical effects of long‐term cardiac contractility modulation (CCM) in subjects with heart failure caused by left ventricular systolic dysfunction. Clin Res Cardiol 2017; 106: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, Abraham WT. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2020; 76: 1–13. [DOI] [PubMed] [Google Scholar]
- 28. Borggrefe M, Mann DL. Cardiac contractility modulation in 2018. Circulation 2018; 138: 2738–2740. [DOI] [PubMed] [Google Scholar]
- 29. Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol‐3‐OH kinase‐γ and PTEN. Nature 2006; 442: 457–460. [DOI] [PubMed] [Google Scholar]
- 30. Macfelda K, Kapeller B, Holly A, Podesser BK, Losert U, Brandes K, Goettel P, Mueller J. Bioelectrical signals improve cardiac function and modify gene expression of extracellular matrix components: bioelectrical signals and heart failure. ESC Heart Fail 2017; 4: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoare JI, Rajnicek AM, McCaig CD, Barker RN, Wilson HM. Electric fields are novel determinants of human macrophage functions. J Leukoc Biol 2016; 99: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 32. Arnold CE, Rajnicek AM, Hoare JI, Pokharel SM, Mccaig CD, Barker RN, Wilson HM. Physiological strength electric fields modulate human T cell activation and polarisation. Sci Rep 2019; 9: 17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, Butcher EC. Lymphocyte electrotaxis in vitro and in vivo. J Immunol 2008; 181: 2465–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart. J Am Coll Cardiol 2012; 60: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simpson LJ, Reader JS, Tzima E. Mechanical regulation of protein translation in the cardiovascular system. Front Cell Dev Biol 2020; 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hedhli N, Pelat M, Depre C. Protein turnover in cardiac cell growth and survival. Cardiovasc Res 2005; 68: 186–196. [DOI] [PubMed] [Google Scholar]
- 37. Jeserich M, Föll D, Olschewski M, Kimmel S, Friedrich MG, Bode C, Geibel A. Evidence of myocardial edema in patients with nonischemic dilated cardiomyopathy. Clin Cardiol 2012; 35: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dongaonkar RM, Stewart RH, Geissler HJ, Laine GA. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc Res 2010; 87: 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciutac AM, Dawson D. The role of inflammation in stress cardiomyopathy. Trends Cardiovasc Med. 2020; S1050–1738(20)30042–6. [DOI] [PubMed] [Google Scholar]
- 40. Gotloib L, Shostak A, Galdi P, Jaichenko J, Fudin R. Loss of microvascular negative charges accompanied by interstitial edema in septic rats' heart. Circ Shock 1992; 36: 45–56. [PubMed] [Google Scholar]
- 41. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006; 86: 279–367. [DOI] [PubMed] [Google Scholar]
- 42. Wiley D, Fimbres Weihs G. Electroosmosis. In: Drioli E., Giorno L. (eds). Encyclopedia of Membranes. Springer Berlin Heidelberg, Berlin, Heidelberg. 2015. 10.1007/978-3-642-40872-4_2079-1 [DOI] [Google Scholar]
- 43. Pietak A, Levin M. Exploring instructive physiological signaling with the bioelectric tissue simulation engine. Front. Bioeng. Biotechnol. 2016; 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischbarg J, Hernandez JA, Rubashkin AA, Iserovich P, Cacace VI, Kusnier CF. Epithelial fluid transport is due to electro‐osmosis (80%), plus osmosis (20%). J Membr Biol 2017; 250: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLaughlin S, Poo MM. The role of electro‐osmosis in the electric‐field‐induced movement of charged macromolecules on the surfaces of cells. Biophys J 1981; 34: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andreev VP. Cytoplasmic electric fields and electroosmosis: possible solution for the paradoxes of the intracellular transport of biomolecules. PLoS One 2013; 8: e61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G‐R, Zhang M, Satin LS, Baumgarten CM. Biphasic effects of cell volume on excitation‐contraction coupling in rabbit ventricular myocytes. Am J Physiol‐Heart Circ Physiol 2002; 282: H1270–H1277. [DOI] [PubMed] [Google Scholar]
- 48. Desai KV, Laine GA, Stewart RH, Cox CS Jr, Quick CM, Allen SJ, Fischer UM. Mechanics of the left ventricular myocardial interstitium: effects of acute and chronic myocardial edema. Am J Physiol‐Heart Circ Physiol 2008; 294: H2428–H2434. [DOI] [PubMed] [Google Scholar]
- 49. Wilcox J, Yancy CW. Stopping medication for heart failure with improved ejection fraction. Lancet 2019; 393: 8–10. [DOI] [PubMed] [Google Scholar]


