Abstract
Mimicking the dynamics of mineral loss and gain involved in dental caries formation can help us evaluate and compare the mineralization efficacy of different treatment agents used in enamel remineralization. Here, we offer an abridged study design outlining the preparation of tooth samples, creation of artificial dental lesions, application of a peptide, and characterization of the regrown enamel-like mineral layer.
Keywords: Tooth enamel, Demineralization, Remineralization, Regrowth, Artificial saliva, Apatite, Leucine-rich amelogenin peptide (LRAP)
1. Introduction
Dental caries is one of the most ubiquitous childhood disease processes whereby net mineral loss from the dental hard tissues compromises tooth structure and function [1]. Mimicking dental lesions through in vitro and in situ studies can help to improve the understanding of the dynamics of mineral loss and gain. A variety of dental lesions and pH-cycling model systems have been established to mimic and investigate the mechanistic aspects of caries formation [2]. Such studies need to be carefully designed in a clean, contamination-free environment and run for a particular duration of time to achieve the best outcomes. Current remineralizing strategies aim to revolutionize the treatment practices in operative dentistry by rebuilding superficial enamel [3, 4]. This bioinspired process is achieved through a critical understanding of the functional role of natural enamel-forming proteins (such as amelogenin) in mediating apatite mineralization in vitro [5–7]. The ultimate objective of these studies is to develop a next-generation dental material that can impede the progression of tooth decay, restore and replace lost enamel tissue, and prolong the integrity of the tooth. In this chapter we provide a simplified protocol to conduct a demineralization-remineralization experiment in evaluating the peptide-mediated remineralization of artificial dental lesions and characterizing the orientation, formation, and composition of the newly formed enamel-like apatite layer. We chose to present the case of leucine-rich amelogenin peptide (LRAP) as a model peptide for the enamel remineralization experiments [4, 8, 9].
2. Materials
2.1. For Tooth Selection, Storage, and Preparation
Sound human 3rd molars.
Ethanol, tweezers, dental probes, and scaler for cleaning molars and removing excess soft tissue.
Phosphate-buffered saline (PBS, pH 7.4) as tooth storage media plus.
0.002% sodium azide (microbial inhibitor).
Ultrasonic bath or cleaner.
400–4000 grit silicon carbide papers.
Nylon adhesive back discs.
0.25 μm diamond or colloidal silica suspension.
Water-resistant nail varnish.
2.2. For Demineralization/Remineralization Buffer and Peptide Sample Preparation
Demineralization buffer: 2 mM CaCl2·2H2O, 2 mM KH2PO4, 50 mM sodium acetate, and 0.879 mL acetic acid adjusted at pH 4.6.
pH probe.
Artificial saliva: 1.2 mM CaCl2·2H2O, 0.72 mM K2HPO4, 16 mM KCl, 0.2 mM MgCl2·6H2O, 50 mM HEPES, and 4.5 mM NH4Cl in DDW adjusted to pH 7.2.
Fluoride (1 ppm) in the form of NaF.
Millex-GV, 0.22 μm disposable filter unit.
Peptide (0.2 mg/mL) and 2% (w/v) chitosan adjusted to pH 6.5.
2.3. For Embedding Tooth Samples in Resin
Self-cure denture base clear powder (G10–0300)/liquid (G10–0136) (Pearson Dental Co.).
Cement spatula.
3. Methods
3.1. Tooth Selection and Storage
Collect sound human molars with no detectable signs of (1) caries, (2) fillings, (3) discoloration (due to fluorosis, amelogenesis imperfecta, etc.), (4) cracks/fracture, or (5) marked erosive or abrasive wear according to a protocol approved by the responsible Institutional Review Board.
Remove excess soft tissue deposits and calculus by scrupulous cleaning, scaling, and pulp extirpation (using dental probe). Rinse teeth in 70% ethanol, and place in distilled water for a 20-min sonication. Wash the cleaned tooth samples thoroughly, and store them in tooth storage media composed of diluted phosphate-buffered saline (PBS, pH 7.4) with 0.002% sodium azide used as a microbial inhibitor at 4 °C [10]. Alternatively, Hank’s Balanced Salt Solution (HBSS) can also be prepared as a storage medium [11] (see Notes 1 and 2).
3.2. Demineralized Enamel Lesion Preparation
Remove the tooth roots (decoronate), and section the remaining crown buccolingually and longitudinally into 2-mm-thick slices using a water-cooled, slow-speed diamond saw.
Polish the tooth slices with a sequential series of wet 400–4000 grit silicon carbide papers and nylon adhesive back discs with 0.25 μm diamond or colloidal silica suspension. Rinse the polished slices thoroughly with distilled water (DDW) three times, sonicate in a water bath for 5 min, rinse again, and allow to air-dry.
Paint the surfaces of the tooth samples with two layers of clear acid-resistant nail varnish, leaving only an exposed window of dimensions 3 × 2mm2. Let the samples air-dry at room temperature (RT) for 3–4 h (see Note 3).
Place the varnish-coated enamel specimens in a demineralization solution (2 mM CaCl2, 2 mM KH2PO4, 50 mM sodium acetate, 0.879 mL acetic acid) adjusted to pH 4.6 using 1 M HCl at 37 °C for 2 h (5 teeth sections/beaker in 50 mL solution).
3.2.1. Preparation of Peptide
Peptides used for enamel remineralization can be easily synthesized commercially using solid phase peptide synthesis [12]. Posttranslational modifications such as phosphorylation can be incorporated in the amino acid sequence. In the case of leucine-rich amelogenin peptide (LRAP, 59 amino acids length), a phosphoryl group is added at Ser16. Use high-performance liquid chromatography (HPLC) and mass spectrometry for peptide purification and determination. The peptides to be used should be ~> 95% pure (see Note 4).
Weigh the peptide sample and dissolve in ultrapure distilled water to yield a stock solution of 2mg/mL, and centrifuge at 10,000 rpm (8944 × g) for 5 min. Place the stock solution in a slow shaker for 4 h at 4 °C, and then divide into aliquots of 100 μL/tube.
Lyophilize the aliquots for 12 h to yield a final peptide concentration of 200 μg per tube (optimized peptide concentration).
Prior to the remineralization cycle, dissolve LRAP (200 μg) in filtered deionized water (DDW) (960 μL) at room temperature with Na2HPO4 (15 μL, 0.1 M) and CaCl2 (25 μL, 0.1 M) to yield a solution of concentration with 0.2 mg/mL LRAP. Adjust the final pH value to ~6.5–7 (close to salivary pH) with 1 M KOH (dilute to 100 mM NaOH if no buffer in peptide solution to avoid sudden changes in the pH value). Centrifuge the sample (10,000 rpm, 5 min) just prior to use.
3.2.2. Preparation of LRAP-Chitosan (CS-LRAP) Hydrogel
Peptides can also be incorporated in a chitosan-based hydrogel and applied on the dental lesions [3, 4]. To prepare chitosan stock solution (CS), dissolve 2% (w/v) chitosan (medium molecular weight, 75–85% deacetylated) in a 1% (v/v) acetic acid solution followed by stirring at 80 °C overnight.
After cooling the solution to room temperature, pass it through a 0.45 μm filter. Adjust the pH value to 6.5 by adding 1 M NaOH solution.
Add LRAP to the chitosan gel: Mix chitosan (medium molecular weight, 75–85% deacetylated, Sigma-Aldrich) solution (960 mL, 2% m/v), Na2HPO4 (15 μL, 0.1 M), CaCl2 (25 μL, 0.1 M), and LRAP (200 μg), followed by stirring at room temperature overnight. Adjust the pH value to 6.5 (~pI of chitosan) by adding 1 M NaOH solution.
3.3. Remineralization Media and Biomimetic Enamel Regrowth
Prepare artificial saliva solution (1 L) with a final concentration of 1.2 mM CaCl2·2H2O, 0.72 mM K2HPO4, 16 mM KCl, 0.2 mM MgCl2·6H2O, 50 mM HEPES, and 4.5 mM NH4Cl in DDW. Adjust to pH 7.2 using 1 M KOH. Stir the solution for 10 min to ensure all the ingredients are dissolved. Filter the stock solution (Millex-GV, 0.22 μm filter unit) three times prior to use.
- To remineralize in artificial saliva: Apply 20–30 μL of prepared protein solution or CS-LRAP hydrogel (see Subheadings 3.2.2) to each enamel window, and let it dry in the desiccator for 10 min at room temperature.
- After incubation (end of remineralization cycle), sonicate the tooth slices in a water bath for 10 min to remove any surface debris or loosely bound crystals, gently rinse with deionized water, and air-dry for further assessment using techniques such as X-ray diffraction, nanoindentation, and scanning electron microscopy. Store the remaining samples in DDW at 4 °C for future use (see Note 6).
Fig. 1.
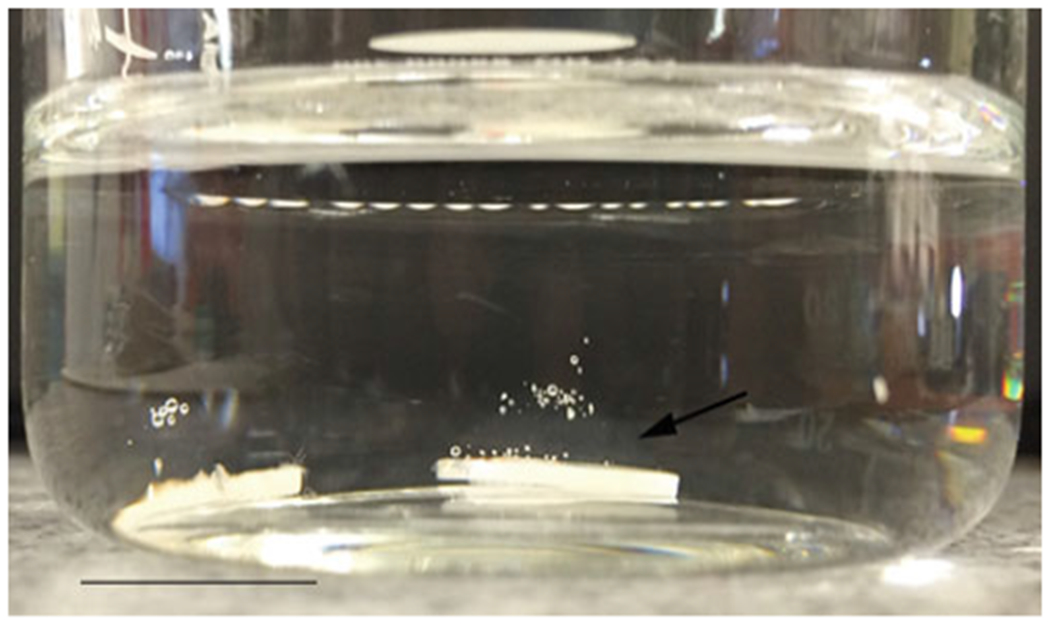
An image demonstrating LRAP-CS hydrogel-coated tooth slice after being immersed in artificial saliva. Note the gel remains adhered (arrow) to the tooth surface forming a shell-like covering
3.4. Assessment and Characterization of Biomimetic Enamel-Like Layer
3.4.1. X-Ray Diffraction (XRD)
XRD is a powerful method employed for crystallographic analysis.
For our studies, we use a diffractometer with monochromatized Cu(Kα) radiation (λ = 0.154 nm) at 40 kV and 44 mA with a sampling step size of 0.08 and 2θ range of 5–65° to analyze the crystal orientation and mineral phase of the newly formed crystals.
- To extrapolate additional details from the XRD data:
- Index the diffraction peaks referring to the standard JCDPS file (#09–0432) using MDI JADE 6.
- Use the R-value (ratio of intensities of 002 and 211) to find the orientation degree of the HAp crystals (higher value indicates preferred orientation).
The degree of crystallinity can be calculated using diffraction peaks (112) or (211) and (300) of HAp with the following equation:
where Xc is defined as the fraction of crystalline phase, I300 is the (300) diffraction peak intensity, and V112/300 is the intensity of the trough between (112) and (300) diffraction peaks of HAp. The range of 2θ where the peaks of interest fall is graphed separately and then fitted using a Gaussian/Lorentzian function.
The Debye-Scherrer equation:
where λ is the wavelength of the monochromatic X-ray beam, B is the full width at half maximum (FWHM) of the peak at the maximum intensity, θ(hkl) is the peak diffraction angle that satisfies Bragg’s law for the (hkl) plane, and t(hkl) is the crystallite size. The (002) reflection peak at 25.9 from the XRD pattern of HAp can be used to calculate the nano-HAp crystallite size.
3.4.2. Scanning Electron Microscopy
To explore the morphology of sound, demineralized, and remineralized enamel surfaces and to investigate the interface between synthetic and native enamel (on the macro-microscopic scale), scanning electron microscopy equipped with an energy-dispersive detector (EDAX) can be used at different levels of magnification:
Mount the tooth specimens on aluminum stubs with a carbon tape, sputter coat with Au/Pt for ~30 s (~5–10 nm thick coating), and observe under an accelerating voltage of 10 kV. Both top-down and side views of the sectioned tooth samples can be observed under SEM after the remineralization cycle (Fig. 2).
- To observe the cross section of the newly formed layers, the tooth slices can be embedded in resin:
- Fill a plastic mold with a thin layer of self-curing resin polymer, and moisten with a drop of the monomer (see Note 7).
- Place each tooth section parallel to the mold space to guarantee the precision of the section, and pour the resin into the remaining space using the salt and pepper method. Allow the resin to cure and harden for up to 2 h in RT.
- Extract the resin block from the mold, and make a longitudinal cut through the window using a water-cooled diamond saw advancing at low speed.
- Polish the cross sections with wet grid papers and nylon cloth (as described above) using gentle force, rinse in ethanol, sonicate for 2 min in distilled water, rinse thoroughly three times, and blast with air to dry the sample, and remove any remaining polishing suspension particles. Repeat step 1 above to image using SEM.
Simultaneously, energy-dispersive X-ray spectroscopy (EDX/EDS/EDXS) can be used for the semiquantitative elemental analysis or chemical characterization of healthy, demineralized, and remineralized tooth samples (Ca/P molar ratio and weight % of elements such as Ca, P, F, Na, Mg, O, C, etc. can be calculated) (Fig. 3a, b).
Fig. 2.
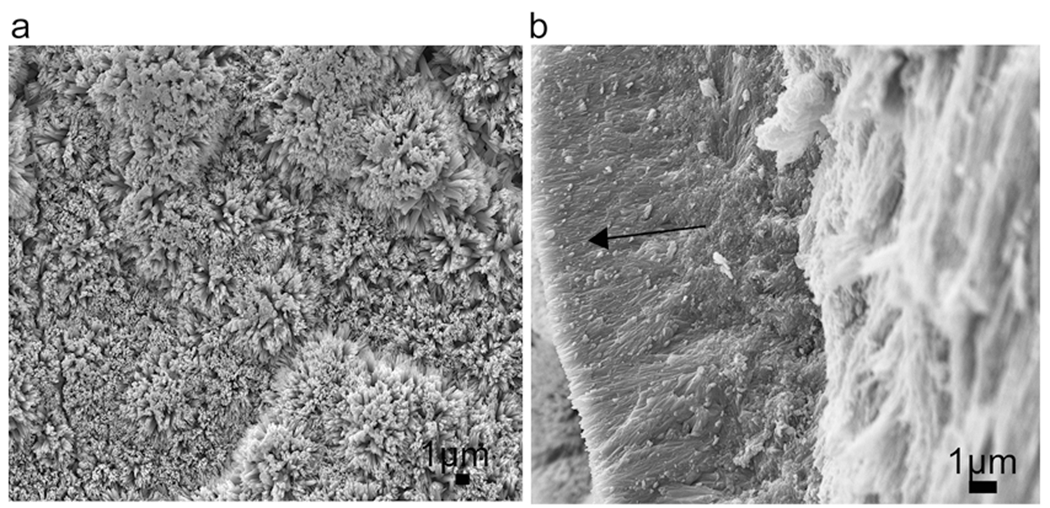
SEM images of the newly grown layer after remineralization in LRAP-CS hydrogel for 3 days (a) top view and (b) cross-sectional view. (arrow shows the newly grown layer)
Fig. 3.
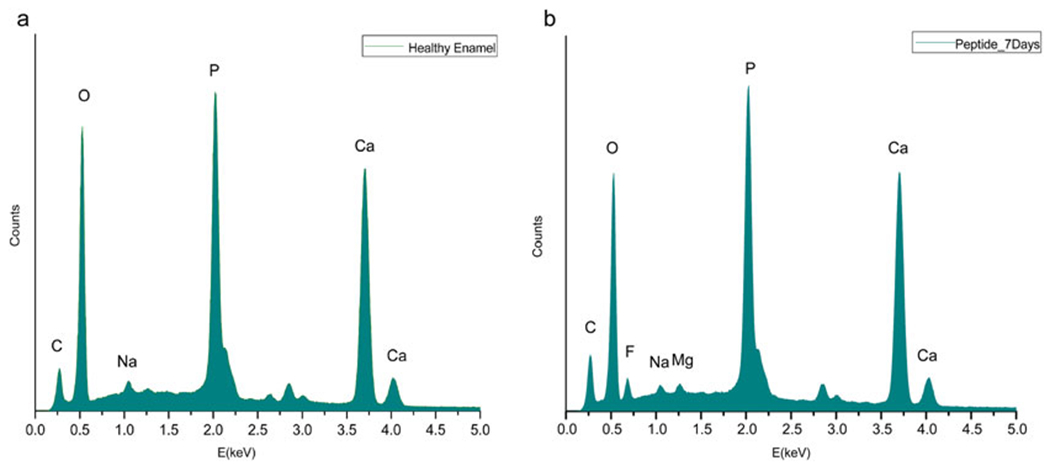
Typical EDXS curve for healthy enamel (a) and the CS-LRAP-treated enamel surface after 7 days of remineralization (b). The chemical composition of the regenerated apatite layer is similar to that of native enamel
3.4.3. Microhardness Tests
The microhardness of repaired enamel is an important indicator of the durability and strength of the regenerated apatite layer. After the remineralization cycle, rinse and sonicate the treated tooth slices for 10 min to ensure all the loosely bound crystals are effectively removed from the enamel surface. The two most routinely used surface microhardness (SMH) methods in tooth enamel studies are (1) Knoop hardness (HK) and (2) Vickers hardness (HV) tests [16]. For HK, only the longer diagonal is measured, and the hardness is calculated by dividing the projected area of indent with the applied force (kgf/mm2). For HV, both the diagonals are measured, and the average is calculated to determine the Vickers pyramid number.
Measure surface microhardness (SMH) with a hardness tester using a load force in the range 25–100 g force and 10–20s dwell time, both before and after the remineralization cycle (see Note 8). For each sample, make 6–10 indentations on the surface spaced 100 μm apart (should be ~2.5 times the indent diagonal). Following the treatment, calculate the degree of hardness recovery as %SMHR = 100 × (SMH2−SMH1)/(SMH0−SMH1), comparing with the microhardness of healthy and demineralized enamel. Here, SMH0 is the surface hardness at baseline (healthy enamel); SMH1 is the surface hardness of the acid-treated demineralized lesion, and SMH2 is the surface hardness after treatment with peptide in artificial saliva. Use the same calibrated machine for before and after treatment measurements (see Note 9). Calculate the average microhardness value for at least five specimens per sample group, and compare the differences in the HK or HV using two-way ANOVA followed by a Tukey test. For thin remineralized coatings, nanoindentation is a more accurate method of assessing changes in the mechanical properties.
Nanoindentation measurement: The nanoindentation technique may be performed to study the mechanical behavior and reliability of dental enamel more accurately. Mount and stabilize the samples on acrylic slabs [17]. Use a Berkovich diamond indentation tip (with a curvature less than 100 nm) to make indents on the sample surface (25 indents/tooth section). A continuous stiffness measurement (CSM) is used to measure the hardness (strength) and the elastic modulus (stiffness) of the regrown apatite layer. Set the following parameters under CSM mode: a target constant strain-rate (CSR) of 0.05s−1, measuring depth range 500nm–1000nm (1000nm depth used in our experiments), and keep the distance between the indents to 100μm to prevent interferences (see Note 10).
Acknowledgments
This research was supported by NIH-NIDCR R01 grants DE-13414 and DE-020099 and the USC Coulter Translational Partnership Program.
4 Notes
Take care to maintain a clean, sterile working environment, exercise lab safety measures, and wear personal protective equipment (mask, lab coat, safety glasses).
Replenish the tooth storage media every 1–2 months as prolonged storage (~12 months) may significantly decrease the microhardness of dental tissues [18]. The significant effect of fluoride (1 ppm) in promoting enamel remineralization is important [19]. Hence, studies adding fluoride to the peptide solution for in vitro enamel remineralization should have a control to demonstrate the effect of fluoride only and peptide-fluoride combination.
The enamel window should be made on the same location for all the tooth specimens (e.g., buccal cusp tips of molars away from the DEJ).
LRAP can be replaced by full-length amelogenin or other peptides for enamel mineralization studies in vitro [3, 20–21].
The number of treatment applications and the duration of the remineralization can be tailored to suit the individual aim and requirement of the experiment. Ensure the inclusion of a validated positive control in all such experiments.
A range of caries remineralization models can be developed to investigate different aspects of the caries process (such as non-cavitated lesions, root caries, role of biofilm, etc.) [22].
Because the resin is a skin irritant, do not handle it with bare hands, avoid skin contact, and wear protective eyeglasses.
The indentation load for the microhardness test can be performed using 1–1000 g and with various loading times. The preferred range has been mentioned in the text. However, the microhardness results on enamel may not be constant at very low loads [16].
In addition to microhardness tests, scratch test may be performed under standard conditions (loading rate 50 N/min and scratching speed 3 mm/min) to demonstrate the adhesion between synthetic and native enamel structure [23].
The penetration depth of the indenter tip may be adjusted depending on the thickness of the regrown layer. For thin remineralized coatings, the depth should be ≤600 nm to minimize the substrate effects arising from the underlying sound enamel. Increasing the penetration depth range on healthy enamel surface from 100 to 2000 nm may drastically decrease the hardness and modulus by almost 30% [24].
References
- 1.Selwitz RH, Ismail AI, Pitts NB (2007) Dental caries. Lancet 369(9555):51–59 [DOI] [PubMed] [Google Scholar]
- 2.Buzalaf MA, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem AC (2010) pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci 18(4):316–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Zhang Y, Yang X, Nutt S, Moradian-Oldak J (2013) An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater 9(7):7289–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee K, Ruan Q, Liberman D, White SN, Moradian-Oldak J (2016) 2016. Repairing human tooth enamel with leucine-rich amelogenin peptide—chitosan hydrogel. J Mater Res 31(5):556–563 [Google Scholar]
- 5.Ruan Q, Moradian-Oldak J (2015) Amelogenin and enamel biomimetics. J Mater Chem 3(16):3112–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beniash E, Simmer JP, Margolis HC (2005) The effect of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J Struct Biol 149(2):182–190 [DOI] [PubMed] [Google Scholar]
- 7.Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E (2011) Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc Natl Acad Sci 108(34):14097–14102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Norcy E, Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC (2011) Leucine-rich amelogenin peptides regulate mineralization in vitro. J Dent Res 90(9):1091–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafiei F, Hossein BG, Farajollahi MM, Fathollah M, Marjan B, Tahereh JK (2015) Leucine-rich amelogenin peptide (LRAP) as a surface primer for biomimetic remineralization of superficial enamel defects: An in vitro study. Scanning 37(3):179–185 [DOI] [PubMed] [Google Scholar]
- 10.Reed R, Xu C, Liu Y, Gorski JP, Wang Y, Walker MP (2015) Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch Oral Biol 60(5):690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habelitz S, Marshall GW, Balooch M, Marshall SJ (2002) Nanoindentation and storage of teeth. J Biomech 35(7):995–998 [DOI] [PubMed] [Google Scholar]
- 12.Amblard M, Fehrentz JA, Martinez J, Subra G (2006) Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol 33(3):239–254 [DOI] [PubMed] [Google Scholar]
- 13.Person A, Bocherens H, Saliège JF, Paris F, Zeitoun V, Gérard M (1995) Early diagenetic evolution of bone phosphate: an X-ray diffractometry analysis. J Archaeol Sci 22(2):211–221 [Google Scholar]
- 14.Poralan GM Jr, Gambe JE, Alcantara EM, Vequizo RM. X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. In IOP conference series: materials science and engineering 79, 1, 012028). IOP Publishing Bristol. 2015 [Google Scholar]
- 15.Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycrystalline and amorphous materials, 2nd edn. Wiley, New York-London, p 689 [Google Scholar]
- 16.Chuenarrom C, Benjakul P, Daosodsai P (2009) Effect of indentation load and time on knoop and vickers microhardness tests for enamel and dentin. Mater Res 12(4):473–476 [Google Scholar]
- 17.Chung HY, Huang KC (2013) Effects of peptide concentration on remineralization of eroded enamel. J Mech Behav Biomed Mater 28:213–221 [DOI] [PubMed] [Google Scholar]
- 18.Aydın B, Pamir T, Baltaci A, Orman MN, Turk T (2015) Effect of storage solutions on microhardness of crown enamel and dentin. Eur J Dent 9(2):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ten Cate JM, Featherstone JDB (1991) Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med 2(3):283–296 [DOI] [PubMed] [Google Scholar]
- 20.Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, Brookes SJ, Aggeli A (2007) Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res 86(5):426–430 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Lv XP, Shi W, Li JY, Li DX, Zhou XD, Zhang LL (2014) 8DSS-promoted remineralization of initial enamel caries in vitro. J Dent Res 93(5):520–524 [DOI] [PubMed] [Google Scholar]
- 22.Cochrane NJ, Zero DT, Reynolds EC (2012) Remineralization models. Adv Dent Res 24(2):129–132 [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Yang J, Li J, Chen L, Tang B, Chen X, Wu W, Li J (2013) Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 34(21):5036–5047 [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Hsiung LL (2007) Depth-dependent mechanical properties of enamel by nanoindentation. J Biomed Mater Res Part A 81(1):66–74 [DOI] [PubMed] [Google Scholar]


