Abstract
Levels of polyunsaturated phosphatidylcholine (PC) influence plasma membrane structure and function. PC is synthesized de novo in the Kennedy pathway and then undergoes extensive deacylation/reacylation remodeling via Lands’ cycle (non-Kennedy pathway). The reacylation is catalyzed by lysophosphatidylcholine acyltransferase (LPCAT). Four LPCAT isoforms are known to date, among which LPCAT3 is the major isoform in adipose tissue, but its exact role in adipogenesis is unclear. Here, we investigated whether LPCAT3 activity affects the adipogenic differentiation potential of 3T3L1 cells to uncover the underlying mechanisms in adipocyte differentiation. Lentivirus-mediated LPCAT3 shRNA expression stably knocked down LPCAT3 in 3T3L1 preadipocytes and LPCAT3 deficiency dramatically reduced the levels of cellular polyunsaturated PCs. Importantly, we found that this deficiency activated the β-catenin dependent Wnt signaling pathway, which suppressed the expression of adipogenesis-related genes, thereby inhibiting 3T3L1 preadipocyte differentiation and lipid accumulation. Moreover, three different Wnt/β-catenin pathway inhibitors reversed the effect of LPCAP3 deficiency, suggesting that Wnt/β-catenin pathway activation is one of the causes for the observed phenotypes. To the best of our knowledge, we show here for the first time that PC remodeling is an important regulator of adipocyte differentiation.
Keywords: lysophosphatidylcholine acyltransferase 3 gene knockdown, phosphatidylcholine remodeling, 3T3L1 adipocytes, Wnt/β-catenin pathway, adipogenesis, lipogenesis
Mammalian cell membrane contains different PCs and exhibit considerable structural diversity [1, 2]. Polyunsaturated PCs have kinks that prevent the molecules from packing tightly, thereby increasing fluidity. Lipid rafts are specific microdomains on the plasma membrane [3]. Caveolae, a caveolin protein-containing lipid rafts, are particularly abundant in adipocytes [4]. Despite the fact of the enrichment of cholesterol and sphingolipids on lipid rafts, various PCs are still the major lipids in raft and non-raft regions. Saturated/monounsaturated PCs are dominant in the former whereas polyunsaturated PCs are dominant in the latter [5, 6].
Cell membrane lipid composition has been related with many biological functions and diseases, including inflammation and insulin resistance [7, 8]. The composition of polyunsaturated PCs in the plasma membrane is regulated by LPCATs [9–11]. Thus, LPCAT activity is important for maintaining plasma membrane structure and function.
LPCAT3 is the major isoform of LPCAT in the small intestine and liver [12, 13]. We observed that Lpcat3 systemic, intestine-specific or liver-specific deficiency in mice affects blood lipid levels, and we attributed these changes to reduced polyunsaturated PCs on cell plasma membrane [12, 13]. Other researchers in the field also reported similar findings [14, 15]. Moreover, LPCAT3 has been directly linked to the biosynthesis of inflammatory lipid mediators in humans [16]. LPCAT3 expression was also associated with a protective effect on endoplasmic reticulum (ER) stress and inflammation in response to saturated fatty acids in vitro and in mouse models [11]. So far, no study has been reported for the function of adipose tissue LPCAT3 (also the major isoform in the tissue) and this is the focus of current study.
Adipogenesis is the process of differentiation of mesenchymal precursor cells into mature adipocytes [17, 18]. Identifying key factors that control adipocyte differentiation is important for understanding adipose tissue biology and pathology. One of the extracellular signaling pathways for affecting adipogenesis is the Wnt/β-catenin pathway [19, 20]. Wnt ligands can bind to low density lipoprotein receptor related protein (LRP) co-receptors and frizzled receptors. After such a binding, cytoplasmic β-catenin is hypophosphorylated, stabilized, and translocated into the nucleus. Then, β-catenin can bind to and co-activates members of the T cell factor/lymphoid-enhancing factor (TCF/LEF) family members to promote target gene expression [21]. Wnt/β-catenin signaling is potent inhibitors of adipogenesis, and the mechanism is related with PPARγ and C/EBPα inactivation [22].
In this study, we also found that LPCAT3 is the major isoform of LPCAT in 3T3L1 preadipocytes, and mature adipocytes, as well as mouse adipose tissue, and we found that LPCAT3 activity–mediated PC remodeling affects adipocyte polyunsaturated PC composition, most like on the cell membrane, thus influencing Wnt/β-catenin signaling pathway and inhibiting pre-adipocyte differentiation and lipogenesis.
MATERIALS AND METHODS
Regents and Cells
3T3L1 pre-adipocytes was from ATCC. Dulbecco's Modified Eagle Medium (DMEM) was from HycloneTM. Fetal bovine serum (FBS,16000-044) was from Gibco®. NBD-lyso PC and arachidonoyl CoA were from Avanti® Polar Lipids. Trizol reagent, Lipofectamine 2000 was from invitrogen (Invitrogen, USA). Puromycin, 3-isobutyl-1-methylxanthine, dexamethasone, Insulin, protease inhibitor cocktails were bought from Sigma-Aldrich®. Protein phosphatase inhibitor cocktail tablet PhosSTOP® was from Roche. Kits the determination of total cholesterol(A111-1), triglyceride(A110-2), and free fatty acid(A042-2) were purchased from Nanjing JianCheng Bioengineering Institute(Nanjing, China). Reverse transcription kit and real-time PCR reagents were from TaKaRa (Dalian, China). RIPA Lysis and Extraction Buffer (89901) were from Thermo ScientificTM. Vector plasmids for lentivirus preparation were a gift from Dr. Yonghua Yang from Fudan University. Various primary and secondary antibodies were listed as follows: rabbit anti beta-catenin antibody (ab6302,Abcam); rabbit anti-FAS antibody (ab103551, Abcam); mouse anti-CD36 antibody (ab133625, Abcam); mouse anti-DGAT1 antibody (SAB4301075, Sigma); mouse anti-HMGCoA reductase antibody (ab174830, Abcam); rabbit anti-SREBP1c antibody (SAB2102992,Sigma); rabbit anti-SREBP2 antibody (SAB2103063,Sigma); rabbit anti-C/EBPα antibody(SAB4500110,Sigma); mouse anti-PPARγ2 antibody (ab45036, Abcam); rabbit anti-phospho-LRP6 antibody (2568T,CST); rabbit anti-LRP6 antibody (3395T,CST); rabbit anti-Dvl2 antibody (3324T,CST); rabbit anti-Axin1 antibody (2087T,CST); rabbit anti-phospho-GSK-3β antibody (ab205709,Abcam); mouse anti β-actin antibody (ab8226,Abcam); rabbit anti histone 3 antibody(4499,CST); goat anti-mouse IgG (ab6789, Abcam); goat anti-rabbit IgG (ab6721, Abcam). Other reagents were all analytical grade purchased from Sinopharm Chemical Reagent Co. L.T.D. (Shanghai, China).
Wnt/ β-catenin inhibitors: IWR-1-endo (Cayman) inhibits Wnt-induced accumulation of β-catenin; PRI-724 (Selleck, USA) and ICG-001 (Selleck, USA) inhibit the recruiting of β-catenin with its coactivator element-binding protein (CBP).
Preparation of lentivirus mediated stable LPCAT3 deficient 3T3L1 preadipocyte
Plasmid pLKD-CMV-EGFP-U6-LPCAT3 shRNA targeting mouse LPCAT3 sequence GGCTTAAGGTGTACAGATC and plasmid pLKD-CMV-EGFP-U6-control shRNA targeting a scrambled sequence TTCTCCGAACGTGTCACGT were constructed firstly. VSVG and PXPAX2, two other packaging vectors, were co-transfected with targeting plasmid into 293T cells by lipofectamine 2000 in a mass ratio of 4:6:3. Culturing medium of 293T cell was collected as lentivirus source which was enriched and purified until a final LPCAT3 knockdown lentivirus titer of 3.06×108 TU/ml and control lentivirus titer of 2.83×109TU/mL was reached. 3T3L1 preadipocytes were infected by both shRNA-LPCAT3 or control lentivirus and screened under the pressure of 0.5 µg/ml puromycin. Finally, puromycin resistant stable LPCAT3 knockdown cells and control cells were collected.
Cell culture and adipocyte differentiation
Stable LPCAT3 deficiency, control or wild type 3T3-L1 pre-adipocytes were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum and 1% antibiotics until confluence and induced to differentiation. In brief, the cells are inoculated in 12 cell culture plate. Three days after inoculation, cells get to 100% confluence. After Two days' post-confluence (Day5 after inoculation), cells were exposed to differentiation medium containing 0.5 mmol/L 3-isobutyl-1-methylxanthine, 1μmol/L dexamethasone, 10 μg/mL insulin and 10% fetal bovine serum for two days. On day 7, culture medium was replaced with DMEM supplemented with 5 μg/mL insulin and 10% FBS, and cultured for two days. On day 9, the cells only cultured in DMEM medium with 10% FBS. After the differentiation process, at least 90% of the cells had accumulated lipid droplets on day 12. Cells on day 14 were regarded as fully differentiated mature adipocytes. All cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Adipocyte LPCAT activity determination
Matured 3T3L1 adipocytes (Day14) were lysed in reaction buffer (75mM Tris-HCl pH7.5, 1mg/mL BSA). A 200μL volume of enzymatic reaction system including 200μg protein and reaction buffer was started by adding 3μL NBD lyso-PC (0.5mg/mL) and 3μL arachidonoyl CoA(2.5mg/mL). The Reaction was maintained for 5 minutes at room temperature and stopped by adding 1 ml of chloroform/methanol (2:1, v/v). Phospholipids were extracted by the method of Bligh and Dyer [23]. The organic phase was air dried and separated by thin layer chromatography using chloroform/methanol/water (65:25:4) as the solvent. The Product was visualized under UV and the product density was determined by Image J software.
Determination of adipocyte triglyceride, free fatty acid, and cholesterol
Matured 3T3L1 adipocytes (Day14) were lysed by 1N sodium hydroxide. Lipids from each sample with the same amount of protein were extracted by chloroform:methanol=2:1. The organic phase was collected and dried under nitrogen. Lipids in each sample were then re-dissolved in 40μL chloroform containing 2% Triton-X100. This extractive was dried again and re-suspended in 40μL of water to achieve a final concentration of 2% triton X-100. Quantitative determination of total cholesterol, triglyceride and free fatty acids were according to the guidance of manufacturer.
Lipid droplet Oil Red-O staining and quantification
Matured 3T3L1 adipocytes (Day 14) were washed with PBS three times and then immersed in a fixative (10% formaldehyde, 1.1% CaCl2, pH7.0) for 30min. After twice wash with PBS and once balance with 60% isopropanol, cells were immersed in Oil Red-O suspension for 20 minutes. For preparation of Oil Red-O suspension, 0.25g Oil Red-O was firstly dissolved in 100mL isopropanol at 56°C for 1 hour. Undissolved particle was removed by filtration after cooling. The oil red-O suspension was prepared freshly by mixing 4mL ddH2O and 6mL filtrated oil red-O solution. After staining, unstained Oil Red-O was removed by wash. The stained cells were photographed under microscope. Size area of lipid droplets in pictures was quantified by Image Pro Plus 6.0 software.
Reverse transcription and real-time PCR
3T3L1 pre-adipocytes(3 wells from 6-well plate), matured adipocytes(3 wells from 6-well plate), and adipose tissue (1mg) were homogenized in 1 mL of Trizol reagent (invitrogen), and total RNA was extracted according to the manufacturer’s instructions. RNA was then reverse transcribed to cDNA followed by Quantitative real-time PCR performed on a Bio-Rad CFX manager version 3.1. GAPDH(glyceraldehyde-3-phosphate dehydrogenase) was used as an endogenous reference. Primer sequences for various target genes are listed as Supplemental Table 1.
Immuno-blotting
The LPCAT3 knockdown and control adipocytes were solubilized in RIPA Lysis and Extraction Buffer containing protease and phosphatase inhibitor cocktails. Protein samples (20μg) were loaded on SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose filter. Filters were blocked in 5% skimmed milk with Tris buffer saline for 1 hour and incubated at 4°C overnight with the primary antibodies. After washed by TBST for at least three times filters were again incubated with species-specific secondary antibody such as goat anti-mouse IgG or goat anti-rabbit IgG for 3 hours at room temperature. Target protein bands were revealed with enhanced chemiluminescence (ECL) substrates liquid and detected by luminescent and fluorescent biological analysis system (Furi Science & Technology Co. L.T.D, Shanghai, China) . Signal density was analyzed with Image J software.
Cell lipid measurement by LC-MS/MS
Liquid chromatography was performed using Eksigent LC100 coupled to a mass spectrometer AB SCIEX Triple TOF 5600+. lipid extract from 3T3L1 adipocytes was separated on chromatography by a pre-column of Phenomenex C18, 4×20mm and a column of Waters XBridge Peptide BEH C18 3.5μm, 2.1×100mm by a gradient elution with mobile phase A(10 mM ammonium acetate +0.1% formic acid water) and B(10 mM ammonium acetate + 0.1%formic acid + 49.95%acetonitrile + 49.95%isopropanol). Lipids were identified by mass spectrometer in the positive ion mode. Four lipids of 19:0 Lyso PC,19:0 PC,17:0 PE, and 12:0 SM were loaded into cell samples before extraction as internal control. By comparing with the identical internal control, subspecies of the lipids were identified with software PeakView1.2 and further quantified with software MultiQuant2.1 to get the peak area data. The amount of certain lipid subspecies was calculated by the peak area normalized with that of the identical internal loading control.
Statistical analysis
Data were expressed as mean ± SD. Statistical analyses were performed by one-way ANOVA for independent or correlated values followed by Students’ t test. P < 0.05 was considered statistically significant. All statistical calculations were performed using Microsoft Excel 2010 (Microsoft) or GraphPad Prism6.0 (GraphPad Software Inc., La Jolla, CA)
RESULTS
Lpcat3 is the major contributor of LPCAT activity in 3T3L1 adipocyte
We measured LPCAT mRNA levels and found that LPCAT3 is the major isoform in C57BL/6 mouse adipose tissue and fully differentiated 3T3L1 adipocyte (Fig. 1A, B). When Lpcat3 is stably knocked down by lenti-virus mediated LPCAT3 shRNA in 3T3L1 preadipocytes, its mRNA level was decreased by 86% (Fig. 1B) and this could be sustained until the cells were fully differentiated (at day 14). Besides LPCAT2 (a minor LPCAT in adipocytes), Lpcat3 knockdown did not change mRNA levels of LPCAT1 and LPCAT4 (Fig. 1B). We then measured total LPCAT activity in 3T3L1 adipocytes and found that it decreased by 74% (Fig. 1C). These results indicated that LPCAT3 is the major contributor of LPCAT activity in 3T3L1 adipocyte, as well as in mouse adipose tissue.
Figure 1.

LPCAT3 is the major isoform of LPCATs in adipocytes. A) mRNA levels of LPCAT1-LPCA4 in mouse epididymal adipose tissue. B) mRNA levels of LPCAT1-LPCA4 in 3T3L1 adipocytes with or without gene knockdown. C) Total LPCAT activity in Lpcat3 knockdown 3T3L1 adipocytes and controls. Values are mean ± SD, n = 5, *P < 0.01.
Previously, we found that Lpcat3-deficiency mediated reduction of polyunsaturated PCs on the plasma membrane of enterocytes and hepatocytes, where LPCAT3 is the major LPCAT too, has important impact on small intestine and liver function [12, 13]. Thus, we next sought to measure cellular lipids by LC-MS/MS in the knockdown and control cells, and we found that polyunsaturated PCs, such as 18:2, 20:4 and 22:6 on the Sn-2 position were significantly decreased (Table 1). In phosphatidylethanolamines (PEs), majority of the subspecies that contains 20:4 or 22:6 on Sn-2 position were significantly decreased, while most of the PEs that contains saturated, monounsaturated acyl group on Sn-2 position were significantly increased (Table 2). The results suggested that, like in small intestine and liver, Lpcat3 deficiency decreases polyunsaturated phospholipid levels which may have impact on adipocyte biology, such as preadipocyte differentiation and lipogenesis.
Table 1.
PC species measured by LC/MS/MS
| Control-shRNA | LPCAT3-shRNA | P value | increase | |
|---|---|---|---|---|
| (virtual unit/mg protein) | ||||
| 16:0* | 22026±1350 | 18411±3758 | NS | |
| 18:0* | 338±65 | 335±47 | NS | |
| 18:2* | 575±103 | 150±32 | <0.001 | −74.1% |
| 20:0* | 24±8 | 29±7 | NS | |
| 22:0* | 13±9 | 6±4 | NS | |
| 16:0-18:1 | 81342±10834 | 65953±18134 | NS | |
| 18:0-20:4 | 21285±2835 | 7922±2265 | <0.001 | −62.8% |
| 18:0-22:6 | 778±98 | 350±53 | <0.01 | −64.3% |
| 18:1-14:0 | 23936±3601 | 25556±6492 | NS | |
| 18:1-18:0 | 38613±5326 | 44148±10038 | NS | |
The amount of PCs was calculated by the Peak Area normalized with that of the internal loading control 19:0 PC.
There PCs share the same acyl group in the sn-1 and sn-2 position. Value, mean±SD, n=6. NS, not significant.
Table 2.
PE species measured by LC/MS/MS
| Control-shRNA | LPCAT3-shRNA | P value | increase | |
|---|---|---|---|---|
| (virtual unit/mg protein) | ||||
| 16:0* | 22±6 | 44±19 | NS | |
| 18:0* | 295±54 | 437±124 | <0.05 | 48% |
| 16:0–18:1 | 2956±613 | 3379±618 | NS | |
| 16:0–18:2 | 650±151 | 975±209 | <0.05 | 50% |
| 16:0–20:4 | 713±171 | 270±53 | <0.001 | −63% |
| 16:0–22:6 | 1481±287 | 259±56 | <0.001 | −82% |
| 18:0–18:1 | 3880±879 | 6269±1342 | <0.01 | 62% |
| 18:0–20:4 | 27±7 | 31±23 | NS | |
| 18:0–22:6 | 688±165 | 224±51 | <0.01 | −67% |
The amount of PEs were calculated by the Peak Area normalized with that of the internal loading control 19:0 PC.
There PCs share the same acyl group in the sn-1 and sn-2 position. Value, mean±SD, n=6. NS, not significant.
Lpcat3 knockdown impairs 3T3L1 preadipocyte adipogenesis
We differentiated Lpcat3 knockdown 3T3L1 preadipocyte and, at day 14, we measured the levels of triglyceride, free fatty acid (FFA), and cholesterol in the cells and found that all these lipids were significantly decreased as compared with the controls, with triglyceride 60%, FFA 68%, and cholesterol 50%, respectively (Fig. 2A). We then stained the lipid droplets (LD) in the adipocytes with Oil Red-O and found that the LD area per microscope vision in the knockdown adipocytes decreased by 58% (Fig. 2B). These results suggested that LPCAT3 deficiency reduces 3T3L1 preadipocyte differentiation and lipid accumulation.
Figure 2.

LPCAT3 KD reduces lipid accumulation in 3T3 L1 adipocytes. A) Adipocyte triglyceride, free fatty acid (FFA), and cholesterol measurement. B) Adipocyte lipid staining with Oil Red O and quantification of lipid droplet (LD). Values are mean ± SD, n = 6, *P < 0.001.
Lpcat3 knockdown diminishes the expression of genes which are involved in lipogenesis
We next sought to investigate the expression of lipogenesis related genes in the fully differentiated Lpcat3 knockdown and control adipocytes. Besides diacylglycerol O-acyltransferase 1 (DGAT1), LPCAT3 deficiency reduced the expression levels of genes involved in lipid synthesis and transportation, such as fatty acid synthase (FAS), Stearoyl-CoA desaturase-1 (SCD1), CD36, and fatty acid transport protein 4(FATP4), HMGCoA reductase (Fig. 3A). We also measured mRNA levels of SREBP-1c, SREBP2, SREBP-1a, C/EBPα, C/EBPβ, C/EBPδ and PPARγ1 and 2, which are trans acting factors responsible for regulating lipogenesis. We found that almost all these genes were down regulated except C/EBPβ in LPCAT3 knockdown adipocytes (Fig. 3B). We further measured protein levels of these factors using immunoblot and found that besides DGAT1, FAS, HMGCoA reductase (Fig. 3C, D), SREBP-1c, SREBP2, C/EBPα, C/EBPδ and PPARγ2 (Fig. 3E, F) were significantly reduced in the knockdown cells compared with the controls.
Figure 3. LPCAT3 KD inhibits the expression of genes which are involved in lipogenesis.
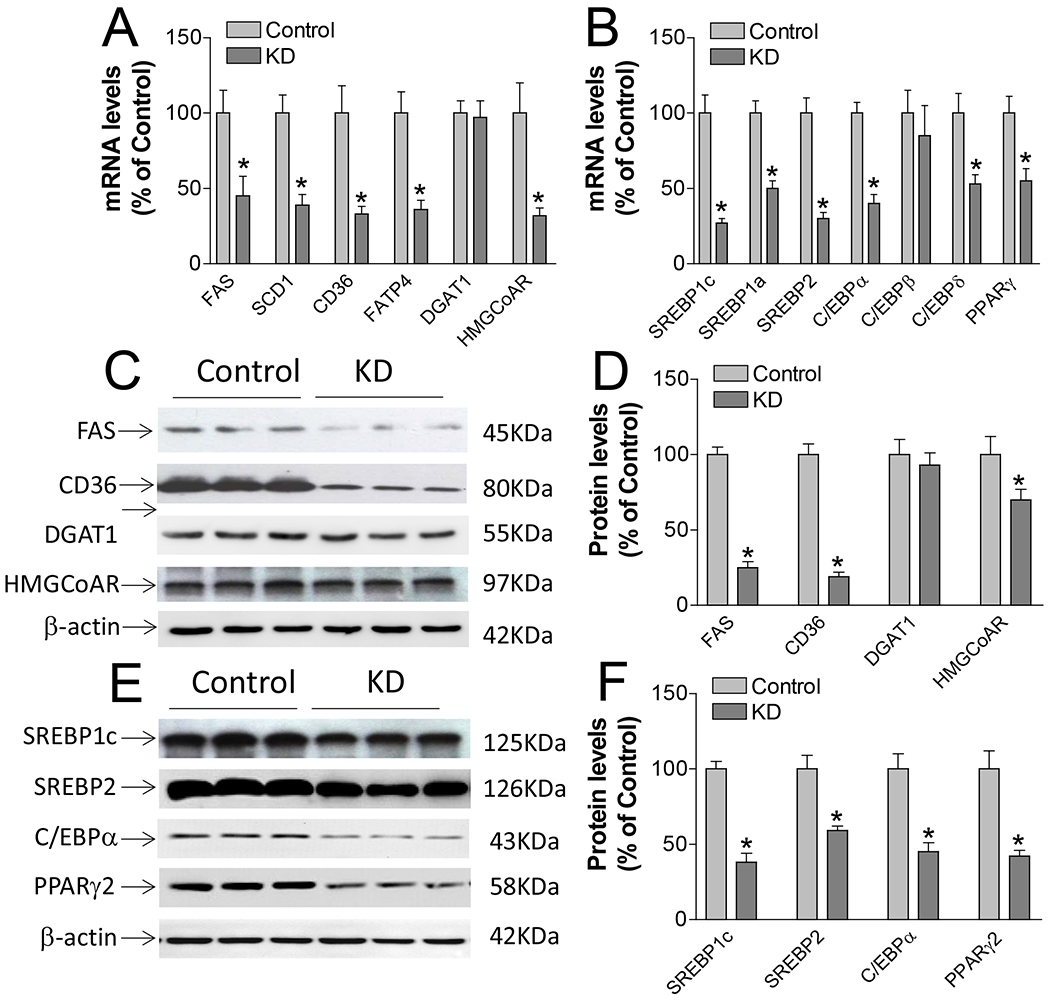
A) mRNA levels of genes which are involved in triglyceride and cholesterol biosynthesis. B) mRNA levels of genes which are involved in regulation of lipid biosynthesis. C) and D) immunoblotting of the enzymes or factors which are involved in triglyceride and cholesterol biosynthesis and quantification. E) and F) immunoblotting of the factors which are involved in regulation of lipid biosynthesis and quantification. FAS, fatty acid synthase; DGAT1, HMGCoAR, HMGCoA reductase; Peroxisome proliferator-activated receptor (PPAR); CCAAT/enhancer-binding protein (C/EBP), sterol regulatory element-binding protein (SREBP). Values are mean ± SD, n = 3, *P < 0.001.
Lpcat3 knockdown promotes active β-catenin accumulated in the nucleus
In search of the mechanism why Lpcat3 deficiency attenuates 3T3L1 preadipocyte adipogenesis, we found that total β-catenin and non-phospho β-catenin (the active form) were upregulated in cell homogenate compared with the control (Fig. 4A, B). Importantly, we found that both total β-catenin and active β-catenin were accumulated in nucleus of Lpcat3 knockdown cells, starting from Day 5 after differentiation in a differentiate-days dependent fashion (Fig. 4C, D). The enrichments of nuclear and cytosol parts were confirmed by immunoblotting of GAPDH and H3, respectively (Fig. 4E). As β-catenin was the center of the canonical Wnt signaling pathway, these results suggested that a β-catenin dependent Wnt signaling pathway could have been activated through Lpcat3 knockdown.
Figure 4.

LPCAT3 KD activates β-catenin and promotes its nucleus accumulation. LPCAT3 KD and control 3T3L1 preadipocytes were collected at different differentiation time points (day0/day5/day9/day14). A) and B) immunoblotting of β-catenin and active form of β-catenin were performed and quantified in cytosol. C) and D) immunoblotting of β-catenin and active form of β-catenin were performed and quantified in nucleus. E) immunoblotting of GAPDH and histone 3 (H3) in purified cytosol and nucleus. Values are mean ± SD, n = 3, *P < 0.01.
We then investigated the Wnt signaling pathway by checking proteins in the signaling cascade. Wnt signaling pathway is initialed by binding of Wnt ligands to frizzled receptors and low density lipoprotein receptor related protein 6 (LRP6) co-receptors, cytoplasmic β-catenin is hypophosphorylated, stabilized, and translocated into the nucleus where it binds to transcription factors to direct target gene expression[21]. Immunoblotting results showed that the co-receptor protein LRP6, phosphorylated LRP6(p-LRP6), and Dvl2 (one of the cytoplasmic relay components) were upregulated by Lpcat3 knockdown at every stage of 3T3L1 differentiation. Furthermore, we checked Axin1 and GSK3β, two protein components in the “β-catenin destruction complex” and found that Lpcat3 knockdown caused downregulation of Axin1 at very beginning of 3T3L1 differentiation, while the level of phosphorylated GSK3β(p-GSK3β), the inactive form of this kinase, was significantly upregulated at day 9 and day 14 of the cell differentiation (Fig. 5A, B, C, D). Collectively, Lpcat3 deficiency stimulates Wnt/β-catenin pathway.
Figure 5.

LPCAT3 KD activates Wnt/β-catenin signaling pathway. LPCAT3 KD and control 3T3L1 preadipocytes were collected at different differentiation time points. A)-D) immunoblotting and quantification of factors in Wnt/β-catenin pathway at day 0, 5, 9, and 14. Values are mean ± SD, n = 3, *P < 0.01
To study whether the activation of Wnt/β-catenin is one of the causes for the reduction of adipogenesis, we treated Lpcat3 knockdown 3T3L1 pre-adipocytes with three well known Wnt/β-catenin pathway inhibitors, IWR-1-endo, PRI-724, and ICG-001. The cells were then differentiated for 9 days. We then measured the levels of triglyceride, free fatty acid and cholesterol, and found that all inhibitors could reverse the effect of Lpcat3 deficiency, i.e. reduction of lipid accumulation (Table 3).
Table 3.
The effect of Wnt/β-catenin pathway inhibitors on lipid accumulation on 3T3L1 knockdown adipocytes
| Triglyceride | Free fatty acids (nmole/mg protein) |
Cholesterol | |
|---|---|---|---|
| IWR-1-endo | |||
| Control | 190±15a | 90±20a | 86±10a |
| KD | 60±9b | 46±11b | 60±8b |
| KD Vehicle | 58±6b | 44±8b | 58±6b |
| KD (36nM) | 120±19c | 80±10a | 69±12a |
| KD (180nM) | 188±22a | 82±15a | 75±7a |
| PRI-724 | |||
| Control | 178±11a | 110±18a | 110±17a |
| KD | 72±7b | 67±14b | 72±6b |
| KD Vehicle | 66±9b | 70±19b | 75±9b |
| KD (30nM) | 156±25a | 103±15a | 93±7a |
| KD (150nM) | 169±18a | 100±10a | 99±10a |
| ICG-001 | |||
| Control | 170±20a | 99±26a | 79±9a |
| KD | 65±5b | 50±6b | 44±4b |
| KD Vehicle | 70±8b | 49±10b | 50±7b |
| KD (0.6μM) | 161±22a | 66±8c | 75±10a |
| KD (3μM) | 158±16a | 73±11c | 77±8a |
Lpcat3 knockdown 3T3L1 pre-adipocytes were treated with different concentration of Wnt/β-catenin pathway inhibitors and then underwent differentiation. At day 9, the adipocytes were collected and lipids in the cells were measured. IWR-1-endo (IC50=180nM) inhibits Wnt-induced accumulation of β-catenin; PRI-724 (IC50=150nM) and ICG-001 (IC50=3μM) inhibit the recruiting of β-catenin with its coactivator element-binding protein (CBP). Values are mean ± S.D., n = 3. Columns labeled with different lowercase letters are statistically different (p < 0.05).
In order to observe an in vivo relevance of current study, we fed C57BL/6 mice (2 months old) with a high fat diet (5286 kcal kg−1; D12331, Research Diets) for 2 weeks. We found that the high fat diet significantly increased LPCAT3 mRNA in adipose tissue (epididymal fat pad), compared with chow diet (Supplemental Fig. S1A). We also measured LPCAT3 mRNA in 10 months-old db/db mouse adipose tissue (subcutaneous fat pad) and found the levels were significantly increased, compared with that of 10 months-old control mice (Supplemental Fig. S1B).
DISCUSSION
In this study, we have demonstrated a novel and pivotal role of LPCAT3 in adipocyte PC remodeling and provide evidence that polyunsaturated phospholipids, PC in particular, are involved in Wnt/β-catenin pathway, thereby influencing pre-adipocyte differentiation and lipogenesis. Our conclusions are based on the following observations: 3T3L1 pre-adipocyte Lpcat3 knockdown leads to 1) less polyunsaturated PCs in the cells; 2) less lipid droplet formation and less lipid accumulation in the adipocytes; 3) more activation of factors in Wnt/β-catenin pathway; and 4) more suppression of Wnt/β-catenin-mediated lipogenesis. Importantly, Wnt/β-catenin pathway inhibitors could reverse lipid phenotype of LPCAP3 deficient adipocytes.
One of the key finding in current study is LPCAT3 is also a major isoform in the adipose tissue. PC is the major phospholipid of mammalian cell membranes[24], including those of adipocytes. Based on our previous work, we have already known LPCAT3, a major isoform in the small intestine and liver, plays an important role in the membrane structure and function of enterocytes and hepatocytes [12, 13]. It is very likely that LPCAT3-mediated PC remodeling (producing polyunsaturated PCs) influences adipocyte membrane integrity, thus helping adipocytes adapt to environmental changes or other physiological needs. Indeed, we found LPCAT3 is one of the key factors for 3T3L1pre-adipocyte differentiation and lipogenesis.
3T3L1 pre-adipocytes are able to differentiate into mature adipocytes in about a week with the treatment of Dexamethasone, isobutylmethylxanthin and insulin [25]. The differentiation and the establishment adipocyte phenotype are characterized by the appearance of early, intermediaten and late markers and accumulation of triglycerides [26]. The triglyceride levels are usually used as an index for the degree of adipocyte differentiation. The decreased triglyceride accumulation in LPCAT3 knockdown adipocyte compared with the controls indicates that LPCAT3 deficiency inhibited adipocyte differentiation (Fig. 2A, B). Besides triglycerides, the amount of fatty acid and cholesterol were also decreased in the LPCAT3 deficient 3T3L1 adipocytes (Fig. 2A). These results indicated that LPCAT3 deficiency induced a comprehensive reduction in lipogenesis in the differentiated adipocytes. HMGCoA reductase is the key enzyme responsible for rate limiting step of cholesterol synthesis. Down-regulation of HMGCoA reductase (Fig. 3A) could contribute to the decreased cholesterol concentration observed in LPCAT3 knockdown adipocytes (Fig. 2A).
Many factors form a network for the adipogenesis. [27]. The sterol regulatory element-binding proteins (SREBPs) are transcription factors that contribute in this network. There are two isoforms, SREBP-1 and SREBP-2 [28, 29]. SREBP1, also known as the adipocyte determination and differentiation dependent factor 1 (ADD1), is tightly related with adipocyte differentiation and cholesterol homeostasis [30]. SREBP-1a and-1c mainly stimulate fatty acid and TG synthesis by activating the expression of lipogenic genes, including acetyl-coenzyme A carboxylase (ACC) and fatty acid synthase (FAS), while SREBP2 regulates genes for cholesterol homeostasis [31]. We found that Lpcat3 deficiency reduces SREBP-1c and SREBP-2 levels, resulting less accumulation of free fatty acid, triglyceride and cholesterol in 3T3L1 adipocytes.
Lpcat3 knockdown downregulates the expression of PPARγ and C/EBP family members (Fig. 3). In mammalian cells, the peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) are the main regulators of adipogenesis and have been shown to have a broad overlap in their downstream targets [32]. PPARγ1 and PPARγ2 are two major isoforms [33] which are abundantly expressed in the adipose tissue [34]. However, evidences suggested that PPARγ2, not PPARγ1, plays the determinant role in adipogenesis [35]. There are six members in CAAT family, C/EBPα, -β, and -δ are closely related with adipogenesis [36, 37]. It has been reported that C/EBPα and PPARγ play co-operatively to induce adipocyte-specific genes that promote adipocyte maturation [38, 39].
In searching of mechanism involved in LPCAT3 deficiency-mediated adipogenesis reduction, we have another key finding of this study. We found that β-catenin is activated and accumulated in the nucleus of LPCAT3 knockdown 3T3L1 preadipocytes and this activation is sustained in all stages of the cell differentiation: growth arrest, mitotic clonal expansion, early differentiation and terminal differentiation (Fig. 4). β-catenin sits in the center of Wnt pathway [40].
Studies illustrated that fluidity is one of the major properties of the membranes [41]. Changing in membrane fluidity can influence the location and function of many embedded proteins or receptors or transporters, since lipids could form transient and stable platforms for protein activity as well as protein–protein interactions [42]. Importantly, membrane fluidity is associated with lipid composition and the increased PC fatty acid unsaturation [43]. The relationship between membrane lipids and the Wnt/β-catenin pathway has been described in different systems [44–46]. LPCAT3 deficiency may inhibit adipocyte membrane fluidity, through the reduction of polyunsaturated PCs (Table 1), and promotes Wnt/β-catenin signaling, thus inhibiting adipogenesis [22]. LPCAT3 deficiency may create bigger lipid rates (with more saturated PCs) where LRP6, a key component of the LRP6/Frizzled co-receptor group involved in canonical Wnt pathway, is located and activated [47]. Thus, in conclusion, LPCAT3 deficiency impairs adipogenesis through activation of the Wnt/β-catenin pathway.
Supplementary Material
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
This work was supported by China Natural Science Fund 31371190 (to Xian-Cheng Jiang), China Natural Science Fund 31300972(to Yue Li), and VA Merit 000900–01 (to Xian-Cheng Jiang)
Abbreviations used: LPCAT3, Lysophosphatidylcholine acyltransferase 3; PC, phosphatidylcholine; Knockdown, KD.
REFERENCES
- [1].Holub BJ, Kuksis A, Metabolism of molecular species of diacylglycerophospholipids, Adv Lipid Res, 16 (1978) 1–125. [DOI] [PubMed] [Google Scholar]
- [2].MacDonald JI, Sprecher H, Phospholipid fatty acid remodeling in mammalian cells, Biochimica et biophysica acta, 1084 (1991) 105–121. [DOI] [PubMed] [Google Scholar]
- [3].Simons K, Ikonen E, Functional rafts in cell membranes, Nature, 387 (1997) 569–572. [DOI] [PubMed] [Google Scholar]
- [4].Razani B, Woodman SE, Lisanti MP, Caveolae: from cell biology to animal physiology, Pharmacological reviews, 54 (2002) 431–467. [DOI] [PubMed] [Google Scholar]
- [5].Hattersley KJ, Hein LK, Fuller M, Lipid composition of membrane rafts, isolated with and without detergent, from the spleen of a mouse model of Gaucher disease, Biochemical and biophysical research communications, 442 (2013) 62–67. [DOI] [PubMed] [Google Scholar]
- [6].de Almeida RF, Fedorov A, Prieto M, Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts, Biophys J, 85 (2003) 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M, Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation, Cell, 147 (2011) 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF, Fatty acid synthesis configures the plasma membrane for inflammation in diabetes, Nature, 539 (2016) 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee HC, Inoue T, Imae R, Kono N, Shirae S, Matsuda S, Gengyo-Ando K, Mitani S, Arai H, Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol, Mol Biol Cell, 19 (2008) 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T, Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 2830–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, Jung ME, Ford DA, Tontonoz P, LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition, Cell metabolism, 18 (2013) 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kabir I, Li Z, Bui HH, Kuo MS, Gao G, Jiang XC, Small Intestine but Not Liver Lysophosphatidylcholine Acyltransferase 3 (Lpcat3) Deficiency Has a Dominant Effect on Plasma Lipid Metabolism, The Journal of biological chemistry, 291 (2016) 7651–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li Z, Jiang H, Ding T, Lou C, Bui HH, Kuo MS, Jiang XC, Deficiency in Lysophosphatidylcholine Acyltransferase 3 Reduces Plasma Levels of Lipids by Reducing Lipid Absorption in Mice, Gastroenterology, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, Young SG, Ford DA, Tontonoz P, Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion, eLife, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, Eto M, Tamura-Nakano M, Yanobu-Takanashi R, Mukumoto Y, Kiyonari H, Okamura T, Kita Y, Shindou H, Shimizu T, Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport, eLife, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ishibashi M, Varin A, Filomenko R, Lopez T, Athias A, Gambert P, Blache D, Thomas C, Gautier T, Lagrost L, Masson D, Liver x receptor regulates arachidonic acid distribution and eicosanoid release in human macrophages: a key role for lysophosphatidylcholine acyltransferase 3, Arteriosclerosis, thrombosis, and vascular biology, 33 (2013) 1171–1179. [DOI] [PubMed] [Google Scholar]
- [17].Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM, Transcriptional regulation of adipogenesis, Genes & development, 14 (2000) 1293–1307. [PubMed] [Google Scholar]
- [18].Rosen ED, MacDougald OA, Adipocyte differentiation from the inside out, Nature reviews. Molecular cell biology, 7 (2006) 885–896. [DOI] [PubMed] [Google Scholar]
- [19].Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA, Regulation of Wnt signaling during adipogenesis, The Journal of biological chemistry, 277 (2002) 30998–31004. [DOI] [PubMed] [Google Scholar]
- [20].Prestwich TC, Macdougald OA, Wnt/beta-catenin signaling in adipogenesis and metabolism, Current opinion in cell biology, 19 (2007) 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moon RT, Bowerman B, Boutros M, Perrimon N, The promise and perils of Wnt signaling through beta-catenin, Science, 296 (2002) 1644–1646. [DOI] [PubMed] [Google Scholar]
- [22].Kawai M, Mushiake S, Bessho K, Murakami M, Namba N, Kokubu C, Michigami T, Ozono K, Wnt/Lrp/beta-catenin signaling suppresses adipogenesis by inhibiting mutual activation of PPARgamma and C/EBPalpha, Biochemical and biophysical research communications, 363 (2007) 276–282. [DOI] [PubMed] [Google Scholar]
- [23].Bligh EG, Dyer WJ, A rapid method of total lipid extraction and purification, Can J Biochem Physiol, 37 (1959) 911–917. [DOI] [PubMed] [Google Scholar]
- [24].Vance JE, Phospholipid synthesis and transport in mammalian cells, Traffic, 16 (2015) 1–18. [DOI] [PubMed] [Google Scholar]
- [25].Scott MA, Nguyen VT, Levi B, James AW, Current methods of adipogenic differentiation of mesenchymal stem cells, Stem cells and development, 20 (2011) 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gregoire FM, Smas CM, Sul HS, Understanding adipocyte differentiation, Physiological reviews, 78 (1998) 783–809. [DOI] [PubMed] [Google Scholar]
- [27].Moseti D, Regassa A, Kim WK, Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules, International journal of molecular sciences, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS, SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene, Cell, 75 (1993) 187–197. [PubMed] [Google Scholar]
- [29].Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS, Horton JD, Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene, The Journal of clinical investigation, 100 (1997) 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim JB, Spiegelman BM, ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism, Genes & development, 10 (1996) 1096–1107. [DOI] [PubMed] [Google Scholar]
- [31].Brown MS, Goldstein JL, The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor, Cell, 89 (1997) 331–340. [DOI] [PubMed] [Google Scholar]
- [32].Lefterova MI, Lazar MA, New developments in adipogenesis, Trends in endocrinology and metabolism: TEM, 20 (2009) 107–114. [DOI] [PubMed] [Google Scholar]
- [33].Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK, Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms, Proceedings of the National Academy of Sciences of the United States of America, 92 (1995) 7921–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM, Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha, Nucleic acids research, 22 (1994) 5628–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS, PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis, Genes & development, 16 (2002) 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Birkenmeier EH, Gwynn B, Howard S, Jerry J, Gordon JI, Landschulz WH, McKnight SL, Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein, Genes & development, 3 (1989) 1146–1156. [DOI] [PubMed] [Google Scholar]
- [37].Yeh WC, Cao Z, Classon M, McKnight SL, Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins, Genes & development, 9 (1995) 168–181. [DOI] [PubMed] [Google Scholar]
- [38].Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM, Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity, Molecular cell, 3 (1999) 151–158. [DOI] [PubMed] [Google Scholar]
- [39].Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM, C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway, Genes & development, 16 (2002) 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Clevers H, Nusse R, Wnt/beta-catenin signaling and disease, Cell, 149 (2012) 1192–1205. [DOI] [PubMed] [Google Scholar]
- [41].Sousa C, Nunes C, Lucio M, Ferreira H, Lima JL, Tavares J, Cordeiro-da-Silva A, Reis S, Effect of nonsteroidal anti-inflammatory drugs on the cellular membrane fluidity, Journal of pharmaceutical sciences, 97 (2008) 3195–3206. [DOI] [PubMed] [Google Scholar]
- [42].Escriba PV, Gonzalez-Ros JM, Goni FM, Kinnunen PK, Vigh L, Sanchez-Magraner L, Fernandez AM, Busquets X, Horvath I, Barcelo-Coblijn G, Membranes: a meeting point for lipids, proteins and therapies, Journal of cellular and molecular medicine, 12 (2008) 829–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stubbs CD, Smith AD, The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function, Biochimica et biophysica acta, 779 (1984) 89–137. [DOI] [PubMed] [Google Scholar]
- [44].Kang DW, Choi KY, Min do S, Phospholipase D meets Wnt signaling: a new target for cancer therapy, Cancer research, 71 (2011) 293–297. [DOI] [PubMed] [Google Scholar]
- [45].Burkhalter RJ, Westfall SD, Liu Y, Stack MS, Lysophosphatidic Acid Initiates Epithelial to Mesenchymal Transition and Induces beta-Catenin-mediated Transcription in Epithelial Ovarian Carcinoma, The Journal of biological chemistry, 290 (2015) 22143–22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pepperl J, Reim G, Luthi U, Kaech A, Hausmann G, Basler K, Sphingolipid depletion impairs endocytic traffic and inhibits Wingless signaling, Mech Dev, 130 (2013) 493–505. [DOI] [PubMed] [Google Scholar]
- [47].Ozhan G, Sezgin E, Wehner D, Pfister AS, Kuhl SJ, Kagermeier-Schenk B, Kuhl M, Schwille P, Weidinger G, Lypd6 enhances Wnt/beta-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains, Developmental cell, 26 (2013) 331–345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


