Abstract
Purpose:
While certain clinical factors suggest a diagnosis of dissociative seizures (DS), otherwise known as functional or psychogenic nonepileptic seizures (PNES), ictal video-electroencephalography monitoring (VEM) is the gold standard for diagnosis. Diagnostic delays were associated with worse quality of life and more seizures, even after treatment. To understand why diagnoses were delayed, we evaluated which factors were associated with delay to VEM.
Methods:
Using data from 341 consecutive patients with VEM-documented dissociative seizures, we used multivariate log-normal regression with recursive feature elimination (RFE) and multiple imputation of some missing data to evaluate which of 76 clinical factors were associated with time from first dissociative seizure to VEM.
Results:
The mean delay to VEM was 8.4 years (median 3 years, IQR 1–10 years). In the RFE multivariate model, the factors associated with longer delay to VEM included more past antiseizure medications (0.19 log-years/medication, standard error (SE) 0.05), more medications for other medical conditions (0.06 log-years/medication, SE 0.03), history of physical abuse (0.75 log-years, SE 0.27), and more seizure types (0.36 log-years/type, SE 0.11). Factors associated with shorter delay included active employment or student status (−1.05 log-years, SE 0.21) and higher seizure frequency (0.14 log-years/log[seizure/month], SE 0.06).
Conclusions:
Patients with greater medical and seizure complexity had longer delays. Delays in multiple domains of healthcare can be common for victims of physical abuse. Unemployed and non-student patients may have had more barriers to access VEM. These results support earlier referral of complex cases to a comprehensive epilepsy center.
Keywords: Functional seizures; psychogenic nonepileptic seizures (PNES); psychogenic nonepileptic attack disorder (PNEA, PNEAD); healthcare disparities; diagnostic delays
1. Introduction:
Dissociative seizures (DS), also known as functional or psychogenic nonepileptic seizures, can be disabling for patients [1–3]. Patients with undiagnosed DS often receive inappropriate treatment for their seizures. This could be because, most frequently, DS are signs and symptoms of a functional neurological disorder (FND) where patients may have alteration in the function but not necessarily structure of the brain where psychological stress may be expressed through physical neurological symptoms [4, 5]. Therefore, treatment aimed to reduce epileptic abnormalities are not effective [6, 7]. Prior to diagnosis, the direct and indirect cost for patients with DS was estimated at 21,000 euros/22,500 US dollars annually due to significant disability incurred by frequent seizures, emergency department visits with risk for intubation for prolonged seizures, and ineffective treatment with anti-seizure medications (ASMs) [8–14]. Cost decreases substantially after diagnosis [15] and an early diagnosis has been associated with improved long term seizure outcomes [15–19].
Based on the importance of a prompt diagnosis, ideally documented with video-EEG monitoring (VEM) [20], we sought to understand which patient-reported factors were associated with longer and shorter delay to VEM. The delay to VEM vary substantially across sites, but have been consistent since 1991 with medians of 3 to 4 years, but averages of 4.3 years to 8.6 years (standard deviations around 8 years) [21–26]. In particular, delay to VEM appears quite different and shorter in children and adolescents, as compared to adults, with prior reported average delay of 18 months (interquartile range 0.5 to 48 months) [27]. In addition to reporting these delays, we evaluated the extent to which patients’ clinical characteristics were associated with delay. We hypothesized that delay to VEM would be associated with less frequent or less severe seizures, seizure characteristics suggestive of epilepsy, more severe comorbid medical and psychiatric disease, and lack of access to healthcare. In addition to those factors that we evaluated, we recognize that delays also are influenced by stigma against FND in patients, loved ones and providers; medically traumatic events associated with poor treatment; and providers’ awareness of or compliance with International League Against Epilepsy (ILAE) guidelines for when to refer to VEM.
2. Methods:
2.1. Patient Population
We sampled all consecutive patients with DS admitted to the UCLA adult VEM unit from January 2006 to December 2019. We excluded patients with comorbid DS and epileptic seizures (ES), as well as patients for whom delay to VEM was not available due to lack of documentation in the patient chart or unknown seizure onset by the patient and loved ones at bedside. Patients’ diagnoses met the criteria for documented DS [20] and were based on expert clinical opinion based on the available clinical history, physical exam, VEM, structural MRI and FDG-PET. While MRI and FDG-PET are not part of the criteria for documented DS, when they are available, they can further rule out localizations of epilepsy with atypical ictal behavior or seizures not readily identifiable on scalp EEG including mesial frontal lobe epilepsy. We included patients who had VEM at other centers because patients were referred for continued uncertainty expressed by the patient or treating provider. A subset of these patients contributed to our prior work on delay to VEM [6] and this manuscript includes expanded modeling of other contributing factors. Other aspects of this dataset have been published elsewhere [28–34].
Patient data were acquired though either retrospective chart review or prospective interview. Delay to VEM was calculated either based on a specific date of first seizure, a statement that seizures started a certain number of months or years ago, or age-of-onset of seizures. Delays longer than 3 years were rounded to the nearest integer year, whereas shorter delays were estimated as precisely as the information allowed. Chart review focused on the earliest, single neurologist’s note that described the patient’s seizures, comorbidities, medication history and allergies. The specific factors abstracted from the notes are listed in Supplemental Table 1 and Kerr and colleagues [28]. Prospective interview occurred during the first 48 hours of VEM and was conducted by a trained non-neurologist or one of the investigators (WTK). Formal psychiatric assessment was not routine during either outpatient or inpatient encounters; therefore, our data reflect patient-reported or neurologist-documented psychiatric comorbidities. If patients were re-admitted after an initial inconclusive initial monitoring, their diagnosis was updated but the delay to video-EEG was based on the time to first admission.
The typical time from VEM referral to VEM admission was 2 to 3 months at our center, although rarely patients had longer within-center time to VEM. These rare cases primarily involve patients who transitioned from pediatric neurology to adult neurology then subsequently pursued VEM. The VEM waitlist was based primarily upon VEM availability and patient schedule requests. Patients were not prioritized by presumptive diagnosis, insurance or other factors. The magnitude of within-center delays was small compared to pre-center delays; therefore we did not analyze or report within-center delays.
2.2. Statistical Modeling
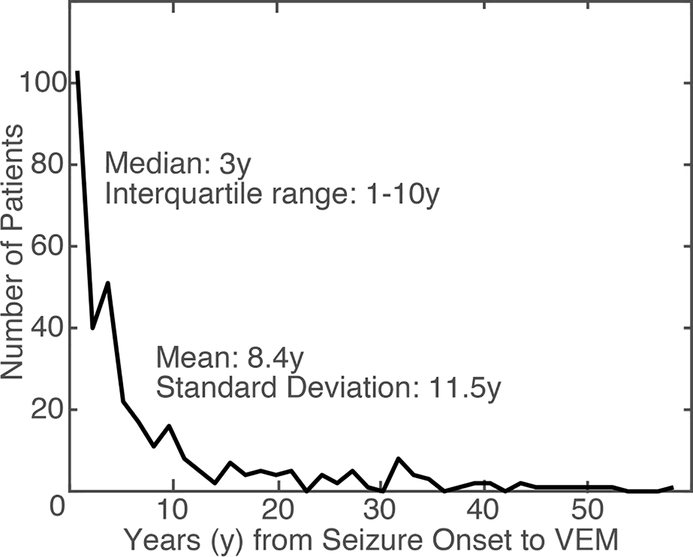
As suggested by prior studies’ standard error being equal to or higher than the mean [21–25], it was clear that delay to VEM was exponentially distributed (Figure 1), therefore we used log-linear multivariate regression to evaluate the extent to which each factor was associated with delay to VEM. The residuals of the log-normal model of delay to VEM were substantially more normal than the linear model of delay to VEM (data not shown). This multivariate model included all 76 studied factors. We log-transformed seizure frequency and seizure duration. Factors not specifically documented were assumed to be absent (e.g. history of sexual abuse). Excluding delay to VEM, missing data that had values but were not documented (e.g. seizure frequency) were imputed based on trends in the retrospective dataset using multiple imputation with 20 independent datasets [35, 36]. Rare missing data in the prospective data was filled in based on retrospective trends.
Figure 1:
Distribution of the delay to video-EEG monitoring (VEM) in our dataset.
To reduce this multivariate model to an interpretable size, we used recursive feature elimination to sequentially exclude the single factor with the largest p-value until the largest p-value was less than 5% [37]. The significance of each factor was based on a combination of the between and within-imputation variance [35, 36].
All patients consented for the use of their records in research, and the UCLA Institutional Review Board approved this study. This work is consistent with Declaration of Helsinki. De-identified raw data and code for this study is available on Mendeley Data.
3. Results:
Delay to VEM was available for 341 of 361 patients with DS that underwent VEM during the time period. The mean delay to VEM was 8.4 years with median of 3 years, minimum of 1 day and maximum of 52 years, and interquartile range of 1 to 10 years. The distribution of delay to VEM is illustrated in Figure 1. Due to the exponential pattern in delay to VEM, our regressions focused on log-delay, which had a robust average of 2.5 years (mean 0.91 log-years, standard error (SE) 2.1 log-years).
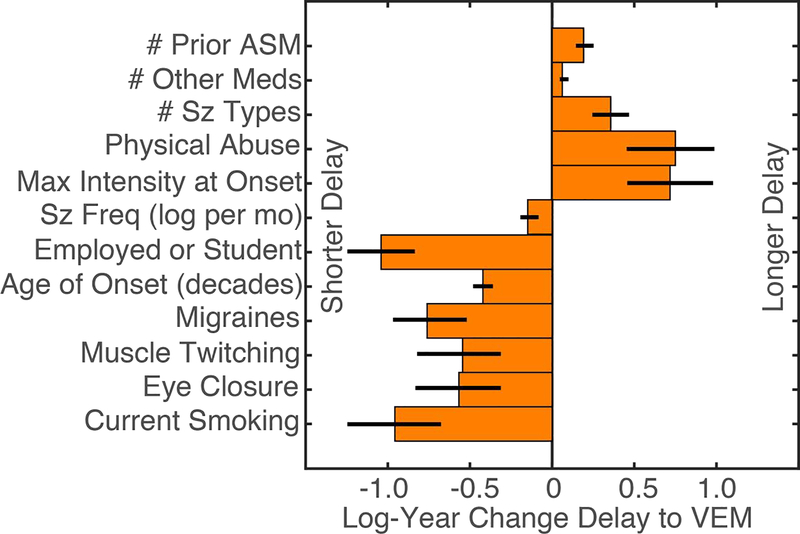
Of the 76 factors considered, there were 12 factors that contributed to the selected regression model with p less than 5% (Figure 2 and Supplemental Table 2). This selected model accounted for significantly more variation than an intercept model (Chi-squared deviance difference, 12 degrees of freedom, p=2×10−95). Controlling for the increased number of factors, the full model accounted for significantly more variation than the selected model (deviance test, 64 degrees of freedom, p=3×10−14).
Figure 2:
Significant multivariate associations between delay to video-EEG monitoring (VEM) in a recursive feature elimination (RFE) log-regression model (p<0.05). Error bars reflect Wald standard error. Abbreviations: number (#), antiseizure medications (ASM), seizure (sz), frequency (freq), month (mo).
The factors that were significantly associated with longer delay to VEM included more prior antiseizure medications (0.19 log-years per medication, standard error (SE) 0.05, p=0.0004), more medications for non-seizure and non-psychiatric conditions (0.06 log-years per medication, SE 0.03, p=0.019), history of physical abuse (0.75 log-years, SE 0.27, p=0.005), more seizure types (0.36 log-years per seizure type, SE 0.11, p=0.001), and each seizure has maximal severity at the time it occurs (0.72 log-years, SE 0.26, p=0.006).
The factors that were significantly associated with a shorter delay to VEM included migraines (−0.76 log-years, SE 0.22, p=0.0007), older age of onset (−0.04 log-years per year of onset, SE 0.006, p=3×10−12), current employment or student status (−1.05 log-years, SE=0.21, p=4×10−7), higher seizure frequency (−0.14 log-years per log-seizure/month, SE 0.06, p=0.007), description of ictal muscle twitching (−0.54 log-years, SE 0.25, p=0.03), ictal eye closure (−0.56 log-years, SE 0.26, p=0.03), and current smoking (−0.95 log-years, SE 0.29, p=0.0008).
4. Discussion:
Despite increased awareness and research about dissociative seizures, the average delay of 8.4 years and median delay of 3 years to VEM was similar to previous reports from the last 20 years across multiple countries with varying healthcare systems. Our data-driven approach identified a smaller set of factors associated with shorter and longer delay to VEM in patients with dissociative seizures. While some of these results are intuitive, other results demonstrated areas that could be targeted to improve time to accurate diagnosis, usually with VEM.
As expected, patients with a higher seizure frequency and current employment or student status had reduced delay to VEM. These early referrals can reflect higher baseline social functional status, thereby motivating clinicians to promptly refer because of a perceived ability to return the patient to work or school. In contrast, clinicians may feel less urgency when fewer seizures occur and when treating patients with lower baseline social functional status, potentially due to the perception of a poorer long-term outcome [38–41].
Alternatively, the shorter delays for patients who were employed or in school may reflect healthcare disparities. Insurance and socio-economic disparities also substantially complicate initiation of targeted psychological treatment after diagnosis, especially due to the limited availability of psychological providers familiar with dissociative seizures or functional neurological disorders [42, 43]. Even after treatment, patients with dissociative seizures who were dependent on social services had worse quality of life and seizure frequency [44]. While we did not have insurance or socio-economic data in this dataset, these findings highlight that one critical factor in diagnostic and treatment delays may be due to disparities in access to healthcare.
While the explanation for why younger patients had a shorter delay to VEM in this study and prior studies is unclear, it may reflect increased availability of family support systems for younger patients [21, 27]. Students frequently are young enough to be covered under their parents’ insurance and many universities provide on-campus access to healthcare, therefore a student status may be an indicator for access to healthcare [45, 46]. Therefore, early in a patient’s course of disease, they may have quality health insurance that later lapses due to a profound impact of the seizures on patient’s ability to work and study. Unfortunately, health insurance and socio-economic data were not available for this study to test this specific hypothesis. We emphasize that prompt referral may reduce the impact of seizures on the patient’s life and productivity.
In addition to recognizing the impact of seizures on the patient’s life, referring providers also may be accurately identifying previously established links between dissociative seizures and migraines, as well as the ictal behaviors of eye closure and muscle twitching. Therefore, our results demonstrate that referring providers may be triaging the severity of the condition appropriately and identifying some but not all important associations [28]. However, other results identify key areas that can be improved.
The International League Against Epilepsy (ILAE) recommends that patients with seizures who have not achieved seizure freedom after adequate trials of two tolerated, appropriately chosen antiseizure medications (ASM) should be referred to a comprehensive epilepsy center for presurgical evaluation because such evaluations improved quality of life, seizure frequency, and are cost-savings [47–49]. One component of a comprehensive epilepsy center evaluation is identifying patients with dissociative seizures; our group identified dissociative seizures in 6% of patients referred for “presurgical” evaluation [34]. Referral for dissociative seizures can occur prior to failure of two ASMs, but our average patient with DS was on the third ASM and 80% of patients were taking or had previously tried an ASM [28, 32]. This may differ across practice locations because, while the CODES trial did not report prior trials of ASMs, only 30% of their patients were actively taking ASMs [22].
We and others found that trials of antiseizure medications were associated with longer delays [21, 26, 50]. Medication trials may be motivated an abnormal interictal EEG, which were present in 26% of older patients with DS [51], or over-interpretation of a normal EEG [52, 53]. Reuber and colleagues also showed that delay to VEM was longer for patients with abnormalities on interictal EEG [21]. Therefore, providers and patients should recognize the cognitive bias of anchoring on a diagnosis based on an abnormal EEG, and they should not require lack of response to antiseizure medications in patients with probable dissociative seizures [7, 50, 54].
One of the counter-intuitive results in this study was the increased delay to VEM in patients taking more medications for non-seizure and non-psychiatric conditions and patients who had more types of seizures. Although increased diagnostic complexity would be thought to be associated with earlier referral for diagnostic clarification, we observed the opposite. This may be because, for patients with epilepsy, one indication for VEM is consideration of surgical intervention. Sometimes patients with multiple seizure semiologies were perceived as less likely to be candidates for focal surgical interventions. Additionally, patients with comorbid epilepsy and dissociative seizures may be inaccurately viewed as poor surgical candidates [55, 56]. Because a patient generally is presumed to have epilepsy prior to VEM, providers may delay referral for patients that were perceived as less ideal surgical candidates.
Additionally, higher numbers of medications likely reflect a higher degree of comorbidity and therefore an increased need to consider other etiologies of transient loss of consciousness including convulsive syncope; complex migraines; and, in older populations, transient ischemic attack [57, 58]. Bodde and colleagues demonstrated that patients with more medical investigations had longer delay to VEM [24]. Due to the high cost of VEM, a healthcare economics perspective might initially prioritize completion of these other outpatient and less intensive evaluations prior to VEM. However, prior evaluations of healthcare utilization suggest that VEM is cost savings for refractory seizures and, after the diagnosis of dissociative seizures, healthcare utilization substantially decreases [19, 59–62]. Therefore, while VEM is resource intensive, these results encourage early referral of these medically complex patients to VEM, as VEM can provide valuable information about the broad differential of paroxysmal events including epilepsy, dissociative seizures and other seizure-like events.
In addition to medical complexity, our results providing valuable insight into the influence of underlying trauma and psychiatric comorbidities. In this dataset, physical abuse was associated with longer delay to VEM, which was similar to prior findings that patients with a history of sexual abuse also had delayed diagnosis [27]. At least in women, overall healthcare use is higher in those with childhood sexual or physical abuse [63]. Yet, victims of physical abuse also delay seeking healthcare for specific conditions, including care for pregnancy and human immunodeficiency virus [64, 65]. Therefore, delays to VEM may reflect another specific condition for which there is lower or delayed healthcare utilization. One explanation is that whereas many general healthcare visits are self-initiated, VEM requires clinician referral; therefore, delay to VEM could reflect clinician behavior rather than patient behavior. Patients with childhood physical or sexual abuse, as well as abuse as an adult, may present with other medical and psychiatric conditions, including pain, fibromyalgia, and prior surgeries [63, 66]. The clinician may prioritize addressing these important and complex comorbidities prior to VEM. As a methodological detail, physical and sexual abuse frequently co-occur in the same patients [30, 67–70]. Our multivariate approach favors selection of a single factor that explains the variation in delay to VEM as compared to two related factors that explain the same variation, therefore we interpret our results that focus on physical abuse likely apply to sexual abuse as described elsewhere [27].
While Bodde and colleagues also demonstrated that more psychiatric treatments and psychological complaints were associated with longer delay to VEM, this was only a trend in our full multivariate model (p=0.052, Supplemental Table 2) and was not selected in our selected multivariate model [24]. While our patients receive formal neuropsychiatric evaluation after diagnosis of dissociative seizures to target treatment, our dataset includes self-reported psychiatric comorbidity as discussed in an interview similar to a neurological interview. This could account for the lower rates of depression and anxiety compared to other datasets from patients with dissociative seizures (30% versus 60% [28, 32, 71]). Therefore, this specific approach may not have been sensitive enough to replicate this prior observation. (However, when we compared factors associated with delay to VEM in patients with epilepsy to dissociative seizures, we did observe this association.)
Lastly, Asadi-Pooya and colleagues previously demonstrated that a history of head trauma was associated with a delay to VEM of more than 3 years [23]. We evaluated the potential association of delay with head injury, head injury with concussion, and head injury with post-concussive symptoms and did not replicate this association.
To facilitate future study of delay to VEM, we also highlight that delay to VEM was exponentially distributed (Figure 1). The high ratio of standard deviation to the mean and the difference between medians and means in other studies of delay to VEM suggests that this also was the case at other centers [21–24]. By modeling this variable as a log-normally distributed variable, we improved the generalizability and interpretability of our results by reducing the contribution of patients with long delays as high leverage points. Therefore, we suggest that future studies report medians, interquartile ranges, and utilize statistical models that are robust to the long right tail seen in exponential distributions.
There are a number of limitations to our data-driven approach that focused on a subset of patient-reported factors. The factors that we evaluated focused primarily on the information that providers use to differentiate dissociative and epileptic seizures and did not evaluate patient-focused factors, racial factors, social determinants of health, prior EEG results, neuroimaging findings, and reason for VEM. The racial factors and social determinants of health likely play a critical role in all aspects of access to specialized neurological care and subspecialized VEM [72]. In addition, local availability of comprehensive epilepsy centers, waiting periods, and insurance likely play a key role in access to VEM. While patients in this sample typically live as far as 300 miles away (e.g. Las Vegas, Nevada), the internal waitlist after referral to VEM is 1–2 months, a broad range of insurance is accepted, and there are multiple comprehensive epilepsy centers nearby. Therefore, this single-center evaluation does not take into account local availability of comprehensive epilepsy centers, waiting periods, and declining some types of insurance.
While the majority of patients with dissociative seizures underwent VEM for characterization of events due to suspicion for dissociative seizures or emergently for a seizure cluster, a substantial portion were admitted for pre-surgical evaluation for presumed epilepsy [34]. While the delay to VEM varied substantially based on reason for VEM, demonstrating that patients referred for characterization had shorter delays simply suggests that referring providers should improve their ability to identify dissociative seizures prior to VEM, perhaps with recently described clinical diagnostic support tools [28, 73, 74]. While our dataset is large and inclusive, the population of patients with DS is heterogeneous, therefore analysis of meaningful subgroups of patients with DS may identify additional trends. Other evaluations cited above have split patients based on ictal behavior, but we identified that patient and caregiver’s subjective descriptions of ictal behavior can be variable, so we did not conduct this extra analysis [24, 29].
While this analysis focuses specifically on delay to VEM in patients with dissociative seizures, prior to VEM, the certainty of the diagnosis of dissociative seizures was lower. Since these levels of diagnostic certainty were established, other methods have been used to risk-stratify or diagnose patients with dissociative seizures prior to inpatient VEM including, but not limited to ambulatory video-EEG, wearable activity monitors, patient-provided videos, and clinical screening scores [28, 73–78]. While none of these have been shown to be equivalent in safety and efficacy to inpatient VEM yet, they may provide alternatives when inpatient VEM is not available. Based on evaluation prior to inpatient VEM, some patients may have had a “probable” or “clinically-established” diagnosis of DS prior to VEM or were diagnosed at an outside center. These patients may have been referred to our center as a confirmation after unsuccessful initial treatment or as a second opinion, which would not represent a true delay to diagnosis. Instead, the need for further evaluation highlights that delays can occur throughout the diagnosis and treatment of dissociative seizures. There may be barriers to initiating and adhering to treatment [79, 80]. Appropriate delivery of the diagnosis of dissociative seizures and techniques like motivational interviewing can improve patient acceptance of diagnosis, as well as improve quality of life after diagnosis [79, 80].
However, there also are healthcare systems barriers to care. In the CODES trial of treatment of dissociative seizures, patients were eligible for the trial if they had been diagnosed in the last 8 weeks and an appointment for a psychiatrist was made for 3 months later, leading to a potential 5-month delay from diagnosis to initiation of treatment [81]. When the goal of diagnosis is identification within two years, such delays after diagnosis also may contribute to patient outcomes. Additionally, one of the main benefits of the CODES trial was improved access to psychological services familiar with dissociative seizures, which was difficult to obtain prior to, and after, the CODES trial [42]. Therefore, while we focus on pre-diagnosis delays, these delays should be considered in the context of the whole process of care of patients from first to last seizure.
This analysis specifically focuses on patients with dissociative seizures, but it is common for seizures to be presumed to be epileptic prior to VEM. Therefore, we hypothesize that factors that contribute to delay to VEM in patients with epilepsy also would apply to patients with dissociative seizures. Because this direct comparison provides insights relevant to the delay to VEM in epilepsy, which warrant further discussion, we perform this direct comparison between patients with epilepsy, dissociative seizures, and both in a companion manuscript in this journal.
Additionally, our data-driven approach identified factors associated with delay to VEM and therefore provides limited information about whether these factors caused the delay. To determine causation, future studies that specifically intervene on some of the factors described here are necessary to evaluate whether those interventions reduce delay to VEM. While our approach of recursive feature elimination resulted in a more interpretable model, this practice can overestimate the influence of each selected factors. Therefore, we focus on general trends and interpretation of the pattern in the model as compared to the specific effect size or p-value.
Conclusion:
This data-driven analysis highlights the association of some patient-reported factors with delay to VEM in patients with dissociative seizures. Patients with higher levels of work and academic functioning, more frequent seizures, and some, but not all, typical signs associated with dissociative seizures had shorter delay to VEM. However, longer delays were associated with more trials of antiseizure medications, physical abuse, more medications for non-seizure and non-psychiatric comorbidities, and more seizure types. Complex patients may benefit from VEM earlier and providers should refer patients with probable dissociative seizures, irrespective of response to antiseizure medications. We expect that targeted interventions to reduce delay to VEM has great potential to improve safety, quality of life, seizure frequency, and healthcare utilization.
Supplementary Material
Highlights:
The mean delay to VEM was 8.4 years (median 3 years)
Delay was associated with polytherapy and more ASM trials prior to VEM
Increased medical complexity was associated with longer delay
Access to healthcare through employment or school shortened delay
High seizure frequency was associated with shorter delay
Acknowledgements:
This work was supported by NIH R25NS065723, the UCLA-California Institute of Technology Medical Scientist Training Program (NIH T32 GM08042), the Neuroimaging Training Program (NIH T90 DA022768, & R90 DA023422), the William M. Keck Foundation, and research grants to JE (R01 NS033310 & P20 NS080181).
Footnotes
Conflicts of Interest & Ethical Publication:
Drs. Engel, Stern, and Kerr have clinical responsibilities that include the diagnosis and treatment of patients with epilepsy and non-epileptic seizures. Drs. Engel, Stern and Kerr have received speaking fees and honoraria for articles on this topic. The remaining authors have no declared conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Asadi-Pooya AA, et al. , Terminology for psychogenic nonepileptic seizures: Making the case for “functional seizures”. Epilepsy Behav, 2020. 104(Pt A): p. 106895. [DOI] [PubMed] [Google Scholar]
- 2.Kerr WT and Stern JM, We need a functioning name for PNES: Consider dissociative seizures. Epilepsy Behav, 2020. 105: p. 107002. [DOI] [PubMed] [Google Scholar]
- 3.Beghi M, Peroni F, and Cornaggia CM, Reply to: We need a functioning name for PNES: Considering dissociative seizures. Epilepsy Behav, 2020. 109: p. 107084. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson P and Looper KJ, Psychogenic nonepileptic seizures: a current overview. Epilepsia, 2012. 53(10): p. 1679–89. [DOI] [PubMed] [Google Scholar]
- 5.Chen DK, Sharma E, and LaFrance WC Jr., Psychogenic Non-Epileptic Seizures. Curr Neurol Neurosci Rep, 2017. 17(9): p. 71. [DOI] [PubMed] [Google Scholar]
- 6.Kerr WT, et al. , Diagnostic delay in psychogenic seizures and the association with anti-seizure medication trials. Seizure, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alessi R and Valente KD, Psychogenic nonepileptic seizures: should we use response to AEDS as a red flag for the diagnosis? Seizure, 2014. 23(10): p. 906–8. [DOI] [PubMed] [Google Scholar]
- 8.Begley CE, et al. , The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia, 2000. 41(3): p. 342–51. [DOI] [PubMed] [Google Scholar]
- 9.Magee JA, et al. , The economic cost of nonepileptic attack disorder in Ireland. Epilepsy Behav, 2014. 33: p. 45–8. [DOI] [PubMed] [Google Scholar]
- 10.Baroni G, et al. , Variables associated with co-existing epileptic and psychogenic nonepileptic seizures: a systematic review. Seizure, 2016. 37: p. 35–40. [DOI] [PubMed] [Google Scholar]
- 11.Dixit R, et al. , Medical comorbidities in patients with psychogenic nonepileptic spells (PNES) referred for video-EEG monitoring. Epilepsy & Behavior, 2013. 28: p. 137–140. [DOI] [PubMed] [Google Scholar]
- 12.Benbadis SR, A spell in the epilepsy clinic and a history of “chronic pain” or “fibromyalgia” independently predict a diagnosis of psychogenic seizures. Epilepsy Behav, 2005. 6(2): p. 264–5. [DOI] [PubMed] [Google Scholar]
- 13.Kapur J, et al. , Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med, 2019. 381(22): p. 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephen CD, et al. , Assessment of Emergency Department and Inpatient Use and Costs in Adult and Pediatric Functional Neurological Disorders. JAMA Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razvi S, Mulhern S, and Duncan R, Newly diagnosed psychogenic nonepileptic seizures: health care demand prior to and following diagnosis at a first seizure clinic. Epilepsy Behav, 2012. 23(1): p. 7–9. [DOI] [PubMed] [Google Scholar]
- 16.Walczak TS, et al. , Outcome after diagnosis of psychogenic nonepileptic seizures. Epilepsia, 1995. 36(11): p. 1131–7. [DOI] [PubMed] [Google Scholar]
- 17.Jones SG, et al. , Clinical characteristics and outcome in patients with psychogenic nonepileptic seizures. Psychosom Med, 2010. 72(5): p. 487–97. [DOI] [PubMed] [Google Scholar]
- 18.LaFrance WC Jr. and Benbadis SR, Avoiding the costs of unrecognized psychological nonepileptic seizures. Neurology, 2006. 66(11): p. 1620–1. [DOI] [PubMed] [Google Scholar]
- 19.Perez DL and LaFrance WC, Nonepileptic seizures: an updated review. CNS Spectr, 2016. 21(3): p. 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaFrance WC Jr., et al. , Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia, 2013. 54(11): p. 2005–18. [DOI] [PubMed] [Google Scholar]
- 21.Reuber M, et al. , Diagnostic delay in psychogenic nonepileptic seizures. Neurology, 2002. 58(3): p. 493–5. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein LH, et al. , Psychological and demographic characteristics of 368 patients with dissociative seizures: data from the CODES cohort. Psychol Med, 2020: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asadi-Pooya AA and Tinker J, Delay in diagnosis of psychogenic nonepileptic seizures in adults: A post hoc study. Epilepsy Behav, 2017. 75: p. 143–145. [DOI] [PubMed] [Google Scholar]
- 24.Bodde NM, et al. , Patients with psychogenic non-epileptic seizures referred to a tertiary epilepsy centre: patient characteristics in relation to diagnostic delay. Clin Neurol Neurosurg, 2012. 114(3): p. 217–22. [DOI] [PubMed] [Google Scholar]
- 25.LaFrance WC Jr., et al. , Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry, 2014. 71(9): p. 997–1005. [DOI] [PubMed] [Google Scholar]
- 26.Bahrami Z, Homayoun M, and Asadi-Pooya AA, Why is psychogenic nonepileptic seizure diagnosis missed? A retrospective study. Epilepsy Behav, 2019. 97: p. 135–137. [DOI] [PubMed] [Google Scholar]
- 27.Valente KD, et al. , Risk Factors for Diagnostic Delay in Psychogenic Nonepileptic Seizures Among Children and Adolescents. Pediatr Neurol, 2017. 67: p. 71–77. [DOI] [PubMed] [Google Scholar]
- 28.Kerr WT, et al. , Objective score from initial interview identifies patients with probable dissociative seizures. Epilepsy Behav, 2020. 2020(113): p. 107525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerr WT, et al. , Reliability of reported peri-ictal behavior to identify psychogenic nonepileptic seizures. Seizure, 2019. 67: p. 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr WT, et al. , An objective score to identify psychogenic seizures based on age of onset and history. Epilepsy Behav, 2018. 80: p. 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr WT, et al. , Diagnostic implications of review-of-systems questionnaires to differentiate epileptic seizures from psychogenic seizures. Epilepsy Behav, 2017. 69: p. 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr WT, et al. , Identifying psychogenic seizures through comorbidities and medication history. Epilepsia, 2017. 58(11): p. 1852–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr WT, et al. , Multimodal diagnosis of epilepsy using conditional dependence and multiple imputation. Int Workshop Pattern Recognit Neuroimaging, 2014: p. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr WT, et al. , Automated diagnosis of epilepsy using EEG power spectrum. Epilepsia, 2012. 53(11): p. e189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin DB, Multiple imputation after 18+ years (with discussion). JASA, 1996. 91: p. 473–489. [Google Scholar]
- 36.Rubin DB, Multiple imputation for non-response in surveys. 1987, New York: John Wiley & Sons. [Google Scholar]
- 37.Guyon I and Elisseeff A, An introduction to variable and feature selection. J Mach Learn Res, 2003. 3: p. 1157–1182. [Google Scholar]
- 38.Tun ZM, et al. , Characteristics of acute febrile illness and determinants of illness recovery among adults presenting to Singapore primary care clinics. BMC Infect Dis, 2016. 16(1): p. 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan R, Razvi S, and Mulhern S, Newly presenting psychogenic nonepileptic seizures: incidence, population characteristics, and early outcome from a prospective audit of a first seizure clinic. Epilepsy Behav, 2011. 20(2): p. 308–11. [DOI] [PubMed] [Google Scholar]
- 40.Wunderink A, et al. , Treatment delay and response rate in first episode psychosis. Acta Psychiatr Scand, 2006. 113(4): p. 332–9. [DOI] [PubMed] [Google Scholar]
- 41.Coste J, et al. , Prognosis and quality of life in patients with acute low back pain: insights from a comprehensive inception cohort study. Arthritis Rheum, 2004. 51(2): p. 168–76. [DOI] [PubMed] [Google Scholar]
- 42.Rawlings GH, et al. , Neurologists’ experiences of participating in the CODES study-A multicentre randomised controlled trial comparing cognitive behavioural therapy vs standardised medical care for dissociative seizures. Seizure, 2019. 71: p. 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pretorius C, Barriers and facilitators to reaching a diagnosis of PNES from the patients’ perspective: Preliminary findings. Seizure, 2016. 38: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 44.Jennum P, Ibsen R, and Kjellberg J, Welfare consequences for people diagnosed with nonepileptic seizures: A matched nationwide study in Denmark. Epilepsy Behav, 2019. 98(Pt A): p. 59–65. [DOI] [PubMed] [Google Scholar]
- 45.Griffith K, Evans L, and Bor J, The Affordable Care Act Reduced Socioeconomic Disparities In Health Care Access. Health Aff (Millwood), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhoades JA, Health Insurance Status of Young Adults, Ages 19–25, 2013, in Statistical Brief (Medical Expenditure Panel Survey (US)). 2001: Rockville (MD).
- 47.Sheikh SR, et al. , Cost-effectiveness of surgery for drug-resistant temporal lobe epilepsy in the US. Neurology, 2020. 95(10): p. e1404–e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwan P, et al. , Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia, 2010. 51(6): p. 1069–77. [DOI] [PubMed] [Google Scholar]
- 49.Engel J Jr., et al. , Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Neurology, 2003. 60(60): p. 538–547. [DOI] [PubMed] [Google Scholar]
- 50.Kerr WT, et al. , Diagnostic delay in psychogenic seizures and the association with anti-seizure medication trials. Seizure, 2016. 40: p. 123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McBride AE, Shih TT, and Hirsch LJ, Video-EEG monitoring in the elderly: a review of 94 patients. Epilepsia, 2002. 43(2): p. 165–9. [DOI] [PubMed] [Google Scholar]
- 52.Kang JY and Krauss GL, Normal Variants Are Commonly Overread as Interictal Epileptiform Abnormalities. J Clin Neurophysiol, 2019. 36(4): p. 257–263. [DOI] [PubMed] [Google Scholar]
- 53.Krauss GL, et al. , Clinical and EEG features of patients with EEG wicket rhythms misdiagnosed with epilepsy. Neurology, 2005. 64(11): p. 1879–83. [DOI] [PubMed] [Google Scholar]
- 54.Saposnik G, et al. , Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak, 2016. 16(1): p. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furlan AER, et al. , Are psychogenic nonepileptic seizures risk factors for a worse outcome in patients with refractory mesial temporal epilepsy submitted to surgery? Results of a retrospective cohort study. Epilepsy Behav, 2019. 93: p. 12–15. [DOI] [PubMed] [Google Scholar]
- 56.Basheikh M, Case report: Epilepsy surgical outcome for epileptic and non epileptic seizures with posttraumatic stress disorder and depression. Epilepsy Behav Case Rep, 2017. 8: p. 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, et al. , Value of witness observations in the differential diagnosis of transient loss of consciousness. Neurology, 2019. [DOI] [PubMed] [Google Scholar]
- 58.Wardrope A, Newberry E, and Reuber M, Diagnostic criteria to aid the differential diagnosis of patients presenting with transient loss of consciousness: A systematic review. Seizure, 2018. 61: p. 139–148. [DOI] [PubMed] [Google Scholar]
- 59.Libbon R, et al. , The feasibility of a multidisciplinary group therapy clinic for the treatment of nonepileptic seizures. Epilepsy Behav, 2019. 98(Pt A): p. 117–123. [DOI] [PubMed] [Google Scholar]
- 60.Seneviratne U, et al. , Medical health care utilization cost of patients presenting with psychogenic nonepileptic seizures. Epilepsia, 2019. 60(2): p. 349–357. [DOI] [PubMed] [Google Scholar]
- 61.Ahmedani BK, et al. , Diagnosis, costs, and utilization for psychogenic non-epileptic seizures in a US health care setting. Psychosomatics, 2013. 54(1): p. 28–34. [DOI] [PubMed] [Google Scholar]
- 62.Martin RC, et al. , Improved health care resource utilization following video-EEG-confirmed diagnosis of nonepileptic psychogenic seizures. Seizure, 1998. 7(5): p. 385–90. [DOI] [PubMed] [Google Scholar]
- 63.Bonomi AE, et al. , Health care utilization and costs associated with childhood abuse. J Gen Intern Med, 2008. 23(3): p. 294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breckenridge JP, et al. , Access and utilisation of maternity care for disabled women who experience domestic abuse: a systematic review. BMC Pregnancy Childbirth, 2014. 14: p. 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pence BW, The impact of mental health and traumatic life experiences on antiretroviral treatment outcomes for people living with HIV/AIDS. J Antimicrob Chemother, 2009. 63(4): p. 636–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finestone HM, et al. , Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse Negl, 2000. 24(4): p. 547–56. [DOI] [PubMed] [Google Scholar]
- 67.Papalia N, et al. , Patterns of Maltreatment Co-Occurrence in Incarcerated Youth in Australia. J Interpers Violence, 2020: p. 886260520958639. [DOI] [PubMed] [Google Scholar]
- 68.Paat YF, Markham C, and Peskin M, Co-occurrence of Dating Violence Victimization Subtypes: Assessing the Influence of Family Factors, Dating Attitudes, Risky Behaviors, and the Moderating Effect of Gender Among School-Aged Teens. Violence Vict, 2020. 35(4): p. 467–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziobrowski HN, et al. , Using latent class analysis to empirically classify maltreatment according to the developmental timing, duration, and co-occurrence of abuse types. Child Abuse Negl, 2020. 107: p. 104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debowska A, et al. , What do we know about child abuse and neglect patterns of co-occurrence? A systematic review of profiling studies and recommendations for future research. Child Abuse Negl, 2017. 70: p. 100–111. [DOI] [PubMed] [Google Scholar]
- 71.Kanner AM, et al. , Depression and epilepsy, pain and psychogenic non-epileptic seizures: clinical and therapeutic perspectives. Epilepsy Behav, 2012. 24(2): p. 169–81. [DOI] [PubMed] [Google Scholar]
- 72.Schiltz NK, et al. , Disparities in access to specialized epilepsy care. Epilepsy Res, 2013. 107(1–2): p. 172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trainor D, et al. , Development and validation of a screening questionnaire for psychogenic nonepileptic seizures. Epilepsy Behav, 2020. 112: p. 107482. [DOI] [PubMed] [Google Scholar]
- 74.Wardrope A, et al. , Machine learning as a diagnostic decision aid for patients with transient loss of consciousness. Neurology: Clinical Practice, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tatum WO, et al. , Assessment of the Predictive Value of Outpatient Smartphone Videos for Diagnosis of Epileptic Seizures. JAMA Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naganur VD, et al. , The utility of an automated and ambulatory device for detecting and differentiating epileptic and psychogenic non-epileptic seizures. Epilepsia Open, 2019. 4(2): p. 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kusmakar S, et al. , Automated Detection of Convulsive Seizures Using a Wearable Accelerometer Device. IEEE Trans Biomed Eng, 2019. 66(2): p. 421–432. [DOI] [PubMed] [Google Scholar]
- 78.Lawley A, et al. , The role of outpatient ambulatory electroencephalography in the diagnosis and management of adults with epilepsy or nonepileptic attack disorder: A systematic literature review. Epilepsy Behav, 2015. 53: p. 26–30. [DOI] [PubMed] [Google Scholar]
- 79.Tolchin B, et al. , Motivational Interviewing Techniques to Improve Psychotherapy Adherence and Outcomes for Patients With Psychogenic Nonepileptic Seizures. J Neuropsychiatry Clin Neurosci, 2020. 32(2): p. 125–131. [DOI] [PubMed] [Google Scholar]
- 80.Tolchin B, et al. , Randomized controlled trial of motivational interviewing for psychogenic nonepileptic seizures. Epilepsia, 2019. 60(5): p. 986–995. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein LH, et al. , Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry, 2020. 7(6): p. 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.