Abstract
Among more than 200 BAP1-mutant families affected by the “BAP1 cancer syndrome,” nearly all individuals inheriting a BAP1 mutant allele developed one or more malignancies during their lifetime, mostly uveal and cutaneous melanoma, mesothelioma, and clear-cell renal cell carcinoma. These cancer types are also those that, when they occur sporadically, are more likely to carry somatic biallelic BAP1 mutations. Mechanistic studies revealed that the tumor suppressor function of BAP1 is linked to its dual activity in the nucleus, where it is implicated in a variety of processes including DNA repair and transcription, and in the cytoplasm, where it regulates cell death and mitochondrial metabolism. BAP1 activity in tumor suppression is cell type– and context-dependent. BAP1 has emerged as a critical tumor suppressor across multiple cancer types, predisposing to tumor development when mutated in the germline as well as somatically. Moreover, BAP1 has emerged as a key regulator of gene–environment interaction.
INTRODUCTION
BAP1 is a ubiquitin carboxy-terminal hydrolase (UCH), a member of the deubiquitylase (DUB) family of proteins. BAP1 was identified and named in 1998 as a nuclear protein that supposedly bound to the RING finger domain of the BRCA1 protein (1). However, the extent and significance of this interaction has been called into question. Subsequent studies suggested that BAP1 may not bind directly to BRCA1 but to BARD1 instead, thereby indirectly influencing BRCA1 activity through disruption of the BRCA1–BARD1 complex, thus downmodulating the E3 ligase function of BRCA1 (2). However, a recent inter-actome study based on an affinity purification mass spectrometry approach identified neither BRCA1 nor BARD1 in association with BAP1 and the human polycomb complex (3). Therefore, the association of BAP1 with BRCA1 and BARD1 is currently unclear and needs to be studied in more detail.
BAP1 maps to human chromosome 3p21.3, and the encoded BAP1 protein is found both in the nucleus and in the cytoplasm (4). BAP1 has a nuclear localization signal at its carboxy-terminus; thus, truncating mutations impair BAP1 nuclear translocation. To translocate from the cytoplasm into the nucleus, BAP1 undergoes self-deubiquitylation, such that mutations in the BAP1 catalytic domain result in BAP1 being sequestered in the cytoplasm (5).
Because of the powerful tumor suppressor activity of BAP1 and of its role in modulating “gene–environment” (GxE) interactions in cancer (6), an increasing number of researchers are investigating the biological mechanisms and medical implications of inherited and acquired BAP1 mutations. This is documented by the mounting number of articles reporting novel BAP1 activities, and clinical studies related to BAP1. As a result, significant progress has been made in recent years. Key mechanisms responsible for BAP1 tumor suppressor activity and its ability to modulate GxE interactions in cancer have been elucidated (Fig. 1). In the clinic, BAP1 testing has become routine. It is an important component of the pathologic diagnosis of mesothelioma (7, 8) and early-detection clinical trials have been established at the NCI and elsewhere for carriers of germline BAP1 mutations (NCT03830229), including trials targeting BAP1 (NCT03207347, NCT03531840). Here we review current understanding of how BAP1 suppresses tumorigenesis and how BAP1 status can inform diagnosis, prognosis, targeted therapy, and cancer prevention in patients with cancer with hereditary and acquired BAP1 mutations. Moreover, we discuss some puzzling questions, such as why germline BAP1 mutations are associated with mesothelioma of low aggressiveness, but very aggressive uveal melanoma.
Figure 1.

BAP1 nuclear and cytoplasmic physiologic activities. BAP1 nuclear activities (top). BAP1 stabilizes and recruits INO80 to replication forks, via the interaction with H2A-Ub, for efficient replication fork progression and DNA replication, thereby ensuring genome stability (21, 22). Nuclear BAP1 regulates gene expression through effects on a number of epigenetic modifications and interaction with transcription factors. The interaction of BAP1–ASXL1 with HCF1–FOXK1 allows deubiquitylation and thereby stabilization of OGT, HCF1, and potentially other unknown targets to regulate transcription. The BAP1–ASXL1–HCF1–OGT complex localizes on numerous gene-regulatory elements, possibly through factors such as FOXK1, and functions as either a gene-specific activator or repressor complex on distinct genes (23–30). BAP1 suppresses tumor development by repressing SLC7A11 expression through regulation of H2A-Ub levels on the SLC7A11 promoter and inducing ferroptosis. SLC7A11 imports extracellular cystine, which is subsequently converted to cysteine in cells; cysteine is a rate-limiting precursor for glutathione (GSH) biosynthesis; GSH is used as a cofactor by glutathione peroxidase 4 to reduce lipid reactive oxygen species (ROS) to lipid alcohols; overproduction of lipid ROS in cells results in ferroptosis (34). By binding BARD1 (2), BAP1 participates in the double-strand DNA break repair process (31, 32). This RAD51-dependent DNA repair pathway is highly regulated and includes many proteins that, in addition to BARD1, may also be substrates for BAP1-mediated ubiquitin hydrolysis. Exposure to DNA-damaging agents, such as asbestos, UV light, and ionizing radiation, induces DNA damage that is rapidly repaired with the help of nuclear BAP1. BAP1 cytoplasmic activities (bottom). The integrity of the IP3R3 ER channels requires the presence of normal amounts of BAP1 that remove ubiquitin from IP3R3. The balance between ubiquitylation mediated by FBXL2 (150) and deubiquitylation mediated by BAP1 (4) maintains a proper amount of IP3R3 required for Ca2+ transfer from the ER to the mitochondria. Mitochondria need Ca2+ for oxidative phosphorylation; however, higher than physiologic Ca2+ concentrations in the mitochondria cause apoptosis, a mechanism used to eliminate cells that accumulate extensive DNA damage that cannot be repaired. This mechanism prevents cells with DNA damage from propagating, thus preventing cancer development (4). MCU, mitochondrial calcium uniporter.
THE DISCOVERY OF THE FAMILIAL BAP1 CANCER SYNDROME
Studying a devastating epidemic in three remote villages in Cappadocia, Turkey, where 50% of villagers died of mesothelioma (9), Carbone and colleagues found that susceptibility to mesothelioma was transmitted in a Mendelian fashion across multiple generations (9, 10). In Cappadocia, the population was exposed to erionite (11), a carcinogenic mineral fiber similar to asbestos (12). However, mesothelioma was detected in some families, but not in others similarly exposed to erionite fibers (9, 13). The investigators proposed that GxE interactions caused the mesothelioma epidemic among genetically predisposed families, challenging the dogma that mesothelioma was an example of a malignancy caused exclusively by exposure to carcinogenic fibers (9). In addition to families in Cappadocia, Carbone and colleagues investigated several U.S. families with multiple cases of mesothelioma, and proposed the existence of a mesothelioma-predisposing gene (9). Those were pre–next-generation sequencing (NGS) years, and the researchers used array-comparative genomic hybridization (aCGH), linkage analyses, and manual Sanger sequencing to screen germline DNA in two unrelated U.S. families (L from Louisiana and W from Wisconsin) with multiple cases of mesothelioma in which family members neither were exposed to erionite nor had occupational exposure to asbestos. However, environmental exposure to asbestos was very likely among L family members (14). The co-occurrence of uveal melanoma and mesothelioma in an “L” family member pointed the investigators in the right direction. Uveal melanoma and mesothelioma are rare cancers with an estimated probability of co-occurrence in the same individual of approximately 1/108. Both tumors have a high frequency of 3p deletions, and, by sequencing 3p, Carbone and colleagues discovered that the individuals affected by mesothelioma, uveal melanoma, or breast cancer in both families carried truncating BAP1 mutations, a condition they named the “BAP1 cancer syndrome” (6, 8, 14, 15). A parallel study by Weisner and colleagues reported that BAP1 germline mutations were causally linked to benign melanocytic tumors developing at a young age, which were initially identified as atypical Spitz tumors (16). Subsequent studies revealed that these benign melanocytic intradermal tumor nodules had unique histologic and molecular characteristics that set them apart from Spitz and other melanocytic lesions, and were thus named “MBAITs” (melanocytic BAP1-associated intradermal tumors; ref. 15). Currently, the detection of MBAITs allows dermatologists to identify potential carriers of germline BAP1 mutations, a diagnosis that is confirmed by germline DNA sequencing (8, 17, 18). Once these independent studies were published (14, 16), the authors of these articles realized that multiple individuals in mesothelioma families L and W carried MBAITs and vice versa that multiple cases of mesothelioma had occurred in the families in which MBAITs had been discovered by dermatologists, thus confirming the importance of BAP1 in these disease contexts. The obvious question was why BAP1 loss was so powerful in causing cancer, including multiple cancers in affected individuals, and why there was a clear prevalence of certain cancer types.
BAP1 BIOLOGICAL ACTIVITIES
BAP1 Nuclear Activities
In the nucleus, BAP1 binds to several proteins (Fig. 1), including HCF1, YY1, OGT, KDM1B, and FOXK1 and 2 (6). These proteins are assembled in multiprotein complexes that in turn may associate with other tissue-specific transcriptional regulators. Different nonoverlapping complexes may form according to tissue specificity, as observed in clear-cell renal cell carcinoma (ccRCC) and elsewhere (19, 20). As a result of these multiple interactions, BAP1 modulates several cellular activities.
BAP1 activity is critical for normal DNA synthesis as well as DNA replication under stress conditions. BAP1 interacts with and deubiquitylates INO80, playing a critical role in stabilizing and targeting the INO80 chromatin-remodeling complex to the replication forks (21). By recruiting INO80 to the stalled forks, BAP1 allows stress-induced stalled replication forks to restart, a mechanism that suppresses genome instability and thus cancer development (22).
BAP1 is associated with transcriptionally active chromatin and interacts with several transcription factors and cofactors, thus acting as a chromatin scaffold for chromatin-remodeling complexes (23, 24). It has been proposed that BAP1 may modulate cell proliferation by deubiquitylating the transcriptional regulator HCF1 (23, 25). BAP1 is found in a ternary complex with HCF1 and the transcription factor YY1 and activates YY1-regulated genes in a DUB-dependent manner (24). BAP1 can also be recruited to the DNA via interaction with FOXK2, which promotes local histone deubiquitylation inducing changes in target gene activity (26). Moreover, BAP1 is part of a ternary complex bridging FOXK2 and HCF1 and represses FOXK2 target genes, an effect requiring BAP1 DUB activity but not the interaction with HCF1 binding (27).
BAP1 binds ASXL1, 2, and 3, human homologs of Drosophila Polycomb group protein ASX, which is an obligate binding partner for the Drosophila BAP1 ortholog Calypso, which catalyzes the monodeubiquitylation of histone H2A at residue K119 (28). BAP1 forms two mutually exclusive complexes with the product of ASXL1, which is frequently mutated in myeloid leukemia (29), and of ASXL2. These interactions help to maintain physiologic protein levels of BAP1. Furthermore, BAP1 deubiquitylates the deubiquitylase adaptor module DEUBAD of ASXL2, leading to its stabilization (30). Therefore, cancer-associated loss of BAP1 expression results in ASXL2 destabilization, and therefore the BAP1–ASXL2 interaction may play a role that remains to be defined in more detail in tumor suppression (28).
BAP1 promotes double-strand DNA repair by homologous recombination (HR), a key process to reduce genetic damage and prevent cancer (31, 32). The involvement of BAP1 in HR may be regulated by the BAP1–BARD1 binding (2).
BAP1 Regulates Cell Death
Recent evidence revealed that in the cytoplasm BAP1 regulates cell death and mitochondrial metabolism (Fig. 1). Studying primary fibroblast cell cultures derived from skin-punch biopsies of individuals from two separate families carrying heterozygous BAP1 mutations (BAP1+/−), and wild-type BAP1 (BAP1+/+) control fibroblasts (matched for sex and age from individuals from the same families), it was found that BAP1 localizes to the endoplasmic reticulum (ER), where it deubiquitylates and thus stabilizes the type 3 inositol-1,4,5-trisphosphate receptor (IP3R3; ref. 4). IP3R3 mediates the release of Ca2+ from the ER into the cytoplasm, in proximity to the mitochondria-associated membranes. Ca2+ is driven into the mitochondrial matrix by the voltage-dependent anion channels (VDAC) and the mitochondrial uniporter channel (MUC), which are located at the outer and inner mitochondrial membranes, respectively (33). The increase in mitochondrial Ca2+ concentration triggers the release of cytochrome c that in turn activates apoptosis (33). In heterozygous BAP1+/− conditions, as in family members affected by the BAP1 cancer syndrome, the reduced BAP1 levels impair both DNA repair, making their cells accumulate more DNA damage, and the apoptotic response. This dual effect favors the accumulation of cells with higher levels of DNA damage so that over time some may become transformed and eventually give rise to cancer (4). These mechanisms were elucidated in human fibroblasts and in mesothelial cells, and it remains to be determined whether they apply to other cell types.
Very recently, studying a human cancer cell line, Zhang and colleagues discovered that BAP1 promotes ferroptosis, a nonapoptotic form of cell death that contributes to the tumor suppression function of BAP1 (34). Using chromatin immunoprecipitation sequencing (ChIP-seq) and RNA sequencing (RNA-seq), Zhang and colleagues identified an array of BAP1-targeted genes, many of them associated with metabolism. Among these genes, SLC7A11 (encoding the Solute Carrier Family 7 Member 11), an antiporter that imports cystine and exports glutamate (35), was repressed by BAP1. SCL7A11 downregulation caused a reduction of the uptake of cystine, a key metabolite for the synthesis of reduced glutathione, which in turn reduced antioxidant activity and lipid peroxidation, inducing ferroptosis. It remains to be studied whether the two distinct types of programmed cell death linked to BAP1, apoptosis and ferroptosis, are controlled in a synchronized or an independent manner (36) and whether BAP1 regulates additional mechanisms of cell death.
Although physiologic BAP1 levels are required for cells to execute apoptosis and ferroptosis, BAP1 may also exert a prosurvival role in response to metabolic stress via repression of the unfolded protein response (UPR). UPR can be induced by glucose starvation, leading to metabolic stress at the ER that, if not resolved, leads to programmed cell death. BAP1 exerts a prosurvival role by suppressing the UPR gene-regulatory network, repressing ATF3 and CHOP. The transcriptional repression of ATF3 and CHOP is dependent upon the deubiquitinylation of H2A (at K119) by BAP1 (37).
By engineering a knock-in mouse model expressing the catalytically inactive C91A Bap1 mutant, He and colleagues showed that the loss of function of BAP1 has a proapoptotic effect in mouse embryonic stem cells, fibroblasts, liver, and pancreas, but not in melanocytes and mesothelial cells, the cells that give rise to the cancer types most commonly associated with BAP1 mutations in humans (38). Studies in Xenopus revealed that BAP1 is required for the epigenetic switch from pluripotency to differentiation in the ectoderm, mesoderm, and neural crest through its regulation of epigenetic marks, such as histone 3 lysine 27 acetylation (H3K27ac). BAP1 loss causes transcriptional silencing and failure of H3K27ac to accumulate at promoters of genes regulating pluripotency-to-commitment transition (39).
These findings suggest a complex role for BAP1 in cancer that is context- and lineage-dependent and may differ among cancer types and species.
BAP1 Modulates Cellular Metabolism
Metabolomics analysis in the serum from BAP1+/− family members and in vitro validation in primary fibroblast cultures from these individuals revealed that a reduction of BAP1 protein levels shifted cell metabolism from oxidative phosphorylation (e.g., Krebs cycle) to aerobic glycolysis (e.g., Warburg effect; ref. 40). Moreover, in primary fibroblasts from individuals with heterozygous BAP1+/− mutations, aerobic glycolysis/lactate secretion were increased and mitochondrial respiration/ATP synthesis were decreased compared with fibroblasts obtained from BAP1+/+ volunteers from the same families that were age- and gender-matched (40). This effect may be linked to the reduced intramitochondrial Ca2+ required by several enzymes that regulate oxidative phosphorylation (ref. 40; Fig. 1). This evidence suggests a potential new tumor-promoting role for the Warburg effect that predates malignancy. In this scenario, BAP1-mutant cells operating in aerobic glycolysis are favored in their invasive growth into the nearby tissues even if they are in a hypoxic environment (40). Moreover, using a genetically engineered inducible Bap1 knockout murine model, it has been demonstrated that the deletion of Bap1 altered several metabolic pathways. Cholesterol biosynthesis was increased, whereas gluconeogenesis and lipid homeostasis proteins were decreased in the liver. The downregulation of mitochondrial proteins in the pancreas was accompanied by pancreatitis (41). Furthermore, through the O-GlcNAc transferase/HCF1 complex, BAP1 regulates gluconeogenesis by modulating the stability of the transcriptional coactivator PGC1α (42). Therefore, BAP1 contributes in maintaining metabolic homeostasis.
BAP1 MUTATIONS AND HUMAN CANCER
In 2010, Harbour and colleagues reported that 26 of 31 metastasizing uveal melanomas carried inactivating somatic BAP1 mutations, and one of the patients also carried a germline BAP1 mutation (43). In 2011, Carbone’s team reported that germline BAP1 mutations predisposed to mesothelioma and uveal melanoma (14), the BAP1 cancer syndrome (6). In 2012, Brugarolas and colleagues reported that 15% of ccRCCs carried somatic BAP1 mutations, and subsequently found that some patients also had inactivating germline mutations (20, 44). Since then, there has been an exponential increase in studies linking BAP1 to human cancer.
BAP1 Germline Mutations
Numerous studies have now confirmed and expanded on the direct link of BAP1 germline mutations to a cancer syndrome characterized by a predisposition to mesothelioma (45–49), uveal melanoma (43), and less frequently cutaneous melanoma (50), as well as ccRCC (20, 44, 51, 52), which are the core cancer types in the BAP1 cancer syndrome (6, 15). Although the term “mutations” has been widely used to encompass different types of genetic damage, most BAP1-mutant families carry truncating BAP1 mutations (8, 53–55). Moreover, breast cancers and basal cell carcinomas are also quite frequent and will likely be included among the “core cancers” as more data accumulate (8, 55–57). Less frequently associated cancers include cholangiocarcinoma (58), meningioma (59), and others (ref. 55; Fig. 2A). Some of the BAP1-associated cancers, such as mesotheliomas and skin melanomas, are strongly linked to environmental carcinogens (60), suggesting GxE interaction in some instances (8, 18, 55, 61).
Figure 2.
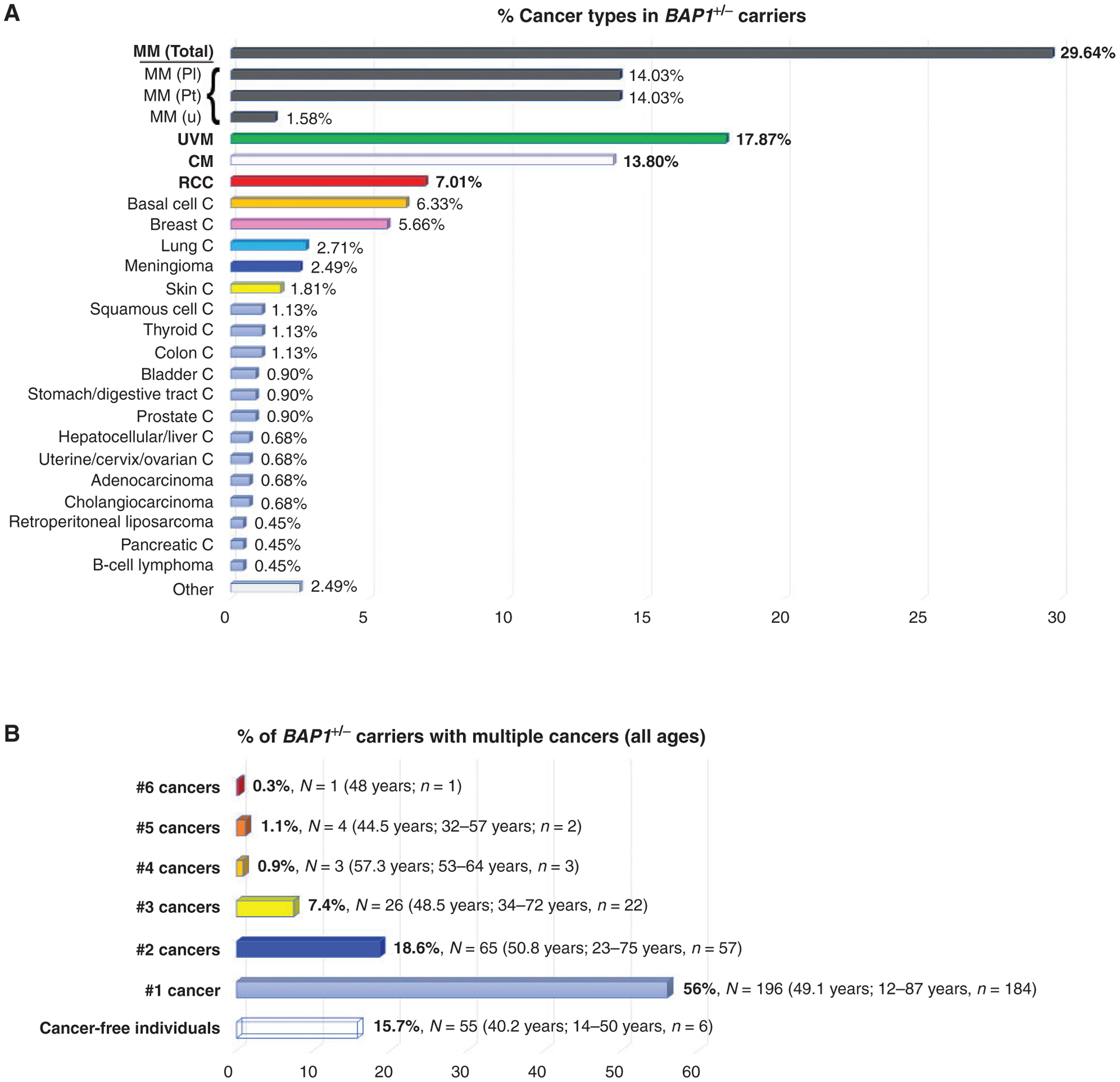
Cancer types and age of onset of tumors presenting in BAP1+/− carriers. A, Occurrence of cancer types expressed as percentage. Data were collected from 45 articles published up to September 30, 2019 (4, 14, 16, 18, 44–47, 50, 51, 53, 54, 56–58, 64, 99, 151–177), for a total of 350 BAP1+/− carriers (all ages); of them, 295 (84.3%) developed cancer, for a total of 442 different cancers (as several of them developed more than one cancer). Note that to avoid the risk of including nonpathogenic BAP1 variants, we used very stringent criteria to select these patients: Only germline BAP1 mutation carriers with a family history of BAP1-core cancers were included. Specifically, the cohort shown in this figure includes cases from 140 published families. Core cancers of the BAP1 cancer syndrome are indicated in bold. CM, cutaneous melanoma; MM, malignant mesothelioma all sites; Pl, pleural malignant mesothelioma; Pt, peritoneal malignant mesothelioma; u, malignant mesothelioma, site not specified; UVM, uveal melanoma; C, carcinoma. B, The percentage of BAP1+/− carriers of all ages with one or multiple cancers. The average age of diagnosis of the first cancer, and relative range in years, are shown in parentheses. Individuals in the group “Cancer-free individuals” are 50 years of age or younger, and thus have not reached the age when cancer has occurred in most BAP1+/−-mutant carriers. N, number of individuals; n, number of individuals whose age at diagnosis was known. Example: 7.4% of patients developed 3 different cancers (total of 26 patients). The age of onset of tumors was known for only 22 of 26 of them; the median age of first tumor onset was 48.5 years; the range of first tumor development was between 34 and 72 years old.
The hypothesis that GxE interaction increased the incidence of mesothelioma (61) was tested by exposing Bap1+/− mice to very low doses of asbestos fibers (total of 0.5 mg, compared with 3–5 mg usually used in such studies). Following this low exposure, Bap1+/− mice developed mesothelioma at a comparable rate to wild-type mice (Bap1+/+ mice) exposed to ten times higher doses of asbestos (62). Parallel studies reported evidence of GxE interaction in mice (63) and also in one BAP1-mutant family (64). Recently, Badhai and colleagues (65) reported that the combined deletion in the mesothelial cell lineage of Bap1, Nf2, and Cdkn2ab caused mesothelioma in 100% of mice. Bap1 deletion alone caused mesothelioma in 5% of unexposed mice, and combined Nf2 and Cdkn2ab deletion alone did not. In summary, inherited BAP1 mutations cause cancer in mice and in humans, and cancer incidence increases upon exposure to asbestos or other carcinogenic fibers and when other mutations are present. However, the spontaneous development of mesotheliomas in Bap1+/− mice not exposed to asbestos (65, 66), and the development of multiple cancer types in carriers of BAP1 mutations (Fig. 2B), including tumor types that have not been associated with known carcinogens (53, 67), suggests that BAP1 mutations also drive tumor growth independently of genotoxic stress, perhaps by favoring the accumulation of age-related DNA damage.
Environmental carcinogens clearly associated with uveal melanoma have not been definitively identified. However, patients with uveal melanoma with germline BAP1 mutations always have an initiating mutation in the G-alpha-q (Gq) pathway, suggesting that additional genetic variants play a critical role in the development of uveal melanoma (68).
Germline mutations are either inherited or de novo mutations. For example, among individuals affected by the Li-Fraumeni syndrome, approximately two thirds were inherited and one third were de novo mutations (69–73). Instead, among more than 200 families with multiple members carrying germline BAP1 heterozygous mutations, all mutations for which family information was available demonstrated heritability, some tracing back more than 600 years (53), suggesting a low rate of de novo germline mutations (8, 67).
In summary, BAP1 is a powerful tumor suppressor gene. Carriers of germline BAP1 mutations often develop multiple cancers during their lifetime. The overall penetrance for cancer is at least 85% and approaches 100% with increasing age (8, 54, 55). The fact that most BAP1-associated cancers arise in middle-age and older individuals, and that the penetrance for any particular cancer type is less than 100%, suggests that genomic aberrations in addition to BAP1 loss are required for cancer formation, for example, mutations in the Gq signaling pathway (68).
Somatic BAP1 Mutations
The first cancer in which somatic (i.e., acquired) BAP1 mutations were found to be common was uveal melanoma, where these mutations are present in approximately 45% of primary tumors and are highly correlated with the poor prognosis class 2 transcriptional signature and metastatic phenotype (ref. 43; Fig. 3A). In uveal melanoma, BAP1 loss may drive malignant progression by removing epigenetic restraints imposed on differentiated cells (Fig. 3B; ref. 39). Other cancers in which acquired somatic BAP1 mutations are common include mesothelioma (60%–70% of them; ref. 8) and ccRCC (15% of them; Fig. 4; ref. 20), the core cancers of the BAP1 cancer syndrome (6, 15). The parallel between the tumor types developing most frequently in carriers of germline BAP1 mutations and the tumor types that most frequently contain somatic BAP1 mutations underscores the increased susceptibility of uveal, mesothelial, and kidney cells to BAP1 loss. Somatic BAP1 mutations are also present in other malignancies (52), although at lower rates: thymic carcinoma (13%), cholangiocarcinoma (7%), cutaneous melanoma (5%), basal cell carcinoma (4%), and others (ref. 74; COSMIC database, https://cancer.sanger.ac.uk/cosmic). The role of BAP1 as a two-hit tumor suppressor gene is underscored by the fact that in humans they are accompanied by monoallelic loss of 3p, or by biallelic deletions of the BAP1 locus (LOH), including broad deletions of 3p21, narrow deletions of several exons, or loss of the entire BAP1 allele (75, 76).
Figure 3.
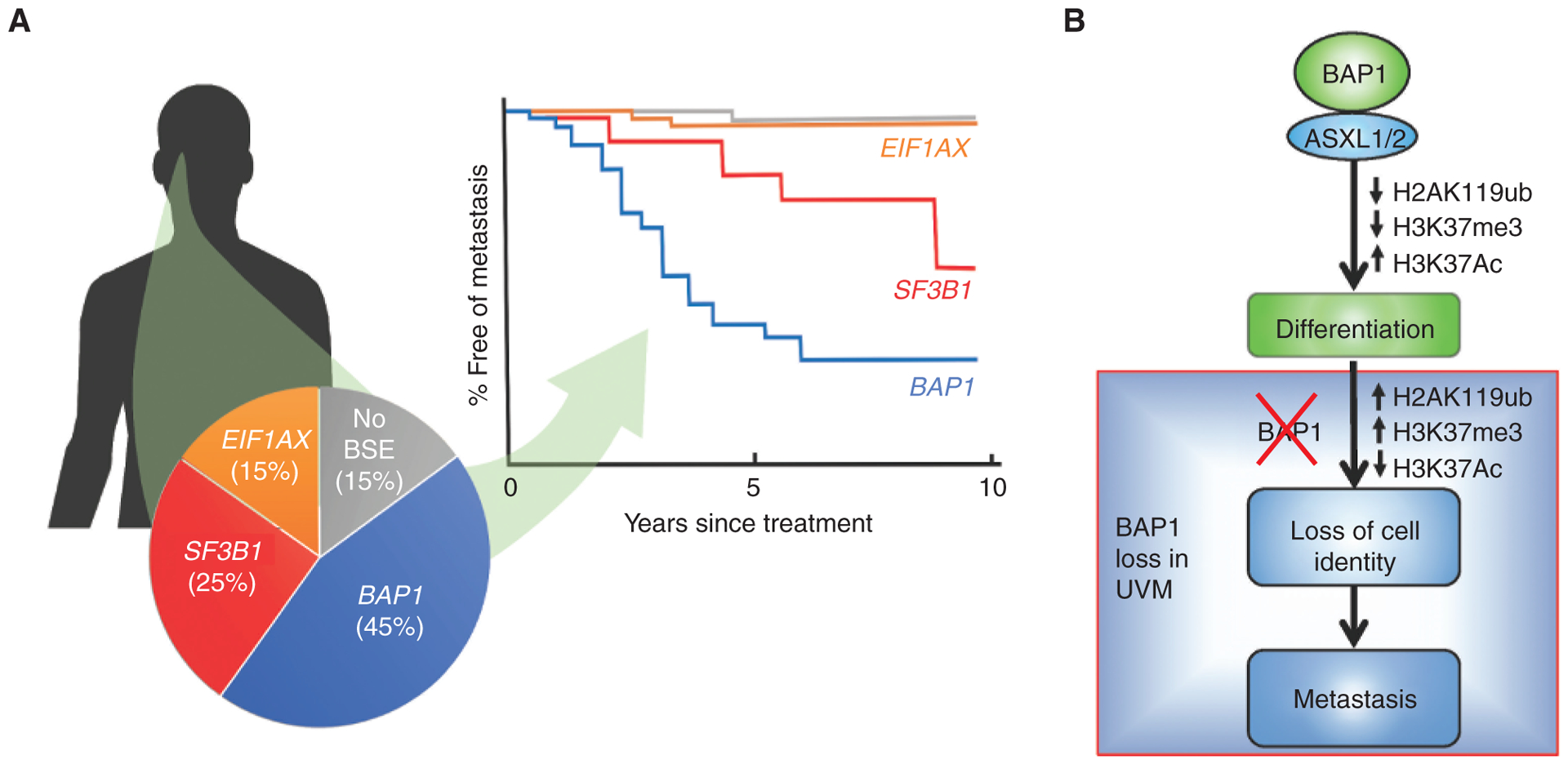
BAP1 mutations in uveal melanoma (UVM). A, Uveal melanomas arise in the iris, ciliary body, and choroid of the uveal tract of the eye. Their metastatic potential is determined by mutually exclusive “BSE” progression mutations in BAP1, SF3B1 (and rarely in other splicing factors), and EIF1AX. Inactivating mutations in BAP1, when coupled with the loss of the other copy of chromosome 3, result in high metastatic risk associated with the class 2 gene-expression profile. Hemizygous mutations in SF3B1 (or rarely in other splicing factors) retain the class 1 gene expression profile and are associated with intermediate metastatic risk. Hemizygous mutations in the translation initiation factor EIF1AX also retain the class 1 gene expression profile and are associated with low metastatic risk. Uveal melanomas without BSE mutations have a prognosis similar to those with EIF1AX mutations. This figure represents a synopsis of published data (68, 101, 178). B, Recent work indicates that BAP1 regulates the switch from progenitor to differentiated cell types in vertebrate development, not only through effects on H2A ubiquitination but perhaps more importantly by repressing HDACs and allowing acetylation of H3K27 to activate genes involved in differentiation in neural crest and other lineages. Loss of BAP1 abrogates this differentiation switch in development that parallels phenotypic and transcriptomic alterations observed in association with BAP1 mutation in uveal melanoma (114).
Figure 4.

BAP1 and PBRM1 establish the foundation for a molecular genetic classification of renal cancer with prognostic implications. ccRCC can be classified into 4 subtypes according to BAP1 and PBRM1 status, and these subtypes are associated with differential kidney cancer–specific survival in patients (20, 98). Targeted disruption of Vhl and either Pbrm1 or Bap1 genes in the mouse kidney induces ccRCC of low and high grade, respectively, similar to human tumors (117). The pie chart shows the inactivation of PBRM1, as shown by loss of protein expression, in 55% of cases. By measuring protein expression, we are able to integrate both mutational and epigenetic mechanisms of gene inactivation.
Initial studies underestimated the frequency of BAP1 mutations in mesothelioma as 22% to 23% (14, 77). A subsequent study using a comprehensive integrated genomic approach that included Sanger sequencing, multiplex ligation–dependent probe amplification (MLPA), copy-number analysis, and cDNA sequencing, combined with IHC and DNA methylation analyses in mesothelioma biopsies, found that >60% carried biallelic somatic BAP1 mutations (78). This study revealed that BAP1 was inactivated both by point mutations (which were detected by Sanger sequencing and not by MLPA) and by larger deletions (which were detected by MLPA but not by Sanger sequencing). This is because of the frequent occurrence of minute BAP1 deletions in the range of 250 to 3,000 kb, which are not reliably detected by targeted NGS (tNGS), by whole-exome sequencing (WES), or by Sanger sequencing, but are instead detected by MLPA. IHC proved to be the most sensitive and specific test to detect BAP1 mutations (78). Surprisingly, DNA methylation analyses found no evidence for epigenetic BAP1 inactivation (78). These findings were supported by a study in which high-density aCGH to detect deletions larger than 250 bp, and tNGS to detect nucleotide level mutations, resulted in a much higher prevalence of BAP1 mutations in human mesothelioma biopsies than either technique alone (~50%). This was because aCGH missed point mutations and tNGS missed larger deletions (79). Additional studies confirmed that BAP1 is the most frequently mutated gene in mesothelioma; however, studies that relied only on tNGS or WES invariably underestimated the true incidence of BAP1 mutations (80–82).
Similarly, Harbour and colleagues used an integrated DNA/RNA-sequencing approach and a customized bioinformatics pipeline to improve the detection of BAP1 mutations in uveal melanoma, identifying BAP1 mutations in approximately 45% of uveal melanomas, twice the rate detected by previous NGS approaches (68). Many of the mutations that were previously undetected consisted of large insertions/deletions (indel) that were missed by standard indel realignment tools (68). In addition to BAP1-mutated cancers, these studies are relevant to all human malignancies where mutations are assessed by tNGS or WES, as these techniques underestimate the extent of genetic damage.
A detailed analysis of 3p deletions in sporadic mesotheliomas revealed that deletions are not contiguous but rather preferentially occur in BAP1 and in some nearby genes (SETD2, PBRM1, and SMARCC1), alternating with segments showing oscillating copy-number changes along the 3p21 chromosome, findings suggestive of chromothripsis (79). The occurrence of chromothripsis in mesothelioma may be favored by BAP1 inactivation (79), and it has been confirmed by mate-pair sequencing (MPseq) analyses (83) and by WES (84). In addition, chromothripsis has been observed in ccRCC where it results in a t(3;5) derivative chromosome, leaving a wild-type chromosome 3 and two copies of wild-type chromosome 5 (85).
A potential clinical interest of BAP1 NGS analysis is the identification of germline mutations not predicted by the clinical guidelines, as in those identified in mesothelioma by a large universal sequencing study conducted in more than 1,000 cases (86). Of note, mesothelioma and uveal melanoma have among the lowest mutational burdens of all cancers in The Cancer Genome Atlas, a finding that is in part a consequence of the technical approach used (NGS) that underestimates the amount of genetic damage (79).
CLINICAL IMPLICATIONS
Diagnosis
BAP1 IHC is now an integral part of the routine of diagnostic pathology of the pleura and peritoneum and it is also extensively used in research (8). About 60% of mesotheliomas show tumor cells with an epithelioid morphology, 10% show a spindle cell sarcomatoid morphology, and 30% a biphasic morphology, that is, a mixture of epithelioid and spindle cells. When clear evidence of invasion is lacking it is not possible to determine whether the lesions are benign/reactive, such as a chronic pleuritis, or malignant. When invasion is present, it is difficult to decide whether the spindle cells are benign or malignant, and thus whether a lesion represents an epitheliod or a biphasic mesothelioma (7, 8). BAP1 IHC is very helpful in both cases. The correct interpretation of BAP1 IHC is critical in cancer diagnosis and also in research; therefore, we will review this issue, which, in our experience, is confusing to many (Fig. 5). Immunostaining produces a nuclear and cytoplasmic stain in stromal cells and in tumor cells containing wild-type BAP1 (Fig. 5A and B). On the other hand, nearly all biallelic BAP1 mutations result in the absence of nuclear staining because either there is no BAP1 protein or the mutated BAP1 protein cannot enter the nucleus (Fig. 5C–F). Negative BAP1 nuclear staining by IHC, regardless of cytoplasmic staining, is found in about 60% to 70% of mesotheliomas and is a reliable, rapid, and economical approach to identify biallelic BAP1 inactivating mutations. Instead, BAP1 nuclear staining is evidence of wild-type BAP1 (8). Note that BAP1 nuclear staining is found in the normal cells of carriers of germline BAP1 mutations because the remaining wild-type allele produces a normal BAP1 protein. Therefore, IHC, which is not a quantitative assay, cannot help to identify carriers of germline BAP1 mutations.
Figure 5.
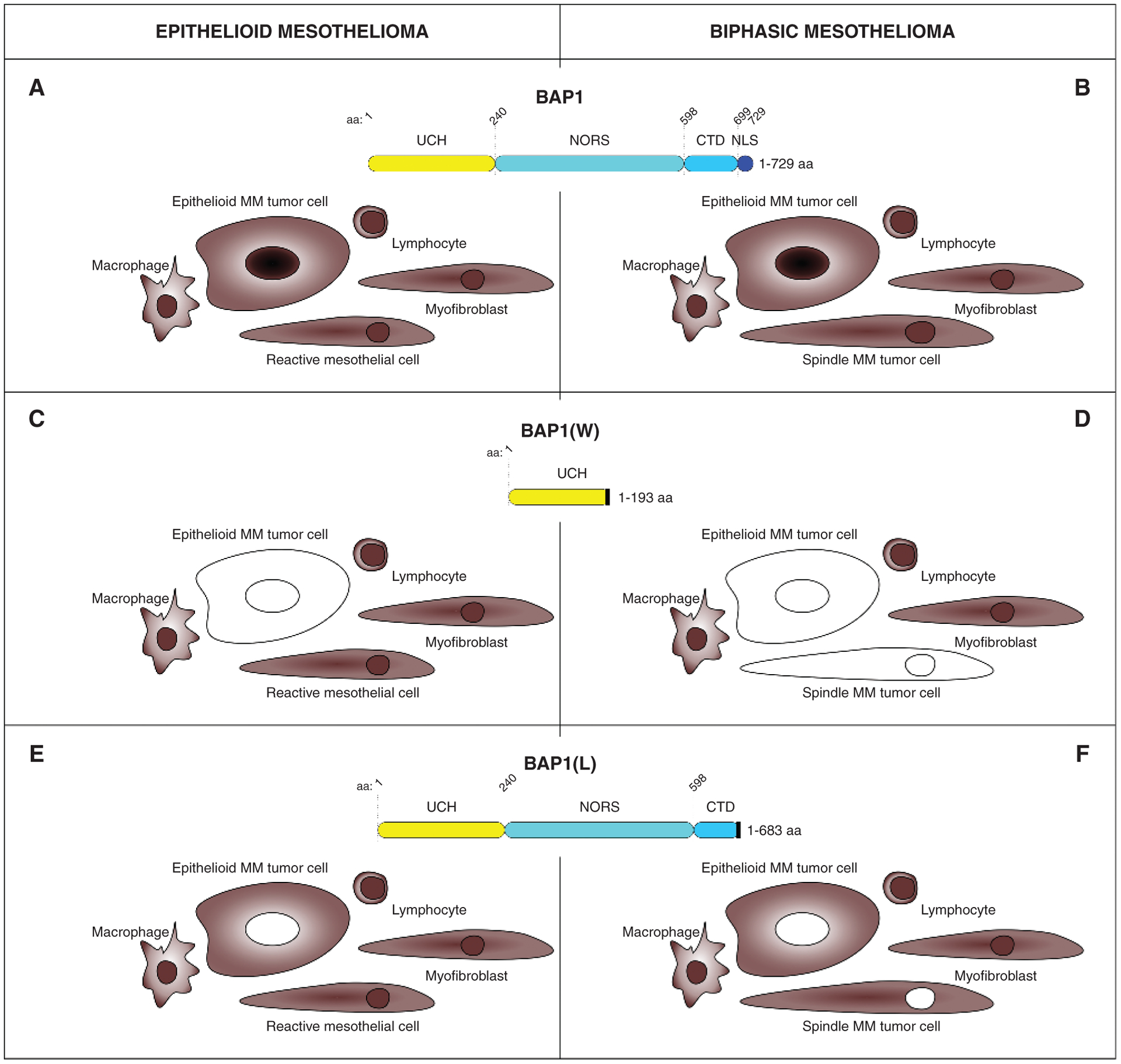
BAP1 immunostaining in mesothelioma. Wild-type BAP1. Epithelioid (A) and biphasic (B) mesotheliomas with wild-type BAP1 found in about 30% of cases show both nuclear and cytoplasmic BAP1 staining, as observed in nearby benign reactive cells. Nuclear and cytoplasmic staining in these cases is strong evidence of wild-type BAP1 but is not helpful in the differential diagnosis. C and D, Mutated BAP1. Negative nuclear and cytoplasmic BAP1 staining is found in about two thirds of the mutated cases, and it is associated with positive staining in nearby stromal and inflammatory cells. This is strong evidence of malignancy and supports the diagnosis of mesothelioma over other cancer types that can metastasize to the pleura/peritoneum. This IHC pattern is seen mostly in tumors carrying truncating mutations resulting in large BAP1 deletions. C, Epithelioid mesothelioma; only the epithelioid mesothelioma cells lost BAP1 staining. D, The presence of spindle tumor cells (BAP1-negative) supports the diagnosis of biphasic mesothelioma. W, representative BAP1 truncating mutation found in the W family. E and F, Mutated BAP1. Negative nuclear staining but positive cytoplasmic BAP1 staining is found in about one third of the mutated cases together with positive nuclear and cytoplasmic staining in nearby stromal and inflammatory cells. As for C and D, this is also strong evidence of malignancy and supports the diagnosis of mesothelioma over other cancer types that can metastasize to the pleura/peritoneum. This IHC pattern is seen mostly in tumors carrying truncating mutations resulting in small BAP1 deletions. E, Epithelioid mesothelioma; only the epithelioid mesothelioma cells lost BAP1 nuclear staining. F, The presence of spindle tumor cells with negative nuclear BAP1 staining supports the diagnosis of biphasic mesothelioma. Note that BAP1 is retained in the nuclei of background reactive benign mesothelial spindle cells; the latter have a slightly smaller size and bland nuclear features. L, representative BAP1 truncating mutation found in the L family.
As for the cytoplasm, two scenarios are possible. In most tumor cells showing no nuclear staining (e.g., mutated BAP1), there is also no staining in the cytoplasm (Fig. 5C and D). At times, however, a mutated, biologically inactive BAP1 (4) can accumulate in the cytoplasm where it forms amyloid (87), and it may produce cytoplasmic staining without accompanying nuclear staining (Fig. 5E and F; ref. 78).
In summary, the absence of BAP1 nuclear staining is a valuable diagnostic test to distinguish benign (positive nuclear staining) from malignant (negative nuclear staining) mesothelial cells, including distinguishing benign pleural effusions, benign atypical mesothelial hyperplasia, and benign chronic pleuritis from mesothelioma (7, 8, 88–91). Moreover, negative BAP1 nuclear staining in both epithelioid and spindle cells confirms the diagnosis of biphasic mesothelioma (Fig. 5F). Negative BAP1 nuclear staining is also helpful in the often difficult differential diagnosis between primary mesothelioma (most of them BAP1-negative) and metastatic lung carcinomas to the pleura, which instead are nearly always BAP1-positive (92, 93).
The IHC assay is similarly effective in ccRCC. In a study of 176 ccRCC at The University of Texas Southwestern (UTSW), where both IHC and DNA sequencing were performed, IHC results were interpretable in 175 tumors (20). Nuclear BAP1 protein was detected in 150 tumors and 148 were wild-type for BAP1. The two discordant samples had missense mutations in the catalytic domain (p.Gly13Val and p.Phe170Leu). Unlike other contexts, however, the mutant BAP1 protein still localized in the nucleus in these two samples. Twenty-five samples were negative by IHC and 22 of these had BAP1 mutations. In addition, Western blot analyses of an IHC-negative sample with wild-type BAP1 failed to reveal detectable BAP1 protein (20), suggesting that BAP1 may have been inactivated through mutations eluding detection by conventional Sanger sequencing, as described in mesothelioma (78, 79). Overall, the positive and negative predictive values of the IHC test for ccRCC were >95% (20). This IHC test has been utilized extensively (more than 3,000 RCCs) at UTSW (94–98) and there is a Clinical Laboratory Improvement Amendments (CLIA)–certified IHC test being implemented in routine clinical practice (7, 8, 78, 90, 91).
Prognosis: Germline Mutations
Baumann and colleagues reported that the presence of germ line BAP1 mutations strikingly increased the 5-year survival rate of patients with mesothelioma by 7-fold [47% (95% confidence interval [CI], 24–67) vs. 6.7% (95% CI, 6.2–7.3)], indicating that mesothelioma is less aggressive when it occurs in the context of the BAP1 cancer syndrome (67). In these individuals, normal cells contain 50% of the BAP1 protein, whereas tumor cells show biallelic BAP1 mutations and thus do not contain a biologically functional BAP1 protein (4, 78). In a follow-up prospective study, this research team tested 79 patients, all tumor stages, diagnosed with mesothelioma at an early age (<50 years of age) and/or with a family history of either mesothelioma or any of the core tumors of the BAP1 cancer syndrome. These characteristics were selected to identify patients who were likely carriers of germline mutations. These 79 patients were tested for germline mutations of BAP1 and of an additional 55 genes that included tumor suppressor genes, oncogenes, genes involved in DNA repair, and genes commonly found mutated in mesothelioma (54). Most subjects (43/79) carried germline BAP1 mutations; 12 of 79 carried germline mutations in other tumor suppressors; and 5 of 79 carried mutations in BAP1 and also in other cancer-related genes. These 79 patients selected for possible cancer heritability had a significantly prolonged survival of 5 to 10+ years (P < 0.001) compared with patients with sporadic mesothelioma from the SEER cohort, who had a median survival of 8 months (all stages combined), and 11 months in patients with stage I mesothelioma (54). These results were supported by two independent and parallel studies. An analysis conducted at the University of Chicago on the germline DNA of 198 patients with mesothelioma using targeted capture and NGS of 85 cancer susceptibility genes revealed that 12% of patients within this cohort carried pathogenic germline mutations; BAP1 was the most commonly mutated gene (47). A similar survey conducted at the NCI on a cohort of 385 patients using a panel of 73 genes involved in DNA repair and tumor suppression demonstrated that 12% of them carried germline mutations (mostly germline BAP1 mutations). The presence of inherited mutations significantly increased median overall survival compared with patients without these mutations (7.9 years vs. 2.4 years, P = 0.001; ref. 99). Together, these studies validate the prognostic significance of germline mutations that confer a significantly improved survival to patients with mesothelioma (Fig. 6).
Figure 6.
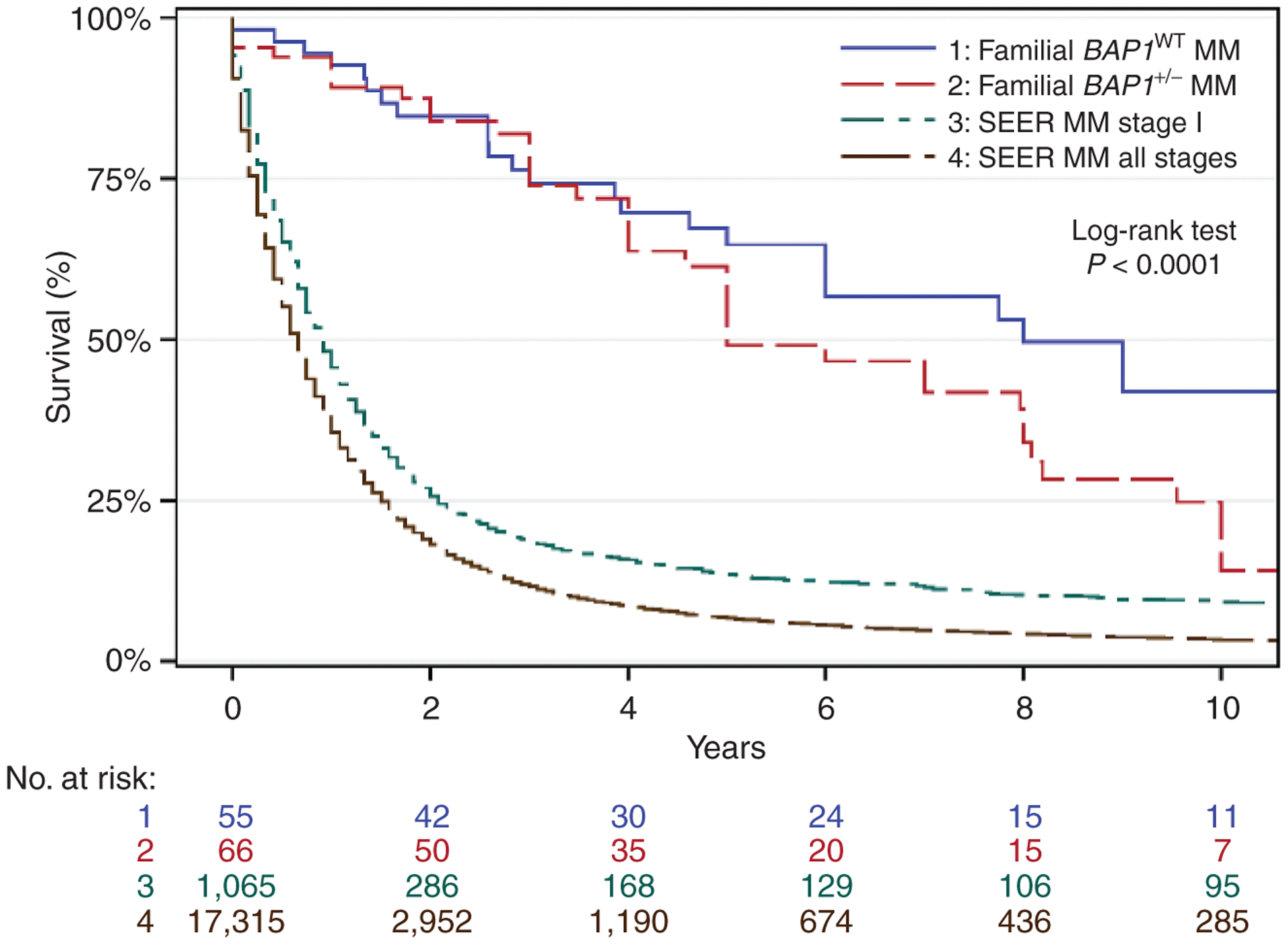
Survival analysis of individuals with sporadic mesothelioma or familial mesothelioma by BAP1 mutation status. Kaplan–Meier survival probability versus years with number at risk. Survival data combine results from references: (54, 67, 99). Blue, familial BAP1WT mesothelioma (median survival, 8 years; 10-year survival, 42.0%); red: familial BAP1+/− mesothelioma (median survival, 5 years; 10-year survival, 14.1%); green: SEER, stage I (median survival, 11 months; 10-year survival, 9.2%); brown: SEER, all stages (median survival, 8 months; 10-year survival, 3.3%). Rows below the graph indicate the number of patients at risk in each cohort per year. BAP1+/−, heterozygous BAP1-inactivating mutations; BAP1WT, wild-type BAP1; MM, malignant mesothelioma.
The improved survival was also observed among patients who, in addition to mesothelioma, developed other aggressive malignancies, which, rather than an exception, is the norm among those carrying germline BAP1 mutations. At times, five aggressive cancers were diagnosed in the same patient, and, surprisingly, they survived 5 to 10 or more years (refs. 53, 54, 67; Fig. 2B). Because almost all BAP1-mutated cancers contain biallelic BAP1 mutations, regardless of whether they are sporadic or occur in carriers of germline mutations, the markedly improved prognosis of mesotheliomas occurring in carriers of germline BAP1 mutations does not seem to be related only to the mutation in the tumor cells. Therefore, the improved prognosis of mesothelioma and other cancer types in carriers of germline BAP1 mutations may be influenced by the microenvironment and/or the immune system. Whether a microenvironment with reduced BAP1 levels would be more effective in controlling tumor growth is an area of intense investigation across multiple laboratories, as it may help to find novel ways to fight cancer.
In contrast to mesothelioma, a study of 8 patients with uveal melanoma with germline BAP1 mutations found an increased risk of metastasis (p.003) compared with uveal melanoma patients with wild-type BAP1 in their germline, confirming that BAP1 mutations induce a metastatic phenotype (100). This study did not compare survival among metastatic uveal melanomas with germline or with somatic mutations. Somatic BAP1 mutations in the tumor biopsy are the strongest known risk factor for uveal melanoma metastatic death (43, 101). These phenotypic differences among cancer types associated with BAP1 mutations suggest that there are cell type- and context-dependent differences in the role of BAP1 in biology and cancer. It is not yet clear whether germline BAP1 mutations are associated with improved survival in RCC.
Prognosis: Somatic Mutations
Mesotheliomas with acquired BAP1 mutations are mostly of the epithelial type and may have a slightly improved prognosis of a few months compared with mesotheliomas of similar histologic type with wild-type BAP1 (102–104); however, some studies did not support this finding (105).
Intriguingly, the opposite correlation with survival was observed in uveal melanoma (43, 100, 101, 106, 107), ccRCC (96, 108, 109), and cholangiocarcinoma (110), where somatic biallelic BAP1 mutations were associated with a metastatic phenotype and poor prognosis. In uveal melanoma, detection of BAP1 mutations directly by sequencing, or indirectly via the class 2 transcriptional signature or other methods, is now a routine part of patient care, with high-risk patients stratified for increased surveillance and clinical trial entry (111–113). The recent finding that BAP1 loss leads to defective differentiation and an arrested primitive phenotype in vertebrate development and uveal melanoma (39) suggests that BAP1 loss can promote tumor progression by inducing cell dedifferentiation and stem-like behavior (Fig. 3B; ref. 114).
Brugarolas and colleagues found that ccRCCs with somatic BAP1 mutations were associated with high-grade tumors. They discovered that mutations in BAP1 were mutually exclusive with mutations in PBRM1 (20, 115). PBRM1, which encodes a switching defective/sucrose nonfermenting (SWI/SNF) nucleosome remodeling complex protein (BAF180), is inactivated in approximately 50% of ccRCC (20, 116). In contrast to BAP1-mutant tumors, PBRM1-mutant tumors tend to be of low grade (20, 98). To determine whether BAP1 and PBRM1 directly affect tumor grade, mice were generated with targeted inactivation of Bap1 or Pbrm1 in the kidney using the same Cre driver (117). Inactivation of Bap1 or Pbrm1, along with Vhl, which is uniformly inactivated in ccRCC, led to the development of ccRCC (117, 118). Similar to humans, BAP1-deficient tumors were of high grade, and PBRM1-deficient tumors were of low grade (Fig. 4). Furthermore, PBRM1-deficient tumors developed after a significantly longer latency period. Overall, these data suggest that the differences in grading observed in ccRCC are directly related to the loss of BAP1 and PBRM1. These differences in grade also translate into differences in survival. Patients with BAP1-deficient tumors have a three-fold higher risk of death than patients with PBRM1-deficient tumors (98, 119). The PBRM1 gene, like the BAP1 and VHL genes, is located on chromosome 3p (19). Following VHL inactivation, which is the signature and initiating event in ccRCC (85, 120, 121), a mutation of the second copy of BAP1 or PBRM1 likely leads to tumors of different grade and prognosis (19). A fourth tumor suppressor gene in the same 3p region, SETD2, is also mutated in ccRCC and is associated with poor prognosis (94). Whereas mutations in BAP1 and PBRM1 tend to be mutually exclusive, mutations in PBRM1 and SETD2 appear to cooperate and are found at higher-than-expected frequencies (115).
Although mutation exclusivity in cancer often identifies genes encoding for proteins that act in the same pathway, where mutations at two different levels may offer little added advantage, this is unlikely the case in ccRCC. Indeed, BAP1- and PBRM1-deficient tumors differ not only in grade and prognosis, but also in gene expression (20, 119), and the mouse models show ccRCCs that are histologically quite different.
In summary, somatic mutations have only mild to no beneficial survival effect in mesothelioma, and are instead associated with a much more aggressive tumor phenotype and reduced survival in uveal melanoma and ccRCC (20, 43, 90, 91, 102–104, 108, 121, 122). These findings are puzzling and if addressed may provide critical information to develop novel therapeutic approaches.
Therapy
Mesothelioma, metastatic uveal melanoma, metastatic RCCs, and other BAP1-related malignancies are resistant to current therapies, and thus it is important to develop novel therapeutic approaches (8, 43, 96, 108, 109, 123–126). Numerous ongoing studies are exploring the possibility of targeting BAP1 mutations or using BAP1 status as a biomarker for sensitivity to different therapies.
The incorporation of the active metabolite of gemcitabine into DNA causes replication arrest and apoptosis, making this drug one of the most used chemotherapeutic agents against several cancers, including mesothelioma, for which it is approved as second-line treatment (125). Two recent independent studies proposed that BAP1 status may be predictive of sensitivity to gemcitabine. Upon treatment with this agent or with hydroxyurea, the viability of mesothelioma spheroids expressing nonfunctional C91A BAP1 was significantly higher compared with wild-type BAP1 counterparts (127). Similarly, mesothelioma cells expressing wild-type BAP1 were more sensitive to gemcitabine-induced apoptosis and cell-cycle derangement, compared with mesothelioma cells expressing nonfunctional BAP1 or silenced for BAP1 (128). These findings underscore the biological relevance in relation to cancer of BAP1 regulation of cell death (Fig. 1). The evidence generated by these studies (127, 128) suggests that BAP1 status is a promising candidate predictor for sensitivity to gemcitabine therapy in patients with mesothelioma and possibly other BAP1-mutated cancers. BAP1 status may equally predict sensitivity to other chemotherapeutic agents that cause DNA damage and cell death.
Recently, Webster and colleagues demonstrated that Bap1 loss cooperated with active oncogenic BRAFV600E in supporting melanoma growth in a mouse model. The mice bearing Bap1-deficient tumors had a complete response to the combination treatment with vemurafenib (BRAF inhibitor) and cobimetinib (MEK inhibitor). This combined therapy is the standard of care for BRAFV600E-mutant human melanoma (129). If the results are confirmed in humans, BAP1 status may help to identify those patients more likely to respond to this therapy. In addition, a large study in more than 100 cases of metastatic RCC demonstrated that BAP1 mutational status did not correlate with clinical benefit upon rapalog therapy (130), despite the significantly higher aggressiveness of RCC in carriers of BAP1 mutations (131).
Histone deacetylases (HDAC), including class I HDAC1 and HDAC2, are epigenetic regulators of gene expression, which can be dysregulated in cancer, and have been proposed as targets by using specific inhibitors such as suberoylanilide hydroxamic acid (SAHA) or vorinostat for mesothelioma (132) and uveal melanoma (133) therapy. Upon BAP1 loss, HDAC1 is increased and HDAC2 is reduced, an effect that was observed across several lung cancer and mesothelioma cell lines (134). This suggested that BAP1 status may help to identify patients who may be responsive to HDAC inhibitors. However, patients with mesothelioma—not selected for BAP1 status—treated in the second or third line in the randomized phase III VANTAGE-014 trial with vorinostat did not show improved survival (135).
HDAC inhibitors can reverse dedifferentiation associated with BAP1 loss and induce cell-cycle exit in uveal melanoma cells (133). BAP1 modulates the development of the neural crest, from which melanocytes arise, and this role is dependent on an indirect effect of BAP1 on acetylation of H3K27 (Fig. 3B), which is associated with increased transcription (39). The BAP1-deficient developmental phenotype could be rescued using SAHA or specific depletion of HDAC4. Although results of HDAC inhibitors in metastatic uveal melanoma have been disappointing, there may be a role for such compounds in uveal melanoma in the adjuvant setting, with the goal of delaying or preventing the outgrowth of micrometastasis in high-risk patients (133).
The chromatin-associated PARP enzyme is involved in the recovery of cells from DNA damage, and PARP inhibitors selectively target cancer cells with defective DNA repair. In the context of mutations in BRCA1 or BRCA2 genes in patients with breast, ovary, prostate, or pancreatic cancers, PARP inhibitors have shown antitumor activity, and three agents are currently in the clinic. BAP1 loss impairs HR and double-strand break repair, promoting error-prone nonhomologous end-joining, with consequent genomic instability (Fig. 1). Therefore, BAP1-deficient cells may be more sensitive to PARP inhibitors (32). BAP1 loss may sensitize ccRCC cells to PARP inhibitors (20). Several studies proposed to test the efficacy of PARP inhibitors in mesothelioma, although patients were not selected for BAP1 status (136, 137). A BAP1 mutant by alternative splicing resulting in a 54-bp deletion increased sensitivity to the PARP inhibitor olaparib (138). A recent study on HR defects in a cohort of patients with mesothelioma showed both in vitro and by digital gene-expression analysis that loss of BAP1 increased sensitivity to PARP inhibitors (139). Presently, two ongoing clinical trials are testing the hypothesis that BAP1 mutations increase sensitivity to PARP inhibitors in mesothelioma (NCT03207347, NCT03531840). However, very recently Hassan and colleagues reported that BAP1 status does not determine sensitivity to PARP inhibitors in patient-derived mesothelioma cell lines (140). We propose that these results may indicate that the increased resistance to cell death caused by BAP1 mutations in tumor cells over-come whatever increased DNA damage may be induced by PARP inhibitors in these same cells, making them resistant to this therapy.
Studies in RCC have led to the identification of a link between BAP1 loss in tumors and an inflammatory microenvironment. To characterize the tumor microenvironment (TME) in RCC, Wang and colleagues (141) undertook an innovative approach involving RNA-seq. RNA-seq datasets were generated from patients’ RCC as well as from the corresponding tumor implanted orthotopically in mice. Subsequently, RNA-seq reads from the tumorgraft corresponding to the murine genome were subtracted. These reads correspond to the tumor stroma, which is replaced by the mouse, as only human tumor cells propagate in the mouse. Comparative analyses were then performed between the patients’ transcriptome, corresponding to tumor and stroma, and the tumorgraft transcriptome, corresponding to the tumor only. By subtracting the tumorgraft transcriptome from the patient tumor transcriptome, Wang and colleagues were able to empirically define the TME in RCC (141). According to this signature, RCCs could be divided into an inflamed subtype and a noninflamed subtype. Interestingly, the inflamed subtype was enriched for BAP1 mutations (P < 0.0001). What drives the inflammation in BAP1-deficient ccRCCs is unclear, but a recent study found a link between BAP1 loss and the expression of endogenous retroviruses (142). In contrast, the noninflamed subtype was enriched for angiogenesis-related genes. These findings may provide cues to help identify the patients with ccRCC most likely to respond to checkpoint versus angiogenesis inhibitors.
Similarly, in peritoneal mesothelioma, two subsets of tumors can be distinguished based on the presence of an inflammatory TME, and this difference correlated with BAP1 haploinsufficiency. BAP1 haploinsufficiency was characterized by a distinct expression profile including genes related to chromatin remodeling, DNA repair, and activation of immune checkpoint receptors, a pattern associated with the inflammatory TME. This evidence makes BAP1 a candidate predictive biomarker for immunotherapy in peritoneal mesothelioma (143) and possibly also in pleural mesothelioma (144). Moreover, based on the structural rearrangements induced by chromothripsis, which may be favored by BAP1 mutations (79), Mansfield and colleagues predicted and validated in vitro the expression of altered peptides that may act as neoantigens and thus potentially increase mesothelioma immunogenicity and responsiveness to immunotherapy (145). BAP1 loss results in increased global trimethylation of histone H3 lysine 27 (H3K27me3), which is catalyzed by the polycomb repressive complex 2 enzyme EZH2 (39, 146). Mesothelioma cells deficient for BAP1 were found to be sensitive to EZH2 inhibitors (146). However, this effect was not seen in neural crest–derived uveal melanoma cells (147). In addition, depletion or pharmacologic inhibition of EZH2 in BAP1-deficient Xenopus embryos did not rescue a neural crest developmental phenotype (39). Thus, the role of EZH2 inhibitors in BAP1-deficient cancers remains unclear, and the results of a clinical trial on tazemetostat in mesothelioma that has been completed are being evaluated (ref. 8; NCT02860286).
CONCLUDING REMARKS
Although the BAP1 cancer syndrome and the driving role of acquired BAP1 mutations in human cancer were discovered less than a decade ago, much progress has been made to elucidate critical mechanisms of BAP1 activities (Fig. 1), and consequently why reduced or absent BAP1 protein levels cause and favor cancer progression. BAP1 plays an important role in regulating both DNA repair by HR and cell death in some cell types. Therefore, reduced levels of BAP1, as observed in carriers of heterozygous BAP1 mutations, increase the amount of genetic damage that occurs spontaneously as cells divide, or that occurs in response to exposure to environmental carcinogens (4). In parallel, the reduced levels of BAP1 impair apoptosis (4), ferroptosis (34), and possibly other mechanisms of cell death, resulting in an accumulation of cells with DNA damage, which normally would be eliminated by these mechanisms. These DNA-damaged cells can eventually become malignant. Moreover, BAP1 loss favors tumor growth by inducing a Warburg effect (i.e., aerobic glycolysis) that provides the metabolic building blocks to support cell division and at the same time helps cancer cells to grow in a hypoxic environment. A 50% reduction of BAP1 protein levels is sufficient to induce a Warburg effect in “normal” cells; thus, cells from those carrying germline mutations are primed to malignant growth once genetic damage causes malignant transformation (40). Therefore, the combined nuclear and cytoplasmic BAP1 activities account for the very high incidence of cancer in carriers of germline BAP1 mutations (Fig. 1).
BAP1 has emerged as a critical regulator of GxE interaction (61). BAP1’s role in increasing susceptibility to asbestos, UV light, and ionizing radiation has been established in primary human fibroblasts and mesothelial cells in culture (4), and it has been demonstrated in mice exposed to asbestos (62, 63). Because BAP1-mutant uveal melanoma does not show strong evidence of DNA damage repair defects or increased mutation burden (148), further work is needed to determine which of the possible functions of BAP1 are relevant to each cancer type in which it is mutated.
All published data support the notion that BAP1 is a potent tumor suppressor, as almost all carriers of pathogenic germline BAP1 mutations developed one or more cancers during their lifetime. LOH for BAP1 is observed in 100% of human tumors developing in carriers of germline BAP1 mutations, as well as in sporadic mesotheliomas with somatic BAP1 mutations, underscoring the potent tumor suppressor activity of BAP1. Intriguingly, LOH for BAP1 is not always observed in tumors developing in mice carrying germline Bap1 mutations (62, 65).
Some of the BAP1 activities may be more or less impactful depending on the cell type and species. This critical question shall be addressed in the coming years to understand why germline BAP1 mutations cause or are present as somatic mutations more frequently in mesothelioma, uveal melanoma, and ccRCC, rather than in other cancer types. This information will help to design more effective preventive and therapeutic strategies for patients carrying germline BAP1 mutations or cancers with somatic BAP1 mutations.
An additional question that will be addressed in coming years is why BAP1 mutations have phenotypic and prognostic implications that are cell type– and context-dependent: Germline mutations confer a better prognosis in mesothelioma (54, 67, 99), whereas somatic mutations induce a worse prognosis in uveal melanoma (42, 100, 101, 106, 107) and ccRCC (96, 108, 109). The difference in survival is quite significant, with median survival of 5 to 7 years for mesothelioma developing in carriers of heterozygous BAP1 mutations compared with 1-year median survival in sporadic mesotheliomas, cancers characteristically resistant to therapy (54, 67, 99). Some of these patients with mesothelioma have a normal life 20 years after diagnosis, their cancers still detectable. They appear to respond to any therapy, which raises the question of whether they respond to therapy or whether these tumors would have remained indolent regardless of therapy.
Adding to this puzzle, in sporadic mesotheliomas acquired biallelic BAP1 mutations are frequent, yet these mesotheliomas are not associated with significantly improved survival (8). These findings in the same tumor type suggest that the improved survival in carriers of germline BAP1 mutations may be linked to the microenvironment, the immune system, and maybe in part to early diagnosis, as family members are being enrolled in early-detection screening programs. However, the improved survival predates screening, as it was also observed in family members who were diagnosed before the discovery of the BAP1 cancer syndrome (67).
What if germline BAP1 mutations render the host capable to fight mesothelioma growth by affecting the tumor microenvironment? There is some preliminary experimental evidence that supports this hypothesis. BAP1 mutations may influence the response to immunotherapy by increasing the propensity to chromothripsis, by deregulating the expression of genes that modulate immune checkpoints, and by promoting a proinflammatory tumor microenvironment (62, 141, 145). By studying patients and mice with germline BAP1 mutations, we may learn how to treat mesotheliomas and maybe other cancers more effectively.
The information about the effects of BAP1 on mitochondrial respiration and cell metabolism has provided researchers with a range of potential targets and tools for prevention and therapy that should be investigated in the coming years. For example, the role of metformin, a drug that reprograms cell metabolism by restraining aerobic glycolysis and promoting mitochondrial respiration (149), could be explored in the existing mouse models carrying heterozygous Bap1 mutations and in xenografts of BAP1-mutated tumors.
Currently, we recommend germline and tumor cell BAP1 testing for all patients with mesothelioma, uveal melanoma, and RCC, as it helps in diagnosis, has prognostic relevance, and may also guide clinicians to target therapies, as several clinical trials are becoming available for patients with BAP1-mutant tumors (see the previous section). Family members of carriers of germline BAP1 mutations should be tested for BAP1 mutations, and those found to carry mutations should be enrolled in early-detection clinical trials (NCT03830229) that can help identify malignancies at an early stage, when they can be cured by surgery (uveal melanoma, RCC, cutaneous melanoma, etc.), or when they may be more susceptible to therapy (mesothelioma). Moreover, BAP1 mutant carriers should limit exposure to diagnostic and therapeutic ionizing radiation that in these individuals may carry a higher cancer risk than in the population at large. Instead, they should be screened preferentially using sonography and MRI (8) according to the same guidelines used for patients affected by the Li-Fraumeni cancer syndrome (73).
In summary, the discoveries made during the past decade allow us to implement preventive and early-detection programs that improve the survival of carriers of BAP1 germline mutations. Furthermore, with future advancements in understanding the fundamental biology of BAP1 and the development of novel technologies, targeted therapies for BAP1-deficient cancers should lead to substantial clinical improvements.
Acknowledgments
We thank all the family members affected by the BAP1 cancer syndrome and the patients with BAP1-mutated tumors who donated their specimens. They allowed us to study and discover how this gene functions in different cancer types and to translate this knowledge to improve cancer prevention, diagnosis, and prognosis (already being implemented), and in the near future, we hope, to improve therapies for patients with BAP1-mutated cancers. M. Carbone and H. Yang report funding from the National Institute of Environmental Health Sciences (NIEHS) 1R01ES030948-01 (to M. Carbone and H. Yang), the NCI 1R01CA237235-01A1 (to M. Carbone and H. Yang) and 1R01CA198138 (to M. Carbone), the U.S. Department of Defense (DoD) CA150671 (to H. Yang, M. Carbone, and H.I. Pass), and from the UH Foundation through donations from the Riviera United-4-a Cure (to M. Carbone and H. Yang), the Melohn Family Endowment, the Honeywell International Inc., the Germaine Hope Brennan Foundation, and the Maurice and Joanna Sullivan Family Foundation (to M. Carbone). M. Carbone is a board-certified pathologist who provides consultation for pleural pathology, including medical-legal. H.I. Pass and H. Yang report funding from the Early Detection Research Network NCI U01CA111295-08. H.I. Pass reports funding from Genentech, and Belluck and Fox LLP. J.W. Harbour reports funding from the Department of Defense W81XWH-15-1-0578, NIH R01 CA125970, P30CA240139 (to Sylvester Comprehensive Cancer Center), and P30EY014801 (to Bascom Palmer Eye Institute), Research to Prevent Blindness Unrestricted Grant (to Bascom Palmer Eye Institute), and a generous gift from Dr. Mark J. Daily. J. Brugarolas reports funding from the NIH 1P50CA196516, R01CA175754, and RP180192.
Footnotes
Disclosure of Potential Conflicts of Interest
M. Carbone has a patent issued for “Methods for Diagnosing a Predisposition to Develop Cancer.” M. Carbone and H. Yang have a patent issued for “Using Anti-HMGB1 Monoclonal Antibody or Other HMGB1 Antibodies as a Novel Mesothelioma Therapeutic Strategy,” and a patent issued for “HMGB1 as a Biomarker for Asbestos Exposure and Mesothelioma Early Detection.” J.W. Harbour has a patent issued for “Method for predicting risk of metastasis” and for “Compositions and methods for detecting cancer metastasis”; has been a paid consultant for Castle Biosciences, licensee of this intellectual property; and is a consultant/advisory board member for Aura Biosciences, TD2, Castle Biosciences, and Immunocore. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16:1097–112. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res 2009;69:111–9. [DOI] [PubMed] [Google Scholar]
- 3.Hauri S, Comoglio F, Seimiya M, Gerstung M, Glatter T, Hansen K, et al. A high-density map for navigating the human polycomb complexome. Cell Rep 2016;17:583–95. [DOI] [PubMed] [Google Scholar]
- 4.Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature 2017;546: 549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mashtalir N, Daou S, Barbour H, Sen NN, Gagnon J, Hammond-Martel I, et al. Autodeubiquitination protects the tumor suppressor BAP1 from cytoplasmic sequestration mediated by the atypical ubiquitin ligase UBE2O. Mol Cell 2014;54:392–406. [DOI] [PubMed] [Google Scholar]
- 6.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer 2013;13:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapel DB, Schulte JJ, Husain AN, Krausz T. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl Lung Cancer Res 2020;9:S3–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone M, Adusumilli PS, Alexander HR Jr, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer 2007;7:147–54. [DOI] [PubMed] [Google Scholar]
- 10.Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet 2001;357:444–5. [DOI] [PubMed] [Google Scholar]
- 11.Carbone M, Baris YI, Bertino P, Brass B, Comertpay S, Dogan AU, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A 2011;108:13618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol 2013;14:576–8. [DOI] [PubMed] [Google Scholar]
- 13.Emri SA. The Cappadocia mesothelioma epidemic: its influence in Turkey and abroad. Ann Transl Med 2017;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbone M, Ferris LK, Baumann F, Napolitano A, Lum CA, Flores EG, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med 2012; 10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piris A, Mihm MC Jr, Hoang MP. BAP1 and BRAFV600E expression in benign and malignant melanocytic proliferations. Hum Pathol 2015;46:239–45. [DOI] [PubMed] [Google Scholar]
- 18.Haugh AM, Njauw CN, Bubley JA, Verzi AE, Zhang B, Kudalkar E, et al. Genotypic and phenotypic features of BAP1 cancer syndrome: a report of 8 new families and review of cases in the literature. JAMA Dermatol 2017;153:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugarolas J Molecular genetics of clear-cell renal cell carcinoma. J Clin Oncol 2014;32:1968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HS, Lee SA, Hur SK, Seo JW, Kwon J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat Commun 2014;5:5128. [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Seo HR, Lee SA, Choi S, Kang D, Kwon J. BAP1 promotes stalled fork restart and cell survival via INO80 in response to replication stress. Biochem J 2019;476:3053–66. [DOI] [PubMed] [Google Scholar]
- 23.Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem 2009;284:34179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 2010;30:5071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol 2009;29:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji Z, Mohammed H, Webber A, Ridsdale J, Han N, Carroll JS, et al. The forkhead transcription factor FOXK2 acts as a chromatin targeting factor for the BAP1-containing histone deubiquitinase complex. Nucleic Acids Res 2014;42:6232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okino Y, Machida Y, Frankland-Searby S, Machida YJ. BRCA1-associated protein 1 (BAP1) deubiquitinase antagonizes the ubiquitin-mediated activation of FoxK2 target genes. J Biol Chem 2015;290: 1580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daou S, Hammond-Martel I, Mashtalir N, Barbour H, Gagnon J, Iannantuono NV, et al. The BAP1/ASXL2 histone H2A deubiquitinase complex regulates cell proliferation and is disrupted in cancer. J Biol Chem 2015;290:28643–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratcorona M, Abbas S, Sanders MA, Koenders JE, Kavelaars FG, Erpelinck-Verschueren CA, et al. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haema-tologica 2012;97:388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daou S, Barbour H, Ahmed O, Masclef L, Baril C, Sen Nkwe N, et al. Monoubiquitination of ASXLs controls the deubiquitinase activity of the tumor suppressor BAP1. Nat Commun 2018;9:4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail IH, Davidson R, Gagne JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res 2014;74:4282–94. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A 2014;111: 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi C, Marchi S, Pinton P. Publisher correction: the machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 2018;19:746. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 2018;20:1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun 2018;38:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Affar EB, Carbone M. BAP1 regulates different mechanisms of cell death. Cell Death Dis 2018;9:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai F, Lee H, Zhang Y, Zhuang L, Yao H, Xi Y, et al. BAP1 inhibits the ER stress gene regulatory network and modulates metabolic stress response. Proc Natl Acad Sci U S A 2017;114:3192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He M, Chaurushiya MS, Webster JD, Kummerfeld S, Reja R, Chaudhuri S, et al. Intrinsic apoptosis shapes the tumor spectrum linked to inactivation of the deubiquitinase BAP1. Science 2019;364:283–5. [DOI] [PubMed] [Google Scholar]
- 39.Kuznetsov JN, Aguero TH, Owens DA, Kurtenbach S, Field MG, Durante MA, et al. BAP1 regulates epigenetic switch from pluripotency to differentiation in developmental lineages giving rise to BAP1-mutant cancers. Sci Adv 2019;5:eaax1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bononi A, Yang H, Giorgi C, Patergnani S, Pellegrini L, Su M, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ 2017;24:1694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baughman JM, Rose CM, Kolumam G, Webster JD, Wilkerson EM, Merrill AE, et al. NeuCode proteomics reveals Bap1 regulation of metabolism. Cell Rep 2016;16:583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan HB, Han X, Li MD, Singh JP, Qian K, Azarhoush S, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab 2012;16: 226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farley MN, Schmidt LS, Mester JL, Pena-Llopis S, Pavia-Jimenez A, Christie A, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res 2013;11: 1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet 2016;89:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kittaneh M, Berkelhammer C. Detecting germline BAP1 mutations in patients with peritoneal mesothelioma: benefits to patient and family members. J Transl Med 2018;16:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panou V, Gadiraju M, Wolin A, Weipert CM, Skarda E, Husain AN, et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol 2018;36: 2863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobrinski DA, Yang H, Kittaneh M. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res 2020;9:S60–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshikawa Y, Emi M, Nakano T, Gaudino G. Mesothelioma developing in carriers of inherited genetic mutations. Transl Lung Cancer Res 2020;9:S67–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Njauw CN, Kim I, Piris A, Gabree M, Taylor M, Lane AM, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One 2012;7:e35295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d’Enghien C, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet 2013;92:974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin M, Zhang L, Hildebrandt MAT, Huang M, Wu X, Ye Y. Common, germline genetic variations in the novel tumor suppressor BAP1 and risk of developing different types of cancer. Oncotarget 2017;8:74936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carbone M, Flores EG, Emi M, Johnson TA, Tsunoda T, Behner D, et al. Combined genetic and genealogic studies uncover a large BAP1 cancer syndrome kindred tracing back nine generations to a common ancestor from the 1700s. PLos Genet 2015;11:e1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, Pagano I, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J Clin Oncol 2018;36: 3485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walpole S, Pritchard AL, Cebulla CM, Pilarski R, Stautberg M, Davidorf FH, et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J Natl Cancer Inst 2018;110:1328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Fouchardiere A, Cabaret O, Savin L, Combemale P, Schvartz H, Penet C, et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet 2015;88:273–7. [DOI] [PubMed] [Google Scholar]
- 57.Wadt KA, Aoude LG, Johansson P, Solinas A, Pritchard A, Crainic O, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet 2015;88:267–72. [DOI] [PubMed] [Google Scholar]
- 58.Pilarski R, Cebulla CM, Massengill JB, Rai K, Rich T, Strong L, et al. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer 2014;53:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankar GM, Abedalthagafi M, Vaubel RA, Merrill PH, Nayyar N, Gill CM, et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol 2017;19:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carbone M, Klein G, Gruber J, Wong M. Modern criteria to establish human cancer etiology. Cancer Res 2004;64:5518–24. [DOI] [PubMed] [Google Scholar]
- 61.Carbone M, Arron ST, Beutler B, Bononi A, Cavenee W, Cleaver JE, et al. Nat Rev Cancer 2012. May 29 [Epub ahead of print]. [Google Scholar]
- 62.Napolitano A, Pellegrini L, Dey A, Larson D, Tanji M, Flores EG, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016;35:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, Kadariya Y, Cheung M, Pei J, Talarchek J, Sementino E, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res 2014;74:4388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung M, Kadariya Y, Talarchek J, Pei J, Ohar JA, Kayaleh OR, et al. Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene-environment interaction. Cancer Lett 2015;369:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Badhai J, Pandey GK, Song JY, Krijgsman O, Bhaskaran R, Chandrasekaran G, et al. Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of malignant mesothelioma in mice. J Exp Med 2020;217:pii:e20191257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadariya Y, Cheung M, Xu J, Pei J, Sementino E, Menges CW, et al. Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res 2016;76:2836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 2018;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalez KD, Buzin CH, Noltner KA, Gu D, Li W, Malkin D, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009;46:689–93. [DOI] [PubMed] [Google Scholar]
- 70.Renaux-Petel M, Charbonnier F, Thery JC, Fermey P, Lienard G, Bou J, et al. Contribution of de novo and mosaic TP53 mutations to Li-Fraumeni syndrome. J Med Genet 2018;55:173–80. [DOI] [PubMed] [Google Scholar]
- 71.Schneider K, Zelley K, Nichols KE, Garber J. Li-Fraumeni Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. Seattle (WA): GeneReviews((R)); 1993. [Google Scholar]
- 72.Malkin D Li-fraumeni syndrome. Genes Cancer 2011;2:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villani A, Shore A, Wasserman JD, Stephens D, Kim RH, Druker H, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol 2016;17:1295–305. [DOI] [PubMed] [Google Scholar]
- 74.Maffeis V, Cappellesso R, Nicole L, Guzzardo V, Menin C, Elefanti L, et al. Loss of BAP1 in pheochromocytomas and paragangliomas seems unrelated to genetic mutations. Endocr Pathol 2019;30: 276–84. [DOI] [PubMed] [Google Scholar]
- 75.Togo Y, Yoshikawa Y, Suzuki T, Nakano Y, Kanematsu A, Zozumi M, et al. Genomic profiling of the genes on chromosome 3p in sporadic clear cell renal cell carcinoma. Int J Oncol 2016;48:1571–80. [DOI] [PubMed] [Google Scholar]
- 76.Emi M, Yoshikawa Y, Sato C, Sato A, Sato H, Kato T, et al. Frequent genomic rearrangements of BRCA1 associated protein-1 (BAP1) gene in Japanese malignant mesothelioma-characterization of deletions at exon level. J Hum Genet 2015;60:647–9. [DOI] [PubMed] [Google Scholar]
- 77.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, Ohmuraya M, Sato A, Tsujimura T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. Proc Natl Acad Sci U S A 2016;113:13432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264–9. [DOI] [PubMed] [Google Scholar]
- 81.Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407–16. [DOI] [PubMed] [Google Scholar]
- 82.Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 2018;8:1548–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mansfield AS, Peikert T, Smadbeck JB, Udell JBM, Garcia-Rivera E, Elsbernd L, et al. Neoantigenic potential of complex chromosomal rearrangements in mesothelioma. J Thorac Oncol 2019;14:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oey H, Daniels M, Relan V, Chee TM, Davidson MR, Yang IA, et al. Whole-genome sequencing of human malignant mesothelioma tumours and cell lines. Carcinogenesis 2019;40:724–34. [DOI] [PubMed] [Google Scholar]
- 85.Mitchell TJ, Turajlic S, Rowan A, Nicol D, Farmery JHR, O’Brien T, et al. Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx Renal. Cell 2018;173:611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs. guideline-based germline testing. JAMA 2017;318:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhattacharya S, Hanpude P, Maiti TK. Cancer associated missense mutations in BAP1 catalytic domain induce amyloidogenic aggregation: a new insight in enzymatic inactivation. Sci Rep 2015;5:18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrici J, Sheen A, Sioson L, Wardell K, Clarkson A, Watson N, et al. Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol 2015;28:1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Önder S, Özogul E, Koksal D, Sarinc Ulasli S, Firat P, Emri S. Diagnostic value of BAP1, GLUT-1 and desmin expression in the discrimination between reactive mesothelial proliferation and malignant mesothelioma in tissues and effusions. Cytopathology 2019;30:592–600. [DOI] [PubMed] [Google Scholar]
- 90.McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670–8. [DOI] [PubMed] [Google Scholar]
- 91.McGregor SM, McElherne J, Minor A, Keller-Ramey J, Dunning R, Husain AN, et al. BAP1 immunohistochemistry has limited prognostic utility as a complement of CDKN2A (p16) fluorescence in situ hybridization in malignant pleural mesothelioma. Hum Pathol 2017;60:86–94. [DOI] [PubMed] [Google Scholar]
- 92.Andrici J, Parkhill TR, Jung J, Wardell KL, Verdonk B, Singh A, et al. Loss of expression of BAP1 is very rare in non-small cell lung carcinoma. Pathology 2016;48:336–40. [DOI] [PubMed] [Google Scholar]
- 93.Carbone M, Shimizu D, Napolitano A, Tanji M, Pass HI, Yang H, et al. Positive nuclear BAP1 immunostaining helps differentiate non-small cell lung carcinomas from malignant mesothelioma. Oncotarget 2016;7:59314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ho TH, Kapur P, Joseph RW, Serie DJ, Eckel-Passow JE, Parasramka M, et al. Loss of PBRM1 and BAP1 expression is less common in non-clear cell renal cell carcinoma than in clear cell renal cell carcinoma. Urol Oncol 2015;33:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kapur P, Christie A, Raman JD, Then MT, Nuhn P, Buchner A, et al. BAP1 immunohistochemistry predicts outcomes in a multi-institutional cohort with clear cell renal cell carcinoma. J Urol 2014;191:603–10. [DOI] [PubMed] [Google Scholar]
- 96.Joseph RW, Kapur P, Serie DJ, Eckel-Passow JE, Parasramka M, Ho T, et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer 2014;120:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eckel-Passow JE, Serie DJ, Cheville JC, Ho TH, Kapur P, Brugarolas J, et al. BAP1 and PBRM1 in metastatic clear cell renal cell carcinoma: tumor heterogeneity and concordance with paired primary tumor. BMC Urol 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joseph RW, Kapur P, Serie DJ, Parasramka M, Ho TH, Cheville JC, et al. Clear cell renal cell carcinoma subtypes identified by BAP1 and PBRM1 expression. J Urol 2016;195:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A 2019;116:9008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta MP, Lane AM, DeAngelis MM, Mayne K, Crabtree M, Gragoudas ES, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol 2015;133:881–7. [DOI] [PubMed] [Google Scholar]
- 101.Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol 2016;134:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arzt L, Quehenberger F, Halbwedl I, Mairinger T, Popper HH. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res 2014;20:145–51. [DOI] [PubMed] [Google Scholar]
- 103.Luchini C, Veronese N, Yachida S, Cheng L, Nottegar A, Stubbs B, et al. Different prognostic roles of tumor suppressor gene BAP1 in cancer: a systematic review with meta-analysis. Genes Chromosomes Cancer 2016;55:741–9. [DOI] [PubMed] [Google Scholar]
- 104.Forest F, Patoir A, Dal Col P, Sulaiman A, Camy F, Laville D, et al. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: prognostic implications. Pathology 2018;50:635–41. [DOI] [PubMed] [Google Scholar]
- 105.Singhi AD, Krasinskas AM, Choudry HA, Bartlett DL, Pingpank JF, Zeh HJ, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol 2016;29: 14–24. [DOI] [PubMed] [Google Scholar]
- 106.van de Nes JA, Nelles J, Kreis S, Metz CH, Hager T, Lohmann DR, et al. Comparing the prognostic value of BAP1 mutation pattern, chromosome 3 status, and BAP1 immunohistochemistry in uveal melanoma. Am J Surg Pathol 2016;40:796–805. [DOI] [PubMed] [Google Scholar]
- 107.Farquhar N, Thornton S, Coupland SE, Coulson JM, Sacco JJ, Krishna Y, et al. Patterns of BAP1 protein expression provide insights into prognostic significance and the biology of uveal melanoma. J Pathol Clin Res 2018;4:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miura Y, Inoshita N, Ikeda M, Miyama Y, Oki R, Oka S, et al. Loss of BAP1 protein expression in the first metastatic site predicts prognosis in patients with clear cell renal cell carcinoma. Urol Oncol 2017;35:386–91. [DOI] [PubMed] [Google Scholar]
- 109.Oka S, Inoshita N, Miura Y, Oki R, Miyama Y, Nagamoto S, et al. The loss of BAP1 protein expression predicts poor prognosis in patients with nonmetastatic clear cell renal cell carcinoma with inferior vena cava tumor thrombosis. Urol Oncol 2018;36:365. [DOI] [PubMed] [Google Scholar]
- 110.Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014;9:e115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudle-man E, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012;119:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aaberg TM Jr, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO III. Current clinical practice: differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol 2014;8:2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Essen TH, van Pelt SI, Versluis M, Bronkhorst IH, van Duinen SG, Marinkovic M, et al. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol 2014;98:1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matatall KA, Agapova OA, Onken MD, Worley LA, Bowcock AM, Harbour JW. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer 2013;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pena-Llopis S, Christie A, Xie XJ, Brugarolas J. Cooperation and antagonism among cancer genes: the renal cancer paradigm. Cancer Res 2013;73:4173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011;469:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gu YF, Cohn S, Christie A, McKenzie T, Wolff N, Do QN, et al. Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade. Cancer Discov 2017;7:900–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang SS, Gu YF, Wolff N, Stefanius K, Christie A, Dey A, et al. Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis. Proc Natl Acad Sci U S A 2014;111:16538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapur P, Pena-Llopis S, Christie A, Zhrebker L, Pavia-Jimenez A, Rathmell WK, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol 2013;14:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gerlinger M, Santos CR, Spencer-Dene B, Martinez P, Endesfelder D, Burrell RA, et al. Genome-wide RNA interference analysis of renal carcinoma survival regulators identifies MCT4 as a Warburg effect metabolic target. J Pathol 2012;227:146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 2014;46: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ewens KG, Lalonde E, Richards-Yutz J, Shields CL, Ganguly A. Comparison of germline versus somatic BAP1 mutations for risk of metastasis in uveal melanoma. BMC Cancer 2018;18:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mutti L, Peikert T, Robinson BWS, Scherpereel A, Tsao AS, de Perrot M, et al. Scientific advances and new frontiers in mesothelioma therapeutics. J Thorac Oncol 2018;13:1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Woodard GA, Jablons DM. Surgery for pleural mesothelioma, when it is indicated and why: arguments against surgery for malignant pleural mesothelioma. Transl Lung Cancer Res 2020;9:S86–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCambridge AJ, Napolitano A, Mansfield AS, Fennell DA, Sekido Y, Nowak AK, et al. Progress in the management of malignant pleural mesothelioma in 2017. J Thorac Oncol 2018;13:606–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Disselhorst MJ, Baas P. Chemotherapy options versus “novel” therapies: how should we treat patients with malignant pleural mesothelioma. Transl Lung Cancer Res 2020;9:S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okonska A, Buhler S, Rao V, Ronner M, Blijlevens M, van der Meulen-Muileman IH, et al. Functional genomic screen in mesothelioma reveals that loss of function of BRCA1-associated protein 1 induces chemoresistance to ribonucleotide reductase inhibition. Mol Cancer Ther 2020;19:552–63. [DOI] [PubMed] [Google Scholar]
- 128.Guazzelli A, Meysami P, Bakker E, Demonacos C, Giordano A, Krstic-Demonacos M, et al. BAP1 status determines the sensitivity of malignant mesothelioma cells to gemcitabine treatment. Int J Mol Sci 2019;20:pii:E429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Webster JD, Pham TH, Wu X, Hughes NW, Li Z, Totpal K, et al. The tumor suppressor BAP1 cooperates with BRAFV600E to promote tumor formation in cutaneous melanoma. Pigment Cell Melanoma Res 2019;32:269–79. [DOI] [PubMed] [Google Scholar]
- 130.Nassar AH, Hamieh L, Gray KP, Thorner AR, Fay AP, Lasseter KD, et al. Mutations and response to rapalogs in patients with metastatic renal cell carcinoma. Mol Cancer Ther 2020;19:690–6. [DOI] [PubMed] [Google Scholar]
- 131.Carlo MI, Hakimi AA, Stewart GD, Bratslavsky G, Brugarolas J, Chen YB, et al. Familial kidney cancer: implications of new syndromes and molecular insights. Eur Urol 2019;76:754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krug LM, Curley T, Schwartz L, Richardson S, Marks P, Chiao J, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer 2006;7:257–61. [DOI] [PubMed] [Google Scholar]
- 133.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012;18: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sacco JJ, Kenyani J, Butt Z, Carter R, Chew HY, Cheeseman LP, et al. Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors. Oncotarget 2015;6:13757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2015;16:447–56. [DOI] [PubMed] [Google Scholar]
- 136.Pinton G, Manente AG, Murer B, De Marino E, Mutti L, Moro L. PARP1 inhibition affects pleural mesothelioma cell viability and uncouples AKT/mTOR axis via SIRT1. J Cell Mol Med 2013;17: 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Srinivasan G, Sidhu GS, Williamson EA, Jaiswal AS, Najmunnisa N, Wilcoxen K, et al. Synthetic lethality in malignant pleural mesothelioma with PARP1 inhibition. Cancer Chemother Pharmacol 2017;80:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parrotta R, Okonska A, Ronner M, Weder W, Stahel R, Penengo L, et al. A novel BRCA1-associated protein-1 isoform affects response of mesothelioma cells to drugs impairing BRCA1-mediated DNA repair. J Thorac Oncol 2017;12:1309–19. [DOI] [PubMed] [Google Scholar]
- 139.Borchert S, Wessolly M, Schmeller J, Mairinger E, Kollmeier J, Hager T, et al. Gene expression profiling of homologous recombination repair pathway indicates susceptibility for olaparib treatment in malignant pleural mesothelioma in vitro. BMC Cancer 2019;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rathkey D, Khanal M, Murai J, Zhang J, Sengupta M, Jiang Q, et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but is enhanced by temozolomide in cells with high-Schlafen 11and low-MGMT expression. J Thorac Oncol 2020;15:843–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang T, Lu R, Kapur P, Jaiswal BS, Hannan R, Zhang Z, et al. An empirical approach leveraging tumorgrafts to dissect the tumor microenvironment in renal cell carcinoma identifies missing link to prognostic inflammatory factors. Cancer Discov 2018;8:1142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Panda A, de Cubas AA, Stein M, Riedlinger G, Kra J, Mayer T, et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight 2018;3:pii:121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med 2019;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gray SG, Mutti L. Immunotherapy for mesothelioma: a critical review of current clinical trials and future perspectives. Transl Lung Cancer Res 2020;9:S100–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mansfield AS, Peikert T, Vasmatzis G. Chromosomal rearrangements and their neoantigenic potential in mesothelioma. Transl Lung Cancer Res 2020;9:S92–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.LaFave LM, Beguelin W, Koche R, Teater M, Spitzer B, Chramiec A, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schoumacher M, Le Corre S, Houy A, Mulugeta E, Stern MH, Roman-Roman S, et al. Uveal melanoma cells are resistant to EZH2 inhibition regardless of BAP1 status. Nat Med 2016;22:577–8. [DOI] [PubMed] [Google Scholar]
- 148.Durante MA, Field MG, Sanchez MI, Covington KR, Decatur CL, Dubovy SR, et al. Genomic evolution of uveal melanoma arising in ocular melanocytosis. Cold Spring Harb Mol Case Stud 2019;5:pii: a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.da Veiga Moreira J, Hamraz M, Abolhassani M, Schwartz L, Jolicoeur M, Peres S. Metabolic therapies inhibit tumor growth in vivo and in silico. Sci Rep 2019;9:3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca2+-mediated apoptosis limiting tumour growth. Nature 2017;546:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011;48:856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wadt K, Choi J, Chung JY, Kiilgaard J, Heegaard S, Drzewiecki KT, et al. A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment Cell Melanoma Res 2012;25:815–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wiesner T, Fried I, Ulz P, Stacher E, Popper H, Murali R, et al. Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol 2012;30:e337–40. [DOI] [PubMed] [Google Scholar]
- 154.Holom GH, Seland H, Strandenes E, Liavaag PG, Lybak S, Loes S, et al. Head and neck reconstruction using microsurgery: a 9-year retrospective study. Eur Arch Otorhinolaryngol 2013;270: 2737–43. [DOI] [PubMed] [Google Scholar]
- 155.Ribeiro C, Campelos S, Moura CS, Machado JC, Justino A, Parente B. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol 2013;24:2147–50. [DOI] [PubMed] [Google Scholar]
- 156.Cheung M, Talarchek J, Schindeler K, Saraiva E, Penney LS, Ludman M, et al. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet 2013;206:206–10. [DOI] [PubMed] [Google Scholar]
- 157.Aoude LG, Wadt K, Bojesen A, Cruger D, Borg A, Trent JM, et al. A BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancers. PLoS One 2013;8:e72144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maerker DA, Zeschnigk M, Nelles J, Lohmann DR, Worm K, Bosser-hoff AK, et al. BAP1 germline mutation in two first grade family members with uveal melanoma. Br J Ophthalmol 2014;98:224–7. [DOI] [PubMed] [Google Scholar]
- 159.Betti M, Casalone E, Ferrante D, Romanelli A, Grosso F, Guarrera S, et al. Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosomes Cancer 2015;54:51–62. [DOI] [PubMed] [Google Scholar]
- 160.Cebulla CM, Binkley EM, Pilarski R, Massengill JB, Rai K, Liebner DA, et al. Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet 2015;36:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aoude LG, Gartside M, Johansson P, Palmer JM, Symmons J, Martin NG, et al. Prevalence of germline BAP1, CDKN2A, and CDK4 mutations in an Australian population-based sample of cutaneous melanoma cases. Twin Res Hum Genet 2015;18:126–33. [DOI] [PubMed] [Google Scholar]
- 162.de la Fouchardiere A, Cabaret O, Petre J, Aydin S, Leroy A, de Potter P, et al. Primary leptomeningeal melanoma is part of the BAP1-related cancer syndrome. Acta Neuropathol 2015;129:921–3. [DOI] [PubMed] [Google Scholar]
- 163.Klebe S, Driml J, Nasu M, Pastorino S, Zangiabadi A, Henderson D, et al. BAP1 hereditary cancer predisposition syndrome: a case report and review of literature. Biomark Res 2015;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ohar JA, Cheung M, Talarchek J, Howard SE, Howard TD, Hes-dorffer M, et al. Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res 2016;76:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abdel-Rahman MH, Rai K, Pilarski R, Davidorf FH, Cebulla CM. Germline BAP1 mutations misreported as somatic based on tumor-only testing. Fam Cancer 2016;15:327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McDonnell KJ, Gallanis GT, Heller KA, Melas M, Idos GE, Culver JO, et al. A novel BAP1 mutation is associated with melanocytic neoplasms and thyroid cancer. Cancer Genet 2016;209:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turunen JA, Markkinen S, Wilska R, Saarinen S, Raivio V, Tall M, et al. BAP1 germline mutations in Finnish patients with uveal melanoma. Ophthalmology 2016;123:1112–7. [DOI] [PubMed] [Google Scholar]
- 168.Betti M, Aspesi A, Biasi A, Casalone E, Ferrante D, Ogliara P, et al. CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett 2016;378:120–30. [DOI] [PubMed] [Google Scholar]
- 169.Bochtler T, Endris V, Reiling A, Leichsenring J, Schweiger MR, Klein S, et al. Integrated histogenetic analysis reveals BAP1-mutated epithelioid mesothelioma in a patient with cancer of unknown primary. J Natl Compr Canc Netw 2018;16:677–82. [DOI] [PubMed] [Google Scholar]
- 170.Watchorn RE, Calonje E, Taibjee SM. Germline BRCA1-associated protein 1 mutation presenting as BAP1 inactivated melanocytic nevi in a child of a father with fatal paraganglioma. Pediatr Dermatol 2018;35:e316–e8. [DOI] [PubMed] [Google Scholar]
- 171.Carlo MI, Mukherjee S, Mandelker D, Vijai J, Kemel Y, Zhang L, et al. Prevalence of germline mutations in cancer susceptibility genes in patients with advanced renal cell carcinoma. JAMA Oncol 2018;4:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ravanpay AC, Barkley A, White-Dzuro GA, Cimino PJ, Gonzalez-Cuyar LF, Lockwood C, et al. Giant pediatric rhabdoid meningioma associated with a germline BAP1 pathogenic variation: a rare clinical case. World Neurosurg 2018;119:402–15. [DOI] [PubMed] [Google Scholar]
- 173.Masoomian B, Shields CL, Mashayekhi A, Ganguly A, Shields JA. Growth of presumed choroidal nevus into melanoma over 4 years in Bap1 tumor predisposition syndrome. Retin Cases Brief Rep 2018. July 6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 174.Wysozan TR, Khelifa S, Turchan K, Alomari AK. The morphologic spectrum of germline-mutated BAP1-inactivated melanocytic tumors includes lesions with conventional nevic melanocytes: a case report and review of literature. J Cutan Pathol 2019;46:852–7. [DOI] [PubMed] [Google Scholar]
- 175.Satolli F, Giudice MB, Bertolani M, Ricci R, Lotti T, Zucchi A, et al. BAP1 tumour predisposition syndrome: a new mutation in one family. Acta Derm Venereol 2019;99:1045–6. [DOI] [PubMed] [Google Scholar]
- 176.Chau C, van Doorn R, van Poppelen NM, van der Stoep N, Mensen-kamp AR, Sijmons RH, et al. Families with BAP1-tumor predisposition syndrome in The Netherlands: path to identification and a proposal for genetic screening guidelines. Cancers 2019;11:pii:E1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.O’Shea SJ, Mitra A, Graham JL, Charlton R, Adlard J, Merchant W, et al. Histopathology of melanocytic lesions in a family with an inherited BAP1 mutation. J Cutan Pathol 2016;43:287–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yavuzyigitoglu S, Mensink HW, Smit KN, Vaarwater J, Verdijk RM, Beverloo B, et al. Metastatic disease in polyploid uveal melanoma patients is associated with BAP1 mutations. Invest Ophthalmol Vis Sci 2016;57:2232–9. [DOI] [PubMed] [Google Scholar]


