Abstract
Objective:
Both animal and human data suggest that noradrenergic stimulation may enhance motor performance after brain damage. We conducted a placebo-controlled, double-blind and crossover design study to investigate the effects of noradrenergic stimulation on the cortical motor system in hemiparetic stroke patients.
Methods:
Stroke patients (n = 11) in the subacute or chronic stage with mild-to-moderate hand paresis received a single oral dose of 6mg reboxetine (RBX), a selective noradrenaline reuptake inhibitor. We used functional magnetic resonance imaging and dynamic causal modeling to assess changes in neural activity and interregional effective connectivity while patients moved their paretic hand.
Results:
RBX stimulation significantly increased maximum grip power and index finger-tapping frequency of the paretic hand. Enhanced motor performance was associated with a reduction of cortical “hyperactivity” toward physiological levels as observed in healthy control subjects, especially in the ipsilesional ventral premotor cortex (vPMC) and supplementary motor area (SMA), but also in the temporoparietal junction and prefrontal cortex. Connectivity analyses revealed that in stroke patients neural coupling with SMA or vPMC was significantly reduced compared with healthy controls. This “hypoconnectivity” was partially normalized when patients received RBX, especially for the coupling of ipsilesional SMA with primary motor cortex.
Interpretation:
The data suggest that noradrenergic stimulation by RBX may help to modulate the pathologically altered motor network architecture in stroke patients, resulting in increased coupling of ipsilesional motor areas and thereby improved motor function.
Focal brain lesions may trigger structural and functional changes in perilesional and remote brain regions.1 Functional neuroimaging studies showed that neural activity in ipsilesional and contralesional cortical areas was pathologically increased when stroke patients moved their paretic hand,2 and that overactivity usually decreases over time, concomitant to clinical recovery.2,3 Modulation of cortical networks by pharmacological means or by noninvasive brain stimulation was demonstrated to ameliorate stroke-induced deficits.4-7 For example, the enhancement of monoaminergic influences via amphetamine in combination with physical therapy facilitates functional improvements after focal brain injury in rats, monkeys, and humans.8-11 Data are, however, inconclusive, as several clinical trials could not confirm positive effects of amphetamine for motor recovery in humans.12,13 The beneficial effects of amphetamine for the facilitation of motor recovery may result, at least in part, from interactions with the noradrenergic system.14-16 Recent studies in healthy humans revealed that modulating the noradrenergic system with reboxetine (RBX), a selective noradrenaline (NA) reuptake inhibitor,17 increases motor cortex excitability and improves motor learning as well as the performance in complex visuomotor tasks.18,19 Likewise, motor performance of stroke patients may be improved via noradrenergic stimulation with RBX.20 The neural mechanisms underlying such NA-mediated motor improvements, however, remain to be elucidated. They may encompass direct interactions with the neurons of the motor system (eg, modulating excitability of motor neurons under the influence of brain stem nuclei) and changes in the functional network architecture (eg, modulation of the influence that one neural system exerts over another; ie, “effective connectivity”).21,22
We investigated the effects of RBX in stroke patients at the behavioral and neural levels using functional magnetic resonance imaging (fMRI). Dynamic causal modeling (DCM) was applied to fMRI data of key motor areas to assess the effects of RBX on interregional connectivity within the cortical motor system.23 We hypothesized that RBX-mediated improvements of motor performance in stroke patients might result from a normalization of abnormally increased neural activity in both hemispheres,3,4 which might be caused by enhanced coupling of neural activity between motor areas mediated by noradrenergic stimulation.24,25
Patients and Methods
The study was approved by the local ethics committee. Informed consent was obtained from each subject.
Subjects
Eleven first-ever stroke patients (Table 1) with persistent motor deficits due to ischemia in the middle cerebral artery territory were recruited from the Department of Neurology, University of Cologne (Cologne, Germany). The patients were included based on the following criteria: (1) stable unilateral motor deficit; (2) absence of aphasia, neglect, and apraxia; and (3) no mirror movements of the unaffected hand. The Beck Depression Inventory (BDI) was used to screen for symptoms of depression. According to the Edinburgh Handedness Inventory,26 9 patients were right-handed and 2 patients were left-handed before stroke. Eleven (3 female) age-matched right-handed healthy subjects (mean ± standard deviation [SD]: 63.2 ± 7.9 years) without any history of neurological, psychiatric, or orthopedic disease served as controls.
TABLE 1:
Clinical Data of Patients
| Patient Number |
Age (yr) |
Sex | Handednessa | Affected Hand |
Site of Lesion |
Lesion Size (cm3) |
Lesion Age (wk) |
NIHSSb | mRSb | BDIb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | R | L | R CR | 0.34 | 6 | 1 | 2 | 2 |
| 2 | 42 | M | R | L | R SPL/IS/STG | 168.36 | 4 | 4 | 4 | 1 |
| 3 | 78 | F | R | L | R Th | 0.31 | 88 | 4 | 2 | 10 |
| 4 | 55 | M | R | R | L CD/CR | 0.56 | 28 | 1 | 1 | 10 |
| 5 | 66 | M | R | L | R Th/BG/IC | 28.59 | 43 | 5 | 3 | 9 |
| 6 | 68 | M | R | L | R Th | 0.68 | 91 | 1 | 1 | 0 |
| 7 | 55 | M | R | L | R CR | 1.30 | 67 | 1 | 1 | 6 |
| 8 | 72 | M | R | L | R Th | 0.40 | 66 | 2 | 2 | 12 |
| 9 | 74 | M | R | R | L Th | 0.27 | 101 | 1 | 1 | 1 |
| 10 | 62 | M | L | R | L GP/IC | 0.68 | 275 | 4 | 3 | 7 |
| 11 | 50 | M | L | R | L SPL/Put | 28.96 | 84 | 1 | 2 | 6 |
| Mean ± SD | 62.4 ± 10.9 | 77.5 ± 73.7 | 2.3 ± 1.6 | 2.0 ± 1.0 | 5.8 ± 4.2 |
Handedness was determined according to the Edinburgh Handedness Inventory.26
These clinical scores were assessed on the first day of examination.
BDI = Beck Depression Inventory; BG = basal ganglia; CD = caudate; CR = corona radiata; GP = globus pallidus; IC = internal capsule; IS = insula; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale; Put = putamen; SPL = superior parietal lobule; STG = superior temporal gyrus; Th = thalamus. L = left; R = right; SD = standard deviation.
Experimental Design
A placebo (PBO)-controlled, double-blind and crossover design was employed. A single oral dose of 6mg RBX (Solvex; Merz Pharmaceuticals, Frankfurt, Germany; tmax ≈ 2 hours; elimination half-life ≈ 13 hours) was used as a noradrenergic stimulant.27 The dose of 6mg RBX had been proven to be sufficient in modulating motor performance of stroke patients.20 Six patients initially received RBX, followed 7 days later by PBO, allowing for a sufficiently long washout phase. The remaining 5 patients initially received PBO, followed 7 days later by RBX. Behavioral performance was tested twice (ie, before [=baseline, BASE session] and about 2.5 hours after drug administration [DRUG session]) on each testing day. Before the second behavioral testing, patients were scanned with fMRI. The control subjects did not receive any drug (neither RBX nor PBO), and underwent only one fMRI testing and one behavioral session using the identical setup as for the patients. These data served as reference for physiological brain activations, brain connectivity, and motor performance.
Behavioral Tasks
The behavioral test battery comprised: (1) the action research arm test (ARAT), (2) maximum hand grip strength measured with a vigorimeter in 3 trials (Martin, Tuttlingen, Germany), (3) maximum index finger-tapping frequency in three 5-second trials, and (4) rapid pointing movements between 2 targets separated by a distance of 30cm as fast and as accurately as possible in two 15-second trials (see Supporting Fig. 1 for more information). Kinematic data of the index finger-tapping and rapid pointing movements were measured with the Zebris CMS20S system (Zebris Medical GmbH, Isny, Germany).18
fMRI
fMRI MOTOR TASK.
For fMRI, subjects were instructed to perform index finger-tapping movements as quickly as possible with the left or right hand (indicated by a visual cue). In a block, 3 cycles of index finger-tapping of either hand (for 4 seconds) were separated by a 2-second pause to prevent muscular fatigue. Movement blocks were separated by baselines (lasting 16 seconds) in which subjects were instructed to relax and keep still. The whole experiment lasted approximately 11 minutes. The motor performance (ie, number of taps per block) of each patient was recorded by an experimenter standing inside the scanner room. No mirror movements were detected when patients moved either the affected or unaffected hand.
IMAGE ACQUISITION.
All fMRI images were acquired with a Siemens Trio 3.0T scanner (Erlangen, Germany) using a gradient echo planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2250 msec; echo time (TE) = 30 msec; field of view (FOV) = 200 mm; axial slices = 37; slice thickness = 3.4 mm; distance factor = 15%; in-plane resolution = 3.1 × 3.1 mm2; and flip angle = 90°. Afterward, high-resolution T1-weighted images and T2 fluid-attenuated inversion recovery images were acquired for all subjects.
Data Analyses
Prior to data analyses, the MRI volumes of 7 patients with right hemispheric lesions were flipped along the midsagittal plane.24 The data of 7 controls were processed in the same manner. As a result, the affected and unaffected hands in the patient group corresponded to the “right” and “left” hands in the control group, respectively.
GENERAL LINEAR MODEL fMRI ANALYSIS.
Images were processed using the Statistical Parametric Mapping (SPM) software package (SPM5; Wellcome Trust Centre for Neuroimaging, University College London, UK) for spatial preprocessing (realignment, spatial normalization, and smoothing) and first-level statistical analysis (see Supporting Information). For spatial normalization, the high-resolution T1-weighted image of each subject was coregistered to the mean realigned EPI image. The coregistered T1-weighted image was spatially normalized to the Montreal Neurological Institute (MNI) single-subject template using the unified segmentation approach,28 in which warping regularization was set to 1 and bias regularization was set to 0.0001. This approach has been proven to be most suitable for spatial normalization of brain images with focal lesions (eg, stroke).29 In addition, a binary lesion mask was created by manually outlining the precise boundaries of the lesion on the T1-weighted image for each patient using MRIcron (Version 7, July 2009; http://www.sph.sc.edu/comd/rorden/mricron). The lesion mask was smoothed (full-width at half-maximum [FWHM] = 8mm [the same FWHM as used for smoothing EPI images], threshold = 0.1%) and was then used for cost function masking during segmentation.30,31 The resulting deformation parameters were then applied to the individual EPI volumes. The quality of the normalization was checked by 2 authors (L.W. and C.G.) using outer brain margins as well as anatomical landmarks (eg, central sulcus, hand knob, insular cortex, and corpus callosum) when comparing the normalized EPI images with the SPM canonical single-subject brain in MNI space. Distances between landmarks on the normalized EPIs and the SPM single-subject template did not exceed 3mm (ie, voxel size of the original EPI volumes), indicating successful spatial normalization results.
For group analysis, the parameters derived from the first-level analysis were compared in 2 full-factorial analyses of variance (ANOVA) with (1) the factors “hand” (right/affected, left/ unaffected) and “group” (controls, patients [PBO]), and (2) the factors “hand” (affected, unaffected) and “agent” (PBO, RBX). The statistical threshold was set at p < 0.05 (family-wise error [FWE] corrected at the cluster level; p < 0.001 uncorrected at the voxel level).
REGION OF INTEREST ANALYSIS.
Region of interest (ROI) analyses were performed on the key motor areas activated during the tapping task, comprising the primary motor cortex (M1), supplementary motor area (SMA), ventral premotor cortex (vPMC), and dorsal premotor cortex (dPMC) (see Supporting Information for more detailed anatomical constraints) using the SPM toolbox “MarsBar” (version 0.41). The cortical and subcortical stroke lesions in the patients’ group did not overlap with any of the assessed ROIs, and did not directly interrupt structural connections between ROIs as inferred from the T1-weighted images. The estimated parameters for hand movements of each motor area were compared between controls and patients (PBO) as well as between PBO and RBX (threshold of p < 0.05).
DYNAMIC CAUSAL MODELING.
Dynamic causal modeling (DCM) is a hypotheses-driven approach to model effective connectivity between distinct brain regions and relies on a priori neurobiological models reflecting the hypothesis on relevant regions, connections and context-dependent modulations thereof.23 We focused our analysis on the core regions of the motor system activated in the finger-tapping task, including bilateral M1, SMA, and vPMC.32 The BOLD time series was extracted from each region at subject specific coordinates (8-mm spheres around individual activation maxima) in the first-level analysis. Four anatomically plausible models differing in effective connectivity (Fig 1) were estimated and compared by Bayesian model selection to determine the most likely model given the data using a random effects approach.33 The statistical significance of the derived coupling parameters was tested by means of one-sample t tests (p < 0.05, false discovery rate [FDR], corrected for multiple comparisons).34 For more detailed information re. the DCM analysis, please see Supporting Information.
FIGURE 1:
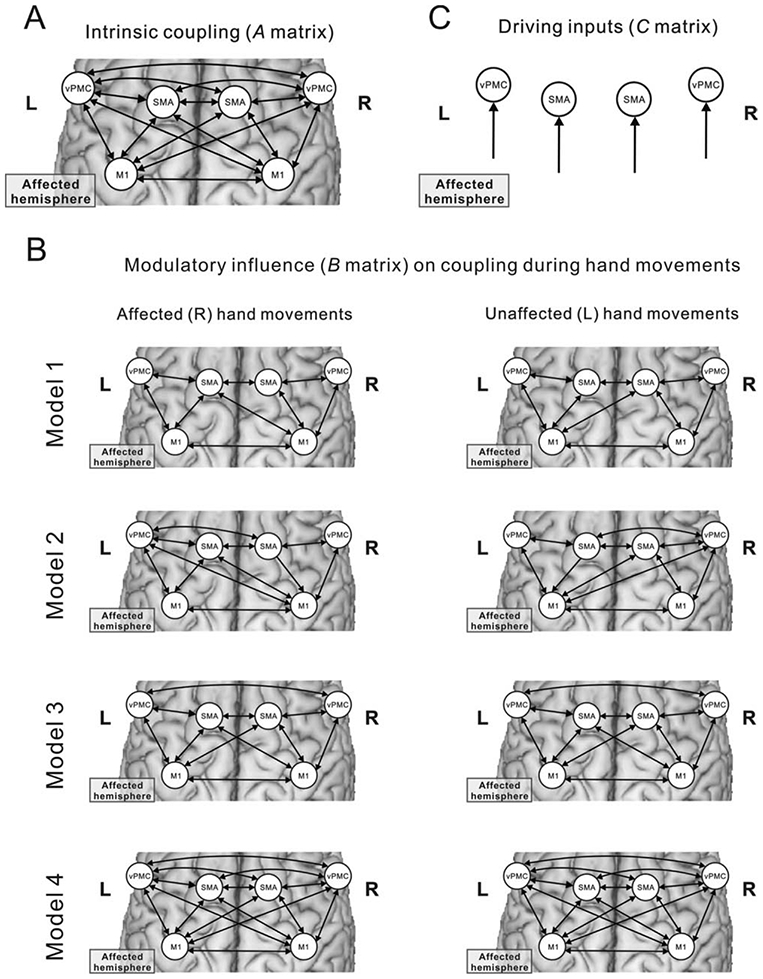
DCM models. (A) Intrinsic (ie, task-independent) connectivity. (B) Alternative models of connectivity modulated by left-hand or right-hand movements used for Bayesian model selection. (C) Driving inputs on the modeled regions. DCM = dynamic causal modeling.
EFFECTS OF LESION LOCATION, SIZE, AND AGE.
To determine whether RBX effects detected at the behavioral, neural, and connectivity levels depended on lesion location, we performed a voxel-based lesion-symptom mapping (VLSM) analysis with MRIcron.35,36 Lesion masks were interactively constructed based on the T1 image of each patient as described above. They were subsequently normalized to MNI space using the SPM software by applying the deformation parameters derived from the respective T1 images. In VLSM analysis, the RBX effects at behavioral, neural, and connectivity levels were treated as continuous variables, respectively. A t test was performed to identify statistically significant differences (FDR corrected, p < 0.05). Furthermore, we correlated the RBX effects with lesion size and age, respectively.
Results
Behavioral Results
CONTROLS vs PATIENTS.
A multivariate analysis of variance with the factors ‘group” (patients [PBO_BASE], controls) and “hand” (affected/right, unaffected/left) on all motor parameters revealed a significant main effect of “hand” (F(5,16) = 8.240, p = 0.001) and a significant interaction (F(5,16) = 4.343, p = 0.011), indicating a hand-specific motor deficit in the patients’ group compared with controls (Table 2).
TABLE 2:
Motor Performance of the Right/Affected Hand
| Tasks | Unit (mean ± SD) |
Controls | Patients_PBO |
Patients_RBX |
Agent |
Session |
Agent × Session |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BASE | DRUG | BASE | DRUG | F(1,10) | p | F(1,10) | p | F(1,10) | p | |||
| ARAT | 57 | 43 | 44 | 42 | 44 | 0.007 | 0.937 | 6.852 | 0.026* | 2.664 | 0.134 | |
| 0 | 11 | 11 | 12 | 12 | ||||||||
| Hand grip strength | kPa | 89.27 | 56.00 | 57.36 | 54.82 | 62.64 | 0.459 | 0.513 | 10.195 | 0.010* | 6.792 | 0.026* |
| 19.79 | 23.50 | 20.88 | 24.50 | 28.43 | ||||||||
| Index finger-tapping | Frequency (Hz) | 4.65 | 2.75 | 2.96 | 2.73 | 3.20 | 1.340 | 0.274 | 20.848 | 0.001** | 8.382 | 0.016* |
| 0.82 | 1.33 | 1.23 | 1.35 | 1.27 | ||||||||
| Amplitude (mm) | 33.23 | 26.31 | 23.09 | 23.59 | 24.72 | 0.164 | 0.694 | 0.425 | 0.529 | 1.925 | 0.195 | |
| 6.17 | 15.47 | 13.89 | 14.60 | 12.48 | ||||||||
| Rapid pointing movements | Frequency (Hz) | 1.81 | 0.95 | 0.99 | 1.00 | 1.09 | 3.354 | 0.097 | 4.227 | 0.067 | 1.595 | 0.235 |
| 0.52 | 0.67 | 0.60 | 0.64 | 0.66 | ||||||||
| Amplitude (mm) | 306.73 | 303.95 | 295.67 | 304.60 | 300.36 | 0.135 | 0.721 | 1.425 | 0.260 | 0.245 | 0.631 | |
| 19.44 | 24.57 | 29.79 | 10.00 | 22.39 | ||||||||
Means and SDs are shown. Post hoc paired t tests showed that compared with the respective BASE session, grip power and index finger-tapping frequency were significantly higher after treatment with RBX (FDR corrected, p < 0.05) but not after PBO administration.
p < 0.05.
p < 0.01.
ARAT = action research arm test; BASE = baseline; DRUG = postadministration session; FDR = false discovery rate; PBO = placebo; RBX = reboxetine; SD = standard deviation.
RBX EFFECTS IN STROKE PATIENTS.
Dry mouth occurred in 4 patients after ingestion of RBX and in 1 patient after PBO administration. Other side effects were not reported.
For the stroke-affected hand, motor performance of the ARAT and the rapid pointing task were unaffected by RBX, as indicated by the nonsignificant interaction effect between “agent” (PBO, RBX) and “session” (BASE, DRUG) in a repeated measures ANOVA (RM-ANOVA) (see Table 2). In contrast, there were a significant interaction effect and a significant main effect of “session” for maximum grip power and index finger-tapping frequency (p < 0.05; Fig 2), indicating that the motor performance of the two tasks was significantly increased by RBX, but not by PBO. A similar RBX effect was observed for the tapping performance during fMRI (PBO [mean ± SD] = 2.83 ± 1.48Hz; RBX = 3.30 ± 1.62Hz; t(10) = 4.058, p < 0.05, FDR corrected).
FIGURE 2:
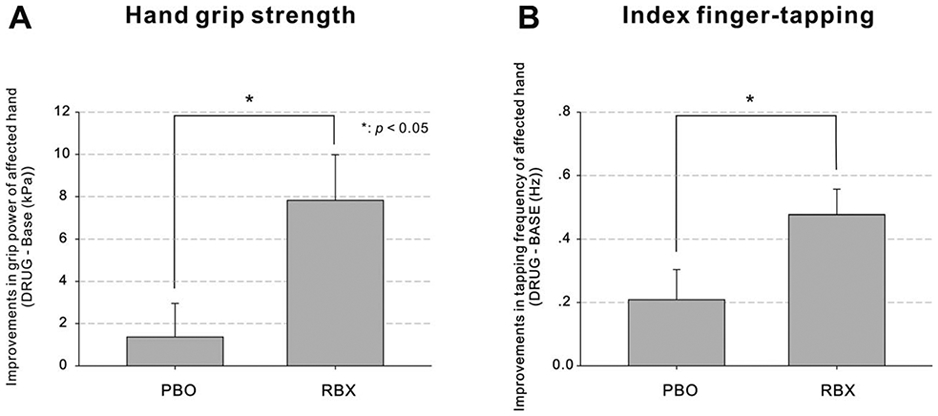
Improvement in motor performance under RBX. (A) Improvements in hand grip strength (DRUG – BASE). (B) Improvements in index finger-tapping frequency (DRUG – BASE). Error bars = SEM. *p < 0.05. BASE = baseline; DRUG = drug administration; RBX = reboxetine; SEM = standard error of the mean.
The VLSM analysis revealed no significant relationship between lesion location and RBX-mediated improvements in grip strength or finger-tapping frequency. In addition, no correlation was found between lesion size and improved motor performance following treatment with RBX. However, a statistically significant inverse relationship (r = −0.65, p < 0.05) between lesion age and improvements in grip power (RBX_[DRUG – BASE] – PBO_[DRUG – BASE]) was identified: The older the lesion, the less effective the grip power enhancement by RBX. In addition, there was no significant difference in motor performance or response to RBX between patients with (ie, the 4 patients with BDI scores ≥ 9) and patients without symptoms of depression (ie, BDI scores < 9) (p > 0.05).
There were no significant RBX effects on any motor parameter of the unaffected hand (p > 0.05). Therefore, the subsequent analyses of fMRI data were focused on movements of the stroke-affected hand.
Changes in BOLD Activity
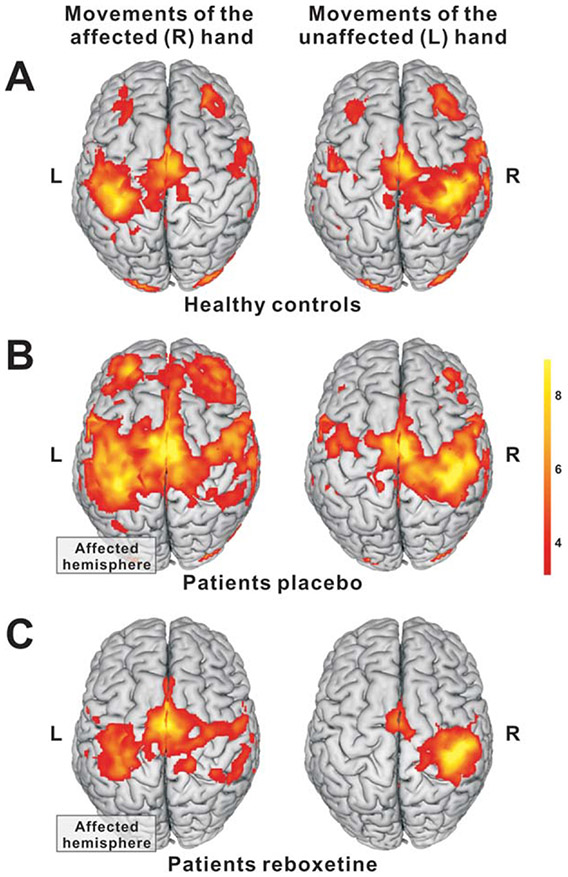
Patients under PBO exhibited a more extended and bilateralized activation pattern when moving the paretic hand compared with healthy subjects (Fig 3A,B). When patients moved their paretic hand after RBX administration, task-related neural overactivity was significantly reduced in a number of areas in both hemispheres including ipsilesional vPMC and dorsolateral prefrontal cortex (Fig 4; Table 3).
FIGURE 3:
BOLD activity during hand movements in healthy controls and patients (FWE corrected; p < 0.05 at the cluster level). Compared with (A) controls, (B) patients had enhanced activity in ipsilesional and contralesional motor areas when moving their affected hand. (C) The overactivity was reduced after administration of RBX compared with PBO. BOLD = blood oxygen level dependent; FWE = family-wise error; PBO = placebo; RBX = reboxetine.
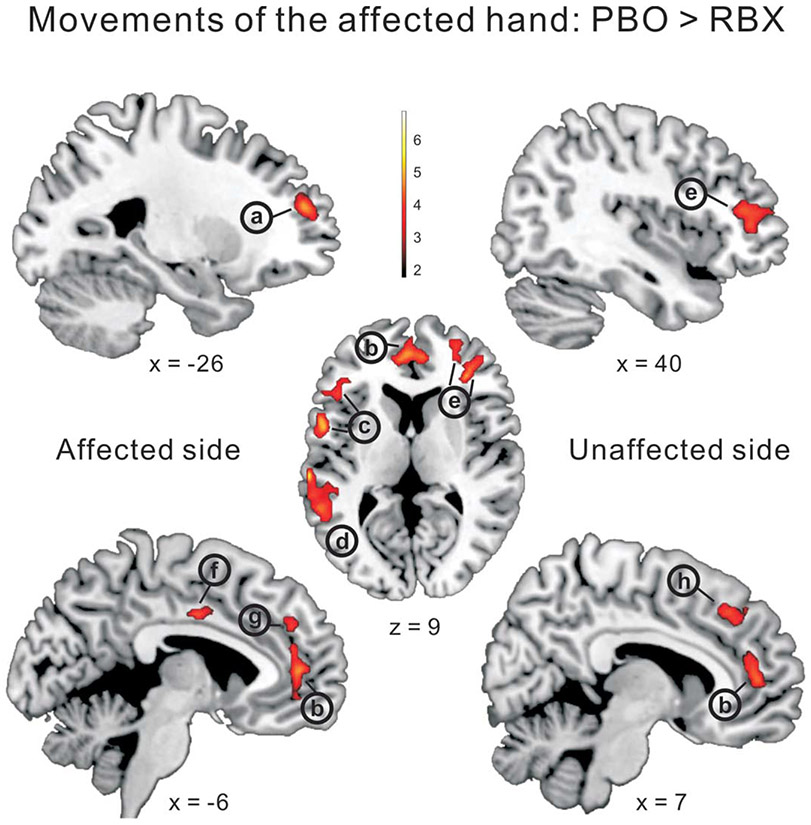
FIGURE 4:
Whole-brain voxelwise analysis. RBX effects during movements of the affected hand (FWE corrected; p < 0.05). Compared with PBO, RBX significantly reduced the BOLD activity in both hemispheres, including (a) ipsilesional middle frontal gyrus, (b) bilateral anterior cingulate cortex, (c) ipsilesional inferior frontal gyrus (ventral premotor cortex), (d) ipsilesional temporoparietal junction/superior temporal gyrus, (e) contralesional superior frontal gyrus, (f) ipsilesional middle cingulate cortex, (g) ipsilesional superior medial frontal gyrus, and (h) contralesional superior medial frontal gyrus. BOLD = blood oxygen level dependent; FWE = family-wise error; PBO = placebo; RBX = reboxetine.
TABLE 3:
Local Maxima of Significantly Activated Regions
| Coordinates | Side | Region | t |
|---|---|---|---|
| −64, −26, 7 | IL | Superior temporal gyrus | 6.87 |
| −56, 6, −9 | IL | Temporal pole | 5.64 |
| −52, 16, −19 | IL | Temporal pole | 5.61 |
| 18, 52, 31 | CL | Superior frontal gyrus | 5.39 |
| −24, 50, 19 | IL | Middle frontal gyrus | 5.21 |
| −2, 44, 31 | IL | Superior medial gyrus | 5.18 |
| −52, 18, 23 | IL | Inferior frontal gyrus, ventral premotor cortex | 5.12 |
| 62, −30, −15 | CL | Inferior temporal gyrus | 4.99 |
| −2, 50, 9 | IL | Anterior cingulate cortex | 4.86 |
| −4, −8, 37 | IL | Middle cingulate cortex | 4.80 |
| 68, −20, −11 | CL | Middle temporal gyrus | 4.75 |
| 64, −24, −21 | CL | Inferior temporal gyrus | 4.75 |
| 6, 48, 7 | CL | Superior medial frontal gyrus | 4.72 |
| 6, 32, 43 | CL | Superior medial frontal gyrus | 4.72 |
| −58, −48, 11 | IL | Middle temporal gyrus | 4.65 |
| 28, 54, 11 | CL | Superior frontal gyrus | 4.58 |
| −60, −32, 17 | IL | Superior temporal gyrus | 4.43 |
| −16, 44, 33 | IL | Superior frontal gyrus | 4.11 |
The above results in this table were from the contrast of PBO_Aff > RBX_Aff (FWE corrected, p < 0.05). RBX_Aff > PBO_Aff, nonsignificant results; PBO_Aff > Controls_R, nonsignificant results; Controls_R > PBO_Aff, nonsignificant results.
Aff = affected hand movements; CL = contralesional side; FWE = family-wise error; IL = ipsilesional side; PBO = placebo; R = right hand movements; RBX = reboxetine.
Furthermore, we investigated BOLD signal changes in a priori defined ROIs representing key areas of the motor system so as to enhance statistical sensitivity. The RM-ANOVA with the factors “group” and “region” on estimated effect sizes during the right/affected finger movements revealed a significant main effect of “group” (F(1,20) = 6.625; p = 0.018). Independent samples t tests (one-tailed; Fig 5) showed that compared with controls, patients had significantly increased BOLD activity in ipsilesional vPMC, dPMC, and M1 as well as in contralesional SMA, dPMC, and M1, and exhibited a trend toward increased activity in ipsilesional SMA (t(20) = 1.456; p = 0.080). Compared with PBO, RBX significantly reduced BOLD activity during movements of the affected hand in ipsilesional SMA and vPMC (see Fig 5). These RBX-mediated changes in BOLD activity, however, did not correlate with the observed increase in finger-tapping frequency of the affected hand (p > 0.05).
FIGURE 5:
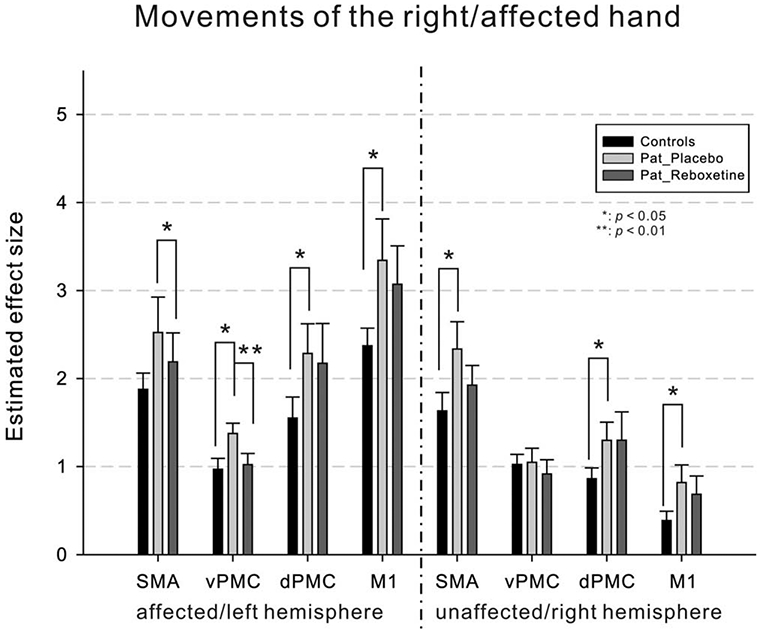
Region of interest analysis. When moving the affected hand, patients (PBO) showed overactivity in ipsilesional/left vPMC, dPMC, and M1, as well as in contralesional/right SMA, dPMC, and M1 compared with controls. After administration of RBX, the overactivity in ipsilesional/left SMA and vPMC was reduced compared with PBO. Error bars = SEM. *p < 0.05; **p < 0.01. dPMC = dorsal premotor cortex; M1 = primary motor cortex; PBO = placebo; RBX = reboxetine; SEM = standard error of the mean; SMA = supplementary motor area; vPMC = ventral premotor cortex.
The VLSM analysis did not show a significant relationship between lesions affecting the deep gray matter and RBX-modulated BOLD activity (p > 0.05). However, we found that patients with lesions in the posterior limb of the internal capsule, corresponding to the corticospinal tract according to the SPM Anatomy toolbox,37 were more likely to exhibit decreases of overactivity in ipsilesional vPMC (p < 0.05). In addition, RBX-mediated changes in BOLD activity during movements of the affected index finger were not significantly different between patients with and without symptoms of depression as indicated by the BDI scores (p > 0.05).
Connectivity Analysis
The Bayesian model selection procedure showed that model 4 (see Fig 1) yielded highest model evidence for representing the intrahemispheric and interhemispheric interactions among SMA, vPMC, and M1 (expected posterior probability and exceedance probability are given in Supporting Fig 2). Importantly, Bayesian model selection results were consistent across both groups and the 2 sessions (ie, PBO and RBX).
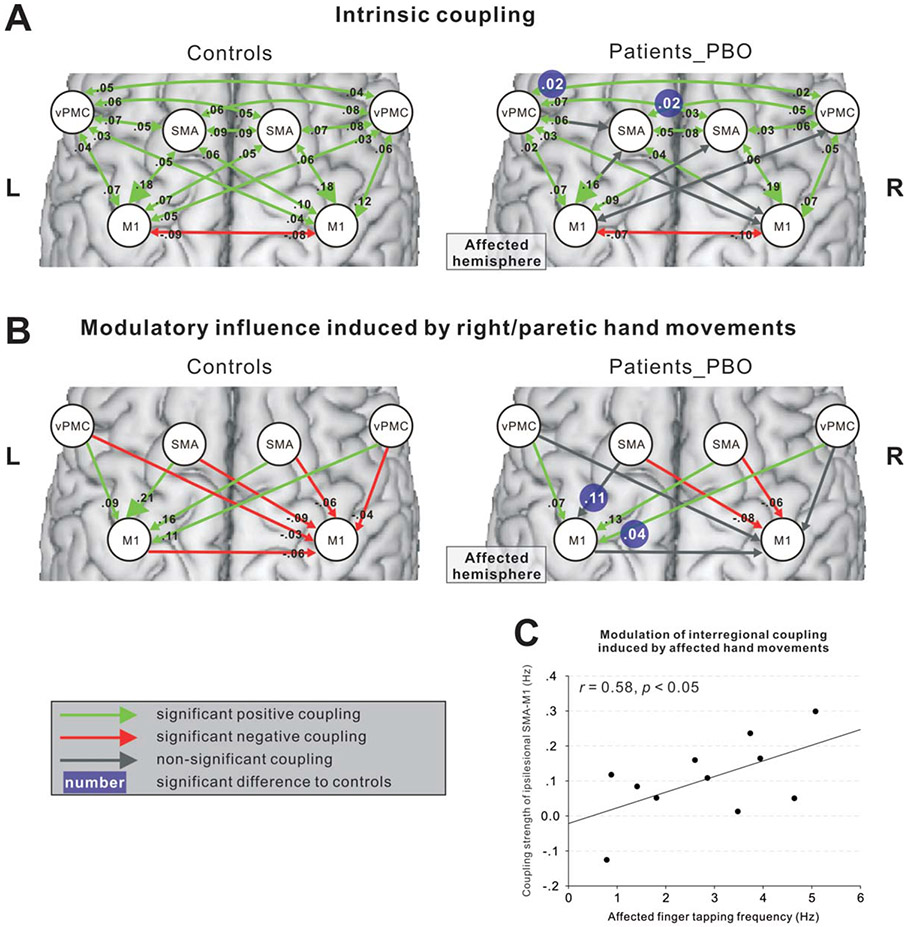
Independent samples t tests for each coupling revealed that intrinsic coupling parameters with SMA or vPMC were significantly reduced in patients compared with controls (Fig 6A; Supporting Table 1). Also the modulatory influence of moving the affected hand on interregional coupling was significantly reduced between ipsilesional SMA and ipsilesional M1 as well between contralesional vPMC and ipsilesional M1 in the patients’ group (see Fig 6B; Supporting Table 2). The “hypoconnectivity” of ipsilesional SMA-M1 was significantly correlated with poorer tapping performance of the paretic hand (see Fig 6C). The equivalent coupling parameter in healthy subjects was not significantly correlated with the absolute maximum tapping performance of the right hand (p > 0.1). Fisher’s z-test confirmed that the correlation coefficient for the patient group differed significantly from that computed for the control group (z = 2.571; p = 0.010). Furthermore, patients showed decreased task-related inhibitory coupling from ipsilesional M1 or contralesional vPMC to contralesional M1.
FIGURE 6:
Effective connectivity in controls and patients (FDR corrected, p < 0.05). (A) Intrinsic coupling. (B) Modulatory influence induced by right/paretic hand movements. The left and right panels show the results of control subjects and stroke patients, respectively. The white numbers with blue background in the right panel indicate the significant difference between patients and controls (FDR corrected, p < 0.05). (C) Correlation between the coupling strength from ipsilesional SMA to ipsilesional M1 and the motor performance of the affected hand (p < 0.05). FDR = false discovery rate; M1 = primary motor cortex; SMA = supplementary motor area.
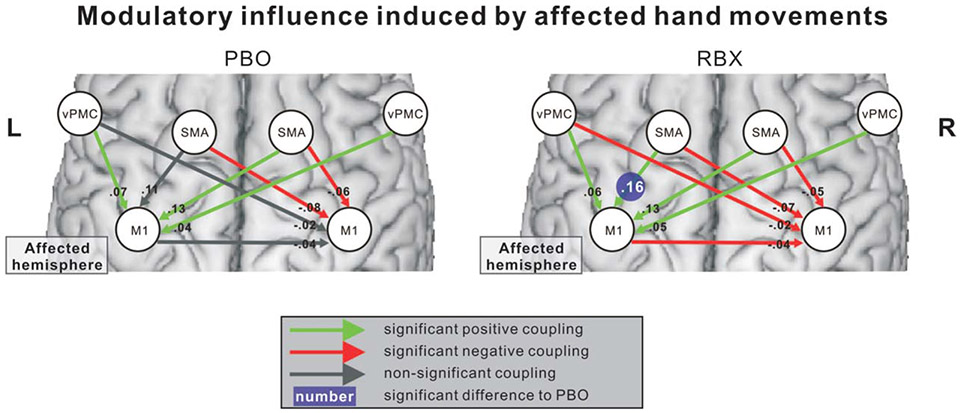
Paired t tests revealed that task-independent (intrinsic) couplings were not statistically different between PBO and RBX (p > 0.05). In contrast, neural coupling parameters during movements of the paretic hand were significantly increased between ipsilesional SMA and ipsilesional M1 after RBX administration compared with PBO (Fig 7). However, there was no significant correlation between increases in ipsilesional SMA-M1 coupling and improvements in finger-tapping frequency (p > 0.01), suggesting that other factors might also influence RBX-mediated improvements. Further analysis revealed that there was a significant correlation between finger-tapping improvements and ARAT scores (rs = 0.83; p < 0.01). Hence, the level of impairment seems to also influence the behavioral responses to RBX. The effects of RBX on interregional coupling were, however, not related to lesion location (as tested in the VLSM analysis), lesion age, or lesion size (p > 0.1).
FIGURE 7:
RBX effects on effective connectivity during affected hand movements. The left and right panels demonstrate the modulatory influence induced by affected hand movements in PBO and RBX sessions, respectively. The coupling strength of ipsilesional SMA-M1 significantly increased after administration of RBX compared with PBO (FDR corrected; p < 0.05). FDR = false discovery rate; M1 = primary motor cortex; PBO = placebo; RBX = reboxetine; SMA = supplementary motor area.
Discussion
RBX-mediated enhancements in motor performance of the stroke-affected hand were associated with a reduction of abnormally increased cortical activity in both hemispheres, resulting in near to normal levels of motor-related activity (compared with healthy controls). Furthermore, connectivity analyses revealed that abnormal “hypoconnectivity” between ipsilesional SMA and M1 was improved under RBX. The data suggest that noradrenergic stimulation via RBX may help to correct abnormal interactions between cortical areas of stroke patients, thereby ameliorating stroke-induced motor deficits.
Noradrenergic Modulation of Motor Performance
Stimulating noradrenergic mechanisms with amphetamine has been shown to facilitate functional improvements in stroke patients.9,10 Evidence from animal studies also supports the role of the noradrenergic system in enhancing motor recovery. For example, depletion of NA by the neurotoxin DSP-4 or by selective lesions of the pontine nucleus locus coeruleus (LC), which is the major source of central noradrenergic projection fibers,21 delayed motor recovery of rats.38,39 In agreement with the findings of Zittel and colleagues,20 we show here that enhancing noradrenergic mechanisms by means of RBX significantly improves index finger-tapping frequency and grip power of the paretic hand. We also found that the shorter the time since stroke, the greater the RBX-mediated improvement in grip strength. This finding indicates that noradrenergic stimulation might be especially effective when neural reorganization processes, for example, changes in transmitter receptor density or expression of transcription factors,40,41 are still upregulated during the first few weeks and months poststroke. However, our and previous studies revealed that RBX did not affect the performance of index finger-tapping in the unaffected hand of stroke patients or in healthy subjects.18,19 This result suggests that RBX does not improve tapping performance per se but could rather enable a better use of neural resources underlying finger-tapping in disturbed networks after stroke. In addition, the RBX effects did not differ between patients with and without symptoms of depression at the behavioral and neural level. As antidepressant drugs (including RBX) usually have a delayed onset of action on symptoms of depression attributed to remodeling effects (which usually also impacts on psychomotor symptoms), patients with higher BDI might show stronger motor improvements when RBX is taken on a regular basis over weeks and months.
RBX-mediated Changes in Neural Activity
Enhanced finger-tapping frequency under RBX was associated with a reduction of overactivity during movements of the paretic hand, especially in ipsilesional vPMC, SMA, and cingulate cortex. This finding resembles the results of prior studies in which improvements in motor functions of stroke patients were associated with a decrease in task-related overactivity.3,42,43 A recent meta-analysis of human imaging data suggests that both SMA and vPMC constitute key motor areas for performing finger-tapping tasks similar to the one used in the present study.32 Studies in monkeys showed that these areas have extensive projections to M1 and, therefore, might play an important role in motor recovery,44-46 for example, by facilitating the motor output of M1 neurons through corticocortical projections.47 The finding that the normalization of overactivity also depended on the integrity of the corticospinal tract underpins its importance for reorganization and functional recovery after stroke.48,49 However, increases in tapping frequency are usually paralleled by an increase in task-related BOLD signal in both healthy subjects and stroke patients.50,51 We, therefore, propose that the here observed RBX-induced reductions in movement-related activity reflect more efficient interactions among the motor areas via stronger neuronal coupling.52 A recent study on motor learning showed that reduced attentional control after movement automatization is accompanied not only by decreases in neural activity but also by increases in effective connectivity in task-related brain areas, suggesting a more efficient neural coding of movement.53 Similar processes could underlie the findings observed in the present study.
In addition to changes in “classical” motor areas, RBX stimulation in this study led to decreases in more “cognitive” regions, such as the dorsolateral prefrontal cortex and the temporal cortex. Neuroimaging studies have shown that these areas are typically involved in tasks relying on motor learning.54 In these studies, practice-induced improvements in task performance are usually associated with a reduction of neural activity in frontotemporal areas, probably owing to less cognitive effort for practiced tasks.55,56 Similar processes might explain the reductions in BOLD signal observed for areas in the prefrontal cortex and the temporal cortex when patients were stimulated with RBX (ie, reduced demands upon motor control as a result of improved motor performance). Although changes in prefrontal activity might also have impacted on interactions with premotor and motor areas (eg, less attentional control of motor areas after RBX),57 we focused our connectivity analysis on the areas of the motor system as we sought to test the hypothesis that stimulating the noradrenergic system may improve the functional network architecture of the motor system thereby inducing improved task performance.
Effects of NA on Cerebral Motor Networks
The DCM data suggest that enhancing noradrenergic transmission in stroke patients induced a rearrangement of motor network interactions. Specifically, the influence of ipsilesional SMA on ipsilesional M1 was significantly enhanced after RBX administration compared with PBO, and correlated with the tapping performance of the affected hand, but did not correlate with the tapping performance in control subjects. Such a difference indicates that disturbed SMA-M1 interactions in stroke patients might contribute to the motor deficits of the paretic hand. As a similar SMA-M1 “hypoconnectivity” has also been found in a different sample of patients performing a different motor task (hand clenching movements),24 a general dysfunction of this connection is likely to constitute a relevant factor for stroke-induced motor impairments. Therefore, the increase in coupling strength of ipsilesional SMA-M1 under noradrenergic stimulation could represent a critical factor mediating behavioral improvements of the stroke-affected hand. Tracer studies in monkeys revealed dense axonal projections from neurons in SMA to neurons in M1, especially between the respective finger representations of the two areas.45,58 Considering the role of the SMA in the generation of internally-driven movements or movement sequences,59 these dense projections toward M1 might be especially important for the task-dependent modulation of M1 activity underlying finger-tapping movements. Furthermore, immunohistochemical studies showed that the sensorimotor areas along the central sulcus and SMA exhibit a high density of NA-containing fibers in both monkeys and humans.60-62 Therefore, manipulating the NA system by means of RBX might be especially effective for connections targeting the sensorimotor regions, such as the SMA-M1 interactions.
Although increased coupling of ipsilesional SMA-M1 was not correlated with improved affected index finger-tapping frequency, the improvement in finger-tapping frequency was correlated with ARAT scores. Hence, the level of impairment seems to influence the behavioral responses to RBX. This hypothesis is supported by results from a clinical trial which showed that patients with less motor impairments showed the best behavioral response to amphetamine.63 Therefore, increases in SMA-M1 coupling might promote improvements in motor performance under RBX stimulation, but this is not the only factor influencing a positive behavioral response to RBX. It is well conceivable that patients with stronger disruption of motor networks (as clinically indicated by lower ARAT) either do not respond to a single dose of RBX or need much higher increases in SMA-M1 coupling to achieve a behavioral gain, as observed in patients with less motor network disruption. One factor that has been shown to influence motor deficits and recovery thereof is the integrity of the corticospinal tract (CST).48,64 The VLSM analysis did not reveal a significant relationship between lesion location (eg, internal capsule) and improvements in motor performance after RBX. However, quantitative data as provided by diffusion tensor imaging techniques might be more sensitive in detecting a relationship between CST damage and response to noradrenergic interventions.64
Transcranial magnetic stimulation studies have shown that pharmacological stimulation of the noradrenergic system in humans may rapidly change neural excitability and practice-dependent plasticity in M1.19,65,66 A number of animal studies have demonstrated that such plasticity-related phenomena can be attributed to the LC-NA system.21 For example, ocular dominance plasticity in kitten visual cortex was abolished by the depletion of NA but was enhanced by the local infusion of NA.67,68 It was further demonstrated that NA facilitates synaptic plasticity via a N-methyl-D-aspartic acid (NMDA) receptor-gated mechanism,69,70 indicating that NA may play a role in supporting activity-dependent modifications of neuronal connections. Studies in rats demonstrated that overall functional connectivity (eg, synchronous activity) among ensembles of neurons may be enhanced with increasing NA efflux, which resembles our finding of enhanced effective connectivity under RBX stimulation.51 Other electrophysiological studies in rodents showed that enhancing NA transmission may increase the signal-to-noise ratio (measured as spike-train activity) in cortical areas by decreasing spontaneous activity.71 Similar mechanisms might underlie the regionally-specific changes in connectivity observed as a result of RBX stimulation: Although RBX may have induced a system-wide increase in NA levels, neuronal processing was especially enhanced among those regions showing pathologically-reduced interregional coupling. Such facilitated gating of neural information after NA stimulation has also been observed in electrophysiological experiments in animals and may underlie enhanced motor performance of the paretic hand after RBX stimulation in the current study.25
Conclusions and Clinical Implications
The current study shows that improvements in motor function observed under RBX result from a rearrangement of cortical interactions, ultimately enhancing the initially reduced motor output of the lesioned hemisphere. It is important to note, however, that the RBX-improved motor performance of stroke patients observed in the current and a previous study represents a short-term effect.20 In animals, repeated administration of RBX induced a stronger increase in extracellular NA than a single dose of reboxetine did,72 and also increased the affinity of alpha-1-adrenergic receptors.73 In addition, repeated administration of the alpha-1 receptor agonist methoxamine increased the density of cortical synapses.74 These long-term administration-induced adaptive changes in the noradrenergic system might consolidate the behavioral improvements induced by a single dose of stimulation and thereby induce long-lasting functional improvements. Therefore, longitudinal studies testing the efficacy and safety of RBX are now needed to further evaluate the potential of this drug to promote functional recovery in stroke patients.
In addition, clinical trials evaluating the chronic administration of amphetamine on facilitating motor recovery in stroke patients yielded both positive and negative results. Factors that might influence the response to amphetaminergic stimulation might be the degree of (initial) motor impairment and the content of physical therapy applied together with pharmacotherapy.63,75 Finally, functional neuroimaging and analyses of connectivity might be useful to identify the differences in brain networks in responders and nonresponders to daily-administered amphetamine. These results might be helpful to decide which patients might especially profit from stimulation by means of amphetamine or RBX.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Human Brain Project (R01-MH074457-01A1 to S.B.E.) and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model to S.B.E.).
We thank all of the patients and subjects who participated in the study. We also thank Dr Marc Tittgemeyer, Dr Michael von Mengershausen, Timm Wetzel, and Kurt Wittenberg for their continued and valuable support.
Footnotes
Additional Supporting Information can be found in the online version of this article
Potential Conflict of Interest
Nothing to report.
References
- 1.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 2008;63:272–287. [DOI] [PubMed] [Google Scholar]
- 2.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke 2003;34:1553–1566. [DOI] [PubMed] [Google Scholar]
- 3.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003;126:2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol 2008;65:741–747. [DOI] [PubMed] [Google Scholar]
- 5.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005;128:490–499. [DOI] [PubMed] [Google Scholar]
- 6.Sparing R, Thimm M, Hesse MD, et al. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain 2009;132:3011–3020. [DOI] [PubMed] [Google Scholar]
- 7.Pariente J, Loubinoux I, Carel C, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol 2001;50:718–729. [DOI] [PubMed] [Google Scholar]
- 8.Barbay S, Zoubina EV, Dancause N, et al. A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair 2006;20:455–458. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo EA, Duncan PW, Propst M, et al. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol 1988;23:94–97. [DOI] [PubMed] [Google Scholar]
- 10.Walker-Batson D, Smith P, Curtis S, et al. Amphetamine paired with physical therapy accelerates motor recovery after stroke-further evidence. Stroke 1995;26:2254–2259. [DOI] [PubMed] [Google Scholar]
- 11.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 1982;217:855–857. [DOI] [PubMed] [Google Scholar]
- 12.Platz T, Kim IH, Engel U, et al. Amphetamine fails to facilitate motor performance and to enhance motor recovery among stroke patients with mild arm paresis: interim analysis and termination of a double blind, randomised, placebo-controlled trial. Restor Neurol Neurosci 2005;23:271–280. [PubMed] [Google Scholar]
- 13.Goldstein LB. Amphetamine trials and tribulations. Stroke 2009;40:S133–S135. [DOI] [PubMed] [Google Scholar]
- 14.Stibick DL, Feeney DM. Enduring vulnerability to transient reinstatement of hemiplegia by prazosin after traumatic brain injury. J Neurotrauma 2001;18:303–312. [DOI] [PubMed] [Google Scholar]
- 15.Boyeson MG, Feeney DM. Intraventricular norepinephrine facilitates motor recovery following sensorimotor cortex injury. Pharmacol Biochem Behav 1990;35:497–501. [DOI] [PubMed] [Google Scholar]
- 16.Feeney DM, De Smet AM, Rai S. Noradrenergic modulation of hemiplegia: facilitation and maintenance of recovery. Restor Neurol Neurosci 2004;22:175–190. [PubMed] [Google Scholar]
- 17.Wong EHF, Sonders MS, Amara SG, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry 2000;47:818–829. [DOI] [PubMed] [Google Scholar]
- 18.Wang LE, Fink GR, Dafotakis M, Grefkes C. Noradrenergic stimulation and motor performance: Differential effects of reboxetine on movement kinematics and visuomotor abilities in healthy human subjects. Neuropsychologia 2009;47:1302–1312. [DOI] [PubMed] [Google Scholar]
- 19.Plewnia C, Hoppe J, Cohen LG, Gerloff C. Improved motor skill acquisition after selective stimulation of central norepinephrine. Neurology 2004;62:2124–2126. [DOI] [PubMed] [Google Scholar]
- 20.Zittel S, Weiller C, Liepert J. Reboxetine improves motor function in chronic stroke. J Neurol 2007;254:197–201. [DOI] [PubMed] [Google Scholar]
- 21.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 2003;42:33–84. [DOI] [PubMed] [Google Scholar]
- 22.Friston KJ. Imaging neuroscience: principles or maps? Proc Natl Acad Sci U S A 1998;95:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage 2003;19:1273–1302. [DOI] [PubMed] [Google Scholar]
- 24.Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008;63:236–246. [DOI] [PubMed] [Google Scholar]
- 25.Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 2000;37:273–82. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 27.Edwards DM, Pellizzoni C, Breuel HP, et al. Pharmacokinetics of reboxetine in healthy volunteers. Single oral doses, linearity and plasma protein binding. Biopharm Drug Dispos 1995;16:443–460. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 29.Crinion J, Ashburner J, Leff A, et al. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage 2007;37:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 2001;14:486–500. [DOI] [PubMed] [Google Scholar]
- 31.Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuroimage 2010;53:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage 2008;42:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephan KE, Penny WD, Daunizeau J, et al. Bayesian model selection for group studies. Neuroimage 2009;46:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 35.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6:448–450. [DOI] [PubMed] [Google Scholar]
- 36.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 37.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005;25:1325–1335. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein LB. Effects of bilateral and unilateral locus coeruleus lesions on beam-walking recovery after subsequent unilateral sensorimotor cortex suction-ablation in the rat. Restor Neurol Neurosci 1997;11:55–63. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein LB, Coviello A, Miller GD, Davis JN. Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restor Neurol Neurosci 1991;3:41–47. [DOI] [PubMed] [Google Scholar]
- 40.Johansson IM, Wester P, Hakova M, et al. Early and delayed induction of immediate early gene expression in a novel focal cerebral ischemia model in the rat. Eur J Neurosci 2000;12:3615–3625. [DOI] [PubMed] [Google Scholar]
- 41.Qü M, Mittmann T, Luhmann HJ, et al. Long-term changes of ionotropic glutamate and GABA receptors after unilateral permanent focal cerebral ischemia in the mouse brain. Neuroscience 1998;85:29–43. [DOI] [PubMed] [Google Scholar]
- 42.Calautti C, Leroy F, Guincestre JY, Baron JC. Dynamics of motor network overactivation after striatocapsular stroke: a longitudinal PET study using a fixed-performance paradigm. Stroke 2001;32:2534–2542. [DOI] [PubMed] [Google Scholar]
- 43.Marshall RS, Perera GM, Lazar RM, et al. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 2000;31:656–661. [DOI] [PubMed] [Google Scholar]
- 44.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci 2005;25:10167–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci 2005;25:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frost SB, Barbay S, Friel KM, et al. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol 2003;89:3205–3214. [DOI] [PubMed] [Google Scholar]
- 47.Shimazu H, Maier MA, Cerri G, et al. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci 2004;24:1200–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward NS, Newton JM, Swayne OB, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006;129:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton JM, Ward NS, Parker GJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain 2006;129:1844–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi MJ, Saito DN, Aramaki Y, et al. Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: a functional magnetic resonance imaging study. Cereb Cortex 2008;18:2932–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riecker A, Grüschel K, Ackermann H, et al. The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp 2010;31:1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grefkes C, Wang LE, Eickhoff SB, Fink GR. Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb Cortex 2010;20:783–797. [DOI] [PubMed] [Google Scholar]
- 53.Wu T, Chan P, Hallett M. Modifications of the interactions in the motor networks when a movement becomes automatic. J Physiol 2008;586:4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res 2006;175:544–555. [DOI] [PubMed] [Google Scholar]
- 55.Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J Neurophysiol 2008;99:1836–1845. [DOI] [PubMed] [Google Scholar]
- 56.Remy F, Wenderoth N, Lipkens K, Swinnen SP. Acquisition of a new bimanual coordination pattern modulates the cerebral activations elicited by an intrinsic pattern: an fMRI study. Cortex 2008;44:482–493. [DOI] [PubMed] [Google Scholar]
- 57.Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol 2009;66:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 2002;77:677–682. [DOI] [PubMed] [Google Scholar]
- 59.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996;6:342–353. [DOI] [PubMed] [Google Scholar]
- 60.Gaspar P, Berger B, Febvret A, et al. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 1989;279:249–271. [DOI] [PubMed] [Google Scholar]
- 61.Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: a fluorescence histochemical analysis. J Comp Neurol 1984;227:23–36. [DOI] [PubMed] [Google Scholar]
- 62.Morrison JH, Foote SL, O’Connor D, Bloom FE. Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-beta-hydroxylase immunohistochemistry. Brain Res Bull 1982;9:309–319. [DOI] [PubMed] [Google Scholar]
- 63.Gladstone DJ, Danells CJ, Armesto A, et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke 2006;37:179–185. [DOI] [PubMed] [Google Scholar]
- 64.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009;30:3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meintzschel F, Ziemann U. Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex 2006;16:1106–1115. [DOI] [PubMed] [Google Scholar]
- 66.Buetefisch CM, Davis BC, Sawaki L, et al. Modulation of use-dependent plasticity by D-amphetamine. Ann Neurol 2002;51:59–68. [DOI] [PubMed] [Google Scholar]
- 67.Pettigrew JD, Kasamatsu T. Local perfusion of noradrenaline maintains visual cortical plasticity. Nature 1978;271:761–763. [DOI] [PubMed] [Google Scholar]
- 68.Kasamatsu T, Pettigrew JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science 1976;194:206–209. [DOI] [PubMed] [Google Scholar]
- 69.Kirkwood A, Rozas C, Kirkwood J, et al. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci 1999;19:1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broecher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res 1992;573:27–36. [DOI] [PubMed] [Google Scholar]
- 71.Hasselmo ME, Linster C, Patil M, et al. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol 1997;77:3326–3339. [DOI] [PubMed] [Google Scholar]
- 72.Page ME, Lucki I. Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology 2002;27:237–247. [DOI] [PubMed] [Google Scholar]
- 73.Rogoz Z, Margas W, Skuza G, et al. Effect of repeated treatment with reboxetine on the central alpha 1-adrenergic and dopaminergic receptors. Pol J Pharmacol 2002;54:593–603. [PubMed] [Google Scholar]
- 74.Nakadate K, Matsukawa M, Okado N. Identification of adrenoceptor subtype-mediated changes in the density of synapses in the rat visual cortex. Neuroscience 2006;138:37–46. [DOI] [PubMed] [Google Scholar]
- 75.Treig T, Werner C, Sachse M, Hesse S. No benefit from D-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin Rehabil 2003;17:590–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.